Escolar Documentos
Profissional Documentos
Cultura Documentos
Electrode Potential Summary
Enviado por
Nooran ShamsDescrição original:
Direitos autorais
Formatos disponíveis
Compartilhar este documento
Compartilhar ou incorporar documento
Você considera este documento útil?
Este conteúdo é inapropriado?
Denunciar este documentoDireitos autorais:
Formatos disponíveis
Electrode Potential Summary
Enviado por
Nooran ShamsDireitos autorais:
Formatos disponíveis
NOCKHARDY
ELECTRODE STANDARD HYDROGEN ELECTRODE
KNOTES POTENTIALS V
A LEVEL CHEMISTRY
AT A G L AN CE Salt Bridge (KCl) Hydrogen
(1 atm.)
THE ELECTROCHEMICAL SERIES Platinum
Zinc
• Species are arranged in order of their standard electrode potentials
• All equations are written as reductions ... gaining electrons
Zn 2+
(aq)
(1M) H +(aq) (1M)
F2(g) + 2e¯ 2F¯(aq) +2.87 V
MnO4¯(aq) + 8H+(aq) + 5e¯ Mn2+(aq) + 4H2O(l) +1.52 V
temperature 298K (25°C)
Cl2(g) + 2e¯ 2Cl¯(aq) +1.36 V
2- solution conc. 1M (1 mol dm-3) with respect to H+ ions
Cr2O7 (aq) + I4H+(aq) + 6e¯ 2Cr3+(aq) + 7H2O(l) +1.33 V
gases 1 atmosphere pressure
Br2(l) + 2e¯ 2Br¯(aq) +1.07 V
E° value 0.00V.
Fe3+(aq) + e¯ Fe2+(aq) +0.77 V
I2(s) + 2e¯ 2I¯(aq) +0.54 V salt bridge filled with saturated potassium chloride solution;
Cu2+(aq) + 2e¯ Cu(s) +0.34 V it enables the circuit to be completed
2H+(aq) + 2e¯ H2(g) 0.00 V
Fe2+(aq) + 2e¯ Fe(s) -0.44 V
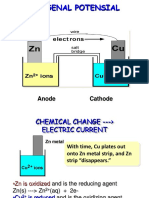
Zn2+ (aq) + 2e¯ Zn(s) -0.76 V CELL DIAGRAMS
These give a diagrammatic representation of what is happening in a cell.
• Highest positive value = best oxidising agent
• A species with a more positive potential (E° value) will oxidise V
one (reverse the equation) with a lower E° value
2+ 2+
Zn Zn Cu Cu
(s) (aq) (aq) (s)
e.g. Cr2O72- (E° = +1.33V ) will oxidise Br¯ to Br2 (E° = +1.07V )
ANODE CATHODE
and I¯ to I2 (E° = +0.54V )
BUT NOT Cl¯ to Cl2 (E° = +1.36V ) • Place the half cell with the more positive E° value on the RHS.
Draw it out as shown to indicate that ...
By combining half equations and their E° values you can predict • the cell reaction goes from left to right
whether, or not, a redox reaction will take place. In theory, a redox • the electrons go round the external circuit from left to right
reaction should proceed if the E° value is positive. In reality, it has to • the cell voltage is E°(RHS) - E°(LHS). In this way it must be positive
be greater than about +0.40V. • oxidation takes place at the anode, reduction at the cathode
© J. L. HOPTON 2000
Você também pode gostar
- Critical Evaluation of Equilibrium Constants Involving 8-Hydroxyquinoline and Its Metal Chelates: Critical Evaluation of Equilibrium Constants in Solution: Part B: Equilibrium Constants of Liquid-Liquid Distribution SystemsNo EverandCritical Evaluation of Equilibrium Constants Involving 8-Hydroxyquinoline and Its Metal Chelates: Critical Evaluation of Equilibrium Constants in Solution: Part B: Equilibrium Constants of Liquid-Liquid Distribution SystemsAinda não há avaliações
- Ib PPT 9 HL PDFDocumento26 páginasIb PPT 9 HL PDFzarna nirmal rawalAinda não há avaliações
- XXIVth International Congress of Pure and Applied Chemistry: Plenary and Main Section Lectures Presented at Hamburg, Federal Republic of Germany, 2–8 September 1973No EverandXXIVth International Congress of Pure and Applied Chemistry: Plenary and Main Section Lectures Presented at Hamburg, Federal Republic of Germany, 2–8 September 1973Ainda não há avaliações
- EPOTPPDocumento28 páginasEPOTPPapi-3706290100% (2)
- Practice Makes Perfect in Chemistry: Oxidation-Reduction with AnswersNo EverandPractice Makes Perfect in Chemistry: Oxidation-Reduction with AnswersAinda não há avaliações
