Escolar Documentos
Profissional Documentos
Cultura Documentos
Tray Drying Report PDF
Enviado por
Joson ChaiTítulo original
Direitos autorais
Formatos disponíveis
Compartilhar este documento
Compartilhar ou incorporar documento
Você considera este documento útil?
Este conteúdo é inapropriado?
Denunciar este documentoDireitos autorais:
Formatos disponíveis
Tray Drying Report PDF
Enviado por
Joson ChaiDireitos autorais:
Formatos disponíveis
UEMK2411 CHEMICAL ENGINEERING LABORATORY I TITLE OF EXPERIMENT Tray Drying OBJECTIVES
GROUP 09
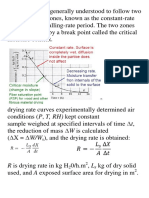
The objectives of this experiment were to perform drying test on solids, to investigate the effects of air velocity on drying rate and to perform heat and mass transfer analysis of a drying process. INTRODUCTION Like evaporation, drying is a mass-transfer process resulting in the removal of water or moisture from a process stream. While evaporation increases the concentration of non-volatile components in solution, in drying processes the final product is a solid. Drying processes reduce the solute or moisture level to improve the storage and handling characteristics of the product, maintain product quality during storage and transportation and reduce freight cost (less water to ship). Drying of solids in certain cases like wood, ceramics and soap has a remarkable fathom of the internal mechanism obtained that allows control of product standard. Surveys of drying of solids have been made from the so-called external viewpoint, wherein the effects of the external drying medium like air velocity, humidity, temperature and wet material shape and subdivision are studied with respect to their influence on the drying rate. Tray dryer is used for drying solids by air or removes the moist vapours which must be supported by trays. Trays are designed to force the air to follow a longer zigzag route which increases the contact time between food and air, thus improve its efficiency. Heating may be by an air current sweeping across the trays, by conduction from heated trays or heated shelves on which the trays lie, or by radiation from heated surfaces. It is most suitable in terms of cost and output when the production rate is small. MATERIAL AND EQUIPMENT The equipment used in this experiment was the tray dryer unit. This unit is designed to demonstrate the theoretical and practical aspects of solids drying.
UEMK2411 CHEMICAL ENGINEERING LABORATORY I
GROUP 09
Axial Fan
Digital scale
Control Panel Drying Chamber RESULTS AND CALCULATIONS Part 1: Initial mass of rice / dry sand mass of tray amount of water added total mass (wet sand + trays + holder) Moisture content percentage cross sectional area of trays Axial fan Frequency Heater temperature 0.632 kg 0.253 kg 0.051 kg 0.936 kg 7.467057101 % 0.0752 m^2 8 Hz 65 deg C
Time (min) 0
Mass, m (kg) 0.936
T1 Dry Bulb Inlet ( ) 41.7
T2 Wet Bulb Inlet ( ) 30.5
T3 Dry Bulb Outlet ( ) 37.2
T4 Wet Bulb Outlet ( ) 27.5 2
UEMK2411 CHEMICAL ENGINEERING LABORATORY I 10 20 30 40 50 60 70 80 90 0.928 0.92 0.904 0.898 0.891 0.884 0.879 0.874 0.868 45.9 45.8 40.0 38.7 47.1 47.5 48.2 48.4 47.9 36.8 38.2 37.0 36.8 38.8 37.5 36.8 35.0 39.2 40.7 40.9 39.0 38.7 42.6 42.9 46.7 45.9 45.3
GROUP 09 29.5 30.5 31.3 30.8 31.3 32.0 32.0 35.6 31.0
Time (min)
Mass of evaporated water (kg)
Product Moisture Content (%)
Air Humidity before tray (%)
Air humidity after tray (%)
drying rate (kg/min)
0 10 20 30 40 50 60 70 80 90
0.000 0.008 0.016 0.032 0.038 0.045 0.052 0.057 0.062 0.068
7.47 6.37 5.25 2.92 2.02 0.94 -0.16 -0.96 -1.77 -2.76
45.10 56.03 62.21 82.46 88.44 59.88 53.35 48.56 41.80 58.63
47.92 44.35 47.71 58.22 57.11 45.36 47.11 36.39 51.31 36.65
0 0.0008 0.0008 0.0016 0.0006 0.0007 0.0007 0.0005 0.0005 0.0006
Calculations: By using the sample from Mass balance: 3 ,
UEMK2411 CHEMICAL ENGINEERING LABORATORY I
GROUP 09
( )
( ( )
Provided that
is the total mass and
is the tray mass.
