Escolar Documentos
Profissional Documentos
Cultura Documentos
Chapter 6 Section 2 Outline
Enviado por
api-2634550560 notas0% acharam este documento útil (0 voto)
120 visualizações4 páginasTítulo original
chapter 6 section 2 outline
Direitos autorais
© © All Rights Reserved
Formatos disponíveis
DOCX, PDF, TXT ou leia online no Scribd
Compartilhar este documento
Compartilhar ou incorporar documento
Você considera este documento útil?
Este conteúdo é inapropriado?
Denunciar este documentoDireitos autorais:
© All Rights Reserved
Formatos disponíveis
Baixe no formato DOCX, PDF, TXT ou leia online no Scribd
0 notas0% acharam este documento útil (0 voto)
120 visualizações4 páginasChapter 6 Section 2 Outline
Enviado por
api-263455056Direitos autorais:
© All Rights Reserved
Formatos disponíveis
Baixe no formato DOCX, PDF, TXT ou leia online no Scribd
Você está na página 1de 4
Name:
Chapter 6 Section 2 Outline
Chemical Reactions
Main Idea: Chemical reactions allow living things to grow, develop, reproduce, and adapt.
Essential Questions:
What are the parts of a chemical reaction?
How can energy changes be related to chemical reactions?
What is the importance of enzymes in living organisms?
I. Reactants and Products
A ___chemical_____ __reaction______ is the process by which atoms or groups
of atoms in substances are reorganized into different substances.
Chemical ___bonds__ are broken or formed during a chemical reaction.
Clues that a chemical reaction has taken place:
Production of ___heat_ or __light___
Formation of a new __, ______, or _____
Substances can also undergo physical changes, which change the appearance but
not the composition.
A. Chemical Equations
In written chemical equations, __chemical______ __formulas______
describe the substances in the reaction and ___arrows___ indicate
the process of change.
___reactants______ are the starting substances, on the left side of
the arrow.
__products______ are the substances formed during the reaction, on
the right side of the arrow.
Reactants Products
The arrow can be read as yields or react to form.
Balanced equations
In chemical reactions, matter cannot be __created
_____ or _________.
This principal is called the _law__ of
__conservation__________ of ___mass_
All chemical equations must show this balance of mass.
The number of _atoms____ of each element on the reactant
side must equal the number of _atoms____ of the same
element on the product side.
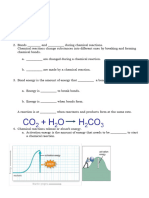
C
6
H
12
O
6
+ 6O
2
6CO
2
+ 6H
2
O
Glucose and oxygen react to form carbon dioxide and water.
Get It? Explain why chemical equations must be balanced. ___________________because
matter cannot be created nor
destroyed______________________________________________________________________
___________________________________________________________________
Calculate the number of atoms of each element in the chemical equation below. Record the
information in the table.
CH
4
+ 2O
2
CO
2
+ 2H
2
O
Element Symbol Element Name Number of Atoms
(reactant side)
Number of Atoms
(product side)
c carbon
h hydrogen
o oxygen
Analyze is the equation balanced? ______cant see no glasses__________
II. Energy of Reactions
Activation Energy
The minimum amount of energy needed for reactants to form products is
called ____activation _______ ___energy___.
Some reactions happen rarely due to the high activation energy required.
Energy change in chemical reactions
Reactions that release energy in the form of heat are
____exothermic______.
As a result, the energy of the product is ____lower_ than the
energy of the reactants.
Reactions that absorb energy in the form of heat are
______endothermic_____.
As a result, the energy of the product is ____higher__ than the
energy of the reactants.
III. Enzymes
All living things are driven by _____chemical ___ ___reactions______.
Additional substances are needed to reduce activation energy and reaction time
in living organisms.
A ___enzyme is a substance that lowers the activation energy needed to start a
chemical reaction.
Catalysts do not change the amount of ___product____ produced, nor are they
used up during the reaction.
