Escolar Documentos
Profissional Documentos
Cultura Documentos
Loudon FM
Enviado por
corneliohd34Descrição original:
Título original
Direitos autorais
Formatos disponíveis
Compartilhar este documento
Compartilhar ou incorporar documento
Você considera este documento útil?
Este conteúdo é inapropriado?
Denunciar este documentoDireitos autorais:
Formatos disponíveis
Loudon FM
Enviado por
corneliohd34Direitos autorais:
Formatos disponíveis
00_BRCLoudonFM_pgs4-2.
qxd
11/20/08
12:51 PM
Page i
Organic Chemistry
F I F T H E D I T I O N
00_BRCLoudonFM_pgs4-2.qxd
11/20/08
12:51 PM
Page ii
00_BRCLoudonFM_pgs4-2.qxd
11/20/08
12:51 PM
Page iii
Organic Chemistry
F I F T H E D I T I O N
Marc Loudon
Purdue University
R O B E RT S A N D C O M PA N Y P U B L I S H E R S Greenwood Village, Colorado
00_BRCLoudonFM_pgs4-2.qxd
11/20/08
12:51 PM
Page iv
ROBERTS AND COMPANY PUBLISHERS 4950 South Yosemite Street, F2 #197 Greenwood Village, Colorado 80111 USA Internet: www.roberts-publishers.com Telephone: (303) 221-3325 Facsimile: (303) 221-3326 ORDER INFORMATION Telephone: (800) 351-1161 or (516) 422-4050 Facsimile: (516) 422-4097 Internet: www.roberts-publishers.com Publisher: Ben Roberts Copyeditor: John Murdzek Proofreader: Gunder Hefta Production Editor: Susan Riley at Side By Side Studios Interior Designer: Mark Ong at Side By Side Studios Cover Concept: Gregory Gatto Cover Rendering: Emiko-Rose Paul and Quade Paul at Echo Medical Media Cover Designer: Mark Ong at Side By Side Studios Compositor: Side By Side Studios Printer: RR Donnelley 2009 by Roberts and Company Publishers Reproduction or translation of any part of this work beyond that permitted by Section 107 or 108 of the 1976 United States Copyright Act without permission of the copyright owner is unlawful. Requests for permission or further information should be addressed to the Permissions Department at Roberts and Company Publishers. ISBN: 978-0-981-5194-3-2
Library of Congress Cataloging-in-Publication Data Loudon, G. Marc. Organic chemistry / Marc Loudon. -- 5th ed. p. cm. Summary: "Introduces organic chemistry through a mechanistic approach within a functional group framework. Contains 1,668 exercises--many of which are taken directly from the scientic literature-that encourage readers to analyze and synthesize chemical concepts. Includes modern topics such as alkene metathesis, Suzuki and Stille cross-coupling reactions, and examples drawn from contemporary medical practice."--Provided by the publisher. Includes index. ISBN 978-0-9815194-3-2 1. Chemistry, Organic--Textbooks. I. Title. QD251.3.L68 2009 547--dc22 2008046105 10 9 8 7 6 5 4 3 2 1
00_BRCLoudonFM_pgs4-2.qxd
11/20/08
12:51 PM
Page v
To Nysa, Seve, and Gray . . . . . . heres to our tomorrows
00_BRCLoudonFM_pgs4-2.qxd
11/20/08
12:51 PM
Page vi
00_BRCLoudonFM_pgs4-2.qxd
11/20/08
12:51 PM
Page vii
Contents
Preface Reviewers and Consultants About the Author xxxi xxxvi xxxix
CHEMICAL BONDING AND CHEMICAL STRUCTURE
1.1 Introduction
A. What Is Organic Chemistry? B. Emergence of Organic Chemistry C. Why Study Organic Chemistry?
1
1 1 1 2 3 3 3 5 9 13 13 14 20 22 23 23 25 28 29 32 32 36 37 37 40
1.2
Classical Theories of Chemical Bonding A. Electrons in Atoms B. The Ionic Bond C. The Covalent Bond D. The Polar Covalent Bond Structures of Covalent Compounds
A. Methods for Determining Molecular Geometry B. Prediction of Molecular Geometry
1.3
1.4 1.5 1.6
Resonance Structures Wave Nature of the Electron Electronic Structure of the Hydrogen Atom A. Orbitals, Quantum Numbers, and Energy B. Spatial Characteristics of Orbitals C. Summary: Atomic Orbitals of Hydrogen Electronic Structures of More Complex Atoms Another Look at the Covalent Bond: Molecular Orbitals A. Molecular Orbital Theory B. Molecular Orbital Theory and the Lewis Structure of H2 Hybrid Orbitals
A. Bonding in Methane B. Bonding in Ammonia
1.7 1.8
1.9
vii
00_BRCLoudonFM_pgs4-2.qxd
11/20/08
12:51 PM
Page viii
viii
CONTENTS
Key Ideas in Chapter 1 Additional Problems
42 43
ALKANES
2.1 2.2 2.3 Hydrocarbons Unbranched Alkanes Conformations of Alkanes
A. Conformation of Ethane B. Conformations of Butane
46
46 48 50 50 53 57 57 58 59 64 66 67 70 70 73 74 76 78 81 81 82 83 83
2.4
Constitutional Isomers and Nomenclature A. Isomers B. Organic Nomenclature C. Substitutive Nomenclature of Alkanes D. Highly Condensed Structures E. Classication of Carbon Substitution Cycloalkanes and Skeletal Structures Physical Properties of Alkanes A. Boiling Points B. Melting Points C. Other Physical Properties Combustion Occurrence and Use of Alkanes Functional Groups, Compound Classes, and the R Notation
A. Functional Groups and Compound Classes B. R Notation
2.5 2.6
2.7 2.8 2.9
Key Ideas in Chapter 2 Additional Problems
ACIDS AND BASES. THE CURVED-ARROW NOTATION
3.1 Lewis AcidBase Association Reactions
A. Electron-Decient Compounds B. Reactions of Electron-Decient Compounds with Lewis Bases C. The Curved-Arrow Notation for Lewis AcidBase Association and Dissociation Reactions
87
87 87 88
89
00_BRCLoudonFM_pgs4-2.qxd
11/20/08
12:51 PM
Page ix
CONTENTS
ix
3.2
Electron-Pair Displacement Reactions A. Donation of Electrons to Atoms That Are Not Electron-Decient B. The Curved-Arrow Notation for Electron-Pair Displacement Reactions Review of the Curved-Arrow Notation
A. Use of the Curved-Arrow Notation to Represent Reactions B. Use of the Curved-Arrow Notation to Derive Resonance Structures
90 90 91 94 94 94 96 96 98 101 103 104 106 108 108 110 111 116 117
3.3
3.4
BrnstedLowry Acids and Bases A. Denition of Brnsted Acids and Bases B. Nucleophiles, Electrophiles, and Leaving Groups C. Strengths of Brnsted Acids D. Strengths of Brnsted Bases E. Equilibria in AcidBase Reactions Free Energy and Chemical Equilibrium Relationship of Structure to Acidity A. The Element Effect B. The Charge Effect C. The Polar Effect Key Ideas in Chapter 3 Additional Problems
3.5 3.6
INTRODUCTION TO ALKENES. STRUCTURE AND REACTIVITY
4.1 Structure and Bonding in Alkenes A. Carbon Hybridization in Alkenes B. The p (Pi) Bond C. Double-Bond Stereoisomers Nomenclature of Alkenes
A. IUPAC Substitutive Nomenclature B. Nomenclature of Double-Bond Stereoisomers: The E,Z System
122
122 123 125 128 131 131 134 139 140 141 141 144 147 147 148 149
4.2
4.3 4.4 4.5
Unsaturation Number Physical Properties of Alkenes Relative Stabilities of Alkene Isomers A. Heats of Formation B. Relative Stabilities of Alkene Isomers Addition Reactions of Alkenes Addition of Hydrogen Halides to Alkenes A. Regioselectivity of Hydrogen Halide Addition B. Carbocation Intermediates in Hydrogen Halide Addition
4.6 4.7
00_BRCLoudonFM_pgs4-2.qxd
11/20/08
12:51 PM
Page x
CONTENTS
C. Structure and Stability of Carbocations D. Carbocation Rearrangement in Hydrogen Halide Addition
151 154
4.8
Reaction Rates A. The Transition State B. The Energy Barrier C. Multistep Reactions and the Rate-Limiting Step D. Hammonds Postulate Catalysis
A. Catalytic Hydrogenation of Alkenes B. Hydration of Alkenes C. Enzyme Catalysis
157 158 160 162 164 166 168 169 172 172 174
4.9
Key Ideas in Chapter 4 Additional Problems
ADDITION REACTIONS OF ALKENES
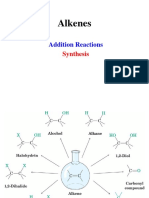
5.1 5.2 An Overview of Electrophilic Addition Reactions Reactions of Alkenes with Halogens A. Addition of Chlorine and Bromine B. Halohydrins Writing Organic Reactions Conversion of Alkenes into Alcohols A. OxymercurationReduction of Alkenes B. HydroborationOxidation of Alkenes C. Comparison of Methods for the Synthesis of Alcohols from Alkenes Ozonolysis of Alkenes Free-Radical Addition of Hydrogen Bromide to Alkenes A. The Peroxide Effect B. Free Radicals and the Fishhook Notation C. Free-Radical Chain Reactions D. Explanation of the Peroxide Effect E. Bond Dissociation Energies Polymers: Free-Radical Polymerization of Alkenes Alkenes in the Chemical Industry Key Ideas in Chapter 5 Additional Problems
178
178 181 181 183 186 187 187 190 194 196 200 200 201 202 207 211 214 216 219 220
5.3 5.4
5.5 5.6
5.7 5.8