- EpotppsDocumento31 páginasEpotppsHelpful HandAinda não há avaliações
- Practice Makes Perfect in Chemistry: Oxidation-ReductionNo EverandPractice Makes Perfect in Chemistry: Oxidation-ReductionNota: 5 de 5 estrelas5/5 (1)
- Lecture 8Documento61 páginasLecture 8Ti GraAinda não há avaliações
- Topic 9 (Galvanic Cell) - Tutorial - Level 1 AnswerDocumento5 páginasTopic 9 (Galvanic Cell) - Tutorial - Level 1 AnswerCheng Xun LeeAinda não há avaliações
- Tutorial 9 - Level 1 Worked SolutionsDocumento11 páginasTutorial 9 - Level 1 Worked SolutionsBloodCypherAinda não há avaliações
- Chemsheets A2 1077 ElectrochemistryDocumento46 páginasChemsheets A2 1077 Electrochemistrytonychenlondon1Ainda não há avaliações
- Electrochemistry 2Documento14 páginasElectrochemistry 2Wella YektiAinda não há avaliações
- Electrochemistry - 2 - 1Documento6 páginasElectrochemistry - 2 - 1Mandeep PediredlaAinda não há avaliações
- Electrochemistry 1Documento74 páginasElectrochemistry 1Vipranshu GuptaAinda não há avaliações
- ElectrochemistryDocumento39 páginasElectrochemistryHaider AliAinda não há avaliações
- Electrochemistry 20222023 (REVIEWED)Documento101 páginasElectrochemistry 20222023 (REVIEWED)alyaainsyirah04Ainda não há avaliações
- Electrochemistry Neeraj BookDocumento24 páginasElectrochemistry Neeraj BookHarsh GuptaAinda não há avaliações
- Standard Hydrogen Electrode PresentationDocumento23 páginasStandard Hydrogen Electrode PresentationKishore KishoreAinda não há avaliações
- Tipos de ElectrodosDocumento10 páginasTipos de ElectrodosluisAinda não há avaliações
- Tutorial 9 - Level 2 Worked SolutionsDocumento11 páginasTutorial 9 - Level 2 Worked SolutionsBloodCypherAinda não há avaliações
- Chapter 17 Electrochemistry ModDocumento68 páginasChapter 17 Electrochemistry ModMichelle AlmendralaAinda não há avaliações
- AS Electrode PotentialsDocumento31 páginasAS Electrode PotentialsRicky LawAinda não há avaliações
- Electrochemistry StudentDocumento88 páginasElectrochemistry StudentCtNabihahAmilaMarminAinda não há avaliações
- Chapter 17 ElectrochemistryDocumento68 páginasChapter 17 ElectrochemistryBeastUnleashed28Ainda não há avaliações
- Week 10 - Electrode PotentialDocumento6 páginasWeek 10 - Electrode PotentialThanni AkanbiAinda não há avaliações
- Chapter 3 ElectrochemistryDocumento8 páginasChapter 3 Electrochemistrymeshal retteryAinda não há avaliações
- Lecture 15.3 - Electrolytic CellsDocumento10 páginasLecture 15.3 - Electrolytic CellsLiam DoranAinda não há avaliações
- Chapter7 Electrochemistry (Part3)Documento13 páginasChapter7 Electrochemistry (Part3)Christoval PandilalaAinda não há avaliações
- UNIT 2 Electrochemistry FinalDocumento25 páginasUNIT 2 Electrochemistry FinalPisces SandAinda não há avaliações
- Topic 9 (Galvanic Cell) - Tutorial - Level 2 AnswerDocumento7 páginasTopic 9 (Galvanic Cell) - Tutorial - Level 2 AnswerCheng Xun LeeAinda não há avaliações
- UNIT 2 Electrochemistry FinalDocumento26 páginasUNIT 2 Electrochemistry FinalA HAinda não há avaliações
- Practice Electrochemistry - 1Documento5 páginasPractice Electrochemistry - 1ervaldiAinda não há avaliações
- 2 Electrochemistry (Voltaic Cells)Documento46 páginas2 Electrochemistry (Voltaic Cells)Gerald Paul SumagpaoAinda não há avaliações
- Lesson 15Documento109 páginasLesson 15anil ariAinda não há avaliações
- Electrolysis in Aqueous SolutionDocumento15 páginasElectrolysis in Aqueous SolutionEdon BediAinda não há avaliações
- Electrochemistry 12 Formula SheetDocumento6 páginasElectrochemistry 12 Formula SheetFranknire IgAinda não há avaliações
- ElectrolysisDocumento12 páginasElectrolysisShofwa AnnisaAinda não há avaliações
- 分析電化學講義1Documento33 páginas分析電化學講義1ylliwqAinda não há avaliações