Special proteins called __enzymes_____ are the biological catalysts that speed
up the rate of chemical reactions in biological processes.
Most enzymes are specific to one reaction.
The reactants that bind to an enzyme are called ___substrates_______.
The specific location where a substrate binds on an enzyme is called the
___active___ ___sight_.
Factors such as _ph._, __temperature_________, and other
___substances_______ affect enzyme activity.
Você também pode gostar
- States of Matter & Phase Equilibria: Essential Chemistry Self-Teaching GuideNo EverandStates of Matter & Phase Equilibria: Essential Chemistry Self-Teaching GuideAinda não há avaliações
- Chapter 6 Section 2 OutlineDocumento4 páginasChapter 6 Section 2 Outlineapi-263455059Ainda não há avaliações
- Stoichiometry: Essential Chemistry Self-Teaching GuideNo EverandStoichiometry: Essential Chemistry Self-Teaching GuideAinda não há avaliações
- Chapter 6 Section 2 OutlineDocumento4 páginasChapter 6 Section 2 Outlineapi-263455041Ainda não há avaliações
- Chapter 7 Chemical ReactionsDocumento10 páginasChapter 7 Chemical Reactionsapi-30718309Ainda não há avaliações
- College Organic Chemistry Semester II: Practice Questions with Detailed ExplanationsNo EverandCollege Organic Chemistry Semester II: Practice Questions with Detailed ExplanationsAinda não há avaliações
- Factors Affecting The Rate of Chemical Reactions Notes Key 1Documento3 páginasFactors Affecting The Rate of Chemical Reactions Notes Key 1api-292000448Ainda não há avaliações
- Chapter+2 4-5+Guided+NotesDocumento3 páginasChapter+2 4-5+Guided+Noteskparsons938512Ainda não há avaliações
- Study Guide Energy and Chemical Change Student EditableDocumento7 páginasStudy Guide Energy and Chemical Change Student EditableRicki HanAinda não há avaliações
- Describing Chemical Reactions Fill inDocumento6 páginasDescribing Chemical Reactions Fill inMelva GuerraAinda não há avaliações
- Chapter 6 Section 2 OutlineDocumento4 páginasChapter 6 Section 2 Outlineapi-263455051Ainda não há avaliações
- Physical Science - Week 28Documento4 páginasPhysical Science - Week 28Mira VeranoAinda não há avaliações
- Q4 W7 8 Sci10 LawDocumento8 páginasQ4 W7 8 Sci10 LawBa BengAinda não há avaliações
- Chapter 6 Section 2 OutlineDocumento4 páginasChapter 6 Section 2 Outlineapi-263455052Ainda não há avaliações
- Chapter 6 Section 2 OutlineDocumento4 páginasChapter 6 Section 2 Outlineapi-263455076Ainda não há avaliações
- Review Chemical Reactions Test Chap 7Documento2 páginasReview Chemical Reactions Test Chap 7townsenr94Ainda não há avaliações
- Enzyme and PH Simulation WorksheetDocumento2 páginasEnzyme and PH Simulation WorksheetPurePureMilkAinda não há avaliações
- Activity SheetDocumento3 páginasActivity Sheetjanice alquizar100% (1)
- S2-2-07 - Balancing Chemical Equations - SimulationDocumento7 páginasS2-2-07 - Balancing Chemical Equations - SimulationVenus IgnacioAinda não há avaliações
- Chemical Energy LabDocumento2 páginasChemical Energy Labapi-251874912Ainda não há avaliações
- Biomolecules Packet 2011Documento6 páginasBiomolecules Packet 2011Nidhi Sisodia100% (1)
- Enzymes: Handbook: MetabolismDocumento2 páginasEnzymes: Handbook: Metabolismsmol ukeleleAinda não há avaliações