00_BRCLoudonFM_pgs4-2.qxd
11/20/08
12:51 PM
Page xi
CONTENTS
xi
PRINCIPLES OF STEREOCHEMISTRY
6.1 Enantiomers, Chirality, and Symmetry
A. Enantiomers and Chirality B. Asymmetric Carbon and Stereocenters C. Chirality and Symmetry
226
226 226 229 229 231 234 235 235 238 239 241 242 246 249 251 253 253 255 257 259 263 263
6.2 6.3
Nomenclature of Enantiomers: The R,S System Physical Properties of Enantiomers: Optical Activity A. Polarized Light B. Optical Activity C. Optical Activities of Enantiomers Racemates Stereochemical Correlation Diastereomers Meso Compounds Enantiomeric Resolution Chiral Molecules without Asymmetric Atoms Conformational Stereoisomers A. Stereoisomers Interconverted by Internal Rotations B. Asymmetric Nitrogen: Amine Inversion Drawing Structures That Contain Three-Dimensional Information The Postulation of Tetrahedral Carbon Key Ideas in Chapter 6 Additional Problems
6.4 6.5 6.6 6.7 6.8 6.9 6.10
6.11 6.12
CYCLIC COMPOUNDS. STEREOCHEMISTRY OF REACTIONS
7.1 7.2 Relative Stabilities of the Monocyclic Alkanes Conformations of Cyclohexane
A. The Chair Conformation B. Interconversion of Chair Conformations C. Boat and Twist-Boat Conformations
268
268 269 269 273 274
00_BRCLoudonFM_pgs4-2.qxd
11/20/08
12:51 PM
Page xii
xii
CONTENTS
7.3 7.4
Monosubstituted Cyclohexanes. Conformational Analysis Disubstituted Cyclohexanes
A. CisTrans Isomerism in Disubstituted Cyclohexanes B. Conformational Analysis C. Use of Planar Structures for Cyclic Compounds D. Stereochemical Consequences of the Chair Interconversion
277 281 281 283 284 285 288 288 289 290 290 292 294 296 298 298 300 301 301 304 305 305 306 308 312 313 314 316
7.5
Cyclopentane, Cyclobutane, and Cyclopropane A. Cyclopentane B. Cyclobutane and Cyclopropane Bicyclic and Polycyclic Compounds
A. Classication and Nomenclature B. Cis and Trans Ring Fusion C. Trans-Cycloalkenes and Bredts Rule D. Steroids
7.6
7.7
Relative Reactivities of Stereoisomers A. Relative Reactivities of Enantiomers B. Relative Reactivities of Diastereomers Reactions That Form Stereoisomers
A. Reactions of Achiral Compounds That Give Enantiomeric Products B. Reactions That Give Diastereomeric Products
7.8
7.9
Stereochemistry of Chemical Reactions A. Stereochemistry of Addition Reactions B. Stereochemistry of Substitution Reactions C. Stereochemistry of Bromine Addition D. Stereochemistry of HydroborationOxidation E. Stereochemistry of Other Addition Reactions Key Ideas in Chapter 7 Additional Problems
INTRODUCTION TO ALKYL HALIDES, ALCOHOLS, ETHERS, THIOLS, AND SULFIDES
8.1 Nomenclature A. Nomenclature of Alkyl Halides B. Nomenclature of Alcohols and Thiols C. Nomenclature of Ethers and Suldes Structures Effect of Molecular Polarity and Hydrogen Bonding on Physical Properties A. Boiling Points of Ethers and Alkyl Halides
323
324 324 326 330 332 333 333
8.2 8.3
00_BRCLoudonFM_pgs4-2.qxd
11/20/08
12:51 PM
Page xiii
CONTENTS
xiii 335 336
B. Boiling Points of Alcohols C. Hydrogen Bonding
8.4
Solvents in Organic Chemistry A. Classication of Solvents B. Solubility Applications of Solubility and Solvation Principles
A. Cell Membranes and Drug Solubility B. Cation-Binding Molecules
339 339 340 346 346 351 355 356 358 358 359 361 361 362 364 364 365 368 371 372 373
8.5
8.6
Acidity of Alcohols and Thiols A. Formation of Alkoxides and Mercaptides B. Polar Effects on Alcohol Acidity C. Role of the Solvent in Alcohol Acidity Basicity of Alcohols and Ethers Grignard and Organolithium Reagents A. Formation of Grignard and Organolithium Reagents B. Protonolysis of Grignard and Organolithium Reagents Industrial Preparation and Use of Alkyl Halides, Alcohols, and Ethers A. Free-Radical Halogenation of Alkanes B. Uses of Halogen-Containing Compounds C. Production and Use of Alcohols and Ethers D. Safety Hazards of Ethers Key Ideas in Chapter 8 Additional Problems
8.7 8.8
8.9
THE CHEMISTRY OF ALKYL HALIDES
9.1 An Overview of Nucleophilic Substitution and b-Elimination Reactions A. Nucleophilic Substitution Reactions B. b-Elimination Reactions C. Competition between Nucleophilic Substitution and b-Elimination Reactions Equilibria in Nucleophilic Substitution Reactions Reaction Rates
A. Denition of Reaction Rate B. The Rate Law C. Relationship of the Rate Constant to the Standard Free Energy of Activation
377
377 377 378 380 381 382 382 383 384 386 386
9.2 9.3
9.4
The SN2 Reaction A. Rate Law and Mechanism of the SN2 Reaction
00_BRCLoudonFM_pgs4-2.qxd
11/20/08
12:51 PM
Page xiv
xiv
CONTENTS
B. Comparison of the Rates of SN2 Reactions and Brnsted AcidBase Reactions C. Stereochemistry of the SN2 Reaction D. Effect of Alkyl Halide Structure on the SN2 Reaction E. Nucleophilicity in the SN2 Reaction F. Leaving-Group Effects in the SN2 Reaction G. Summary of the SN2 Reaction
388 388 390 392 398 399
9.5
The E2 Reaction A. Rate Law and Mechanism of the E2 Reaction B. Why the E2 Reaction Is Concerted C. Leaving-Group Effects on the E2 Reaction D. Deuterium Isotope Effects in the E2 Reaction E. Stereochemistry of the E2 Reaction F. Regioselectivity of the E2 Reaction G. Competition between the E2 and SN2 Reactions: A Closer Look H. Summary of the E2 Reaction The SN1 and E1 Reactions
A. Rate Law and Mechanism of SN1 and E1 Reactions B. Rate-Limiting and Product-Determining Steps C. Reactivity and Product Distributions in SN1E1 Reactions D. Stereochemistry of the SN1 Reaction E. Summary of the SN1 and E1 Reactions
400 400 400 402 402 404 406 407 411 412 412 414 416 418 420 420 424 424 426 428 429
9.6
9.7 9.8
Summary of Substitution and Elimination Reactions of Alkyl Halides Carbenes and Carbenoids A. a-Elimination Reactions B. The SimmonsSmith Reaction Key Ideas in Chapter 9 Additional Problems
10
THE CHEMISTRY OF ALCOHOLS AND THIOLS
10.1 10.2 10.3 Dehydration of Alcohols Reactions of Alcohols with Hydrogen Halides Sulfonate and Inorganic Ester Derivatives of Alcohols
A. Sulfonate Ester Derivatives of Alcohols B. Alkylating Agents C. Ester Derivatives of Strong Inorganic Acids D. Reactions of Alcohols with Thionyl Chloride and Phosphorus Tribromide
436
436 440 443 443 447 448 449 450
10.4
Conversion of Alcohols into Alkyl Halides: Summary
00_BRCLoudonFM_pgs4-2.qxd
11/20/08
12:51 PM
Page xv
CONTENTS
xv
10.5
Oxidation and Reduction in Organic Chemistry A. Half-Reactions and Oxidation Numbers B. Oxidizing and Reducing Agents Oxidation of Alcohols
A. Oxidation to Aldehydes and Ketones B. Oxidation to Carboxylic Acids
452 452 456 459 459 461 462 465 465 469 471 474 474 476 477
10.6
10.7 10.8
Biological Oxidation of Ethanol Chemical and Stereochemical Group Relationships
A. Chemical Equivalence and Nonequivalence B. Stereochemistry of the Alcohol Dehydrogenase Reaction
10.9 10.10 10.11
Oxidation of Thiols Synthesis of Alcohols Design of Organic Synthesis Key Ideas in Chapter 10 Additional Problems
11
THE CHEMISTRY OF ETHERS, EPOXIDES, GLYCOLS, AND SULFIDES
11.1 Synthesis of Ethers and Suldes A. Williamson Ether Synthesis B. AlkoxymercurationReduction of Alkenes C. Ethers from Alcohol Dehydration and Alkene Addition Synthesis of Epoxides
A. Oxidation of Alkenes with Peroxycarboxylic Acids B. Cyclization of Halohydrins
482
482 482 484 485 488 488 491 492 495 495 497 500 503 503 506 508 508 509
11.2
11.3 11.4
Cleavage of Ethers Nucleophilic Substitution Reactions of Epoxides
A. Ring-Opening Reactions under Basic Conditions B. Ring-Opening Reactions under Acidic Conditions C. Reaction of Epoxides with Organometallic Reagents
11.5
Preparation and Oxidative Cleavage of Glycols A. Preparation of Glycols B. Oxidative Cleavage of Glycols Oxonium and Sulfonium Salts
A. Reactions of Oxonium and Sulfonium Salts B. S-Adenosylmethionine: Natures Methylating Agent
11.6
00_BRCLoudonFM_pgs4-2.qxd
11/20/08
12:51 PM
Page xvi
xvi
CONTENTS
11.7
Intramolecular Reactions and the Proximity Effect A. Neighboring-Group Participation B. The Proximity Effect and Effective Molarity C. Stereochemical Consequences of Neighboring-Group Participation Oxidation of Ethers and Suldes The Three Fundamental Operations of Organic Synthesis Synthesis of Enantiomerically Pure Compounds: Asymmetric Epoxidation Key Ideas in Chapter 11 Additional Problems
