Escolar Documentos
Profissional Documentos
Cultura Documentos
LSM1101 Practical 1
Enviado por
givena2ndchanceDescrição original:
Direitos autorais
Formatos disponíveis
Compartilhar este documento
Compartilhar ou incorporar documento
Você considera este documento útil?
Este conteúdo é inapropriado?
Denunciar este documentoDireitos autorais:
Formatos disponíveis
LSM1101 Practical 1
Enviado por
givena2ndchanceDireitos autorais:
Formatos disponíveis
Results (45%) The data is organized in a clear manner.
Figures and/or tables are effective and accompanied by titles and legends. Describe your results in text.
Discussion (35%) (Interprets the results and reaches a conclusion) Explanation of basic principles
Explanation of the experimental logic Explanation of nay difference between your results with others, or with your
expectations. Writing (10%) Text is clear, concise and easy to read.
U0900023J U084997N Practical 1: pH & Buffers
Introduction
Objective:
To understand buffers, their buffering regions and the Henderson-Hasselbalch equation.
Background:
Buffers work by resisting changes in pH. Only weak acids and bases, which can form conjugate bases and acids respectively can be buffers. A pH meter is used to measure the H+ concentration in any given solution, the result is temperature dependent, as such, the machine must be calibrated at a known pH and temperature.
Materials & Methods:
Various buffers, namely, Histidine Monohydrochloride, Potassium Phosphate and Tris-HCl, are used in 3 separate experiments. Titration was used not only to determine buffering capacity but also Ka values and the effect of temperature.
3.1.4
Data handling and questions
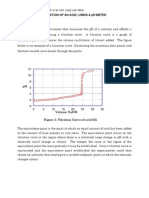
(a) Number of moles of Histidine present in solution: 9.73 x 10-4 mol (b) (c) (d) (e) See graph below pKa values: 5.94 & 9.94 It shows maximal buffering capacity at pH 6. The effective buffering range is pH 6 to 9. Using method (i) will give a more accurate estimate of pKa, since it is assumed the NaOH has not been prepared accurately, then any calculations based on initial known values would be off. However, in the case of the graph, it is merely a rough estimate, and hence any discrepancies will not be significant enough to give false readings. (i) for 5ml of NaOH: pH is 6.20, as compared to 6.14 in the experiment. (ii) for 12ml of NaOH: pH is 8.90 as compared to 8.73 in the experiment. Differences in calculated pH values and experimental pH values differ. This might be due to experimental errors and errors in substance preparation. (i) Number of ionisable groups in Histidine at initial pH: 2 (ii) Groups responsible for observed pKas: NH3+ and =NH+
(f)
(g)
(h) Structures of ionic species in histidine that participate in buffering:
Results
Part 1:
pH 4.82 5.17 5.37 5.53 5.68 5.78 5.86 5.94 6.04 6.14 6.22 6.33 6.42 6.54 6.65 6.75 6.95 7.17 7.33 7.66 Vol of NaOH /ml 0.5 1 1.5 2 2.5 3 3.5 4 4.5 5 5.5 6 6.5 7 7.5 8 8.5 9 9.5 10 mol NaOH 0.000025 0.00005 0.000075 0.0001 0.000125 0.00015 0.000175 0.0002 0.000225 0.00025 0.000275 0.0003 0.000325 0.00035 0.000375 0.0004 0.000425 0.00045 0.000475 0.0005 mol NaOH/mol Hist 0.025693731 0.051387461 0.077081192 0.102774923 0.128468654 0.154162384 0.179856115 0.205549846 0.231243577 0.256937307 0.282631038 0.308324769 0.334018499 0.35971223 0.385405961 0.411099692 0.436793422 0.462487153 0.488180884 0.513874615