- ds24 Enzyme Review AssessmentDocumento8 páginasds24 Enzyme Review Assessmentapi-110789702Ainda não há avaliações
- q2 Law Science 9 Weeks 5 6Documento8 páginasq2 Law Science 9 Weeks 5 6Haydee Penalosa AunzoAinda não há avaliações
- The Functional Groups: Can You See Me?Documento4 páginasThe Functional Groups: Can You See Me?coalie galaxyAinda não há avaliações
- Ds35-Enzyme Laboratory Formative AssessmentDocumento7 páginasDs35-Enzyme Laboratory Formative Assessmentapi-110789702Ainda não há avaliações
- Bio 024 - Session 11 Sas Nursing (New Format) - WatermarkDocumento7 páginasBio 024 - Session 11 Sas Nursing (New Format) - WatermarkMaria Vannesa Anne SalvacionAinda não há avaliações
- Shs Stem - Biology 1: Quarter 1 - Module 14Documento21 páginasShs Stem - Biology 1: Quarter 1 - Module 14Grace RellosoAinda não há avaliações
- BIIODocumento4 páginasBIIOTimothy RandallAinda não há avaliações
- Student Exploration: Chemical EquationsDocumento10 páginasStudent Exploration: Chemical EquationsBen Campbell0% (1)
- Unit 2B Guided Notes 2022Documento6 páginasUnit 2B Guided Notes 2022Victoria WarrenAinda não há avaliações
- Physical Science: Quarter 3module 8/week 5 Collision Theory and Chemical Reaction RateDocumento16 páginasPhysical Science: Quarter 3module 8/week 5 Collision Theory and Chemical Reaction RateJennie KimAinda não há avaliações
- hssb0400s StudygdbDocumento12 páginashssb0400s StudygdbMohamed HassaneinAinda não há avaliações
- STM 005: General Chemistry 1 SAS Module #12Documento8 páginasSTM 005: General Chemistry 1 SAS Module #12Feedback Or BawiAinda não há avaliações
- PSCH 0814 KeyDocumento2 páginasPSCH 0814 KeyJan Ira RenolayanAinda não há avaliações
- Student Exploration: Collision TheoryDocumento7 páginasStudent Exploration: Collision TheoryNicholas CrowellAinda não há avaliações
- The Particle Theory of Matter: Chemistry: Atoms, Elements and CompoundsDocumento22 páginasThe Particle Theory of Matter: Chemistry: Atoms, Elements and CompoundsISTEBREK TAHERAinda não há avaliações
- Chemical ReactionDocumento4 páginasChemical ReactionABDULRAHMAN ABDRABBOAinda não há avaliações
- Gizmo - Chemical EquationsDocumento6 páginasGizmo - Chemical EquationsAngela0% (5)
- Sas 8Documento12 páginasSas 8Reizel GaasAinda não há avaliações
- Science Grade 10: Department of EducationDocumento17 páginasScience Grade 10: Department of EducationLeiAinda não há avaliações
- Photosynthesis GR 2017Documento2 páginasPhotosynthesis GR 2017api-440268289Ainda não há avaliações
- Student Exploration: Cell Energy CycleDocumento5 páginasStudent Exploration: Cell Energy Cyclevaleli123Ainda não há avaliações
- Chemical Reactions Notes and Problem SetsDocumento2 páginasChemical Reactions Notes and Problem SetsAlAr-JohnTienzoTimeniaAinda não há avaliações
- Physci Q1 Week5 2023Documento6 páginasPhysci Q1 Week5 2023MIKU ChanAinda não há avaliações
- Cell Energy SEDocumento7 páginasCell Energy SEFakunle TimileyinAinda não há avaliações
- Inv5 WhatDocumento3 páginasInv5 Whatapi-237500650Ainda não há avaliações
- BioGT - Enzyme Notes - Digging Deeper in The Class of ProteinsDocumento4 páginasBioGT - Enzyme Notes - Digging Deeper in The Class of Proteinsanna rothAinda não há avaliações
