Escolar Documentos
Profissional Documentos
Cultura Documentos
Case Study - Intestinal Resection and Anastomosis in A Dog
Enviado por
api-301746262Descrição original:
Título original
Direitos autorais
Formatos disponíveis
Compartilhar este documento
Compartilhar ou incorporar documento
Você considera este documento útil?
Este conteúdo é inapropriado?
Denunciar este documentoDireitos autorais:
Formatos disponíveis
Case Study - Intestinal Resection and Anastomosis in A Dog
Enviado por
api-301746262Direitos autorais:
Formatos disponíveis
Running head: CANINE INTESTINAL SURGERY
Case Study:
Intestinal Resection and Anastomosis in a Dog
Rebecca M. England
4325 Companion Animal Anesthesia and Surgical Nursing
Tarleton State University
CANINE INTESTINAL SURGERY
Elf is a one-year-old spayed female Beagle cross that presented with acute vomiting for
less than 24 hours (Rockett & Christensen, 2010). The owner was concerned that it may be due
to the ingestion of part of a pillow that she had recently torn apart. When the owner returned
home two days ago, she was unable to find locate most of the stuffing. Elf started vomiting about
24 hours later (Rockett & Christensen, 2010).
Her physical exam findings:
BCS: 3/5 at 44 lbs.
Temperature: 102.1 F
HR 120 bpm
RR panting
Mucous membranes: pale pink and dry
Respiratory: no abnormalities heard
Gastrointestinal: Resistance during abdominal palpation, discomfort seen, no F.B. detected
Dehydration assessment: 6% deficit
Due to the clinical history and presentation of Elf at this examination, the veterinarian
recommended abdominal radiographs be performed to assist in ruling out the possibility of a
foreign body obstruction. The owner consented. Survey radiographs, in addition to contrast
radiographs, were taken. In looking at the study, delayed gastric emptying was noted (Rockett &
Christensen, 2010). The veterinarian diagnosed vomiting secondary to foreign body ingestion, so
an abdominal exploratory was scheduled (Rockett & Christensen, 2010).
The veterinarian requested an ECG prior to induction of anesthesia (Rockett &
Christensen, 2010). A technician started the procedure, and it was quickly noted that it was being
performed incorrectly. The pictures in this text show two techniques, and Figure 5-9a is
obviously correct, shown on the next page (Rockett & Christensen, 2010). This placement is
correct in that the dog is placed in right lateral recumbency (not left) and the leads are placed
appropriately, slightly proximal to the elbows and stifles and on the correct limbs (Thomas &
Lerche, 2011). A fifth lead may sometimes be used at the apex of the heart on the left side of the
CANINE INTESTINAL SURGERY
thorax to obtain additional information. It appears to be appropriately placed in this picture. The
lead placement is often remembered by a common rhyme used among technicians, white on the
right (or snow over grass), and smoke over fire. This allows quick placement of the leads using
the colors of the leads. There is also a color photograph for comparison, but without the chest
and green leads, as it is difficult to determine if the leads are placed on the proper limbs due to
the black and white nature of the photograph.
Figure 5-9a: Obtaining an ECG on a dog. Correct positioning shown.
From Rockett & Christensen, 2010
Standard 3-lead ECG commonly used in veterinary patients, retrieved from personal photography library.
CANINE INTESTINAL SURGERY
In Figure 5-9b below, positioning of the patient and application of the leads is incorrect.
The patient is in left lateral recumbency and the leads have been placed distal to the elbows and
stifles. Again, there is also a color photograph for comparison, but without the chest and green
leads, as it is difficult to determine if the leads are placed on the proper limbs due to the black
and white nature of the photograph.
Figure 5-9b: Obtaining an ECG on a dog. Incorrect positioning shown.
From Rockett & Christensen, 2010
Standard 3-lead ECG commonly used in veterinary patients, retrieved from personal photography library.
CANINE INTESTINAL SURGERY
After the ECG is performed, the technician starts to gather equipment and supplies
needed for the induction and monitoring of anesthesia (Rockett & Christensen, 2010). The
technician gets out an esophageal stethoscope, shown below. An esophageal stethoscope is used
to manually auscultate and monitor heart rate and rhythm (Tear, 2012). If the end of the tubing is
adjacent to the lung fields, breath sounds may also be auscultated (Tear, 2012).
