Escolar Documentos
Profissional Documentos
Cultura Documentos
Alkyne Metathesis & Enyne Metathesis
Enviado por
polypeptide0 notas0% acharam este documento útil (0 voto)
8 visualizações3 páginasorganometallic chemistry notes
Direitos autorais
© © All Rights Reserved
Formatos disponíveis
DOCX, PDF, TXT ou leia online no Scribd
Compartilhar este documento
Compartilhar ou incorporar documento
Você considera este documento útil?
Este conteúdo é inapropriado?
Denunciar este documentoorganometallic chemistry notes
Direitos autorais:
© All Rights Reserved
Formatos disponíveis
Baixe no formato DOCX, PDF, TXT ou leia online no Scribd
0 notas0% acharam este documento útil (0 voto)
8 visualizações3 páginasAlkyne Metathesis & Enyne Metathesis
Enviado por
polypeptideorganometallic chemistry notes
Direitos autorais:
© All Rights Reserved
Formatos disponíveis
Baixe no formato DOCX, PDF, TXT ou leia online no Scribd
Você está na página 1de 3
- alkyne metathesis cleaves the C-C triple bonds in two alkynes to form two new alkynes;
it is used to make conjugated polymers with interesting electronic properties and to
synthesize macrocycles with E or Z alkenes after reduction ; it is catalyzed by metal
alkylidenes
- the intermediate of alkyne metathesis involves a metallacyclobutadiene complex
- alkyne cross metathesis can be used to synthesize unsymmetrical alkynes
- ring closing alkyne metathesis + reduction can be used to synthesize macrocyclic natural
products containing cis olefins
- enyne metathesis generates dienes from alkynes and alkenes by splitting the alkene in
half and adding them across the alkyne ; high heterofunction tolerance when used with
Grubbs catalyst
- enyne metathesis can be used with olefin metathesis to form bicyclic products
- the mechanism of enyne metathesis involves the formation of an alkylidene complex and
the subsequent formation of a metallocyclobutene complex
Você também pode gostar
- Hidden Figures: The American Dream and the Untold Story of the Black Women Mathematicians Who Helped Win the Space RaceNo EverandHidden Figures: The American Dream and the Untold Story of the Black Women Mathematicians Who Helped Win the Space RaceNota: 4 de 5 estrelas4/5 (895)
- Chemical Equilibrium (Final Review)Documento20 páginasChemical Equilibrium (Final Review)polypeptideAinda não há avaliações
- Never Split the Difference: Negotiating As If Your Life Depended On ItNo EverandNever Split the Difference: Negotiating As If Your Life Depended On ItNota: 4.5 de 5 estrelas4.5/5 (838)
- Homework 3Documento13 páginasHomework 3polypeptideAinda não há avaliações
- The Yellow House: A Memoir (2019 National Book Award Winner)No EverandThe Yellow House: A Memoir (2019 National Book Award Winner)Nota: 4 de 5 estrelas4/5 (98)
- Glass Care Safe Handling PDFDocumento16 páginasGlass Care Safe Handling PDFAdinda SaraswatiAinda não há avaliações
- The Subtle Art of Not Giving a F*ck: A Counterintuitive Approach to Living a Good LifeNo EverandThe Subtle Art of Not Giving a F*ck: A Counterintuitive Approach to Living a Good LifeNota: 4 de 5 estrelas4/5 (5794)
- NMR Yield CalculationDocumento4 páginasNMR Yield CalculationpolypeptideAinda não há avaliações
- Glass Care Safe Handling PDFDocumento16 páginasGlass Care Safe Handling PDFAdinda SaraswatiAinda não há avaliações