510 510 513 516 518 520 522 527 528
11.8 11.9 11.10
12
INTRODUCTION TO SPECTROSCOPY. INFRARED SPECTROSCOPY AND MASS SPECTROMETRY
12.1 Introduction to Spectroscopy
A. Electromagnetic Radiation B. Absorption Spectroscopy
536
536 536 538 540 540 542 544 545 548 552 552 552 553 556 557 558 558 560 563 566 569 571 571
12.2
Infrared Spectroscopy A. The Infrared Spectrum B. Physical Basis of IR Spectroscopy Infrared Absorption and Chemical Structure
A. Factors That Determine IR Absorption Position B. Factors That Determine IR Absorption Intensity
12.3
12.4
Functional-Group Infrared Absorptions A. IR Spectra of Alkanes B. IR Spectra of Alkyl Halides C. IR Spectra of Alkenes D. IR Spectra of Alcohols and Ethers Obtaining an Infrared Spectrum Introduction to Mass Spectrometry A. Electron-Impact Mass Spectra B. Isotopic Peaks C. Fragmentation D. The Molecular Ion. Chemical-Ionization Mass Spectra E. The Mass Spectrometer Key Ideas in Chapter 12 Additional Problems
12.5 12.6
00_BRCLoudonFM_pgs4-2.qxd
11/20/08
12:51 PM
Page xvii
CONTENTS
xvii
13
NUCLEAR MAGNETIC RESONANCE SPECTROSCOPY
13.1 13.2 13.3 An Overview of Proton NMR Spectroscopy Physical Basis of NMR Spectroscopy The NMR Spectrum: Chemical Shift and Integral A. Chemical Shift B. Chemical Shift Scales C. Relationship of Chemical Shift to Structure D. The Number of Absorptions in an NMR Spectrum E. Counting Protons with the Integral F. Using the Chemical Shift and Integral to Determine Unknown Structures The NMR Spectrum: SpinSpin Splitting A. The n | 1 Splitting Rule B. Why Splitting Occurs C. Solving Unknown Structures with NMR Spectra Involving Splitting Complex NMR Spectra
A. Multiplicative Splitting B. Breakdown of the n | 1 Rule
578
578 581 583 583 585 586 589 592 593 595 596 599 601 603 603 607 611 612 612 614 616 616 619 622 629 632 634 635 636
13.4
13.5
13.6 13.7
Use of Deuterium in Proton NMR Characteristic Functional-Group NMR Absorptions A. NMR Spectra of Alkenes B. NMR Spectra of Alkanes and Cycloalkanes C. NMR Spectra of Alkyl Halides and Ethers D. NMR Spectra of Alcohols NMR Spectroscopy of Dynamic Systems Carbon NMR Solving Structure Problems with Spectroscopy The NMR Spectrometer Other Uses of NMR Key Ideas in Chapter 13 Additional Problems
13.8 13.9 13.10 13.11 13.12
14
THE CHEMISTRY OF ALKYNES
14.1 14.2 Nomenclature of Alkynes Structure and Bonding in Alkynes
644
644 646
00_BRCLoudonFM_pgs4-2.qxd
11/20/08
12:51 PM
Page xviii
xviii
CONTENTS
14.3
Physical Properties of Alkynes A. Boiling Points and Solubilities B. IR Spectroscopy of Alkynes C. NMR Spectroscopy of Alkynes Introduction to Addition Reactions of the Triple Bond Conversion of Alkynes into Aldehydes and Ketones A. Hydration of Alkynes B. HydroborationOxidation of Alkynes Reduction of Alkynes
A. Catalytic Hydrogenation of Alkynes B. Reduction of Alkynes with Sodium in Liquid Ammonia
649 649 649 650 652 654 654 657 659 659 660 662 662 665 666 668 670 671 671
14.4 14.5
14.6
14.7
Acidity of 1-Alkynes A. Acetylenic Anions B. Acetylenic Anions as Nucleophiles Organic Synthesis Using Alkynes Pheromones Occurrence and Use of Alkynes Key Ideas in Chapter 14 Additional Problems
14.8 14.9 14.10
15
DIENES, RESONANCE, AND AROMATICITY
15.1 Structure and Stability of Dienes
A. Stability of Conjugated Dienes. Molecular Orbitals B. Structure of Conjugated Dienes C. Structure and Stability of Cumulated Dienes
676
677 677 680 682 684 684 686 687 690 690 694 696 700 700 702 705
15.2
UltravioletVisible Spectroscopy A. The UVVis Spectrum B. Physical Basis of UVVis Spectroscopy C. UVVis Spectroscopy of Conjugated Alkenes The DielsAlder Reaction
A. Reaction of Conjugated Dienes with Alkenes B. Effect of Diene Conformation on the DielsAlder Reaction C. Stereochemistry of the DielsAlder Reaction
15.3
15.4
Addition of Hydrogen Halides to Conjugated Dienes A. 1,2- and 1,4-Additions B. Allylic Carbocations. The Connection between Resonance and Stability C. Kinetic and Thermodynamic Control
00_BRCLoudonFM_pgs4-2.qxd
11/20/08
12:51 PM
Page xix
CONTENTS
xix
15.5 15.6
Diene Polymers Resonance
A. Drawing Resonance Structures B. Relative Importance of Resonance Structures C. Use of Resonance Structures
708 709 710 711 714 716 717 718 721 721 728 730 731
15.7
Introduction to Aromatic Compounds A. Benzene, a Puzzling Alkene B. Structure of Benzene C. Stability of Benzene D. Aromaticity and the Hckel 4n | 2 Rule E. Antiaromatic Compounds Key Ideas in Chapter 15 Additional Problems
16
THE CHEMISTRY OF BENZENE AND ITS DERIVATIVES
16.1 16.2 16.3 Nomenclature of Benzene Derivatives Physical Properties of Benzene Derivatives Spectroscopy of Benzene Derivatives A. IR Spectroscopy B. NMR Spectroscopy C. 13C NMR Spectroscopy D. UV Spectroscopy Electrophilic Aromatic Substitution Reactions of Benzene
A. Halogenation of Benzene B. Electrophilic Aromatic Substitution C. Nitration of Benzene D. Sulfonation of Benzene E. FriedelCrafts Alkylation of Benzene F. FriedelCrafts Acylation of Benzene
740
740 743 743 743 744 748 748 750 751 753 754 755 756 759 762 762 768 772 776 777 780 780
16.4
16.5
Electrophilic Aromatic Substitution Reactions of Substituted Benzenes A. Directing Effects of Substituents B. Activating and Deactivating Effects of Substituents C. Use of Electrophilic Aromatic Substitution in Organic Synthesis Hydrogenation of Benzene Derivatives Source and Industrial Use of Aromatic Hydrocarbons Key Ideas in Chapter 16 Additional Problems
16.6 16.7
00_BRCLoudonFM_pgs4-2.qxd
11/20/08
12:51 PM
Page xx
xx
CONTENTS
17
ALLYLIC AND BENZYLIC REACTIVITY
17.1 17.2 17.3 Reactions Involving Allylic and Benzylic Carbocations Reactions Involving Allylic and Benzylic Radicals Reactions Involving Allylic and Benzylic Anions A. Allylic Grignard Reagents B. E2 Eliminations Involving Allylic or Benzylic Hydrogens Allylic and Benzylic SN2 Reactions Allylic and Benzylic Oxidation A. Oxidation of Allylic and Benzylic Alcohols B. Benzylic Oxidation of Alkylbenzenes Terpenes A. The Isoprene Rule B. Biosynthesis of Terpenes Key Ideas in Chapter 17 Additional Problems
788
789 793 798 799 801 802 803 803 805 807 807 810 813 814
17.4 17.5
17.6
18
THE CHEMISTRY OF ARYL HALIDES, VINYLIC HALIDES, AND PHENOLS. TRANSITION-METAL CATALYSIS 822
18.1 18.2 18.3 18.4 18.5 Lack of Reactivity of Vinylic and Aryl Halides under SN2 Conditions Elimination Reactions of Vinylic Halides Lack of Reactivity of Vinylic and Aryl Halides under SN1 Conditions Nucleophilic Aromatic Substitution Reactions of Aryl Halides Introduction to Transition-Metal Catalyzed Reactions A. Transition Metals and Their Complexes B. Oxidation State C. The dn Notation D. Electron Counting: The 16- and 18-Electron Rules E. Fundamental Reactions of Transition-Metal Complexes Examples of Transition-Metal-Catalyzed Reactions
A. The Heck Reaction B. The Suzuki Coupling
823 825 826 828 831 832 835 836 836 839 845 845 848
18.6
00_BRCLoudonFM_pgs4-2.qxd
11/20/08
12:51 PM
Page xxi
CONTENTS
xxi 852 857
C. Alkene Metathesis D. Other Examples of Transition-Metal-Catalyzed Reactions
18.7
Acidity of Phenols A. Resonance and Polar Effects on the Acidity of Phenols B. Formation and Use of Phenoxides Oxidation of Phenols to Quinones Electrophilic Aromatic Substitution Reactions of Phenols Reactivity of the ArylOxygen Bond
A. Lack of Reactivity of the ArylOxygen Bond in SN1 and SN2 Reactions B. Substitution at the ArylOxygen Bond: The Stille Reaction
858 858 861 862 867 870 870 872 874 875 876
18.8 18.9 18.10
18.11
Industrial Preparation and Use of Phenol Key Ideas in Chapter 18 Additional Problems
19
THE CHEMISTRY OF ALDEHYDES AND KETONES. CARBONYL-ADDITION REACTIONS