8.09 8.39 8.57 8.73 8.84 8.97 9.07 9.17 9.26 9.35 9.43 9.53 9.61 9.71 9.81 9.94 10.07 10.22 10.41 10.65 10.88 11.07 11.21 11.34 11.42 11.48 11.5
10.5 11 11.5 12 12.5 13 13.5 14 14.5 15 15.5 16 16.5 17 17.5 18 18.5 19 19.5 20 20.5 21 21.5 22 22.5 23 23.5
0.000525 0.00055 0.000575 0.0006 0.000625 0.00065 0.000675 0.0007 0.000725 0.00075 0.000775 0.0008 0.000825 0.00085 0.000875 0.0009 0.000925 0.00095 0.000975 0.001 0.001025 0.00105 0.001075 0.0011 0.001125 0.00115 0.001175
0.539568345 0.565262076 0.590955807 0.616649538 0.642343268 0.668036999 0.69373073 0.71942446 0.745118191 0.770811922 0.796505653 0.822199383 0.847893114 0.873586845 0.899280576 0.924974306 0.950668037 0.976361768 1.002055498 1.027749229 1.05344296 1.079136691 1.104830421 1.130524152 1.156217883 1.181911614 1.207605344
Q 3.2.4 Part 1 Tris-Hcl buffer seems to be better at buffering when an alkaline solution is added and Potassium Phosphate buffer seems to be better when an acidic solution is added. Part 2 Effective buffering range for Potassium Phosphate buffer is pKa 1 that is pH range 5.8 - 7.8. Effective buffering range for Tris-Hcl buffer is from pH 7.1 9.1. So for the phosphate which functions optimally at pH7.2 Tris-Hcl buffer should be used. Although it is not better than the potassium phosphate buffer in the case if large quantities of alkali is added its effective buffering range shows that is will still be a buffer is an acid added. In the case of the potassium phosphate buffer it is not a good offer if the solution starts to become acidic. Q 3.3.4 Temperature of Solution(oC) 25 oC 4 oC pH of Tris-Hcl Buffer pH of Potassium Phosphate Buffer 7.11 7.13
1.47 1.87
From these results it can be concluded that lowering the temperature of the solutions causes the pH of the respective solutions to rise. Tris-HCl buffer and potassium phosphate buffer both contain weak acids. HA H+ + ADissociation is a bond breaking process thus it requires energy in order to proceed. Thus the forward reaction is endothermic and the reverse reaction is therefore exothermic. When the buffer solutions were cooled to 40C, the buffer tried to remove the disturbance characterized
as the loss of heat energy by shifting the equilibrium towards producing more undissociated acid. This is to counter the energy lost as heat so as to restore the equilibrium in a way. With decreased dissociation, [H+] in solution would be lower accounting for an increase in pH.
Você também pode gostar
- MACD - 5 Profitable Trading StrategiesDocumento10 páginasMACD - 5 Profitable Trading StrategiesPeter FrankAinda não há avaliações
- Experiment 1 Preparation of Buffer SolutionsDocumento16 páginasExperiment 1 Preparation of Buffer Solutionsmohamad ashaziq89% (56)
- Advanced Pharmaceutical analysisNo EverandAdvanced Pharmaceutical analysisNota: 4.5 de 5 estrelas4.5/5 (2)
- Potentiometric TitrationDocumento9 páginasPotentiometric Titrationiah_guevarraAinda não há avaliações
- Determination of Ka Value of A Weak AcidDocumento15 páginasDetermination of Ka Value of A Weak AcidMyaIdzaharAinda não há avaliações
- An Analysis of The Finite Element Method1Documento320 páginasAn Analysis of The Finite Element Method1VICTOR CHAVEZAinda não há avaliações