Esophageal stethoscope. From Tear, 2012
The veterinarian also wants Elfs percent oxygen saturation (Rockett & Christensen,
2010). The monitoring equipment needed to obtain this data is called a pulse oximeter (Tear,
2012), as seen below.
CANINE INTESTINAL SURGERY
The assistant prepared to use a non-rebreathing anesthetic set-up for this patient, seen
below. This tubing, however, is not appropriate for a patient of Elfs size, being that she is 44
pounds. Non-rebreathing circuits are for use in patients weighing less than 15 pounds.
A rebreathing circuit such as a universal F rebreathing circuit is appropriate for Elf, seen below.
It is ideal for patients larger in size weighing more than 15 pounds.
The size of rebreathing reservoir bag to be used must be calculated based on the patients weight
and tidal volume of the lungs. See the formula and calculation below:
CANINE INTESTINAL SURGERY
Tidal volume = body weight in pounds x 30 = bag size in milliliters (mL)
44 pounds x 30 = 1,320 mL
Tidal volume > 1,000 mL but < 2,000 mL = 2-liter bag
A 2-liter rebreathing reservoir bag is appropriate for Elf. A picture is shown below:
The veterinarian wants to use Olsen-Hegar needle drivers for the surgical procedure
(Rockett & Christensen, 2010). In the text (Rockett & Christensen, 2010), this instrument is
labeled A, seen below. This instrument in particular has scissor blades set behind the jaws,
whereas the Mayo-Hegar needle drivers have no scissor blades.
CANINE INTESTINAL SURGERY
The veterinary assistant heats up 0.9% sodium chloride in the incubator. Is this type of
fluid appropriate for flushing the abdomen? Yes, it is. 0.9% sodium chloride is an isotonic fluid,
which closely matches the bodys fluids. It is primarily used in the event that intestinal contents
leaked into the abdominal cavity (Tear, 2012). Flushing the abdomen will decrease the chance of
peritonitis from occurring.
The veterinarian needs a specific type of towel clamp that would be appropriate for holding the
suction tubing in place. The other clamp choices are not appropriate as they are sharp and could
potentially puncture the drape material or the suction tubing. This clamp is identified as C in the text
(Rockett & Christensen, 2010), and shown below. It is blunt and will not penetrate materials.
CANINE INTESTINAL SURGERY
Prior to anesthetizing Elf for her surgical procedure, an IV catheter is placed so that she
can receive fluids to replace her dehydration deficit. She is considered to be 6% dehydrated. The
volume of fluid she will need to replace the deficit is calculated below:
Dehydration deficit calculation:
Dehydration % x BWlb x 500
6% x 44 lbs. x 500 = 1,320 ml to replace the deficit.
After receiving fluids, Elf was induced using propofol at a dose of 2 mg/lb. She received
8.8 ml of propofol IV for induction. See the calculation below:
Propofol is 10 mg/ml (Plumb, 2011)
44 lbs. * 2 mg/lb. = 88 mg
88 mg divided by 10 mg/ml = 8.8 ml propofol
CANINE INTESTINAL SURGERY
10
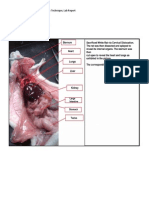
A large amount of pillow batting was removed from the proximal portion of the jejunum
(Rockett & Christensen, 2010). The assistant asks about the location of the jejunum in
relationship to the stomach. Here is a drawing below, depicting the gastrointestinal system of a
dog:
Once the surgery is complete, Elf is monitored in the recovery room. Extubation should
be performed until the patients primary reflexes return. These include a palpebral reflex
response and the ability to swallow. The ability to swallow is most crucial for the patient. If the
patient is unable to swallow, and then subsequently extubated too early, the patient will not be
able to protect the airway. This could lead to aspiration, an inability to breath, and even death.