- Science 10 Worksheet Week5 8Documento4 páginasScience 10 Worksheet Week5 8Ren AkiraAinda não há avaliações
- Module - 1 - CO1-2 - StoichiometryDocumento6 páginasModule - 1 - CO1-2 - StoichiometryEmanuel JheadAinda não há avaliações
- AP Biology - Chapter 3 Water (Worksheet) Lookabaugh - Campbell Seventh EditionDocumento3 páginasAP Biology - Chapter 3 Water (Worksheet) Lookabaugh - Campbell Seventh EditionHenrique De LaraAinda não há avaliações
- Cabarles, K Las 9Documento9 páginasCabarles, K Las 9Krystyn Hope CabarlesAinda não há avaliações
- Biomolecules CornellDocumento4 páginasBiomolecules CornellDivineDoctorAinda não há avaliações
- Activity 12 Enzyme ActivityDocumento4 páginasActivity 12 Enzyme ActivityEllen May Diaz-ToringAinda não há avaliações
- HANDOUT - Photosynthesis and Cellular Respiration Review Sheet 2017Documento3 páginasHANDOUT - Photosynthesis and Cellular Respiration Review Sheet 2017ezraburrosAinda não há avaliações
- Na + CL ! Nacl: Guided NotesDocumento4 páginasNa + CL ! Nacl: Guided NotesSalina SalujaAinda não há avaliações
- Con Review WKSHTDocumento216 páginasCon Review WKSHTgkapsAinda não há avaliações
- Cape Biology Unit 1Documento3 páginasCape Biology Unit 1krypleAinda não há avaliações
- Bayla (Las 8)Documento12 páginasBayla (Las 8)Zeian Jacob BaylaAinda não há avaliações
- A8 KineticsDocumento6 páginasA8 KineticsGabby TanakaAinda não há avaliações
- Method For Preparing Anhydrous Iron ChloridesDocumento4 páginasMethod For Preparing Anhydrous Iron ChloridesVioleta GrigorasAinda não há avaliações
- Exogenic ProcessesDocumento19 páginasExogenic ProcessesMARIA LOURDES MENDOZAAinda não há avaliações
- MNL 67-2009 PDFDocumento128 páginasMNL 67-2009 PDFlolo100% (2)
- Neopuff Tech ManDocumento6 páginasNeopuff Tech ManoechimAinda não há avaliações
- CHEM 202 Ch7 Lec SlidesDocumento31 páginasCHEM 202 Ch7 Lec SlidesEilaf BadrAinda não há avaliações
- APARECE REYES Comparison of The Effectiveness of Activated Carbon From Cocos Nucifera Chemically Activated by Lemon Juice and Sodium Chloride For Water PurificationDocumento32 páginasAPARECE REYES Comparison of The Effectiveness of Activated Carbon From Cocos Nucifera Chemically Activated by Lemon Juice and Sodium Chloride For Water PurificationellaAinda não há avaliações
- 1.what The Principle of Potentiometric Methods, Voltametry, Polarography Coulometry Conductometry and ElectrophoresisDocumento18 páginas1.what The Principle of Potentiometric Methods, Voltametry, Polarography Coulometry Conductometry and ElectrophoresisarizkaAinda não há avaliações
- 06-02 Formation Damage Concepts and MechanismsDocumento22 páginas06-02 Formation Damage Concepts and MechanismsKader BakourAinda não há avaliações
- June 2020 (R) MSDocumento16 páginasJune 2020 (R) MSmrasin92Ainda não há avaliações
- FullDocumento1.175 páginasFull43 Trần Công VinhAinda não há avaliações
- Properties of Potash AlumDocumento8 páginasProperties of Potash AlumAminu TeslimAinda não há avaliações
- Allylic 1, 3Documento32 páginasAllylic 1, 3Pinaki MandalAinda não há avaliações
- 2012 CJC CH h2 p2 PromoDocumento12 páginas2012 CJC CH h2 p2 PromoDaniel ChuAinda não há avaliações