- Physical Chemistry 2 - Kinetics of Electrochemical ProcessesDocumento44 páginasPhysical Chemistry 2 - Kinetics of Electrochemical ProcessesNguyễn Thu HàAinda não há avaliações
- Electrochemistry GR 12 - TheoryDocumento45 páginasElectrochemistry GR 12 - TheoryPepsiAinda não há avaliações
- Standard Cell Potentials PracticesDocumento3 páginasStandard Cell Potentials PracticeservaldiAinda não há avaliações
- ElectrochemistryDocumento74 páginasElectrochemistryVipranshu GuptaAinda não há avaliações
- Physical Chemistry Chapter 4 - ElectrochemistryDocumento36 páginasPhysical Chemistry Chapter 4 - Electrochemistryjatropos6810Ainda não há avaliações
- Ex Cell NotationDocumento2 páginasEx Cell NotationveemueAinda não há avaliações
- Matriculation Chemistry (Electrochemistry)Documento77 páginasMatriculation Chemistry (Electrochemistry)ridwan100% (3)
- Electrochemistry: Syllabus Theme 5Documento63 páginasElectrochemistry: Syllabus Theme 5Jack WilliamsAinda não há avaliações
- R20 Applied Chemistry - UNIT-2 (Ref-2)Documento31 páginasR20 Applied Chemistry - UNIT-2 (Ref-2)pkAinda não há avaliações
- Standard Electrode Potentials & CellsDocumento3 páginasStandard Electrode Potentials & Cellsmy nameAinda não há avaliações
- Chapter 20Documento77 páginasChapter 20XYRUS MARAMOTAinda não há avaliações
- Electrode PotentialDocumento31 páginasElectrode PotentialseekforheavenAinda não há avaliações
- Electrochemistry - ApplicationsDocumento18 páginasElectrochemistry - ApplicationsMAinda não há avaliações
- 1st Yr 2007 RedoxDocumento66 páginas1st Yr 2007 RedoxAriyanti NaissaissAinda não há avaliações
- 1501 Electrode Potential: The Spontaneity of Electron Transfer Relationship Between E, GandkDocumento21 páginas1501 Electrode Potential: The Spontaneity of Electron Transfer Relationship Between E, GandkJuan Martínez0% (1)
- Subject: Chemistry Electrochemistry: Decreases PH of Solution (D) Electrolysis of CusoDocumento28 páginasSubject: Chemistry Electrochemistry: Decreases PH of Solution (D) Electrolysis of CusoQwertyAinda não há avaliações
- Electrochem Understanding - AnswersDocumento11 páginasElectrochem Understanding - AnswersSiva NeshAinda não há avaliações
- When A Non-Spontaneous Redox Reaction Is Made To Occur by Putting Electrical Energy Into The SystemDocumento11 páginasWhen A Non-Spontaneous Redox Reaction Is Made To Occur by Putting Electrical Energy Into The SystemArdit QerimiAinda não há avaliações
- POTENSIAL KorosiDocumento33 páginasPOTENSIAL KorosiLisa AndrianiAinda não há avaliações
- POTENSIAL KorosiDocumento33 páginasPOTENSIAL Korosilisa andrianiAinda não há avaliações
- Lecture 15.2 - E0 & EDocumento10 páginasLecture 15.2 - E0 & ELiam DoranAinda não há avaliações
- Electro Chemistry One PageDocumento2 páginasElectro Chemistry One Pageshankaranand200517Ainda não há avaliações
- Links Lel AssignmentDocumento1 páginaLinks Lel AssignmentNooran ShamsAinda não há avaliações
- Drug AddictionDocumento1 páginaDrug AddictionNooran ShamsAinda não há avaliações
- Manual For The Surveillance of Vaccine-Preventable Diseases (4th Edition, 2008)Documento2 páginasManual For The Surveillance of Vaccine-Preventable Diseases (4th Edition, 2008)Nooran ShamsAinda não há avaliações
- Aryl Halides (Halogenoarenes) : The Structure of ChlorobenzeneDocumento3 páginasAryl Halides (Halogenoarenes) : The Structure of ChlorobenzeneNooran ShamsAinda não há avaliações
- Inductive Sensor For Temperature Measurement in Induction Heating Applications PDFDocumento8 páginasInductive Sensor For Temperature Measurement in Induction Heating Applications PDFNjabulo XoloAinda não há avaliações