Therefore, a patient should not be extubated until swallowing well. Five minutes after
extubation, Elf starts to vomit. It is important in a situation like this that the patient be propped
up in a sternal position to minimize the chances of aspirating fluid or other contents from the
CANINE INTESTINAL SURGERY
11
gastrointestinal tract. If severe or the patient has complications breathing, reintubation may be
necessary. Otherwise, monitor and keep the patient sternal until the vomiting has subsided. It
may be necessary to administer antinausea medication per the veterinarians orders if it persists.
Now that the surgery room is no longer being used, it must be prepared for the next
patient. There are several things that should be done to clean and maintain the room properly
(Tear, 2012):
1. The O.R. should be cleaned daily, even if it is not used
2. Equipment used to clean the O.R. should only be used in the O.R to prevent crosscontamination
3. All surfaces of the room should be damp-dusted and wiped down before and after
each patient
4. Any equipment and furniture that is visibly soiled needs to be cleaned at the end of
each procedure
5. All instruments should be removed from the O.R. and washed/cleaned/repackaged in
a designated area
6. Surfaces should be cleaned with an appropriate disinfectant (this will vary depending
on the hospitals need and exposure to diseases)
7. When cleaning surfaces, a scrubbing motion should be used
8. Examples of surfaces to clean include:
a. Surgical lights
b. Tables
c. Glass sliding cabinets
d. Wheels, step stools, foot pedals, wall telephones, light switches
e. All handles (doors, drawers, and cabinets)
f. Anesthesia machine
g. Ventilation faceplates and doorway ledges
9. The floor should be swept and mopped with a fresh bucket of appropriate disinfectant
and a clean mop head
10. The surgery door should be kept closed at all times to reduce the chances of
contamination
Based on hospitals standards, there may be additional things to perform. Overall, however, the
concept is the same.
This case study reveals the wide variety of knowledge that is necessary to carry out a
surgical procedure such as this one. A variety of equipment is needed, and one must be familiar
with its function and how to apply it to patient use. Several calculations are also necessary, from
CANINE INTESTINAL SURGERY
12
induction to the post-operative period. Patient anatomy is especially important to understand, not
only from a procedural standpoint, but also in order to educate owners. Maintaining the O.R. is
also an vital daily task.
CANINE INTESTINAL SURGERY
13
References
Colville, T. and Bassert, J. (2007). Clinical Anatomy and Physiology for Veterinary Technicians, 2nd ed.
St. Louis, MO: Mosby.
Plumb, D. C. (2011). Plumbs Veterinary Drug Handbook, 7th ed. PharmVet Inc.
Rockett and Christensen (2010). Case Studies in Veterinary Technology: A Scenario-Based Critical
Thinking Approach. Heyburn, ID: Rockett House Publishing LLC.
Sonsthagen, T. (2005). Veterinary Instruments and Equipment: A Pocket Guide. St. Louis, MO: Mosby.
Tear, M. (2012). Small Animal Surgical Nursing: Skills and Concepts, 2nd ed. St. Louis, MO: Mosby
Elsevier.
Thomas, J. A. and Lerche, P. (2011). Anesthesia and Analgesia for Veterinary Technicians, 4th ed. St.
Louis, MO: Elsevier.