( ( ) [ (
) )]
for sand is obtained from Table A-14 from the Fundamentals of Heat and Mass Transfer 6th ed. by Frank P.Incropera.
UEMK2411 CHEMICAL ENGINEERING LABORATORY I
GROUP 09
Graph of Moisture Content(%) vs Time(min)
8 Moisture Content(%) 6 4 2 0 -2 -4 0 20 40 60 80 100
Time(min)
UEMK2411 CHEMICAL ENGINEERING LABORATORY I
GROUP 09
Graph of Drying Rate(g/min) vs Moisture Content(%)
1.8 1.6 1.4 1.2 1 0.8 0.6 0.4 0.2 0 -2 0 2 4 Moisture Content(%) 6 8 Drying Rate(g/min) -4
Part 2: Initial mass of rice / dry sand mass of tray amount of water added total mass (wet sand + trays + holder) Moisture content percentage cross sectional area of trays Axial fan Frequency Heater temperature 0.678 kg 0.253 kg 0.053 kg 0.931 kg 7.250341997 % 0.0752 m^2 10 min 65 ( )
Time (min)
Mass, m (kg)
T1 Dry Bulb Inlet ( )
T2 Wet Bulb Inlet ( ) 36.2 39.0 32.6 34.3 37.5
T3 Dry Bulb Outlet ( )
T4 Wet Bulb Outlet ( )
8 9 10 11 12
0.924 0.917 0.91 0.908 0.902
50.2 47.0 38.7 43.3 46.2
47.6 44.2 33.7 40.4 42.5
32.1 30.8 29.5 30.0 30.5
UEMK2411 CHEMICAL ENGINEERING LABORATORY I Fan Frequency (Hz) Mass of evaporated water (kg) Product Moisture Content (%) 8 9 10 11 12 0.007 0.014 0.021 0.023 0.029 6.29 5.34 4.38 4.10 3.28 41.00 61.07 65.75 55.02 57.81 Air Humidity before tray (%) Air humidity after tray (%) 34.55 38.86 73.60 47.35 42.63
GROUP 09 Drying rate (kg/min) g/min
0.0007 0.0007 0.0007 0.0002 0.0006
0.7 0.7 0.7 0.2 0.6
The calculation for the mass of evaporated water, moisture content and drying rate is the same and values will be provided in the tables.
( ( ) [ (
) )]
for sand is obtained from Table A-14 from the Fundamentals of Heat and Mass Transfer 6th ed. by Frank P.Incropera.
