Escolar Documentos
Profissional Documentos
Cultura Documentos
BK10110302-Shuler Problems PDF
Enviado por
VPrasarnth RaajTítulo original
Direitos autorais
Formatos disponíveis
Compartilhar este documento
Compartilhar ou incorporar documento
Você considera este documento útil?
Este conteúdo é inapropriado?
Denunciar este documentoDireitos autorais:
Formatos disponíveis
BK10110302-Shuler Problems PDF
Enviado por
VPrasarnth RaajDireitos autorais:
Formatos disponíveis
V.Prasarnth Raaj SOLUTIONS Problem 3.
12
Shuler Problems
BK10110302
1. Is Harrys reasoning right? Do you agree with him? Harrys reasoning is right. Immobilization often prolongs the life of the protein. Thus I agree with Harry that, immobilization can prolong the active lifespan of enzymes (although it can also kill enzyme with certain linkages). 2. Why is that so? Aggregation is often a problem with proteins in solution, the higher the concentration of enzyme, the quicker the aggregation and it can lead the enzymes to die faster. This can be further increased if redox sites are involved, at least in part due to cysteine reactivity and divalent bonds forming between enzymes leading to inactive sludge Additionally, enzymes which undergo conformational changes during their catalysis also can become more prone to denature in a purified state denatured proteins also tend to glom up more readily, rendering dead enzyme quite quickly. Certain enzymes (those designed to chew up other molecules) also will exhibit some activity against themselves (even if low, this adds up quickly in the high concentration, low other-substrate type environment of storage). Immobilization solves several of these problems - enzymes are at a relatively low concentration for aggregation and inter-enzyme reactions with each other, while they can still be at a high relative concentration of reaction with substrate flowed through the beads. From the description the type of beads is Poros-type beads
V.Prasarnth Raaj Problem 3.14
Shuler Problems
BK10110302
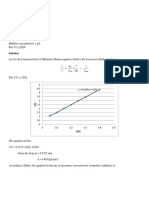
a. Because the reaction rate is almost the same for the 0.1 and 0.2 cm particle diameter, we can assume that the rate of reaction without immobilizing uricase enzyme is 200 mg l-1 h1 . 100 (Dp = 0.5cm) = = 0.5 200 (Dp = 0.7cm) = b. Applying Lineweaver-Burk plot, 1 1 1 = + [] 0 (mg UA 1 ) 10 25 50 100 200 250 1/0 0.1 0.04 0.02 0.01 0.005 0.004 (mg UA 1 h1 ) 10 20 30 40 45 46 1/ 0.1 0.05 0.033333 0.025 0.022222 0.021739 50 = 0.25 200
Lineweaver-Burk Plot
0.12 0.1 0.08 y = 0.8217x + 0.0175
1/v
0.06 0.04 0.02
-0.03
0 -0.01
0.01
0.03 1/S
0.05
0.07
0.09
0.11
1 = 58.82 mg UA 1 h1 0.017
= 0.821 58.82 = 48.29 mg UA 1
V.Prasarnth Raaj Problem 3.15 a. d=2mm ; r=1mm
Shuler Problems
BK10110302
[Sb]=0.5mM ; neglect liquid film resistance, therefore [Sb]= [Ss] v=10mM h-1 = 2.78x10-3mM s-1 De=1.5x10-5cm2/sec Km' = 0.2 mM 4 = 3 3 = 4.18 103 3 , = 2.78 103 1 4.18 103 3
= 0.665 3 1 = [ ] + [ ] ( )(0.5) 0.2 + 0.5
0.665 3 1 =
3 1 = 0.931
0.931 3 10.2 = = 0.1 1.5 105 2 1 = 55.7 = = 3
3 55.7
= 0.0538 b. d=4mm ; r=2mm ; r=0.2cm 4 = 3 3 = 0.0343
V.Prasarnth Raaj