19.1 Nomenclature of Aldehydes and Ketones A. Common Nomenclature B. Substitutive Nomenclature Physical Properties of Aldehydes and Ketones Spectroscopy of Aldehydes and Ketones A. IR Spectroscopy B. Proton NMR Spectroscopy C. Carbon NMR Spectroscopy D. UV Spectroscopy E. Mass Spectrometry Synthesis of Aldehydes and Ketones Introduction to Aldehyde and Ketone Reactions Basicity of Aldehydes and Ketones Reversible Addition Reactions of Aldehydes and Ketones A. Mechanisms of Carbonyl-Addition Reactions B. Equilibria in Carbonyl-Addition Reactions C. Rates of Carbonyl-Addition Reactions Reduction of Aldehydes and Ketones to Alcohols
888
890 890 892 894 895 895 897 898 899 901 903 903 904 907 907 910 913 914
19.2 19.3
19.4 19.5 19.6 19.7
19.8
00_BRCLoudonFM_pgs4-2.qxd
11/20/08
12:51 PM
Page xxii
xxii
CONTENTS
19.9 19.10
Reactions of Aldehydes and Ketones with Grignard and Related Reagents Acetals and Their Use as Protecting Groups
A. Preparation and Hydrolysis of Acetals B. Protecting Groups
918 921 921 925 926
926 929
19.11
Reactions of Aldehydes and Ketones with Amines A. Reaction with Primary Amines and Other Monosubstituted Derivatives of Ammonia B. Reaction with Secondary Amines Reduction of Carbonyl Groups to Methylene Groups The Wittig Alkene Synthesis Oxidation of Aldehydes to Carboxylic Acids Manufacture and Use of Aldehydes and Ketones Key Ideas in Chapter 19 Additional Problems
19.12 19.13 19.14 19.15
931 933 936 938 939 940
20
THE CHEMISTRY OF CARBOXYLIC ACIDS
20.1 Nomenclature of Carboxylic Acids A. Common Nomenclature B. Substitutive Nomenclature Structure and Physical Properties of Carboxylic Acids Spectroscopy of Carboxylic Acids A. IR Spectroscopy B. NMR Spectroscopy AcidBase Properties of Carboxylic Acids
A. Acidity of Carboxylic and Sulfonic Acids B. Basicity of Carboxylic Acids
948
948 948 951 953 955 955 955 957 957 960 960 963 964 965 965 968
20.2 20.3
20.4
20.5 20.6 20.7 20.8
Fatty Acids, Soaps, and Detergents Synthesis of Carboxylic Acids Introduction to Carboxylic Acid Reactions Conversion of Carboxylic Acids into Esters
A. Acid-Catalyzed Esterication B. Esterication by Alkylation
00_BRCLoudonFM_pgs4-2.qxd
11/20/08
12:51 PM
Page xxiii
CONTENTS
xxiii
20.9
Conversion of Carboxylic Acids into Acid Chlorides and Anhydrides A. Synthesis of Acid Chlorides B. Synthesis of Anhydrides Reduction of Carboxylic Acids to Primary Alcohols Decarboxylation of Carboxylic Acids Key Ideas in Chapter 20 Additional Problems
970 970 972 974 976 978 979
20.10 20.11
21
THE CHEMISTRY OF CARBOXYLIC ACID DERIVATIVES
21.1 Nomenclature and Classication of Carboxylic Acid Derivatives A. Esters and Lactones B. Acid Halides C. Anhydrides D. Nitriles E. Amides, Lactams, and Imides F. Nomenclature of Substituent Groups G. Carbonic Acid Derivatives Structures of Carboxylic Acid Derivatives Physical Properties of Carboxylic Acid Derivatives A. Esters B. Anhydrides and Acid Chlorides C. Nitriles D. Amides Spectroscopy of Carboxylic Acid Derivatives
A. IR Spectroscopy B. NMR Spectroscopy
986
986 986 988 988 989 989 991 991 992 994 994 994 995 995 996 996 997 1000 1003 1004 1004 1008 1009 1011 1011
21.2 21.3
21.4
21.5 21.6 21.7
Basicity of Carboxylic Acid Derivatives Introduction to the Reactions of Carboxylic Acid Derivatives Hydrolysis of Carboxylic Acid Derivatives A. Hydrolysis of Esters B. Hydrolysis of Amides C. Hydrolysis of Nitriles D. Hydrolysis of Acid Chlorides and Anhydrides E. Mechanisms and Reactivity in Nucleophilic Acyl Substitution Reactions
00_BRCLoudonFM_pgs4-2.qxd
11/20/08
12:51 PM
Page xxiv
xxiv
CONTENTS
21.8
Reactions of Carboxylic Acid Derivatives with Nucleophiles A. Reactions of Acid Chlorides with Nucleophiles B. Reactions of Anhydrides with Nucleophiles C. Reactions of Esters with Nucleophiles Reduction of Carboxylic Acid Derivatives
A. Reduction of Esters to Primary Alcohols B. Reduction of Amides to Amines C. Reduction of Nitriles to Primary Amines D. Reduction of Acid Chlorides to Aldehydes E. Relative Reactivities of Carbonyl Compounds
1016 1016 1019 1020 1022 1022 1023 1025 1027 1028 1029 1029 1031 1032 1034 1034 1036 1037 1038
21.9
21.10
Reactions of Carboxylic Acid Derivatives with Organometallic Reagents A. Reaction of Esters with Grignard Reagents B. Reaction of Acid Chlorides with Lithium Dialkylcuprates Synthesis of Carboxylic Acid Derivatives Use and Occurrence of Carboxylic Acids and Their Derivatives A. Nylon and Polyesters B. Waxes, Fats, and Phospholipids Key Ideas in Chapter 21 Additional Problems
21.11 21.12
22
THE CHEMISTRY OF ENOLATE IONS, ENOLS, AND a,b-UNSATURATED CARBONYL COMPOUNDS
22.1 Acidity of Carbonyl Compounds A. Formation of Enolate Anions B. Introduction to Reactions of Enolate Ions Enolization of Carbonyl Compounds a-Halogenation of Carbonyl Compounds A. Acid-Catalyzed a-Halogenation B. Halogenation of Aldehydes and Ketones in Base: The Haloform Reaction C. a-Bromination of Carboxylic Acids D. Reactions of a-Halo Carbonyl Compounds Aldol Addition and Aldol Condensation
A. Base-Catalyzed Aldol Reactions B. Acid-Catalyzed Aldol Condensation C. Special Types of Aldol Reactions D. Synthesis with the Aldol Condensation
1047
1048 1048 1051 1053 1057 1057 1059 1060 1062 1063 1063 1066 1067 1070
22.2 22.3
22.4
00_BRCLoudonFM_pgs4-2.qxd
11/20/08
12:51 PM
Page xxv
CONTENTS
xxv
22.5
Condensation Reactions Involving Ester Enolate Ions A. Claisen Condensation B. Dieckmann Condensation C. Crossed Claisen Condensation D. Synthesis with the Claisen Condensation Biosynthesis of Fatty Acids Alkylation of Ester Enolate Ions A. Malonic Ester Synthesis B. Direct Alkylation of Enolate Ions Derived from Monoesters C. Acetoacetic Ester Synthesis Conjugate-Addition Reactions
A. Conjugate Addition to a,b-Unsaturated Carbonyl Compounds B. Conjugate Addition Reactions versus Carbonyl-Group Reactions C. Conjugate Addition of Enolate Ions
1072 1072 1076 1076 1078 1081 1084 1084 1086 1089 1092 1092 1095 1097 1100 1101 1101 1102 1103 1105 1106
22.6 22.7
22.8
22.9 22.10
Reduction of a,b-Unsaturated Carbonyl Compounds Reactions of a,b-Unsaturated Carbonyl Compounds with Organometallic Reagents A. Addition of Organolithium Reagents to the Carbonyl Group B. Conjugate Addition of Lithium Dialkylcuprate Reagents Organic Synthesis with Conjugate-Addition Reactions Key Ideas in Chapter 22 Additional Problems
22.11
23
THE CHEMISTRY OF AMINES
23.1 Nomenclature of Amines A. Common Nomenclature B. Substitutive Nomenclature Structure of Amines Physical Properties of Amines Spectroscopy of Amines
A. IR Spectroscopy B. NMR Spectroscopy
1116
1117 1117 1117 1119 1120 1121 1121 1121 1122 1122 1123 1127 1128 1129
23.2 23.3 23.4
23.5
Basicity and Acidity of Amines A. Basicity of Amines B. Substituent Effects on Amine Basicity C. Separations Using Amine Basicity D. Acidity of Amines E. Summary of Acidity and Basicity
00_BRCLoudonFM_pgs4-2.qxd
11/20/08
12:51 PM
Page xxvi
xxvi
CONTENTS
23.6 23.7
Quaternary Ammonium and Phosphonium Salts Alkylation and Acylation Reactions of Amines
A. Direct Alkylation of Amines B. Reductive Amination C. Acylation of Amines
1129 1131 1131 1133 1135 1136 1138 1139 1139 1142 1144 1145 1145 1146 1147 1150 1154 1155 1155 1155 1157 1158
23.8 23.9 23.10
Hofmann Elimination of Quaternary Ammonium Hydroxides Aromatic Substitution Reactions of Aniline Derivatives Diazotization; Reactions of Diazonium Ions A. Formation and Substitution Reactions of Diazonium Salts B. Aromatic Substitution with Diazonium Ions C. Reactions of Secondary and Tertiary Amines with Nitrous Acid Synthesis of Amines
A. Gabriel Synthesis of Primary Amines B. Reduction of Nitro Compounds C. Amination of Aryl Halides and Aryl Triates D. Curtius and Hofmann Rearrangements E. Synthesis of Amines: Summary
23.11
23.12
Use and Occurrence of Amines A. Industrial Use of Amines and Ammonia B. Naturally Occurring Amines Key Ideas in Chapter 23 Additional Problems
24
CARBOHYDRATES
24.1 24.2 24.3 Classication and Properties of Carbohydrates Fischer Projections Structures of the Monosaccharides A. Stereochemistry and Conguration B. Cyclic Structures of the Monosaccharides Mutarotation of Carbohydrates Base-Catalyzed Isomerization of Aldoses and Ketoses Glycosides Ether and Ester Derivatives of Carbohydrates Oxidation and Reduction Reactions of Carbohydrates