UEMK2411 CHEMICAL ENGINEERING LABORATORY I
GROUP 09
Graph of Drying rate(g/min) vs Fan frequency(Hz)
0.8 0.7 0.6 0.5 0.4 0.3 0.2 0.1 0 0 2 4 6 8 10 12 14 Fan Frequency(Hz) Drying rate(g/min)
DISCUSSIONS After conducting the experiment, the results obtained were tabulated and plotted into graphs. For the part 1 of the experiment, we were required to dry the sands in the tray drying equipment for about an interval of . Results were recorded in
. When plotting the graph of Moisture Content VS Drying
Time, the graph obtained found out to be having negative moisture content against drying time. Theoretically, the graph will be having a trend of linearly decreasing. However, the graph we obtained was decreasing and decreased further along the drying time. This might due the moisture of the sand initially contained some water. So when we dried the sand in the drying chamber for more than , we
obtained a negative value for the moisture content. This is due to the initial rate of moisture that we took as a reference point. Asides that, the graph of drying rate VS moisture content seems to be fluctuating along the -axis. Theoretically, the graph should be having a trend that will increase dramatically to a certain period. Then after that it will remain constant. However, the plots we got were like unstable drying rate. This might due to some reason that caused it to be. While conducting the experiment, the major problem that we encountered was the heater on the tray drier. According to the lab manual, the heater is supposed to heat up the air that being sucked into the chamber by the axial fan. Therefore, it has a sensor that will cause it to automatically control the power supply when the desired temperature reached and heat up against once the 8
UEMK2411 CHEMICAL ENGINEERING LABORATORY I
GROUP 09
temperature drops. However, the malfunction of the heater might leads to the inaccuracy of the experimental results whereby the heater did not heat up when the temperatures of the air flow drops until it has a very big gap with the set value. This decreased the accuracy of the dry bulb temperature and the wet bulb temperature that we need for calculations. Based on the results tabulated, we need to find the percentage of air humidity in the tray dryer. By having the values of wet bulb temperature and dry bulb temperature, with the help of psychrometric chart, the air humidity can be found. In our results, we used psychrometric calculator provided by Sugartech to find the air humidity. Based on the calculations, we calculated that the dryer has only an efficiency of which was extremely low for the efficiency.
For the Part 2 of the experiment, we manipulated the fan frequency to see the effect of fan frequency on the drying rate. The results were recorded down for an interval of for each set of fan frequency starting from to .
From the results obtained, a graph of Drying Rate VS Fan Frequency was plotted. The graph plotted shows a constant drying rate across fan frequency. But when the fan frequency was set to , the drying rate decrease instantaneously to .
This is because at that particular time, the tray dryer equipment became malfunction again. The heater did not heat up the air flow to the temperature that we set which was . This cause the air temperature to drop and thus the humidity inside the
chamber at that moment increase. When the air humidity in the chamber increased, the evaporation of water from the sand hard to occur, thus the drying rate was low. However, the heater automatically turned on again when we change the frequency to . That was why the drying rate increase again after . Thus, after
performing the calculations, we obtained that the efficiency of the same tray drying unit has a value of Part 1. There were some precautions that need to be taken into account during the experiment. Firstly, the heater must be switched on and the fan must be turned on. Next, the wet bulb temperature sensor must always being make sure it was wet enough. In addition, when measuring the mass of the tray, sands and water, the weigh balance reading must be stable before the reading was recorded as the balance 9 . It was much better compared to its efficiency of dryer in
UEMK2411 CHEMICAL ENGINEERING LABORATORY I
GROUP 09
is too sensitive until a single movement on it will affect the results. Safety measure had been taken when dealing with the tray dryer unit as it might be a very hot surface especially the heating element, thus gloves are prepared in order to prevent burn injuries. CONCLUSION As a conclusion, the objectives of the experiment were achieved. The drying test was successfully performed on the solids used sands and the effect of air velocity on drying rate were studied. Asides that, the heat and mass transfer analyses of a drying process were performed by obtaining the values in the calculations.