Shuler Problems 2.78 103 1 0.0343
BK10110302
, =
= 0.082 3 1 = [ ] + [ ] ( )(0.5) 0.2 + 0.5
0.082 3 1 =
3 1 = 0.1148
0.1148 3 10.2 = = 0.2 1.5 105 2 1 = 39.12 = = 3
3 39.12
= 0.0767
V.Prasarnth Raaj Problem 3.17
Shuler Problems
BK10110302
V.Prasarnth Raaj Problem 3.18
Shuler Problems
BK10110302
V.Prasarnth Raaj Problem 6.15 (a)
Shuler Problems
BK10110302
V.Prasarnth Raaj Problem 6.15 (b)
Shuler Problems
BK10110302
V.Prasarnth Raaj Problem 6.17
Shuler Problems
BK10110302
V.Prasarnth Raaj Problem 6.17
Shuler Problems
BK10110302
V.Prasarnth Raaj Problem 6.19 (a)
Shuler Problems
BK10110302
V.Prasarnth Raaj Problem 6.19 (b)
Shuler Problems
BK10110302
Two graph need to be plotted in order to find the optimum dilution rate Plot 1 DX vs D Plot the table below using this equation DX = 0.1-((0.004*D)/(0.2-D)) D 40 30 20 10 0 DX 0.10402 0.104027 0.10404 0.104082 0.1
DX vs D
0.105 0.1045 0.104 0.1035 0.103 0.1025 0.102 0.1015 0.101 0.1005 0.1 0.0995 0 5 10
12.5
Productivity of Biomass, DX
15
20
25
30
35
40
45
Dilution Rate, D
Optimum dilution rate maximizing productivity of biomass, Dopt = 12.5
V.Prasarnth Raaj Plot 2 DP vs D Plot the table below using this equation DP = 0.2-((0.008*D)/(0.2-D))
Shuler Problems
BK10110302
D 0 20 40 60 80
DP 0.2 0.208081 0.20804 0.208027 0.20802
DP vs D
0.21 0.209 0.208 0.207 0.206 0.205 0.204 0.203 0.202 0.201 0.2 0.199 0 10 20
25
Productivity of Product, DP
30
40
50
60
70
80
90
Dilution Rate, D
Optimum dilution rate maximizing productivity of product, Dopt = 25
Você também pode gostar
- Nptel: Multicomponent Mass Transfer - Video CourseDocumento4 páginasNptel: Multicomponent Mass Transfer - Video CourseucAinda não há avaliações
- TugasDocumento23 páginasTugasAnisa Ramadhani Sahlan100% (1)
- Sample Problem #14Documento7 páginasSample Problem #14DozdiAinda não há avaliações
- Sample Problem ThermoDocumento25 páginasSample Problem ThermoJonnah Faye Mojares0% (1)
- Chemical Engineering Day 1 PDFDocumento13 páginasChemical Engineering Day 1 PDFCharles Arthel ReyAinda não há avaliações
- Microbial Cell Growth and Kinetics PhasesDocumento58 páginasMicrobial Cell Growth and Kinetics PhasesKIL170051 STUDENTAinda não há avaliações
- Palaganas Hw2 Che 197Documento5 páginasPalaganas Hw2 Che 197Elah Palaganas100% (1)
- Applied Chemical Engineering CalculationsDocumento7 páginasApplied Chemical Engineering Calculationsmbolantenaina100% (1)
- Theory of The Chemostat Cambridge Studies in Mathematical BiologyDocumento330 páginasTheory of The Chemostat Cambridge Studies in Mathematical BiologyChristian HuertaAinda não há avaliações
- 2 Cell Kinetics and Fermenter Design Part 2 DiscussionDocumento4 páginas2 Cell Kinetics and Fermenter Design Part 2 DiscussionEzekielAinda não há avaliações
- Tugas 2 - Kelompok 1 - Problem 2.1 - RevisiDocumento3 páginasTugas 2 - Kelompok 1 - Problem 2.1 - RevisiDelyana RatnasariAinda não há avaliações
- Exercise Flow of FluidsDocumento2 páginasExercise Flow of FluidsJD6 AgarbAinda não há avaliações
- Tutorial Group 1Documento6 páginasTutorial Group 1Sanjeev NehruAinda não há avaliações