- Identifying An Unknown Weak Acids ExperimentDocumento18 páginasIdentifying An Unknown Weak Acids Experimentgeek3112100% (5)
- 06 and 07 Standardization of NaOH and Acid Base TitrationDocumento16 páginas06 and 07 Standardization of NaOH and Acid Base TitrationTyler Hardy80% (5)
- Discussion On Potentiometric TitrationsDocumento16 páginasDiscussion On Potentiometric TitrationsKcirtap Zketh60% (5)
- Determination of Ka of Unknown AcidDocumento23 páginasDetermination of Ka of Unknown AcidShasha0% (1)
- GRR Study MSA TemplateDocumento21 páginasGRR Study MSA TemplaterajarajanAinda não há avaliações
- Potentiometric Titration CurvesDocumento5 páginasPotentiometric Titration CurvesDavid GrahamAinda não há avaliações
- LAB REPORT - Determination of Concentration Acetic Acid in VinegarDocumento12 páginasLAB REPORT - Determination of Concentration Acetic Acid in Vinegarhisham100% (3)
- The Ka & Molar Mass of A Monoprotic Weak AcidDocumento7 páginasThe Ka & Molar Mass of A Monoprotic Weak AcidLeslie Sarah100% (1)
- Physical Organic Chemistry—Ii: Specially Invited Lectures Presented at the Second IUPAC Conference on Physical Organic Chemistry Held at Noordwijkerhout, Netherlands, 29 April–2 May 1974No EverandPhysical Organic Chemistry—Ii: Specially Invited Lectures Presented at the Second IUPAC Conference on Physical Organic Chemistry Held at Noordwijkerhout, Netherlands, 29 April–2 May 1974Th. J. De BoerAinda não há avaliações
- Acid-Base Titrations Curve Formal LabDocumento9 páginasAcid-Base Titrations Curve Formal LabAshley StraubAinda não há avaliações
- Lab Report DETERMINATION OF THE CONCENTRATION OF ACETIC ACID IN VINEGARDocumento27 páginasLab Report DETERMINATION OF THE CONCENTRATION OF ACETIC ACID IN VINEGARمحمد ازوادي100% (1)
- Potentiometric Titration of An Acid MixtureDocumento9 páginasPotentiometric Titration of An Acid MixtureRuth Danielle GasconAinda não há avaliações
- Case Analysis - PACADIDocumento12 páginasCase Analysis - PACADINidhi GuptaAinda não há avaliações
- Lab Experiment 3 Ka Determination Through PH TitrationDocumento4 páginasLab Experiment 3 Ka Determination Through PH TitrationxmusiqaAinda não há avaliações
- Quantitative Determination of Potassium Acid Phthalate KHPDocumento17 páginasQuantitative Determination of Potassium Acid Phthalate KHPMichelle Cruz AbrilAinda não há avaliações
- Sample Chemistry Undergraduate Laboratory ReportDocumento14 páginasSample Chemistry Undergraduate Laboratory ReportApril TapayanAinda não há avaliações
- KaDocumento5 páginasKaSonu DubeyAinda não há avaliações
- 8 - Lab8-Potentiometric Titration of Acid MixtureDocumento6 páginas8 - Lab8-Potentiometric Titration of Acid MixtureHoang Huong TraAinda não há avaliações
- Lab Report Experiment 2 Determination of Ka Value of A Weak AcidDocumento17 páginasLab Report Experiment 2 Determination of Ka Value of A Weak AcidarisyahariffAinda não há avaliações
- Lab Report 3 KotDocumento15 páginasLab Report 3 KotNikMuhammadIzzatAinda não há avaliações
- Sample Lab Report For Experiment 2Documento2 páginasSample Lab Report For Experiment 2Ashfaq AhmadAinda não há avaliações
- Che485 Lab1 Mac2023 Ceeh2202fDocumento19 páginasChe485 Lab1 Mac2023 Ceeh2202f2023389329Ainda não há avaliações
- Chemistry Lab Report1Documento22 páginasChemistry Lab Report1RoseAnne BellaAinda não há avaliações