- Devil in the Grove: Thurgood Marshall, the Groveland Boys, and the Dawn of a New AmericaNo EverandDevil in the Grove: Thurgood Marshall, the Groveland Boys, and the Dawn of a New AmericaNota: 4.5 de 5 estrelas4.5/5 (266)
- Chem 14A Discussion Section: Coordination ChemistryDocumento3 páginasChem 14A Discussion Section: Coordination ChemistrypolypeptideAinda não há avaliações
- The Little Book of Hygge: Danish Secrets to Happy LivingNo EverandThe Little Book of Hygge: Danish Secrets to Happy LivingNota: 3.5 de 5 estrelas3.5/5 (400)
- Units of Measurement: Introduction To ChemistryDocumento4 páginasUnits of Measurement: Introduction To ChemistrypolypeptideAinda não há avaliações
- Elon Musk: Tesla, SpaceX, and the Quest for a Fantastic FutureNo EverandElon Musk: Tesla, SpaceX, and the Quest for a Fantastic FutureNota: 4.5 de 5 estrelas4.5/5 (474)
- Midterm RecitationDocumento12 páginasMidterm RecitationpolypeptideAinda não há avaliações
- Lecture of Zoonoses at NTU 2015Documento159 páginasLecture of Zoonoses at NTU 2015polypeptideAinda não há avaliações
- A Heartbreaking Work Of Staggering Genius: A Memoir Based on a True StoryNo EverandA Heartbreaking Work Of Staggering Genius: A Memoir Based on a True StoryNota: 3.5 de 5 estrelas3.5/5 (231)
- Homework 3Documento13 páginasHomework 3polypeptideAinda não há avaliações
- Aic I HW7Documento2 páginasAic I HW7polypeptideAinda não há avaliações
- The Emperor of All Maladies: A Biography of CancerNo EverandThe Emperor of All Maladies: A Biography of CancerNota: 4.5 de 5 estrelas4.5/5 (271)
- Chemistry GRE SampleDocumento0 páginaChemistry GRE Sampleyoostan100% (2)
- The Unwinding: An Inner History of the New AmericaNo EverandThe Unwinding: An Inner History of the New AmericaNota: 4 de 5 estrelas4/5 (45)
- Animal ConditioningDocumento27 páginasAnimal ConditioningpolypeptideAinda não há avaliações
- General Chemistry C Homework 6 Due On 12/11 Name: ID: DepartmentDocumento2 páginasGeneral Chemistry C Homework 6 Due On 12/11 Name: ID: DepartmentpolypeptideAinda não há avaliações
- The Hard Thing About Hard Things: Building a Business When There Are No Easy AnswersNo EverandThe Hard Thing About Hard Things: Building a Business When There Are No Easy AnswersNota: 4.5 de 5 estrelas4.5/5 (344)
- Pka TablesDocumento6 páginasPka TablesAlessandro MoreniAinda não há avaliações
- Team of Rivals: The Political Genius of Abraham LincolnNo EverandTeam of Rivals: The Political Genius of Abraham LincolnNota: 4.5 de 5 estrelas4.5/5 (234)
- Mechanism of Microbial InfectionsDocumento122 páginasMechanism of Microbial InfectionspolypeptideAinda não há avaliações
- Viral Diseases - Mechanisms of Microbial InfectionsDocumento105 páginasViral Diseases - Mechanisms of Microbial InfectionspolypeptideAinda não há avaliações
- Functional Groups in Organic ChemistryDocumento1 páginaFunctional Groups in Organic ChemistrygznmemberAinda não há avaliações
- The Gifts of Imperfection: Let Go of Who You Think You're Supposed to Be and Embrace Who You AreNo EverandThe Gifts of Imperfection: Let Go of Who You Think You're Supposed to Be and Embrace Who You AreNota: 4 de 5 estrelas4/5 (1090)
- Chapter 28 LCDocumento5 páginasChapter 28 LCpolypeptideAinda não há avaliações