A. Oxidation to Aldonic Acids
1166
1167 1168 1173 1173 1178 1183 1186 1188 1191 1193 1194
24.4 24.5 24.6 24.7 24.8
00_BRCLoudonFM_pgs4-2.qxd
11/20/08
12:51 PM
Page xxvii
CONTENTS
xxvii 1195 1196 1197
B. Oxidation to Aldaric Acids C. Periodate Oxidation D. Reduction to Alditols
24.9 24.10
KilianiFischer Synthesis Proof of Glucose Stereochemistry
A. Which Diastereomer? The Fischer Proof B. Which Enantiomer? The Absolute Conguration of D-(+)-Glucose
1198 1199 1199 1203 1205 1205 1209 1212 1213
24.11
Disaccharides and Polysaccharides A. Disaccharides B. Polysaccharides Key Ideas in Chapter 24 Additional Problems
25
THE CHEMISTRY OF THE AROMATIC HETEROCYCLES
25.1 Nomenclature and Structure of the Aromatic Heterocycles A. Nomenclature B. Structure and Aromaticity Basicity and Acidity of the Nitrogen Heterocycles
A. Basicity of the Nitrogen Heterocycles B. Acidity of Pyrrole and Indole
1220
1220 1220 1221 1224 1224 1226 1226 1226 1229 1230 1231 1231 1234 1238 1239 1240 1245 1245 1248 1253 1255 1257 1258
25.2
25.3
The Chemistry of Furan, Pyrrole, and Thiophene A. Electrophilic Aromatic Substitution B. Addition Reactions of Furan C. Side-Chain Reactions The Chemistry of Pyridine
A. Electrophilic Aromatic Substitution B. Nucleophilic Aromatic Substitution C. N-Alkylpyridinium Salts and Their Reactions D. Side-Chain Reactions of Pyridine Derivatives E. Pyridinium Ions in Biology: Pyridoxal Phosphate
25.4
25.5
Nucleosides, Nucleotides, and Nucleic Acids A. Nucleosides and Nucleotides B. The Structures of DNA and RNA C. DNA Modication and Chemical Carcinogenesis Other Biologically Important Heterocyclic Compounds Key Ideas in Chapter 25 Additional Problems
25.6
00_BRCLoudonFM_pgs4-2.qxd
11/20/08
12:51 PM
Page xxviii
xxviii
CONTENTS
26
AMINO ACIDS, PEPTIDES, AND PROTEINS
26.1 Nomenclature of Amino Acids and Peptides
A. Nomenclature of Amino Acids B. Nomenclature of Peptides
1265
1266 1266 1267 1270 1271 1271 1273 1277 1279 1279 1279 1280 1281 1282 1283 1292 1292 1295 1296 1299 1303 1305 1305 1308 1308 1310 1315 1315 1318 1323 1324
26.2 26.3
Stereochemistry of the a-Amino Acids Acid-Base Properties of Amino Acids and Peptides A. Zwitterionic Structures of Amino Acids and Peptides B. Isoelectric Points of Amino Acids and Peptides C. Separations of Amino Acids and Peptides Using AcidBase Properties Synthesis and Enantiomeric Resolution of a-Amino Acids A. Alkylation of Ammonia B. Alkylation of Aminomalonate Derivatives C. Strecker Synthesis D. Enantiomeric Resolution of a-Amino Acids Acylation and Esterication Reactions of Amino Acids Solid-Phase Peptide Synthesis Hydrolysis of Peptides A. Complete Hydrolysis and Amino Acid Analysis B. Enzyme-Catalyzed Peptide Hydrolysis Primary Structure of Peptides and Proteins A. Peptide Sequencing by Mass Spectrometry B. Peptide Sequencing by the Edman Degradation C. Protein Sequencing D. Posttranslational Modication of Proteins Higher-Order Structures of Proteins
A. Secondary Structure B. Tertiary and Quaternary Structure
26.4
26.5 26.6 26.7
26.8
26.9
26.10
Enzymes: Biological Catalysts A. The Catalytic Action of Enzymes B. Enzymes as Drug Targets: Enzyme Inhibition Key Ideas in Chapter 26 Additional Problems
27
PERICYCLIC REACTIONS
27.1 Molecular Orbitals of Conjugated p-Electron Systems A. Molecular Orbitals of Conjugated Alkenes
1333
1336 1336
00_BRCLoudonFM_pgs4-2.qxd
11/20/08
12:51 PM
Page xxix
CONTENTS
xxix 1340 1343
B. Molecular Orbitals of Conjugated Ions and Radicals C. Excited States
27.2
Electrocyclic Reactions A. Ground-State (Thermal) Electrocyclic Reactions B. Excited-State (Photochemical) Electrocyclic Reactions C. Selection Rules and Microscopic Reversibility Cycloaddition Reactions Thermal Sigmatropic Reactions A. Classication and Stereochemistry B. Thermal [3,3] Sigmatropic Reactions C. Summary: Selection Rules for Thermal Sigmatropic Reactions Fluxional Molecules Biological Pericyclic Reactions: The Formation of Vitamin D Key Ideas in Chapter 27 Additional Problems
1343 1343 1346 1347 1349 1353 1353 1360 1362 1364 1365 1367 1368
27.3 27.4
27.5 27.6
APPENDICES
Appendix I. Appendix II. Appendix III. Substitutive Nomenclature of Organic Compounds Infrared Absorptions of Organic Compounds Proton NMR Chemical Shifts in Organic Compounds A. Protons within Functional Groups B. Protons Adjacent to Functional Groups
13
A-1
A-1 A-2 A-5 A-5 A-5 A-7 A-7 A-7 A-8 A-8 A-9 A-9 A-9 A-9 A-10 A-10 A-10 A-10 A-10
APPENDIX IV.
C NMR Chemical Shifts in Organic Compounds
A. Chemical Shifts of Carbons within Functional Groups B. Chemical Shifts of Carbons Adjacent to Functional Groups
APPENDIX V.
Summary of Synthetic Methods A. Synthesis of Alkanes and Aromatic Hydrocarbons B. Synthesis of Alkenes C. Synthesis of Alkynes D. Synthesis of Alkyl, Aryl, and Vinylic Halides E. Synthesis of Grignard Reagents and Related Organometallic Compounds F. Synthesis of Alcohols and Phenols G. Synthesis of Glycols H. Synthesis of Ethers, Acetals, and Suldes I. Synthesis of Epoxides J. Synthesis of Disuldes
00_BRCLoudonFM_pgs4-2.qxd
11/20/08
12:51 PM
Page xxx
xxx
CONTENTS
K. Synthesis of Aldehydes L. Synthesis of Ketones M.Synthesis of Sulfoxides and Sulfones N. Synthesis of Carboxylic and Sulfonic Acids O. Synthesis of Esters P. Synthesis of Anhydrides Q. Synthesis of Acid Chlorides R. Synthesis of Amides S. Synthesis of Nitriles T. Synthesis of Amines U. Synthesis of Nitro Compounds
A-11 A-11 A-11 A-11 A-12 A-12 A-12 A-12 A-12 A-12 A-13
APPENDIX VI. APPENDIX VII.
Reactions Used to Form CarbonCarbon Bonds Typical Acidities and Basicities of Organic Functional Groups
A. Acidities of Groups That Ionize to Give Anionic Conjugate Bases B. Basicities of Groups That Protonate to Give Cationic Conjugate Acids
A-13 A-14 A-14 A-15 C-1 I-1
Credits Index
00_BRCLoudonFM_pgs4-2.qxd
11/20/08
12:51 PM
Page xxxi
Preface
A PREVIEW OF THE FIFTH EDITION
I believe, and research in chemical education shows, that students who make the effort to learn but still have trouble in organic chemistry are in many cases trying to memorize their way through the subject. One of the keys to students success, then, is to provide them with help in relating one part of the subject to the nextto help them see how various reactions that seem very different are tied together by certain fundamentals. An overarching goal of my text is to help students achieve a relational understanding of organic chemistry. Here are some of the ways that I have tried to help students meet this goal.
Use of an AcidBase Framework Is a Key to Understanding Mechanisms
Although I have organized Organic Chemistry 5th Edition by functional group, I have used mechanistic reasoning to help students understand the why of reactions. Mechanisms alone, however, do not provide the relational understanding that students need. Left to their own devices, many students view mechanisms as something else to memorize, and they are bafed by the curved-arrow notation. I believe passionately that an understanding of acidbase chemistry is the key that can unlock the door to a mechanistic understanding of much organic chemistry. In Organic Chemistry 5th Edition, I use both Lewis acids and bases and Brnsted acids and bases as the foundations for mechanistic reasoning. Although students have memorized the appropriate denitions in general chemistry, few have developed real insight about the implications of these concepts for a broader range of chemistry. I have dedicated Chapter 3 to these fundamental acidbase concepts. The terms nucleophile, electrophile, and leaving group then spring easily from Lewis and Brnsted acidbase concepts, and the curvedarrow notation makes sense. I have provided a substantial number of drill problems to test how well students have mastered these principles. I have reinforced these ideas repeatedly with each new reaction type. Free-radical reactions are also covered, but not until the electron-pair concepts are fully established.