REFERENCES 1. Drying of solids. (n.d.). Retrieved July 29, 2011, from Classof1: http://classof1.com/homework_answers/chemical_engineering/drying_of_soli ds/ 2. Henley, E. J., Seader, J., & Roper, D. (2011). Separation Process Principles 3rd Edition. Asia: John Wiley & Sons Pte Ltd. 3. Incropera, F. P., Dewitt, D. P., Bergman, T. L., & Lavine, A. S. (2005). Fundamentals of Heat and Mass Transfer. Asia: John Wiley & Son Inc. 4. Solids Drying. (n.d.). Retrieved July 29, 2011, from GEA Barr Rosin: http://www.barr-rosin.com/applications/solids_drying.asp 5. Fellows, P.J. (2000). Food Processing Technology - Principles and Practice (2nd Edition). (pp: 309-340). Woodhead Publishing. Online version available at: http://www.knovel.com/web/portal/browse/display?_EXT_KNOVEL_DISPL AY_bookid=213&VerticalID=0
10
Você também pode gostar
- 13 Test BankDocumento45 páginas13 Test BankJonathan HuAinda não há avaliações
- Packed Bed Distillation Column Lab ReportDocumento13 páginasPacked Bed Distillation Column Lab ReportShamini Sathivel100% (6)
- (Lab Report Operation Unit) Experiment 5: INTRODUCTION TO DRYING PROCESS: DRYING A SOLIDDocumento9 páginas(Lab Report Operation Unit) Experiment 5: INTRODUCTION TO DRYING PROCESS: DRYING A SOLIDFazsroul84% (19)
- Rhodes Solutions Ch13Documento1 páginaRhodes Solutions Ch13wtss123100% (1)
- CMT 450 Tray Drier B Laboratory ReportDocumento16 páginasCMT 450 Tray Drier B Laboratory ReportAyish MataAinda não há avaliações
- Catalyst Deactivation Models & MitigationDocumento96 páginasCatalyst Deactivation Models & MitigationJoson ChaiAinda não há avaliações
- LAB REPORT-Gas AbsorptionDocumento16 páginasLAB REPORT-Gas Absorptionmizizasbonkure90100% (1)
- Fluidisation ReportDocumento29 páginasFluidisation ReportBenjamin Jie100% (2)
- Nucleate BoilingDocumento376 páginasNucleate Boilingrzlisk011713100% (1)
- Rhodes Solutions Ch4Documento19 páginasRhodes Solutions Ch4Joson Chai100% (4)
- Cooling Tower Exp 3Documento16 páginasCooling Tower Exp 3mahmudAinda não há avaliações
- Lab Report On Fitting LossDocumento13 páginasLab Report On Fitting LossJyiou Yimushi100% (1)
- Rhodes Solutions Ch12Documento7 páginasRhodes Solutions Ch12Joson ChaiAinda não há avaliações
- Designing Large Package BoilersDocumento3 páginasDesigning Large Package BoilersdemonarundoAinda não há avaliações
- Experiment About Air-Conditioning UnitDocumento32 páginasExperiment About Air-Conditioning UnitVerlon Vincent100% (1)
- Rhodes Solutions Ch10Documento15 páginasRhodes Solutions Ch10Joson Chai100% (5)
- Rhodes Solutions Chapter 2Documento16 páginasRhodes Solutions Chapter 2niquee9ner78% (9)
- Permeability of Porous Media Using Liquid ParameterDocumento18 páginasPermeability of Porous Media Using Liquid ParameterIdham ArifAinda não há avaliações
- Climbing FilmDocumento34 páginasClimbing FilmTunji Aminu100% (1)
- Laboratory Report 10 (Drag On A Sphere)Documento16 páginasLaboratory Report 10 (Drag On A Sphere)Wang WeiXinAinda não há avaliações
- Report Tray DryerDocumento15 páginasReport Tray DryerSharing Caring75% (4)
- Lab Report Distillation Column PDFDocumento26 páginasLab Report Distillation Column PDFGebrina RizkiaAinda não há avaliações
- LAB 3 Radiation Heat TrasferDocumento16 páginasLAB 3 Radiation Heat TrasferMastura Ahmad Termizi89% (19)
- Full Report Fluid MixingDocumento21 páginasFull Report Fluid Mixingsyafiq100% (1)
- Heat Transfer Lab Report Exp 1Documento14 páginasHeat Transfer Lab Report Exp 1Hafizuddin Adzhar100% (2)