- E Bio Che SampleDocumento9 páginasE Bio Che SamplePiyush NandanAinda não há avaliações
- Enzyme KineticsDocumento72 páginasEnzyme Kineticsitokki otoya100% (1)
- RK4 ODE Method ExamplesDocumento6 páginasRK4 ODE Method ExamplesDheiver SantosAinda não há avaliações
- Stoichiometry of Microbial Growth and Product FormationDocumento30 páginasStoichiometry of Microbial Growth and Product FormationMark GerardAinda não há avaliações
- Physical Property Data-KoretskyDocumento8 páginasPhysical Property Data-KoretskyLMVM927919Ainda não há avaliações
- ProblemsDocumento31 páginasProblemsAhmed AdhamAinda não há avaliações
- Sample Problem 2Documento2 páginasSample Problem 2Sata AjjamAinda não há avaliações
- Solution: For A First-Order Reaction, The Following Rate Coefficients Were FoundDocumento16 páginasSolution: For A First-Order Reaction, The Following Rate Coefficients Were FoundDeepak SharmaAinda não há avaliações
- Activity Number 2Documento9 páginasActivity Number 2Mariella SingsonAinda não há avaliações
- Mass Transfer Sample ChaptersDocumento29 páginasMass Transfer Sample ChaptersGurunath EpiliAinda não há avaliações
- Batangas State University ChE Course on Enzyme KineticsDocumento53 páginasBatangas State University ChE Course on Enzyme KineticsNasser Gemina PantaoAinda não há avaliações
- C H O + A O + B NH C C H NO + D H O+eCO: InstructionsDocumento4 páginasC H O + A O + B NH C C H NO + D H O+eCO: InstructionsJohn Paul Jandayan33% (3)
- CHE 304 (Spring 2010) Problem Set #6 SolutionsDocumento4 páginasCHE 304 (Spring 2010) Problem Set #6 SolutionsNatália FerreiraAinda não há avaliações
- BioprocessProblem 3Documento1 páginaBioprocessProblem 3AshenafiAinda não há avaliações
- Soln Sa Adsorption PDFDocumento2 páginasSoln Sa Adsorption PDFRee ValeraAinda não há avaliações
- Diffusion Mass TransferDocumento18 páginasDiffusion Mass TransferbhuniakanishkaAinda não há avaliações
- Lec 7Documento7 páginasLec 7Vinicius Noronha100% (1)
- Day 3Documento10 páginasDay 3ize_angel14Ainda não há avaliações
- Classification of BioreactorsDocumento2 páginasClassification of BioreactorsisabelelmhAinda não há avaliações
- Heat Capacity Data and Formulas for 49 Inorganic and Organic CompoundsDocumento7 páginasHeat Capacity Data and Formulas for 49 Inorganic and Organic CompoundsGabriel SugayaAinda não há avaliações
- Tutorial 4 Solution PDFDocumento6 páginasTutorial 4 Solution PDFSalihah AbdullahAinda não há avaliações
- Enzym ESDocumento21 páginasEnzym ESpoopnoodlemanAinda não há avaliações
- Lec 7-1Documento37 páginasLec 7-1Caterina BarrettaAinda não há avaliações
- Bioprocess Engineering Shuler Kargi Solution Manual PDFDocumento1 páginaBioprocess Engineering Shuler Kargi Solution Manual PDFjestineAinda não há avaliações
- 9A23401 Mass Transfer OperationsDocumento8 páginas9A23401 Mass Transfer OperationssivabharathamurthyAinda não há avaliações
- 3811 Acids Bases WanswersDocumento2 páginas3811 Acids Bases WanswersClark Ivan TorresAinda não há avaliações
- Solving Enzyme Kinetics Problems: Sucrose Transport, Vmax vs Km Effects, Carbonic Anhydrase ReactionDocumento4 páginasSolving Enzyme Kinetics Problems: Sucrose Transport, Vmax vs Km Effects, Carbonic Anhydrase Reactionbiotech_vidhya100% (1)