Tiered Topic Development Provides Reinforcement of Important Ideas
I have introduced complex subjects in tiers. This means that students will see many concepts introduced initially in a fairly simply way, then reviewed with another layer of complexity added, and reviewed again at a greater level of sophistication. Acidbase chemistry, discussed above, is an example of tiered development. After the initial chapter on acidbase chemistry and the curved-arrow notation, these concepts are revisited in detail as they are used in the early examples of reactions and mechanisms, and again with the introduction of each new reaction type.
xxxi
00_BRCLoudonFM_pgs4-2.qxd
11/20/08
12:51 PM
Page xxxii
xxxii
PREFACE
The presentation of stereochemistry is another example. The basic idea of stereoisomers is introduced in Chapter 4 (Alkenes). A full chapter on stereochemistry comes two chapters later. Cyclic compounds and the stereochemistry of reactions follow subsequently. Then the ideas of group equivalence and nonequivalence are introduced even later, both in the context of enzyme catalysis and NMR spectroscopy. The approach to organic synthesis is yet another example. I start with simple reactions and then show students how to think about them in reverse. Then, later, I introduce the idea of multistep synthesis using relatively simple two- and three-step sequences. Later still, we have another discussion in which stereochemistry comes into play. Even later, the use of protecting groups is introduced. This tiered presentation of key topics requires some repetition. Although the repetition of key points might be considered inefcient, I believe that it is crucial to the learning process. When a topic is considered after its rst introduction, I have provided detailed cross-referencing to the original material. Students are never cast adrift with terminology that has not been completely dened and reinforced.
Everyday Analogies Help Students to Construct Their Own Knowledge
I believe in the constructivist theory of learning, which holds that students construct learning in their own minds by relating each new idea to something they already know. This is why the relational approach to learning organic chemistry is so important. For the same reason, I have provided common analogies from everyday experience for many of the discussions of chemical principles so that students can relate a new idea to something they already know. One of many examples can be found in the sidebar on p. 164.
Biological Examples Motivate Students Interested in the Allied Health Sciences
Many organic chemistry classes are populated largely by premedical students, prepharmacy students, and other students interested in the life sciences. Biological examples help to motivate these students. I have provided a number of examples from modern biochemistry and medicine throughout the book. Amino acids and proteins have a dedicated chapter that has been completely rewritten in light of modern developments. Carbohydrates also have a dedicated chapter that has been moved so that it now follows carbonyl and amine chemistry. I have integrated many other biological examples into discussions of the relevant chemistry. The ultimate goal of these examples is to reinforce the chemistry being discussed with material that students should nd particularly relevant. Among these are discussions of cell membranes, bioorganic stereochemistry, pheromones, imaging agents, nucleic acids, coenzyme mechanisms, and many, many more. One of many such discussions, for example, is found in the sidebar on pp. 396398 and the accompanying illustration on p. 399.
Students in an Introductory Course Should See Examples of Contemporary Organic Chemistry
The canon of undergraduate organic chemistry necessarily contains many classical reactions, but this text introduces some very modern chemistry as well. For example, the 4th edition introduced a section on transition-metal catalysis, a eld that has literally exploded in the last few years. This section carefully explains the conventions used in the eld for electron counting and calculating oxidation states. This edition builds on that introduction, which previously included
00_BRCLoudonFM_pgs4-2.qxd
11/20/08
12:51 PM
Page xxxiii
PREFACE
xxxiii
the Heck and Stille reactions, by adding sections on the Suzuki coupling, alkene metathesis, and BuchwaldHartwig amination. Asymmetric epoxidation is also introduced, and a modern approach to understanding the rate accelerations observed for many intramolecular reactions has been developed. There is a somewhat higher level of molecular orbital theory than in previous editions. Accompanied by detailed explanations and illustrations, MO theory is related to practical considerations, such as the meaning of resonance structures, the basis of aromaticity, and the understanding of reaction stereochemistry.
Solving Problems Is an Essential Component of the Learning Process
We all know that solving problems is a key to learning organic chemistry. I have provided 1672 problems, many of them multipart, ranging from drill problems to problems that will challenge the most astute students. Many are based directly on material in the literature. The 840 problems within the body of the text are typically drill problems that test whether students understand the current material. The 832 problems at the end of the chapters cover material from the entire chapter and, in many cases, integrate material from earlier chapters. Additionally, I have interspersed 123 Study Problems throughout the text. Each of these problems has a worked-out solution that carefully shows students the logic involved in the problem-solving process. Many students rely too heavily on the Solutions Manual. To help avoid this problem, I have reintroduced in this edition a paired problems approach. This means that the solutions to about 60% of the problems are provided in the Study Guide and Solutions Manual that accompanies the text (see below), but the solutions to the rest of the problems are not provided to the students. Instructors will receive complete solutions to these unsolved problems in PDF format. An instructor who would like to make these solutions available to his or her students can simply post them on a course Web site or hand them out. I have experimented with different ways to use these additional solutions. For example, I have provided the relevant solutions to students just prior to major exams; this strategy encourages them to try these problems without immediate help from the solutions manual, but also allows them to check their answers later. Alternatively, an instructor could these additional problems as the basis for exam questions. Many of the unsolved problems are adjacent to at least one solved problem of the same type.
A Full-Color Presentation Improves Pedagogy
With this edition, this textbook makes its rst appearance in full color. As I have redesigned the art program to make use of color, I have kept a few things uppermost in my mind: color should be used solely for functional, pedagogical purposes; and both gratuitous illustrations and excessive color should be avoided. (I believe students have enough to worry about rather than trying to gure out whats important in a textbook.) The use of color, the presentation of the art, and the text design itself ow from the ideas in The Psychology of Everyday Things, a book by Don Norman. The core idea is that these elements in the text should provide subliminal cues to students that facilitate the learning process.
Supplements Provide Additional Help for Both Students and Instructors
1. The Study Guide and Solutions Manual presents chapter summaries, glossaries of terms, reaction summaries, solutions to selected problems, Study Guide Links, and Further
00_BRCLoudonFM_pgs4-2.qxd
11/20/08
12:51 PM
Page xxxiv
xxxiv
PREFACE
Explorations. The Study Guide Links, which are called out with margin icons in the text, are additional discussions of certain topics with which, in my experience, many students require additional assistance. Examples are How to Study Organic Reactions, and Solving Structure Problems. The Further Explorations, also called out with margin icons in the text, are short discussions that move beyond the text material. An example is Fourier-Transform NMR. 2. Molecular models (Model 1013) from Maruzen International are available as a bundle with the textbook. For more information and pricing, please contact Ben Roberts: bwr@roberts-publishers.com. 3. We will provide, to adopting instructors, a PDF image of the problem solutions that are not provided in the Study Guide and Solutions Manual (see the discussion of paired problems above), as well as full-color images of all gures in conventional formats so that they can be used in classroom presentations. Instructors at adopting institutions who want this material should request it from the publisher at bwr@roberts-publishers.com. 4. As with the fourth edition, we will maintain, on the World-Wide Web, an up-to-date list of errata in PDF format for both the text and the Study Guide and Solutions Manual supplement. These lists of errors will be generally available to instructors and students alike.
A BOOK WITH A SCHOLARLY HISTORY
The rst edition of Organic Chemistry, published in 1984, required 72 years of development, because each topic was researched back to the original or review literature. Subsequent editions, including this one, have continued this scholarly development process. Almost every reaction example is taken from the literature. Each edition has beneted from a thorough peer review.