Você também pode gostar
- Human Anatomy Physiology Laboratory Manual Fetal Pig Version 12th Edition Marieb Solution ManualDocumento5 páginasHuman Anatomy Physiology Laboratory Manual Fetal Pig Version 12th Edition Marieb Solution Manualsritrader89% (9)
- Harmonizing A MelodyDocumento6 páginasHarmonizing A MelodyJane100% (1)
- Necropsy Guide for Dogs, Cats, and Small MammalsNo EverandNecropsy Guide for Dogs, Cats, and Small MammalsSean P. McDonoughNota: 4 de 5 estrelas4/5 (1)
- Histo Tech Lab Report 1Documento4 páginasHisto Tech Lab Report 1Hendry RaoAinda não há avaliações
- How To Anaesthetize A GiraffeDocumento4 páginasHow To Anaesthetize A Giraffeltrathie3087100% (2)
- Tomato & Tomato Products ManufacturingDocumento49 páginasTomato & Tomato Products ManufacturingAjjay Kumar Gupta100% (1)
- DSA Interview QuestionsDocumento1 páginaDSA Interview QuestionsPennAinda não há avaliações
- DetoxificationDocumento20 páginasDetoxificationapi-301746262100% (2)
- Julian's GodsDocumento162 páginasJulian's Godsअरविन्द पथिक100% (6)
- Anaesthesia NotesDocumento61 páginasAnaesthesia NotessuggaplumAinda não há avaliações
- SCIENCE 5 PPT Q3 W6 - Parts of An Electric CircuitDocumento24 páginasSCIENCE 5 PPT Q3 W6 - Parts of An Electric CircuitDexter Sagarino100% (1)
- Case Study - Seizuring DogDocumento8 páginasCase Study - Seizuring Dogapi-301746262Ainda não há avaliações
- Case Study - Seizuring DogDocumento8 páginasCase Study - Seizuring Dogapi-301746262Ainda não há avaliações
- Icu Design and SetupDocumento9 páginasIcu Design and SetupNorfaidah AliAinda não há avaliações
- FleasDocumento27 páginasFleasapi-301746262100% (6)
- C - TS4CO - 2021: There Are 2 Correct Answers To This QuestionDocumento54 páginasC - TS4CO - 2021: There Are 2 Correct Answers To This QuestionHclementeAinda não há avaliações
- Neonatal IntubationDocumento7 páginasNeonatal Intubationdrsalil82Ainda não há avaliações
- Ultrasonography For The Veterinary Nurse - Frank BuschDocumento2 páginasUltrasonography For The Veterinary Nurse - Frank BuschFrank Busch100% (1)
- Factors Affecting Job Satisfaction of EngineersDocumento35 páginasFactors Affecting Job Satisfaction of Engineerslingg8850% (2)
- Anestesia em Animais Silvestres Capitulo 1Documento24 páginasAnestesia em Animais Silvestres Capitulo 1Daniel CostaAinda não há avaliações
- Canadian C-Spine Rule Could Help Trauma Patients, Ease Overcrowding in Emergency Departments, Study FindsDocumento4 páginasCanadian C-Spine Rule Could Help Trauma Patients, Ease Overcrowding in Emergency Departments, Study FindsAmrie HeussaffAinda não há avaliações
- Group B: Romero D. Romero P. Rufino L. Saddi E. Sanchez D. Santos ADocumento19 páginasGroup B: Romero D. Romero P. Rufino L. Saddi E. Sanchez D. Santos APam RomeroAinda não há avaliações
- An Alternative Method of Endotracheal Intubation of Common MarmosetsDocumento6 páginasAn Alternative Method of Endotracheal Intubation of Common Marmosetssolxmar tmAinda não há avaliações
- Endotracheal SuctionDocumento11 páginasEndotracheal SuctionKeshav Singhmaar AryaAinda não há avaliações
- Case Scenario ArfDocumento3 páginasCase Scenario ArfPam RomeroAinda não há avaliações
- J Exp Med-1922-Warren-187-202Documento16 páginasJ Exp Med-1922-Warren-187-202Anggun PermatasariAinda não há avaliações
- Total Injectable Anesthesia of Dogs and Cats For Remote Location Veterinary Sterilization Clinic BMC 2020Documento6 páginasTotal Injectable Anesthesia of Dogs and Cats For Remote Location Veterinary Sterilization Clinic BMC 2020Daniel DanielAinda não há avaliações
- Rapid Sequence Intubation W Checklist 2022Documento4 páginasRapid Sequence Intubation W Checklist 2022drulilalbbAinda não há avaliações