- Chapter1s StatMechDocumento22 páginasChapter1s StatMechpolypeptideAinda não há avaliações
- Chapter 7 Intro To Optical InstrumentsDocumento10 páginasChapter 7 Intro To Optical InstrumentspolypeptideAinda não há avaliações
- The World Is Flat 3.0: A Brief History of the Twenty-first CenturyNo EverandThe World Is Flat 3.0: A Brief History of the Twenty-first CenturyNota: 3.5 de 5 estrelas3.5/5 (2259)
- Chapter24 Materials ChemDocumento25 páginasChapter24 Materials ChempolypeptideAinda não há avaliações
- Protective GroupDocumento26 páginasProtective Groupchhan4Ainda não há avaliações
- FLUXIONALITYDocumento7 páginasFLUXIONALITYpolypeptideAinda não há avaliações
- Alkyne Metathesis & Enyne MetathesisDocumento3 páginasAlkyne Metathesis & Enyne MetathesispolypeptideAinda não há avaliações
- C H ActivationDocumento55 páginasC H Activationpolypeptide100% (1)
- The Sympathizer: A Novel (Pulitzer Prize for Fiction)No EverandThe Sympathizer: A Novel (Pulitzer Prize for Fiction)Nota: 4.5 de 5 estrelas4.5/5 (121)
- Test Taking StrategiesDocumento193 páginasTest Taking StrategiesAmy Kennedy100% (1)
- E26 QuestionsDocumento5 páginasE26 QuestionspolypeptideAinda não há avaliações
- Literature Report 2Documento12 páginasLiterature Report 2polypeptideAinda não há avaliações
- Est-Ce QueDocumento6 páginasEst-Ce QuepolypeptideAinda não há avaliações
- Scheme 2: C H (COOH)Documento4 páginasScheme 2: C H (COOH)Guilheme GomesAinda não há avaliações
- CHM 510 Experiment 3Documento12 páginasCHM 510 Experiment 3Nabilah100% (1)
- Lehninger Ch26Documento81 páginasLehninger Ch26AMAN KUMAR SINGH100% (1)
- Indian Association of Chemistry Teachers: National Standard Examination in Chemistry 2007-2008Documento10 páginasIndian Association of Chemistry Teachers: National Standard Examination in Chemistry 2007-2008Srinivas VenkataramanAinda não há avaliações
- Chemical Composition of Everyday ProductsDocumento220 páginasChemical Composition of Everyday Productsquythanck81% (16)
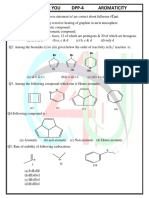
- Aromaticity DPP 4Documento4 páginasAromaticity DPP 4SubhadeepAinda não há avaliações
- IJSO Biology Module - 3Documento207 páginasIJSO Biology Module - 3Ikhbaat Atiqur RehmanAinda não há avaliações
- Msds BenzeneDocumento32 páginasMsds BenzeneMertcan AslanAinda não há avaliações
- Matovu Henry. Phytochemical Profiling and Larvicidal Activity of Synadenium GrantiiDocumento8 páginasMatovu Henry. Phytochemical Profiling and Larvicidal Activity of Synadenium GrantiiJohn KakemboAinda não há avaliações
- Controlled Delivery of Peptides and ProteinsDocumento19 páginasControlled Delivery of Peptides and ProteinsVanessya Oscar PermatasariAinda não há avaliações
- PolymerDocumento95 páginasPolymerG M Ali KawsarAinda não há avaliações
- Doe Handbook: Primer On Spontaneous Heating and PyrophoricityDocumento75 páginasDoe Handbook: Primer On Spontaneous Heating and PyrophoricitySarah TrawinskiAinda não há avaliações
- Prepartion of 4 Bromo 2 ChloroacetanildeDocumento11 páginasPrepartion of 4 Bromo 2 ChloroacetanildeAmil Patel0% (1)
- Types of Sealants: CaulkingDocumento10 páginasTypes of Sealants: Caulkinglouie roderosAinda não há avaliações