- Energy Balance On DecanterDocumento6 páginasEnergy Balance On DecanterShahid IsmailAinda não há avaliações
- CHE 312 Problem Set #2Documento4 páginasCHE 312 Problem Set #2rkz93Ainda não há avaliações
- 2 πN 60 = 2 π (1200 rev) 60 = 40 π 18 μDocumento3 páginas2 πN 60 = 2 π (1200 rev) 60 = 40 π 18 μNUR ASYIQIN BINTI AZAHARAinda não há avaliações
- Chapter 5 AdsorptionDocumento46 páginasChapter 5 AdsorptionSyahmiAinda não há avaliações
- (Differential Calculus, Integral Calculus, Differential Equations, Probability and StatisticsDocumento7 páginas(Differential Calculus, Integral Calculus, Differential Equations, Probability and Statisticskimuel demesaAinda não há avaliações
- May 2015 Che Board ExaminationDocumento12 páginasMay 2015 Che Board ExaminationIvan Jio Revilla SanchezAinda não há avaliações
- Biochemical EngineeringDocumento37 páginasBiochemical EngineeringPepy PeachAinda não há avaliações
- Tutorial 1Documento2 páginasTutorial 1eddy50% (2)
- Pre Board Day 3 Exam October 2017 PDFDocumento3 páginasPre Board Day 3 Exam October 2017 PDFMarvin ParasAinda não há avaliações
- 2015 NovemberDocumento13 páginas2015 NovemberAkiAinda não há avaliações
- Thermal Analysis TechniquesDocumento59 páginasThermal Analysis TechniquesBhagyashree PaniAinda não há avaliações
- CHEMICAL ENGINEERING INSTRUMENTATION AND PROCESS CONTROL PROBLEMSDocumento4 páginasCHEMICAL ENGINEERING INSTRUMENTATION AND PROCESS CONTROL PROBLEMSMarco SarmientoAinda não há avaliações
- Corr 2018 SRC Analytical Chemistry Module 5 JGDocumento5 páginasCorr 2018 SRC Analytical Chemistry Module 5 JGpaula lunaAinda não há avaliações
- Rr410802 Chemical Reaction Engineering IIDocumento8 páginasRr410802 Chemical Reaction Engineering IISrinivasa Rao G100% (3)
- Resumen CH04 FelderDocumento45 páginasResumen CH04 Feldercuberbill1980Ainda não há avaliações
- Chapter 7 - Analysis of BioreactorDocumento52 páginasChapter 7 - Analysis of BioreactorاشرفاللساميAinda não há avaliações
- ShareDocumento1 páginaShareRicardo VelozAinda não há avaliações
- Lab Report Iodine Clock ReactionDocumento6 páginasLab Report Iodine Clock ReactionYoonseo (Elin) ChaAinda não há avaliações
- Analytical Modeling of Solute Transport in Groundwater: Using Models to Understand the Effect of Natural Processes on Contaminant Fate and TransportNo EverandAnalytical Modeling of Solute Transport in Groundwater: Using Models to Understand the Effect of Natural Processes on Contaminant Fate and TransportAinda não há avaliações
- Mach CC App Form Jun16 Eng 20160629Documento6 páginasMach CC App Form Jun16 Eng 20160629ZulIzzamreeZolkepliAinda não há avaliações
- Hyundai AccentDocumento10 páginasHyundai AccentVPrasarnth RaajAinda não há avaliações
- CarDocumento1 páginaCarVPrasarnth RaajAinda não há avaliações
- Map To CBJ OfficeDocumento1 páginaMap To CBJ OfficeVPrasarnth RaajAinda não há avaliações
- Basic Well Log Analysis - Introduction - Oct2013Documento35 páginasBasic Well Log Analysis - Introduction - Oct2013Jorge PirelaAinda não há avaliações