- Potentiometric Titration of A Weak Acid: Chemistry 135 Clark CollegeDocumento14 páginasPotentiometric Titration of A Weak Acid: Chemistry 135 Clark CollegeMay LeeAinda não há avaliações
- Acid Base TitrationDocumento12 páginasAcid Base TitrationMsfaeza HanafiAinda não há avaliações
- AcidinjuiceandsodalabrubricDocumento11 páginasAcidinjuiceandsodalabrubricapi-302408024Ainda não há avaliações
- Lab Report Experiment 1Documento12 páginasLab Report Experiment 1afifiAinda não há avaliações
- Determination Acetic AcidDocumento21 páginasDetermination Acetic Acidameyakem100% (1)
- No. Pages: Table of ContentDocumento18 páginasNo. Pages: Table of ContentAzzian AriffinAinda não há avaliações
- Chemistry Laboratory (CY1094D) : Dr. Mausumi ChattopadhyayaDocumento18 páginasChemistry Laboratory (CY1094D) : Dr. Mausumi ChattopadhyayaSita kumarAinda não há avaliações
- Lab Report 1Documento27 páginasLab Report 1szulkipeliAinda não há avaliações
- Che485 Lab1 Mac2023 Ceeh2202f 2023389329Documento17 páginasChe485 Lab1 Mac2023 Ceeh2202f 2023389329Wan AfiqAinda não há avaliações
- Chem Term 1Documento42 páginasChem Term 1gwx4zxbnxnAinda não há avaliações
- 62 Experiment #5. Titration of An Acid Using A PH MeterDocumento7 páginas62 Experiment #5. Titration of An Acid Using A PH MeteryumnatehreemAinda não há avaliações
- CPB 30103 Biochemical Engineering UniKL MICET Experiment 1: Preparation of Buffer Solution Full Lab ReportDocumento10 páginasCPB 30103 Biochemical Engineering UniKL MICET Experiment 1: Preparation of Buffer Solution Full Lab ReportSiti Hajar Mohamed0% (1)
- Result Discussion Conclusion Exp 2 Physic ChemDocumento6 páginasResult Discussion Conclusion Exp 2 Physic Chemarif arifin100% (1)
- Experiment 1: Determination of Total Acidity of Vinegar: Final Laboratory ReportDocumento13 páginasExperiment 1: Determination of Total Acidity of Vinegar: Final Laboratory ReportEunice OpinioAinda não há avaliações
- ProjectDocumento2 páginasProjectCindy Mae A. PogoyAinda não há avaliações
- AP Chemistry - Titration Curves of Strong and Weak Acids and BasesDocumento5 páginasAP Chemistry - Titration Curves of Strong and Weak Acids and BasesJonathan Chen100% (2)
- Lab Report 2Documento11 páginasLab Report 2afnan_lion94Ainda não há avaliações
- Acid-Base Titrations: Standardization of Naoh and Antacid AnalysisDocumento5 páginasAcid-Base Titrations: Standardization of Naoh and Antacid AnalysisJohn KenoAinda não há avaliações
- Formal Report Exp 1Documento5 páginasFormal Report Exp 1Nick Austin BayotAinda não há avaliações
- BI309 Lab 3 Ryan CarrollDocumento4 páginasBI309 Lab 3 Ryan CarrollRyan CarrollAinda não há avaliações
- Experiment 1 Preparation of Buffer SolutionsDocumento16 páginasExperiment 1 Preparation of Buffer SolutionsNAEEM MALIKAinda não há avaliações
- Enviromental Engineering Lab CE463: Name:omar Hayel Darabseh ID:103118 SEC#: 6Documento8 páginasEnviromental Engineering Lab CE463: Name:omar Hayel Darabseh ID:103118 SEC#: 6Omar H DarabsehAinda não há avaliações
- ACID - BASE TITRATIONS Determination of Purity of Potassium AcidDocumento4 páginasACID - BASE TITRATIONS Determination of Purity of Potassium Acidhilary0622Ainda não há avaliações
- Carbonate-Bicarbonate Mixture Anal Chem Post LabDocumento7 páginasCarbonate-Bicarbonate Mixture Anal Chem Post LabKennedy OrtegaAinda não há avaliações