- Datasheet en 20170526Documento9 páginasDatasheet en 20170526LODELBARRIO RDAinda não há avaliações
- Linear Motion4Documento9 páginasLinear Motion4Jai GaizinAinda não há avaliações
- 2008 MuchiriDocumento20 páginas2008 MuchiriBushra AzharAinda não há avaliações
- Opc Da Client ManualDocumento29 páginasOpc Da Client ManualantoAinda não há avaliações
- M31M 1 1Documento13 páginasM31M 1 1DannyChaconAinda não há avaliações
- sbd0105 33 71 1BBE0 000011Documento1 páginasbd0105 33 71 1BBE0 000011NetflixAinda não há avaliações
- Created by C. Mani, Education Officer, KVS RO Silchar: ST ND RD ST ND RD ST ND RDDocumento51 páginasCreated by C. Mani, Education Officer, KVS RO Silchar: ST ND RD ST ND RD ST ND RDjaindevansh100% (2)
- OneTen-Frontend Web Development FundamentalsDocumento5 páginasOneTen-Frontend Web Development FundamentalsOkpetah Chioma christabelAinda não há avaliações
- Steel and Timber Structures Part Two:: Design of Structural Steel MembersDocumento28 páginasSteel and Timber Structures Part Two:: Design of Structural Steel MembersDhinesh KalaimaranAinda não há avaliações
- Eddy AxialDocumento20 páginasEddy Axialandrea19711971Ainda não há avaliações
- For More ACCA Study Materials, Tutor Support, Exam Tips VisitDocumento2 páginasFor More ACCA Study Materials, Tutor Support, Exam Tips VisitNeel KostoAinda não há avaliações
- EO Gaddis Java Chapter 06 6e-ClassesObjectsPart2Documento48 páginasEO Gaddis Java Chapter 06 6e-ClassesObjectsPart2nyle90009Ainda não há avaliações
- 05 - A Statistical Analysis of Body Measurements of Filipino WomenDocumento15 páginas05 - A Statistical Analysis of Body Measurements of Filipino WomenJenberrose G TrelinskiAinda não há avaliações
- Radar Pulse CompressionDocumento19 páginasRadar Pulse Compressionmalek1p2Ainda não há avaliações
- Seizmic Design of RE WallsDocumento49 páginasSeizmic Design of RE Wallsljubomirjocic@yahoo.com100% (1)
- 03 Traversing Gear SlaveDocumento69 páginas03 Traversing Gear SlaveDeMen NguyenAinda não há avaliações
- En 2014 New Brochure WebDocumento20 páginasEn 2014 New Brochure WebSasa NackovicAinda não há avaliações
- ACT Geometry - PolygonsDocumento8 páginasACT Geometry - PolygonsaftabAinda não há avaliações
- Linear Programming: Simplex Method: Dr. R. K Singh Professor, Operations Management MDI, GurgaonDocumento58 páginasLinear Programming: Simplex Method: Dr. R. K Singh Professor, Operations Management MDI, GurgaonvsyoiAinda não há avaliações
- Windy Hill Middle School - Trumpet Warm Up BookDocumento61 páginasWindy Hill Middle School - Trumpet Warm Up BookGleyce VieiraAinda não há avaliações
- Os Module1Documento37 páginasOs Module1lingaraj_superstarAinda não há avaliações
- Conveyor Chain and SprocketsDocumento5 páginasConveyor Chain and Sprocketsmartc35Ainda não há avaliações
- Dosing Technology: eco-CONTROL EC200-KDocumento2 páginasDosing Technology: eco-CONTROL EC200-KMario Vargas VegaAinda não há avaliações
- PLC Programming With RSLogix 500Documento132 páginasPLC Programming With RSLogix 500kemo_750252831Ainda não há avaliações
- Fuse Link KDocumento6 páginasFuse Link KABam BambumAinda não há avaliações
- Electronic - Banking and Customer Satisfaction in Greece - The Case of Piraeus BankDocumento15 páginasElectronic - Banking and Customer Satisfaction in Greece - The Case of Piraeus BankImtiaz MasroorAinda não há avaliações
- Art Appreciation Learning ModulesDocumento32 páginasArt Appreciation Learning ModulesJonah ChoiAinda não há avaliações
- Lecture Slides: Shigley's Mechanical Engineering DesignDocumento95 páginasLecture Slides: Shigley's Mechanical Engineering DesignpurnaAinda não há avaliações
- Chapter 3Documento21 páginasChapter 3aloneirAinda não há avaliações