- Reasoning Questions From Organic Chemistry by Manoj Kumar KV KishtwarDocumento5 páginasReasoning Questions From Organic Chemistry by Manoj Kumar KV KishtwarShivesh Singh100% (1)
- Biochem CH 25 Nucleotide BiosynthesisDocumento6 páginasBiochem CH 25 Nucleotide BiosynthesisSchat ZiAinda não há avaliações
- 2018 May TZ2 Paper 2 HL Chemistry MarkschemeDocumento26 páginas2018 May TZ2 Paper 2 HL Chemistry MarkschememounishadmAinda não há avaliações
- Synthesis of Amide and BetaineDocumento5 páginasSynthesis of Amide and BetainejaboerboyAinda não há avaliações
- New Microsoft Word DocumentDocumento7 páginasNew Microsoft Word Documentuzzal ahmedAinda não há avaliações
- Post Lab Activity 5Documento4 páginasPost Lab Activity 5Shaira Soriano CincoAinda não há avaliações
- Introduction To Inherently Safer Design: Prepared For Safety and Chemical Engineering Education (SACHE) byDocumento71 páginasIntroduction To Inherently Safer Design: Prepared For Safety and Chemical Engineering Education (SACHE) bySebastian iacopiAinda não há avaliações
- Universitas Indonesia: Report Assignment 3Documento35 páginasUniversitas Indonesia: Report Assignment 3Dennis ChanAinda não há avaliações
- 2015 Chandra - Basic Concepts of BiotechnologyDocumento518 páginas2015 Chandra - Basic Concepts of BiotechnologyPavani ReddyAinda não há avaliações
- OIV-Methods Vol 1 en 2012Documento488 páginasOIV-Methods Vol 1 en 2012Violeta IP100% (1)
- Prof. Rohan Shenoy's Test-Series For MHT-CET Biology - 2009 - Archived Question Paper For PracticeDocumento3 páginasProf. Rohan Shenoy's Test-Series For MHT-CET Biology - 2009 - Archived Question Paper For PracticeBiologyForMHTCETAinda não há avaliações
- Structure: 1. SolubilityDocumento3 páginasStructure: 1. SolubilityFlori StoicaAinda não há avaliações
- 11 Micro NutritionDocumento71 páginas11 Micro NutritionSumit RaiAinda não há avaliações
- Pectin/LDPEDocumento27 páginasPectin/LDPEHansinee SitinamaluwaAinda não há avaliações
- Lipid Digestion and AbsorptionDocumento37 páginasLipid Digestion and AbsorptionIMDCBiochem100% (1)
- All About Screening For Milk Adulteration: A Guide To Opportunities With Analytical SolutionsDocumento23 páginasAll About Screening For Milk Adulteration: A Guide To Opportunities With Analytical SolutionsKingshuk DasAinda não há avaliações
- Is That a Fact?: Frauds, Quacks, and the Real Science of Everyday LifeNo EverandIs That a Fact?: Frauds, Quacks, and the Real Science of Everyday LifeNota: 5 de 5 estrelas5/5 (4)
- Periodic Tales: A Cultural History of the Elements, from Arsenic to ZincNo EverandPeriodic Tales: A Cultural History of the Elements, from Arsenic to ZincNota: 3.5 de 5 estrelas3.5/5 (137)
- The Nature of Drugs Vol. 1: History, Pharmacology, and Social ImpactNo EverandThe Nature of Drugs Vol. 1: History, Pharmacology, and Social ImpactNota: 5 de 5 estrelas5/5 (5)
- Sodium Bicarbonate: Nature's Unique First Aid RemedyNo EverandSodium Bicarbonate: Nature's Unique First Aid RemedyNota: 5 de 5 estrelas5/5 (21)
- Taste: Surprising Stories and Science About Why Food Tastes GoodNo EverandTaste: Surprising Stories and Science About Why Food Tastes GoodNota: 3 de 5 estrelas3/5 (20)
- Guidelines for Chemical Process Quantitative Risk AnalysisNo EverandGuidelines for Chemical Process Quantitative Risk AnalysisNota: 5 de 5 estrelas5/5 (1)