- Pressure Vessel Plates, Carbon Steel, For Moderate-And Lower-Temperature ServiceDocumento4 páginasPressure Vessel Plates, Carbon Steel, For Moderate-And Lower-Temperature ServiceGerman FavelaAinda não há avaliações
- BuratDocumento5 páginasBuratFreya AvellanoAinda não há avaliações
- Amu 2 (2017)Documento23 páginasAmu 2 (2017)Deep JattAinda não há avaliações
- Salcare Super 7 at 1Documento1 páginaSalcare Super 7 at 1xeon585100% (1)
- Plants: Phytochemicals: Extraction, Isolation, and Identification of Bioactive Compounds From Plant ExtractsDocumento23 páginasPlants: Phytochemicals: Extraction, Isolation, and Identification of Bioactive Compounds From Plant ExtractsPemi SusiskaAinda não há avaliações
- 9701 s06 QP 1Documento26 páginas9701 s06 QP 1G M Ali KawsarAinda não há avaliações
- F F O P L: Epofine - 740 / Finehard - 918 / Accelerator - 062Documento4 páginasF F O P L: Epofine - 740 / Finehard - 918 / Accelerator - 062Karishma Prabhu100% (1)
- Kathon LXDocumento15 páginasKathon LXIvanovich RuizAinda não há avaliações
- Systematic Analysis of Simple Inorganic SaltsDocumento6 páginasSystematic Analysis of Simple Inorganic Saltspavan AckermanAinda não há avaliações
- Atkins, P. Dan Paula, J.D., 2009, Elements of Physical Chemistry,, Fifth Edition, W. H. Freeman and Company, New York.-194-214-DikonversiDocumento34 páginasAtkins, P. Dan Paula, J.D., 2009, Elements of Physical Chemistry,, Fifth Edition, W. H. Freeman and Company, New York.-194-214-DikonversiNurul Qalby DikhaesaAinda não há avaliações
- Chemist 2014 Pharmacy Question PaperDocumento10 páginasChemist 2014 Pharmacy Question PaperKeshavVashisthaAinda não há avaliações
- Production: Ethers, Aliphatic)Documento6 páginasProduction: Ethers, Aliphatic)Harsh ShahAinda não há avaliações
- 6 8 S8 LAS Q4 Week 6 PempenaDocumento5 páginas6 8 S8 LAS Q4 Week 6 PempenaAyela Kim PiliAinda não há avaliações
- PH Measurement and Buffer PreparationDocumento3 páginasPH Measurement and Buffer PreparationaayaaaAinda não há avaliações
- Fall 2023 Chapter 7, 9 & 10 CHEM 517 Bioreactor Processing & ControlDocumento78 páginasFall 2023 Chapter 7, 9 & 10 CHEM 517 Bioreactor Processing & ControlskyliairojoAinda não há avaliações
- University of Cambridge International Examinations International General Certificate of Secondary Education Chemistry Paper 1 Multiple Choice May/June 2005 45 MinutesDocumento16 páginasUniversity of Cambridge International Examinations International General Certificate of Secondary Education Chemistry Paper 1 Multiple Choice May/June 2005 45 MinutesFranca OkechukwuAinda não há avaliações
- ICH Quality Guidelines: An Implementation GuideNo EverandICH Quality Guidelines: An Implementation GuideAndrew TeasdaleAinda não há avaliações
- Chemistry for Breakfast: The Amazing Science of Everyday LifeNo EverandChemistry for Breakfast: The Amazing Science of Everyday LifeNota: 4.5 de 5 estrelas4.5/5 (14)
- Periodic Tales: A Cultural History of the Elements, from Arsenic to ZincNo EverandPeriodic Tales: A Cultural History of the Elements, from Arsenic to ZincNota: 3.5 de 5 estrelas3.5/5 (137)
- It's Elemental: The Hidden Chemistry in EverythingNo EverandIt's Elemental: The Hidden Chemistry in EverythingNota: 4 de 5 estrelas4/5 (10)