- Reeves 2001Documento11 páginasReeves 2001Luis José Hume QuirozAinda não há avaliações
- Table 1 Indications, Contraindications and Hazards of Suctioning Indications of SuctioningDocumento4 páginasTable 1 Indications, Contraindications and Hazards of Suctioning Indications of SuctioningRika RukmanaAinda não há avaliações
- Animal Facility Videoendoscopic Intubation Station: Tips and Tricks From Mice To RabbitsDocumento4 páginasAnimal Facility Videoendoscopic Intubation Station: Tips and Tricks From Mice To RabbitsJavier Martin Bartolo GarciaAinda não há avaliações
- Linsenmeier 2020Documento6 páginasLinsenmeier 2020Novita ApramadhaAinda não há avaliações
- Nursing Facts in Brief Bio Physical Concept in Nursing and Rehabilitation/laboratory TestsDocumento9 páginasNursing Facts in Brief Bio Physical Concept in Nursing and Rehabilitation/laboratory TestsDhine Dhine ArguellesAinda não há avaliações
- Monitoring Peri Ajnestesi DepiDocumento19 páginasMonitoring Peri Ajnestesi DepijoyfullAinda não há avaliações
- Fast Localised Abdominal Sonography Protocol for Horses with Colic (FLASHDocumento6 páginasFast Localised Abdominal Sonography Protocol for Horses with Colic (FLASHludiegues752Ainda não há avaliações
- Anesthesia, Chemical Restraint and Pain Management in Snakes (Serpentes) - A Review Seven Mustafa, Nadya ZlatevaDocumento8 páginasAnesthesia, Chemical Restraint and Pain Management in Snakes (Serpentes) - A Review Seven Mustafa, Nadya ZlatevaFirdaus BillyAinda não há avaliações
- Tabije, Arvie Jayselle PDocumento8 páginasTabije, Arvie Jayselle PJaysellePuguonTabijeAinda não há avaliações
- English For Professional Nurse 2Documento7 páginasEnglish For Professional Nurse 2Anonymous HWsv9pBUhAinda não há avaliações
- Nursing Management in Abdominal SurgeryDocumento19 páginasNursing Management in Abdominal Surgeryejguy7777100% (2)
- IV-C Safety Guidelines For Emergency Drugs, Endotracheal Set-Up and Defibrillator UseDocumento21 páginasIV-C Safety Guidelines For Emergency Drugs, Endotracheal Set-Up and Defibrillator UseRio DanteAinda não há avaliações
- Safe Recovery From AnaesthesiaDocumento7 páginasSafe Recovery From AnaesthesiaKarthikAinda não há avaliações
- Chapter 7 HandoutDocumento5 páginasChapter 7 HandoutFrance KennethAinda não há avaliações
- Et IntubationDocumento3 páginasEt IntubationCherry L. M. JaramilloAinda não há avaliações
- Operasi Pada TikusDocumento8 páginasOperasi Pada Tikuslathifa_nurAinda não há avaliações
- LaryngectomyDocumento7 páginasLaryngectomyŞahin ŞahinAinda não há avaliações
- Anesthesia Rabbits PreDocumento4 páginasAnesthesia Rabbits PreAgungLailatulKurniawanAinda não há avaliações
- Resume Nasogastric Tube InstallationDocumento17 páginasResume Nasogastric Tube InstallationRedmy LasmanaAinda não há avaliações
- Nebulization Nursing Procedure at Ateneo de Zamboanga UniversityDocumento9 páginasNebulization Nursing Procedure at Ateneo de Zamboanga UniversityMitzi BelamideAinda não há avaliações
- Pronation Therapy: DR Ahmed Abdelhady MD Critical Care ICU ConsultantDocumento30 páginasPronation Therapy: DR Ahmed Abdelhady MD Critical Care ICU Consultantlayla alyamiAinda não há avaliações
- Rodent Euthanasia Adult NIHDocumento2 páginasRodent Euthanasia Adult NIHgabriela_mariangela5929Ainda não há avaliações
- Goligorsky M.S. - Renal Disease. Techniques and Protocols (2003)Documento485 páginasGoligorsky M.S. - Renal Disease. Techniques and Protocols (2003)NMC NEPHROLOGYAinda não há avaliações
- Case ReportDocumento9 páginasCase ReportAnanta IpoAinda não há avaliações