- Finaldissertation NoRestrictionDocumento120 páginasFinaldissertation NoRestrictionVPrasarnth RaajAinda não há avaliações
- C19115I2901Documento1 páginaC19115I2901VPrasarnth RaajAinda não há avaliações
- Job satisfaction in human resource managementDocumento102 páginasJob satisfaction in human resource managementMed ElbanadiAinda não há avaliações
- HP PDS Con EngDocumento3 páginasHP PDS Con EngVPrasarnth RaajAinda não há avaliações
- Proton On The Road Price After Gst-20160215Documento1 páginaProton On The Road Price After Gst-20160215VPrasarnth RaajAinda não há avaliações
- Windscreen Report Claim FormDocumento3 páginasWindscreen Report Claim FormVPrasarnth RaajAinda não há avaliações
- ResignationDocumento1 páginaResignationAlan KhorAinda não há avaliações
- Pchardware StartecDocumento1 páginaPchardware StartecVPrasarnth RaajAinda não há avaliações
- 308 - November 2015 Version 29Documento19 páginas308 - November 2015 Version 29VPrasarnth RaajAinda não há avaliações
- 4 TH SOGCEDirectoryDocumento40 páginas4 TH SOGCEDirectoryVPrasarnth RaajAinda não há avaliações
- List of Tables, Figures & Executive SummaryDocumento4 páginasList of Tables, Figures & Executive SummaryVPrasarnth RaajAinda não há avaliações
- 2011 AccentDocumento8 páginas2011 AccentVPrasarnth RaajAinda não há avaliações
- Prototype Development To Test EOR Methods: Joana - Sanches@ist - Utl.ptDocumento10 páginasPrototype Development To Test EOR Methods: Joana - Sanches@ist - Utl.ptVPrasarnth RaajAinda não há avaliações
- Petroleum Home Assignment OutlineDocumento3 páginasPetroleum Home Assignment OutlineVPrasarnth RaajAinda não há avaliações
- Jadual Waktu MOG Semester 2, Sesi 2014.2015Documento1 páginaJadual Waktu MOG Semester 2, Sesi 2014.2015VPrasarnth RaajAinda não há avaliações
- All It DesktopDocumento4 páginasAll It DesktopNatasya WillmoreAinda não há avaliações
- LCADocumento1 páginaLCAVPrasarnth RaajAinda não há avaliações
- Bioprocess QuizDocumento4 páginasBioprocess QuizVPrasarnth RaajAinda não há avaliações
- British PetroleumDocumento10 páginasBritish PetroleumVPrasarnth RaajAinda não há avaliações
- Petroleum Assignment - UOP Q-Max Cumene Process (FULL)Documento436 páginasPetroleum Assignment - UOP Q-Max Cumene Process (FULL)VPrasarnth Raaj100% (12)
- Rajni and ManagementDocumento12 páginasRajni and ManagementVPrasarnth RaajAinda não há avaliações
- Milestone 5 Outline KC40203 2012Documento1 páginaMilestone 5 Outline KC40203 2012VPrasarnth RaajAinda não há avaliações
- Comparison of Different Collection Efficiency Models For Venturi ScrubbersDocumento10 páginasComparison of Different Collection Efficiency Models For Venturi ScrubbersPassmore DubeAinda não há avaliações
- Packed Tower AbsorberDocumento58 páginasPacked Tower AbsorberCelestino Montiel MaldonadoAinda não há avaliações
- Vitamins and MineralsDocumento4 páginasVitamins and MineralsNoreen Orro BernalAinda não há avaliações
- Vitamins: (DR Pauling Recommendation, Svaki Dan Uzeti)Documento3 páginasVitamins: (DR Pauling Recommendation, Svaki Dan Uzeti)Veky VictoriaAinda não há avaliações