- Acetone DiffusionDocumento15 páginasAcetone DiffusionArmaan Hussain40% (5)
- Chemical Engineering Laboratory For Unit Operations 2 (Adamson University and de La Salle University)Documento91 páginasChemical Engineering Laboratory For Unit Operations 2 (Adamson University and de La Salle University)Micahmae Morbs100% (1)
- .Lab Report Tray DryerDocumento22 páginas.Lab Report Tray Dryernajwasyafiqah_1Ainda não há avaliações
- Cooling Tower LabDocumento33 páginasCooling Tower Labkeckstand100% (2)
- Tubular Heat Exchanger Lab ReportDocumento21 páginasTubular Heat Exchanger Lab ReportMuhammad Zafrulhafiz100% (4)
- Drying 2 Class Notes PDFDocumento18 páginasDrying 2 Class Notes PDFFarouk Bassa100% (1)
- Lab Report Spray DryerDocumento4 páginasLab Report Spray DryerOh Hui XuanAinda não há avaliações
- CPE533 Gas Absorption Full Lab ReportDocumento30 páginasCPE533 Gas Absorption Full Lab ReportFazsroul82% (11)
- Tray Dryer ExperimentDocumento13 páginasTray Dryer Experimentgeek311295% (37)
- Evaporation (Lab Report)Documento5 páginasEvaporation (Lab Report)Ynno0% (1)
- Moisture Content and Drying Rate CalculationsDocumento47 páginasMoisture Content and Drying Rate Calculationshels245100% (2)
- Drying of Solids (Sand)Documento15 páginasDrying of Solids (Sand)Mahe Rukh75% (4)
- Gas Diffusion CoefficientDocumento17 páginasGas Diffusion CoefficientJames Edwards82% (11)
- Tray DryerDocumento16 páginasTray Dryermirdza94Ainda não há avaliações
- Heat Exchanger Lab ReportDocumento5 páginasHeat Exchanger Lab ReportRam Krishna Singh79% (29)
- Drying Pineapple in a Tray DryerDocumento17 páginasDrying Pineapple in a Tray DryerJaymacAinda não há avaliações
- Friction Losses and Pump HorsepowerDocumento4 páginasFriction Losses and Pump HorsepowerChristian Lucio RanadaAinda não há avaliações
- Tray Drying Experiment: Effects of Air Velocity on Drying RateDocumento13 páginasTray Drying Experiment: Effects of Air Velocity on Drying RateSrinyanavel ஸ்ரீஞானவேல்75% (4)
- Tray Dryer - Lab ReportDocumento9 páginasTray Dryer - Lab Reportinurhadi1350% (2)
- Energy BalanceDocumento16 páginasEnergy BalancewizlanAinda não há avaliações
- Result & Discussion Exp Tray DryerDocumento6 páginasResult & Discussion Exp Tray Dryerfatin noraini71% (7)
- Discussion Tray DryerDocumento3 páginasDiscussion Tray DryerIskandar ZulkarnainAinda não há avaliações
- TRAY DRYER EXPERIMENT: DRYING CURVES FOR WET SANDDocumento8 páginasTRAY DRYER EXPERIMENT: DRYING CURVES FOR WET SANDcrumsy100% (2)
- Effects of Particle Size On DryingDocumento4 páginasEffects of Particle Size On DryingAshley Perida50% (2)
- Lab Report - Distillation of Bubble CapDocumento21 páginasLab Report - Distillation of Bubble Capratish100% (1)
- Lab Report Performance of A Steam Plant LatestDocumento16 páginasLab Report Performance of A Steam Plant LatestM Asrar SidonAinda não há avaliações
- Apparatus, Procedure, Recommendation Tray DryerDocumento4 páginasApparatus, Procedure, Recommendation Tray DryerillyzlAinda não há avaliações
- Heat ExchangerDocumento21 páginasHeat ExchangersedamyrulAinda não há avaliações
- Particle DragDocumento21 páginasParticle DragKHAIRUNISAAinda não há avaliações
- Investigation of Liquid-Solid and Gas-Solid Fluidized BedDocumento18 páginasInvestigation of Liquid-Solid and Gas-Solid Fluidized Bedmahbub1332100% (1)
- Lab Report TPP Experiment 3Documento10 páginasLab Report TPP Experiment 3Nurul Najwa100% (1)