- Managing Eclampsia: A Step-by-Step GuideDocumento4 páginasManaging Eclampsia: A Step-by-Step GuiderhtyehAinda não há avaliações
- QS EndotrachealIntubationDocumento4 páginasQS EndotrachealIntubationChandra HalimAinda não há avaliações
- Ja Alas 2009000292Documento4 páginasJa Alas 2009000292MihEugenAinda não há avaliações
- Training Facilities in Gastrointestinal EndoscopyDocumento4 páginasTraining Facilities in Gastrointestinal EndoscopyAlberto Kenyo Riofrio PalaciosAinda não há avaliações
- Als ProceduresDocumento3 páginasAls Proceduresapi-296884857Ainda não há avaliações
- Assisting For Endotracheal IntubationDocumento16 páginasAssisting For Endotracheal IntubationSREEDEVI T SURESH100% (1)
- OSCE Marksheet 1Documento7 páginasOSCE Marksheet 1Elias PetrouAinda não há avaliações
- Dwnload Full Human Anatomy and Physiology Laboratory Manual Fetal Pig Version Update 10th Edition Marieb Solutions Manual PDFDocumento36 páginasDwnload Full Human Anatomy and Physiology Laboratory Manual Fetal Pig Version Update 10th Edition Marieb Solutions Manual PDFpeiwenhlmel100% (9)
- Adult Rodents - Chemical Methods 1. CO Asphyxiation: EUTHANASIA in Laboratory AnimalsDocumento3 páginasAdult Rodents - Chemical Methods 1. CO Asphyxiation: EUTHANASIA in Laboratory Animalsjekapu simwingaAinda não há avaliações
- The Development, Concept and Use of Medical Equipment As Science and Technology AdvancesDocumento34 páginasThe Development, Concept and Use of Medical Equipment As Science and Technology Advancesmatt_21_luiAinda não há avaliações
- Ovrio Salpingo Histerectomiavideolaparoscpicaemgatosdomsticos TcnicacomdoisportaisDocumento6 páginasOvrio Salpingo Histerectomiavideolaparoscpicaemgatosdomsticos TcnicacomdoisportaisDra MeiAinda não há avaliações
- Solution Manual For Human Anatomy Laboratory Manual With Cat Dissections 8Th Edition Marieb 9780134255583 9780134255583 Full Chapter PDFDocumento29 páginasSolution Manual For Human Anatomy Laboratory Manual With Cat Dissections 8Th Edition Marieb 9780134255583 9780134255583 Full Chapter PDFleo.kitchen421100% (11)
- HP May03 CapsuleDocumento7 páginasHP May03 CapsuleManeesh RainaAinda não há avaliações
- ThyroidectomyDocumento51 páginasThyroidectomyPopey Moore100% (1)
- Bennett - 2011 - The Chelonian Respiratory SystemDocumento15 páginasBennett - 2011 - The Chelonian Respiratory SystemdsouzapeixotoAinda não há avaliações
- Single Incision, Laparoscopic-Assisted Ovariohysterectomy For Mucometra and Pyometra in DogsDocumento5 páginasSingle Incision, Laparoscopic-Assisted Ovariohysterectomy For Mucometra and Pyometra in Dogsalfi fadilah alfidruAinda não há avaliações
- Respiratory EmergencyDocumento6 páginasRespiratory Emergencyapi-235947247Ainda não há avaliações
- Laparoscopic Colopexy in Dogs: Full Paper SurgeryDocumento6 páginasLaparoscopic Colopexy in Dogs: Full Paper SurgeryrianperoAinda não há avaliações
- Rebeccas Research PaperDocumento33 páginasRebeccas Research Paperapi-301746262Ainda não há avaliações
- Final Case Study - The Complete Surgical Nursing ExperienceDocumento11 páginasFinal Case Study - The Complete Surgical Nursing Experienceapi-301746262Ainda não há avaliações
- Educational Program 2 - Importance of Post-Operative CareDocumento9 páginasEducational Program 2 - Importance of Post-Operative Careapi-301746262Ainda não há avaliações
- MriDocumento21 páginasMriapi-301746262Ainda não há avaliações
- Retrogade CystographyDocumento16 páginasRetrogade Cystographyapi-301746262Ainda não há avaliações
- Client Education - Preventative Dental Care PPT 2Documento40 páginasClient Education - Preventative Dental Care PPT 2api-301746262Ainda não há avaliações