1
ACKNOWLEDGMENTS
I am indebted to my dean, Craig Svensson, and my department head, Rick Borch, for providing a sabbatical leave in 20072008, as well as a climate in which this edition could be completed successfully. The electronic resources of the Purdue Library have streamlined the research process for this text in a way that was unimaginable 25 years ago, and I would like to thank Emily Mobley, Purdues Dean of Libraries Emerita, for bringing the electronic library to fruition, and Jim Mullins, the present dean, for fostering its continued improvement. Thanks to my faculty and staff colleagues at Purdue and beyondJohn Grutzner, Don Bergstrom, Mark Green, Chris Rochet, Ross Weatherman, Phil Fuchs, Mark Lipton, Ei-ichi Negishi, Markus Lill, David Nichols, Mark Cushman, Arun Ghosh, John Bartmess (University of Tennessee), Bob Hammer (formerly of Louisiana State University), Karl Wood, David Allen, and Susan Holladayfor advice, assistance, and suggestions. Special thanks go to two very special teaching assistants, Lisa Bonner and Animesh Aditya, for their hard work, their advice, and their effective and inspiring teaching. The reviewers named in the list that follows this preface provided invaluable assistance in polishing this text. I am particularly indebted to Prof. David Hansen of Amherst College, Prof. Paul Rablen of Swarthmore College, and Prof. Carolyn Anderson of Calvin College, who provided invaluable suggestions through all or most of the project. Prof. Ahamindra Jain of Harvard University, a dedicated teacher and a delightful collaborator, made some very valuable suggestions in the early going, and I was very much looking forward to working with him as my coauthor on the Study Guide and Solutions
00_BRCLoudonFM_pgs4-2.qxd
11/20/08
12:51 PM
Page xxxv
PREFACE
xxxv
Manual supplement. Sadly, Ahamindra passed away in 2008, and is sorely missed. I would also like to thank the many students from all over the country who made suggestions, offered comments, and reported errors. I welcome correspondence with the students using this edition. I can be reached by e-mail at marc.loudon.1@purdue.edu. My relationship with the professionals at Side By Side Studios in San Francisco, Mark Ong and Susan Riley, has been particularly gratifying. Not only has their composition work been superb, but also their advice has been invaluable. Side By Side gets ve stars! Working with Ben Roberts at Roberts and Company Publishers has also been a delight, and I hope our association continues far into the future. I very much appreciate the hard work, advice, and attention to detail of the copy editor, John Murdzek, and the proofreader, Gunder Hefta. I would particularly like to thank those acknowledged separately in the Credits section for their willingness to allow me to reproduce their materials. I could not have completed this project without the love and support of Judy and my family, for which I am grateful beyond words. My wish is that the students who use this text will see the amazing diversity and beauty of science through their study of organic chemistry, and that they will benet from using this book as much as I have enjoyed writing it. October 2008 West Lafayette, Indiana Marc Loudon
00_BRCLoudonFM_pgs4-2.qxd
11/20/08
12:51 PM
Page xxxvi
Reviewers and Consultants
The author and publisher wish to acknowledge with gratitude the extensive support received from the organic chemistry community in the development of this edition. Our reviewers and consultants are listed below; some people served in multiple roles. Editorial Consultants The following editorial consultants read chapters, evaluated the art for style and pedagogy, and advised on many important, large-scale editorial decisions. Carolyn Anderson, Calvin College John Bartmess, University of Tennessee Marcia B. France, Washington and Lee University Robert Hammer, Louisiana State University David Hansen, Amherst College Ahamindra Jain, Harvard University James Nowick, University of California, Irvine Paul R. Rablen, Swarthmore College General Reviewers The following general reviewers helped us generate a detailed plan for changes from the fourth edition to the fth edition. Angela Allen, University of Michigan, Dearborn Don Bergstrom, Purdue University David Brown, Davidson College Scott Bur, Gustavus Adolphus College Joyce Blair Easter, Virginia Wesleyan University Tom Evans, Denison College Natia Frank, University of Victoria Phillip Fuchs, Purdue University Ronald Magid, University of Tennessee Tim Minger, Mesa Community College William Ojala, University of St. Thomas Andy Phillips, University of Colorado, Boulder Jetze J. Tepe, Michigan State University Scott Ulrich, Ithaca College Chapter Reviewers The following reviewers read chapters in detail. Carolyn E. Anderson, Calvin College John Bartmess, University of Tennessee Marcia B. France, Washington and Lee University David Hansen, Amherst College xxxvi
00_BRCLoudonFM_pgs4-2.qxd
11/20/08
12:51 PM
Page xxxvii
REVIEWERS AND CONSULTANTS
xxxvii
Ahamindra Jain, Harvard University Joseph Konopelski, University of California, Santa Cruz Paul LePlae, Wabash College Dewey G. McCafferty, Duke University (and his students) Nasri Nesnas, Florida Institute of Technology Paul R. Rablen, Swarthmore College Christian M. Rojas, Barnard College Barry Snider, Brandeis University Scott A. Snyder, Columbia University Dasan M. Thamattoor, Colby College Lawrence T. Scott, Boston College Scott Ulrich, Ithaca College Richard G. Weiss, Georgetown University Focus Group Participants The following consultants took part in an important focus group at which we discussed the price of textbooks, the trends in student learning, and the value of supplements. Cynthia McGowan, Merrimack College Eriks Rozners, Northeastern University David Hansen, Amherst College Ahamindra Jain, Harvard University Bela Torok, University of Massachusetts, Boston Robert Hanson, Northeastern University
00_BRCLoudonFM_pgs4-2.qxd
11/20/08
12:51 PM
Page xxxviii
00_BRCLoudonFM_pgs4-2.qxd
11/20/08
12:51 PM
Page xxxix
About the Author
Marc Loudon received his BS (magna cum laude) in chemistry in 1964 from Louisiana State University and his PhD in organic chemistry in 1968 from the University of California, Berkeley, where he worked with Professor Donald S. Noyce. After two years of postdoctoral study with Professor Daniel E. Koshland in the Biochemistry Department at Berkeley, Dr. Loudon joined the chemistry faculty at Cornell University, where he taught organic chemistry to both preprofessional students and science majors. He received the Clark Teaching Prize of Cornells College of Arts and Sciences in 1976. Since 1977, Dr. Loudon has been Professor of Medicinal Chemistry at Purdue University. From 19882007, Dr. Loudon served as Associate Dean for Research and Graduate Programs of the College of Pharmacy, Nursing, and Health Sciences. Dr. Loudon teaches organic chemistry to pharmacy and prepharmacy students at Purdue, where he has twice won the School of Pharmacys Henry Heine Outstanding Teacher Award. In 1988, he received the Class of 1922 Helping Students Learn Award. In 1996, Dr. Loudon was among three faculty at Purdue who were the rst to be named Distinguished Professor on the basis of teaching and teaching scholarship; as result of that award, Dr. Loudon became the Gustav Cwalina Distinguished Professor of Medicinal Chemistry. In 1999, Dr. Loudon won Purdues university-wide Charles B. Murphy Award for undergraduate teaching and, in the same year, was listed in Purdues permanent Book of Great Teachers. In 2000, Dr. Loudon was named Indiana Professor of the Year by the Carnegie Foundation. In 2001, Dr. Loudon served as a member of the Chemistry Panel of BIO2010, which was commissioned by the National Academy of Sciences to make national recommendations for the biology curriculum. Dr. Loudon, in collaboration with Professor George Bodner at Purdue, has developed techniques for teaching organic chemistry to large classes using cooperative learning methods. Dr. Loudon has presented numerous public lectures on his techniques. Dr. Loudon and his wife Judy have two grown sons and three grandchildren. He is an accomplished pianist and organist and has performed professionally in the San Francisco Bay area, at Cornell University, and in Indiana. He also enjoys playing competitive tennis, but success in competition is yet to catch up with his ambition.
xxxix
00_BRCLoudonFM_pgs4-2.qxd
11/20/08
12:51 PM
Page xl
Você também pode gostar
- A New Introduction To Organic Chemistry by G.I. BrownDocumento193 páginasA New Introduction To Organic Chemistry by G.I. Brownkiddho100% (1)
- Enzymes: A Practical Introduction to Structure, Mechanism, and Data AnalysisNo EverandEnzymes: A Practical Introduction to Structure, Mechanism, and Data AnalysisNota: 4 de 5 estrelas4/5 (2)
- Organic 2 PDFDocumento864 páginasOrganic 2 PDFaisyahAinda não há avaliações
- 2013 Sample QuestionsDocumento262 páginas2013 Sample QuestionsNorhafiza RoslanAinda não há avaliações
- Mod 4 Revision Guide 10 Synthetic RoutesDocumento2 páginasMod 4 Revision Guide 10 Synthetic RoutesdufraiscAinda não há avaliações
- Aliphatic Organic SynthesisDocumento30 páginasAliphatic Organic SynthesisrationalwikiAinda não há avaliações
- ALKANES2Documento41 páginasALKANES2Shiki Asagami BrunestedAinda não há avaliações
- Chapter 19. Aldehydes and Ketones: Nucleophilic Addition ReactionsDocumento64 páginasChapter 19. Aldehydes and Ketones: Nucleophilic Addition Reactionsaggelisgeorge8546Ainda não há avaliações
- Organic Chemistry 32-235 Practice Questions For Exam #2: 2. Consider The SDocumento9 páginasOrganic Chemistry 32-235 Practice Questions For Exam #2: 2. Consider The Ssweta KushwahaAinda não há avaliações
- Hetero-Cyclic CompoundsDocumento69 páginasHetero-Cyclic CompoundsNaveed SajidAinda não há avaliações