- Liquid Diffusion CoefficientDocumento15 páginasLiquid Diffusion CoefficientmuhdfadzlihadiAinda não há avaliações
- Literature Review of Distillation ColumnDocumento15 páginasLiterature Review of Distillation ColumnAhmad Ifwat100% (3)
- Experiment 2 - Study of Packed Column DistillationDocumento7 páginasExperiment 2 - Study of Packed Column DistillationAdawiyah Az-zahra100% (1)
- Refrigeration Unit Lab Report FKKDocumento28 páginasRefrigeration Unit Lab Report FKKKicauan KataAinda não há avaliações
- Lab Report 5Documento12 páginasLab Report 5Norhanisah Zamri Rcsu100% (1)
- CHE504 - Lab Report On Distillation ColuDocumento27 páginasCHE504 - Lab Report On Distillation ColuMuhammad Irfan MalikAinda não há avaliações
- Lab 2 Full Report PDFDocumento20 páginasLab 2 Full Report PDFmuhammad ilyas100% (1)
- Optimized Solids Suspension: Achieving Uniform Dispersion is Critical to Product QualityDocumento7 páginasOptimized Solids Suspension: Achieving Uniform Dispersion is Critical to Product QualitymichsantosAinda não há avaliações
- Tray DryerDocumento22 páginasTray DryerjuaxxoAinda não há avaliações
- Heat Transfer Experiment ObjectivesDocumento16 páginasHeat Transfer Experiment Objectivesfaranimohamed75% (4)
- Unit Operation Laboratory 2 (CCB 3062)Documento7 páginasUnit Operation Laboratory 2 (CCB 3062)Carl Erickson100% (1)
- Tray Dryer PDFDocumento17 páginasTray Dryer PDFAdibah AyuniAinda não há avaliações
- Tray DryerDocumento15 páginasTray Dryerfaez94Ainda não há avaliações
- Respiration Calorimeters for Studying the Respiratory Exchange and Energy Transformations of ManNo EverandRespiration Calorimeters for Studying the Respiratory Exchange and Energy Transformations of ManAinda não há avaliações
- Rhodes Solutions Ch14Documento7 páginasRhodes Solutions Ch14Joson ChaiAinda não há avaliações
- Coloumetric Titration - Gamry InstrumentDocumento3 páginasColoumetric Titration - Gamry InstrumentJoson ChaiAinda não há avaliações
- Rhodes Solutions Ch9Documento9 páginasRhodes Solutions Ch9Joson ChaiAinda não há avaliações
- Rhodes Solutions Ch15Documento4 páginasRhodes Solutions Ch15Joson ChaiAinda não há avaliações
- Rhodes Solutions Ch11Documento6 páginasRhodes Solutions Ch11Joson Chai100% (1)
- Lab 5 Heat ExchangerDocumento4 páginasLab 5 Heat ExchangerJoson ChaiAinda não há avaliações
- Example Event Tree AnalysisDocumento28 páginasExample Event Tree AnalysisJoson ChaiAinda não há avaliações
- 5 Industr HygieneDocumento89 páginas5 Industr HygieneJoson ChaiAinda não há avaliações
- UKMM1043 Basic Economic, Accounting and Management May 2012 QuestionDocumento2 páginasUKMM1043 Basic Economic, Accounting and Management May 2012 QuestionJoson ChaiAinda não há avaliações
- Drying Lab 1Documento6 páginasDrying Lab 1Joson ChaiAinda não há avaliações
- Determination of Gas Diffusivity - ResultsDocumento5 páginasDetermination of Gas Diffusivity - ResultsJoson ChaiAinda não há avaliações
- Title of Experiment: Gas Absorption Objective of Experiment: To Calculate The Mass Transfer Coefficient of Oxygen Through TheDocumento2 páginasTitle of Experiment: Gas Absorption Objective of Experiment: To Calculate The Mass Transfer Coefficient of Oxygen Through TheJoson ChaiAinda não há avaliações
- UEME3213 Assignment 1 2013Documento2 páginasUEME3213 Assignment 1 2013Joson ChaiAinda não há avaliações
- Lect 10 - Mechanism DescriptionDocumento18 páginasLect 10 - Mechanism DescriptionJoson ChaiAinda não há avaliações