- Week 11 Case Study - Dog With PruritusDocumento9 páginasWeek 11 Case Study - Dog With Pruritusapi-301746262Ainda não há avaliações
- Midterm Case Study PPT - Heartworm Infection in A DogDocumento33 páginasMidterm Case Study PPT - Heartworm Infection in A Dogapi-301746262Ainda não há avaliações
- Week 5 Nursing Plan - Lymphoma PatientDocumento3 páginasWeek 5 Nursing Plan - Lymphoma Patientapi-301746262Ainda não há avaliações
- ESSAYSDocumento5 páginasESSAYSDGM RegistrarAinda não há avaliações
- Dreams FinallDocumento2 páginasDreams FinalldeeznutsAinda não há avaliações
- IC 4060 Design NoteDocumento2 páginasIC 4060 Design Notemano012Ainda não há avaliações
- Absenteeism: It'S Effect On The Academic Performance On The Selected Shs Students Literature ReviewDocumento7 páginasAbsenteeism: It'S Effect On The Academic Performance On The Selected Shs Students Literature Reviewapi-349927558Ainda não há avaliações
- Prep - VN: Where Did The Polo Family Come From?Documento1 páginaPrep - VN: Where Did The Polo Family Come From?Phương LanAinda não há avaliações
- Lifting Plan FormatDocumento2 páginasLifting Plan FormatmdmuzafferazamAinda não há avaliações
- Daft Presentation 6 EnvironmentDocumento18 páginasDaft Presentation 6 EnvironmentJuan Manuel OvalleAinda não há avaliações
- Towards Emotion Independent Languageidentification System: by Priyam Jain, Krishna Gurugubelli, Anil Kumar VuppalaDocumento6 páginasTowards Emotion Independent Languageidentification System: by Priyam Jain, Krishna Gurugubelli, Anil Kumar VuppalaSamay PatelAinda não há avaliações
- Extra Vocabulary: Extension Units 1 & 2Documento1 páginaExtra Vocabulary: Extension Units 1 & 2CeciBravoAinda não há avaliações
- Lucy Wang Signature Cocktail List: 1. Passion Martini (Old Card)Documento5 páginasLucy Wang Signature Cocktail List: 1. Passion Martini (Old Card)Daca KloseAinda não há avaliações
- Kepler's Law 600 Years Before KeplerDocumento7 páginasKepler's Law 600 Years Before KeplerJoe NahhasAinda não há avaliações
- Career Guidance Program Modules MonitoringDocumento7 páginasCareer Guidance Program Modules MonitoringJevin GonzalesAinda não há avaliações
- 100 Bedded Hospital at Jadcherla: Load CalculationsDocumento3 páginas100 Bedded Hospital at Jadcherla: Load Calculationskiran raghukiranAinda não há avaliações
- Bias in TurnoutDocumento2 páginasBias in TurnoutDardo CurtiAinda não há avaliações
- Corti Et Al., 2021Documento38 páginasCorti Et Al., 2021LunaAinda não há avaliações
- Kenneth L. Campbell - The History of Britain and IrelandDocumento505 páginasKenneth L. Campbell - The History of Britain and IrelandKseniaAinda não há avaliações
- M2M RF - RHNDocumento3 páginasM2M RF - RHNNur Nadia Syamira Bt SaaidiAinda não há avaliações
- Experiment No. 11 Fabricating Concrete Specimen For Tests: Referenced StandardDocumento5 páginasExperiment No. 11 Fabricating Concrete Specimen For Tests: Referenced StandardRenAinda não há avaliações
- Part 4: Implementing The Solution in PythonDocumento5 páginasPart 4: Implementing The Solution in PythonHuỳnh Đỗ Tấn ThànhAinda não há avaliações
- Forouzan MCQ in Error Detection and CorrectionDocumento14 páginasForouzan MCQ in Error Detection and CorrectionFroyd WessAinda não há avaliações
- Zsoka PDFDocumento13 páginasZsoka PDFMasliana SahadAinda não há avaliações
- History of Philippine Sports PDFDocumento48 páginasHistory of Philippine Sports PDFGerlie SaripaAinda não há avaliações
- What Is Innovation and Its Characteristics of InnovationDocumento4 páginasWhat Is Innovation and Its Characteristics of InnovationMohd TauqeerAinda não há avaliações