- AQA AS Level Chemistry Data SheetDocumento4 páginasAQA AS Level Chemistry Data SheetA100% (1)
- Module8 PDFDocumento40 páginasModule8 PDFFaizan AhmadAinda não há avaliações
- XI Chemistry Chapterwise Advanced Study MaterialDocumento537 páginasXI Chemistry Chapterwise Advanced Study MaterialregisAinda não há avaliações
- MSCCH 604Documento203 páginasMSCCH 604Gourav Biju100% (1)
- Inorganic Qualitative Analysis Acidic RadicalDocumento24 páginasInorganic Qualitative Analysis Acidic RadicalShivani ShreshthaAinda não há avaliações
- GRE Sub 化学题 (太傻整理)Documento30 páginasGRE Sub 化学题 (太傻整理)Alisa100% (1)
- Vollhardt 6e Lecture PowerPoints - Chapter 11Documento58 páginasVollhardt 6e Lecture PowerPoints - Chapter 11superfr3shmAinda não há avaliações
- Hein Chem12 Ch14 AnsDocumento9 páginasHein Chem12 Ch14 AnsAlex KingsAinda não há avaliações
- Books Suggested For M.sc. Semester 2Documento1 páginaBooks Suggested For M.sc. Semester 2gsv988Ainda não há avaliações
- Organic Compound Nomenclature and CharacteristicDocumento8 páginasOrganic Compound Nomenclature and CharacteristictasneemAinda não há avaliações
- Revised Organic ChemistryDocumento90 páginasRevised Organic ChemistryMinh TieuAinda não há avaliações
- Structure and Synthesis of Alcohols: Organic Chemistry, 7Documento52 páginasStructure and Synthesis of Alcohols: Organic Chemistry, 7haha_le12100% (1)
- Organic Chemistry 2Documento298 páginasOrganic Chemistry 2ariel100% (1)
- Alcohols, Phenols and EthersDocumento99 páginasAlcohols, Phenols and EthersSanya VermaAinda não há avaliações
- Organic ChemistryDocumento14 páginasOrganic ChemistryStuteeAinda não há avaliações
- Alcohol, Phenol, and Ethers:: "Their Structures, Physical Properties and Nomenclature"Documento33 páginasAlcohol, Phenol, and Ethers:: "Their Structures, Physical Properties and Nomenclature"AmanAinda não há avaliações
- 18.3 Lack of Reactivity of Vinylic and Aryl Halides Under S 1 ConditionsDocumento3 páginas18.3 Lack of Reactivity of Vinylic and Aryl Halides Under S 1 Conditionsrish rajAinda não há avaliações
- Midterm II Key Chem 2312-003 F '12Documento7 páginasMidterm II Key Chem 2312-003 F '12acb4039Ainda não há avaliações
- ALKANESDocumento73 páginasALKANESChona TuyAinda não há avaliações
- Oxidation Numbers: Chemistry For The Gifted and Talented 61Documento14 páginasOxidation Numbers: Chemistry For The Gifted and Talented 61EricAinda não há avaliações
- C F C CL C - BR: HalogenoalkanesDocumento11 páginasC F C CL C - BR: HalogenoalkanesMufaro MutotiAinda não há avaliações
- Alkenes ReactionsDocumento69 páginasAlkenes ReactionsAhmad SayyedahmadAinda não há avaliações
- Student - S Guide - Chapter 4 - Q & ADocumento70 páginasStudent - S Guide - Chapter 4 - Q & AmoastAinda não há avaliações
- 12 Chemistry Impq CH08 D and F Block Elements 02Documento8 páginas12 Chemistry Impq CH08 D and F Block Elements 02srivathson7Ainda não há avaliações
- Mekanisme RX OrganikDocumento82 páginasMekanisme RX OrganikAdzimahAinda não há avaliações
- Washburn, E.W. - International Critical Tables of Numerical Data, Physics, Chemistry and Technology (1926 - 1930 - 20, Knovel)Documento1.055 páginasWashburn, E.W. - International Critical Tables of Numerical Data, Physics, Chemistry and Technology (1926 - 1930 - 20, Knovel)Guillermo Aquino100% (1)
- Organic Chemistry II Practice Exam #3A Answer KeyDocumento8 páginasOrganic Chemistry II Practice Exam #3A Answer Keyhiep237Ainda não há avaliações
- Isomerism in Coordination Compounds PDFDocumento7 páginasIsomerism in Coordination Compounds PDFmf720383270100% (1)
- Organic Chem U-2 AlcoholDocumento33 páginasOrganic Chem U-2 Alcoholsinte beyuAinda não há avaliações
- 5 Key Factors That Affect Acidity in Organic Chemistry - Master Organic ChemistryDocumento23 páginas5 Key Factors That Affect Acidity in Organic Chemistry - Master Organic ChemistryAhmad NisarAinda não há avaliações
- WTF Bharat Panchal FinalDocumento135 páginasWTF Bharat Panchal FinalDhruv KarheAinda não há avaliações
- Problem Ver 2Documento133 páginasProblem Ver 2NuteLLa Gaming (EFL)Ainda não há avaliações
- Reactions of Alcohols: Organic Chemistry, 7Documento53 páginasReactions of Alcohols: Organic Chemistry, 7haha_le12100% (1)
- ChemistryDocumento34 páginasChemistryraghuram_allaAinda não há avaliações
- Chapter 4 With Video LinksDocumento37 páginasChapter 4 With Video LinksDoom RefugeAinda não há avaliações
- 05 Conformational Anal 2Documento11 páginas05 Conformational Anal 2Swati GautamAinda não há avaliações
- Notes Lecture 1 Conformational AnalysisDocumento18 páginasNotes Lecture 1 Conformational AnalysisDianing Wismarani Putri100% (1)
- MSCCH07Documento385 páginasMSCCH07capdesuro100% (1)
- Carboxylic AcidDocumento21 páginasCarboxylic AcidMuhammad AjmalAinda não há avaliações
- PDFDocumento155 páginasPDFHifza shairwani100% (1)
- 12th Chemistry PracticalDocumento88 páginas12th Chemistry Practicalsavitristiching100% (1)
- Chemistry: NO Judul PengarangDocumento10 páginasChemistry: NO Judul Pengarangkartini11Ainda não há avaliações
- Organic Chemistry PDFDocumento3 páginasOrganic Chemistry PDFChuran SinghAinda não há avaliações
- Thermochemistry: - Petrucci, Herring Madura and BissonnetteDocumento49 páginasThermochemistry: - Petrucci, Herring Madura and BissonnetteYousif Khalid100% (1)
- Physics II Problems PDFDocumento1 páginaPhysics II Problems PDFBOSS BOSSAinda não há avaliações
- Lesson 1 - Section 5.1 - Chemical and Physical ChangeDocumento4 páginasLesson 1 - Section 5.1 - Chemical and Physical ChangeshanlbyAinda não há avaliações
- Cumicrete: Conventional Dense Castables (Calcined Clay Based)Documento6 páginasCumicrete: Conventional Dense Castables (Calcined Clay Based)Devanathan ChinnasamyAinda não há avaliações
- Lecture 4 On Fatigue Failure RDocumento39 páginasLecture 4 On Fatigue Failure RSelam SamsonAinda não há avaliações
- Hawe Pressure Dt11 d5440t2 enDocumento4 páginasHawe Pressure Dt11 d5440t2 enАндрей дронAinda não há avaliações
- A Design Procedure For High-Efficiency and Compact-Size 5-10-W MMIC Power Amplifiers in GaAs pHEMT TechnologyDocumento12 páginasA Design Procedure For High-Efficiency and Compact-Size 5-10-W MMIC Power Amplifiers in GaAs pHEMT Technologyln zAinda não há avaliações
- Esas Ayuda 3Documento7 páginasEsas Ayuda 3Friendrich FriedchickenAinda não há avaliações
- Natural ConvectionDocumento7 páginasNatural ConvectionLuthfy AditiarAinda não há avaliações
- Diffusional Other TransformationsDocumento51 páginasDiffusional Other TransformationsAnil Kumar TAinda não há avaliações
- Notice Phase Test - Stu - 25.10.20Documento1 páginaNotice Phase Test - Stu - 25.10.20Ayush ChouhanAinda não há avaliações
- Determine The EAN of Central Metal Ion of ComplexesDocumento10 páginasDetermine The EAN of Central Metal Ion of ComplexesArnab KumarAinda não há avaliações
- Curriculum Vitae S.V.Raghava Reddy DEG: Manager - Production, Company: Dasami Lab Private Limited, Choutuppal, IndiaDocumento3 páginasCurriculum Vitae S.V.Raghava Reddy DEG: Manager - Production, Company: Dasami Lab Private Limited, Choutuppal, IndianagarjunaAinda não há avaliações
- Physic MCQ Nuclear PhysicsDocumento5 páginasPhysic MCQ Nuclear PhysicsArshad TanoliAinda não há avaliações
- Disclosure To Promote The Right To InformationDocumento13 páginasDisclosure To Promote The Right To InformationVakkanthula SaikarthikAinda não há avaliações
- Stauff Englisch BT 6 2023Documento4 páginasStauff Englisch BT 6 2023PeterAinda não há avaliações
- CIVDES2 Lecture Notes - 1Documento18 páginasCIVDES2 Lecture Notes - 1Anthony TangAinda não há avaliações
- VLL Separator - Train1Documento9 páginasVLL Separator - Train1yamen-691904Ainda não há avaliações
- 8250 - Series Relief Valves PDFDocumento16 páginas8250 - Series Relief Valves PDFcasandraAinda não há avaliações
- Student Exploration: PH Analysis: Lemon Juice Tastes Sour. When You Get Lemon Juice in Your Eye It BurnsDocumento3 páginasStudent Exploration: PH Analysis: Lemon Juice Tastes Sour. When You Get Lemon Juice in Your Eye It BurnsHailey’s WoodAinda não há avaliações
- Time-Resolved Imaging and Spectroscopy of Atmospheric Pressure Plasma Bullet Propagation and RONS ProductionDocumento19 páginasTime-Resolved Imaging and Spectroscopy of Atmospheric Pressure Plasma Bullet Propagation and RONS ProductionAndrei VasileAinda não há avaliações
- A Comprehensive Review of Asphaltene Deposition in Petroleum Reservoirs Theory, Challenges, and TipsDocumento39 páginasA Comprehensive Review of Asphaltene Deposition in Petroleum Reservoirs Theory, Challenges, and TipsSoleiman ChatrsimabAinda não há avaliações
- General Engineering Science) /: Question BookletDocumento8 páginasGeneral Engineering Science) /: Question BookletErPriyeRanjanAinda não há avaliações
- LANDFILLDocumento37 páginasLANDFILLdevash100% (1)
- Chapter 1 Introduction & Fick's LawDocumento19 páginasChapter 1 Introduction & Fick's LawRenu SekaranAinda não há avaliações
- Physics P2 SPM 2014 A Modul Melaka GemilangDocumento9 páginasPhysics P2 SPM 2014 A Modul Melaka GemilangCikgu FaizalAinda não há avaliações
- MM102 NotesDocumento7 páginasMM102 NotesHassan AliAinda não há avaliações
- TABOND 3043 Adhesive Resin: CharacteristicsDocumento1 páginaTABOND 3043 Adhesive Resin: CharacteristicsVictor CastrejonAinda não há avaliações
- A Note of Caution On Numerical Scheme Selection Evidence From Cyclone Separator CFD Simulations With Appropriate Near-Wall Grid SizesDocumento23 páginasA Note of Caution On Numerical Scheme Selection Evidence From Cyclone Separator CFD Simulations With Appropriate Near-Wall Grid SizesTanweer HussainAinda não há avaliações
- Full Paper APM 2012 68Documento5 páginasFull Paper APM 2012 68bishnu ppAinda não há avaliações
- Cu CR 1 ZRDocumento38 páginasCu CR 1 ZRDaško MedenjaškoAinda não há avaliações