- Muhga35vb PDFDocumento36 páginasMuhga35vb PDFMihaela CondratAinda não há avaliações
- Chapter 6 - Assignment 3Documento2 páginasChapter 6 - Assignment 3Arielle Emperador De GuzmanAinda não há avaliações
- Dialnet CalculationOfMarineAirConditioningSystemsBasedOnEn 6769359Documento15 páginasDialnet CalculationOfMarineAirConditioningSystemsBasedOnEn 6769359Om Parkash SharmaAinda não há avaliações
- Gen. Chemistry 2 - LAS NO. 2 - JohnJosephS - Castro - VERSION 4Documento5 páginasGen. Chemistry 2 - LAS NO. 2 - JohnJosephS - Castro - VERSION 4Hannah DennisehAinda não há avaliações
- Quiz - Thermochem PRACTICE ANSWERSDocumento2 páginasQuiz - Thermochem PRACTICE ANSWERSliana.mirlohi4Ainda não há avaliações
- Psychrometrics: Moist Air PropertiesDocumento12 páginasPsychrometrics: Moist Air PropertiesMotaz H OthmanAinda não há avaliações
- Lecture34 1Documento38 páginasLecture34 1vamsikrishna14Ainda não há avaliações
- Chemical Process CalculationsDocumento3 páginasChemical Process CalculationsRusheenRathore0% (1)
- Study of Hybrid Solar Desalination SystemDocumento2 páginasStudy of Hybrid Solar Desalination SystemDrEmadEl-SaidAinda não há avaliações
- Bulletin Manifold and Drip-Leg Heaters 121212Documento2 páginasBulletin Manifold and Drip-Leg Heaters 121212Hamada ElsharawyAinda não há avaliações
- Psychrometric Chart PDFDocumento2 páginasPsychrometric Chart PDFvitaliskcAinda não há avaliações
- Thermodynamics Chapter on Energy, Work, Heat & First LawDocumento42 páginasThermodynamics Chapter on Energy, Work, Heat & First Lawn4alpacaAinda não há avaliações
- Bimetallic Thermometer PDFDocumento3 páginasBimetallic Thermometer PDFHesti Nur AiniAinda não há avaliações
- Tadao AndoDocumento42 páginasTadao AndoAlexandra TanaseAinda não há avaliações
- Embraco Standard Aftermarket Portfolio PDFDocumento2 páginasEmbraco Standard Aftermarket Portfolio PDFJOR4CHAinda não há avaliações
- Increase in Energy Efficiency of A Steel Billet Reheating Furnace by Heat Balance Study and Process ImprovementDocumento8 páginasIncrease in Energy Efficiency of A Steel Billet Reheating Furnace by Heat Balance Study and Process ImprovementRaheel NadeemAinda não há avaliações
- RAC SyllabusDocumento2 páginasRAC SyllabusjaiAinda não há avaliações
- JB34 v1-0 14apr10 W502 Slides Day 2Documento148 páginasJB34 v1-0 14apr10 W502 Slides Day 2Janardhan Rao MalakapalliAinda não há avaliações
- Condensing Unit Technical Data Sheet CMLT90LD_NDocumento2 páginasCondensing Unit Technical Data Sheet CMLT90LD_NNaqqash SajidAinda não há avaliações
- Types of Ac SystemsDocumento26 páginasTypes of Ac SystemsVarsha PatelAinda não há avaliações
- DataSheet - StorMaxx NP Storage TankDocumento4 páginasDataSheet - StorMaxx NP Storage TankSunMaxx SolarAinda não há avaliações
- Clase 3 y 4Documento34 páginasClase 3 y 4EdinberSPAinda não há avaliações
- Equilibrium Lab: Gizmo Simulations for Chemical EquilibriumDocumento11 páginasEquilibrium Lab: Gizmo Simulations for Chemical EquilibriumGabriela PopaAinda não há avaliações
- Chem 2 Q1 Module 1 Attractive ForcesDocumento9 páginasChem 2 Q1 Module 1 Attractive ForcesPrincess Venita BerganteAinda não há avaliações
- Freezing Point Depression 2Documento2 páginasFreezing Point Depression 2Joon Bok NamleeAinda não há avaliações
- Experiment 1 Linear and Radial Heat Conduction (Spreadsheet)Documento10 páginasExperiment 1 Linear and Radial Heat Conduction (Spreadsheet)Jing ZeAinda não há avaliações