- Transcription PPTDocumento69 páginasTranscription PPTEllieAinda não há avaliações
- Protein Targeting: After This Chapter, You Should Be Able ToDocumento12 páginasProtein Targeting: After This Chapter, You Should Be Able ToAmeena SherinAinda não há avaliações
- HW As Cie Biological MoleculesDocumento17 páginasHW As Cie Biological Moleculestdmvq7yhggAinda não há avaliações
- Cara Kerja Kimia Klinik SpektrofotometriDocumento1 páginaCara Kerja Kimia Klinik SpektrofotometrilabrsiabcmAinda não há avaliações
- 9 Csomes DNADocumento6 páginas9 Csomes DNAKenth Roger A. MaquilingAinda não há avaliações
- Enzymes PowerpointDocumento39 páginasEnzymes PowerpointRizky Yudha IrawanAinda não há avaliações
- Catalog - Actosome WhitenolDocumento1 páginaCatalog - Actosome Whitenolanh.tranAinda não há avaliações
- Bioc 470 Notes Lecture 1Documento3 páginasBioc 470 Notes Lecture 1anon_900161280Ainda não há avaliações
- Dr. H. Bambang Ermanadji, MM, AkupunkturisDocumento19 páginasDr. H. Bambang Ermanadji, MM, AkupunkturisImam AzharAinda não há avaliações
- Janeway's Immunobiology 8thDocumento892 páginasJaneway's Immunobiology 8thSergio Falcon Rivera92% (12)
- Epigenetic SDocumento19 páginasEpigenetic SNoor SabahAinda não há avaliações
- Detection of Nucleic Acid Sequences by The Polymerase Chain Reaction TechniqueDocumento9 páginasDetection of Nucleic Acid Sequences by The Polymerase Chain Reaction TechniqueEric GozzerAinda não há avaliações
- Origami Protein HandoutDocumento4 páginasOrigami Protein HandoutRaajeswaran BaskaranAinda não há avaliações
- Whole Life Sciences GlossaryDocumento108 páginasWhole Life Sciences Glossaryramakrishnansatish100% (4)
- Amino Acids NotesDocumento17 páginasAmino Acids NotesNguyễn SunAinda não há avaliações
- Tema 3 - Inmune Responses To ImplantsDocumento18 páginasTema 3 - Inmune Responses To ImplantsIsrael GonzálezAinda não há avaliações
- DNAmazing Challenge (Protein Synthesis)Documento6 páginasDNAmazing Challenge (Protein Synthesis)DivineAinda não há avaliações
- Syllabus BMB 401 US2012Documento3 páginasSyllabus BMB 401 US2012ChiOfGreeAinda não há avaliações
- Ramji 2015Documento13 páginasRamji 2015AlexandraAinda não há avaliações
- 2023 - Enzymes Worksheet NotesDocumento9 páginas2023 - Enzymes Worksheet NotesFishy Sap100% (1)
- BlottingDocumento4 páginasBlottingSai Sridhar100% (1)
- 02 Enzyme CatalysisDocumento42 páginas02 Enzyme CatalysisOscar BravoAinda não há avaliações
- Bio MoleculesDocumento18 páginasBio MoleculessofhiabiancagolezAinda não há avaliações
- Detergent EnzymesDocumento2 páginasDetergent EnzymesAmer KasidehAinda não há avaliações
- Antipurinergic Therapy For Autism-An In-Depth Review.Documento15 páginasAntipurinergic Therapy For Autism-An In-Depth Review.Miguel Romero100% (1)
- MBI, BT Apps Mastering NcertDocumento20 páginasMBI, BT Apps Mastering NcertDurgadeviAinda não há avaliações
- Factors Affecting DNA Denaturation and RenaturationDocumento4 páginasFactors Affecting DNA Denaturation and RenaturationVenkateswarlu Yadavalli100% (2)
- Clinically Significant EnzymesDocumento3 páginasClinically Significant EnzymesNoreen B. BañagadoAinda não há avaliações