- The Regenerative Grower's Guide to Garden Amendments: Using Locally Sourced Materials to Make Mineral and Biological Extracts and FermentsNo EverandThe Regenerative Grower's Guide to Garden Amendments: Using Locally Sourced Materials to Make Mineral and Biological Extracts and FermentsNota: 5 de 5 estrelas5/5 (3)
- Monkeys, Myths, and Molecules: Separating Fact from Fiction, and the Science of Everyday LifeNo EverandMonkeys, Myths, and Molecules: Separating Fact from Fiction, and the Science of Everyday LifeNota: 4 de 5 estrelas4/5 (1)
- Taste: Surprising Stories and Science About Why Food Tastes GoodNo EverandTaste: Surprising Stories and Science About Why Food Tastes GoodNota: 3 de 5 estrelas3/5 (20)
- The Nature of Drugs Vol. 1: History, Pharmacology, and Social ImpactNo EverandThe Nature of Drugs Vol. 1: History, Pharmacology, and Social ImpactNota: 5 de 5 estrelas5/5 (5)
- AP® Chemistry Crash Course, For the 2020 Exam, Book + Online: Get a Higher Score in Less TimeNo EverandAP® Chemistry Crash Course, For the 2020 Exam, Book + Online: Get a Higher Score in Less TimeNota: 5 de 5 estrelas5/5 (1)
- The Periodic Table: A Very Short IntroductionNo EverandThe Periodic Table: A Very Short IntroductionNota: 4.5 de 5 estrelas4.5/5 (3)
- Chemistry for Breakfast: The Amazing Science of Everyday LifeNo EverandChemistry for Breakfast: The Amazing Science of Everyday LifeNota: 4.5 de 5 estrelas4.5/5 (90)
- Guidelines for Defining Process Safety Competency RequirementsNo EverandGuidelines for Defining Process Safety Competency RequirementsNota: 3 de 5 estrelas3/5 (1)
- Handbook of Formulating Dermal Applications: A Definitive Practical GuideNo EverandHandbook of Formulating Dermal Applications: A Definitive Practical GuideAinda não há avaliações
- AP Chemistry Flashcards, Fourth Edition: Up-to-Date Review and PracticeNo EverandAP Chemistry Flashcards, Fourth Edition: Up-to-Date Review and PracticeAinda não há avaliações
- The Production of Volatile Oils and Perfumery Plants in the United StatesNo EverandThe Production of Volatile Oils and Perfumery Plants in the United StatesAinda não há avaliações
- The Nature of Drugs Vol. 1: History, Pharmacology, and Social ImpactNo EverandThe Nature of Drugs Vol. 1: History, Pharmacology, and Social ImpactNota: 5 de 5 estrelas5/5 (1)
- Formulating, Packaging, and Marketing of Natural Cosmetic ProductsNo EverandFormulating, Packaging, and Marketing of Natural Cosmetic ProductsAinda não há avaliações
- Essential Chemistry for Formulators of Semisolid and Liquid DosagesNo EverandEssential Chemistry for Formulators of Semisolid and Liquid DosagesNota: 5 de 5 estrelas5/5 (2)
- Chemistry All-in-One For Dummies (+ Chapter Quizzes Online)No EverandChemistry All-in-One For Dummies (+ Chapter Quizzes Online)Ainda não há avaliações
- The Billion-Dollar Molecule: The Quest for the Perfect DrugNo EverandThe Billion-Dollar Molecule: The Quest for the Perfect DrugNota: 5 de 5 estrelas5/5 (2)
- Is That a Fact?: Frauds, Quacks, and the Real Science of Everyday LifeNo EverandIs That a Fact?: Frauds, Quacks, and the Real Science of Everyday LifeNota: 5 de 5 estrelas5/5 (4)
- Organic Chemistry for Schools: Advanced Level and Senior High SchoolNo EverandOrganic Chemistry for Schools: Advanced Level and Senior High SchoolAinda não há avaliações