- Problem Set 1 BCDocumento4 páginasProblem Set 1 BCakshookannanAinda não há avaliações
- Lab Report 4Documento22 páginasLab Report 4wilhelminaanimAinda não há avaliações
- Acid and Base Chemistry LabDocumento7 páginasAcid and Base Chemistry LabChrist ElleAinda não há avaliações
- 5: PH Measurement and Its Applications (Experiment) : ObjectivesDocumento19 páginas5: PH Measurement and Its Applications (Experiment) : ObjectivesNajmi NasirAinda não há avaliações
- Summary: Phthalate (KHP) Solution Which The Molarity Is Already Known. Expressing The Chemical ReactionDocumento15 páginasSummary: Phthalate (KHP) Solution Which The Molarity Is Already Known. Expressing The Chemical ReactionDayledaniel SorvetoAinda não há avaliações
- Exp 1 Concentration of Acetic AcidDocumento18 páginasExp 1 Concentration of Acetic AcidMatt CerosAinda não há avaliações
- Lab Report (Vinegar)Documento17 páginasLab Report (Vinegar)SazrinaMohdSafar100% (4)
- Practical Report On The PkaDocumento13 páginasPractical Report On The PkaRohit50% (2)
- Thermometric Titrimetry: International Series of Monographs in Analytical ChemistryNo EverandThermometric Titrimetry: International Series of Monographs in Analytical ChemistryAinda não há avaliações
- Acid-Base Disorders: Clinical Evaluation and ManagementNo EverandAcid-Base Disorders: Clinical Evaluation and ManagementAinda não há avaliações
- GEK1532 Optics of The EyeDocumento42 páginasGEK1532 Optics of The Eyegivena2ndchanceAinda não há avaliações
- GEK1532 Nerve PulsesDocumento35 páginasGEK1532 Nerve Pulsesgivena2ndchanceAinda não há avaliações
- GEK1532 Color Perception: Thorsten Wohland Dep. of Chemistry S8-03-06 Tel.: 6516 1248 E-Mail: Chmwt@nus - Edu.sgDocumento43 páginasGEK1532 Color Perception: Thorsten Wohland Dep. of Chemistry S8-03-06 Tel.: 6516 1248 E-Mail: Chmwt@nus - Edu.sggivena2ndchanceAinda não há avaliações
- GEK1532 Causes of ColourDocumento44 páginasGEK1532 Causes of Colourgivena2ndchanceAinda não há avaliações
- GEK1045 EssayDocumento10 páginasGEK1045 Essaygivena2ndchanceAinda não há avaliações
- GEK1045 Essay Questions and Guidelines 0910Documento4 páginasGEK1045 Essay Questions and Guidelines 0910givena2ndchanceAinda não há avaliações
- LSM1102 - Tutorial Questions On DNA ReplicationDocumento2 páginasLSM1102 - Tutorial Questions On DNA Replicationgivena2ndchanceAinda não há avaliações
- LSM1102 - Widespread Lateral Gene Transfer From Intracellular Bacteria To Multi Cellular EukaryotesDocumento5 páginasLSM1102 - Widespread Lateral Gene Transfer From Intracellular Bacteria To Multi Cellular Eukaryotesgivena2ndchanceAinda não há avaliações
- LSM 1102 Continual Assessment 2 Sem 2 2005-6 Answer SchemebDocumento6 páginasLSM 1102 Continual Assessment 2 Sem 2 2005-6 Answer Schemebgivena2ndchanceAinda não há avaliações
- LSM1102 - Answers of Tutorial 1& 2Documento3 páginasLSM1102 - Answers of Tutorial 1& 2givena2ndchanceAinda não há avaliações
- Course Outline: ECOR 2606Documento3 páginasCourse Outline: ECOR 2606f22archrerAinda não há avaliações
- Regression Statistics: AnovaDocumento2 páginasRegression Statistics: AnovaAbigail VAinda não há avaliações
- 02 Engineering ResearchDocumento19 páginas02 Engineering ResearchjeffersonAinda não há avaliações
- Symbolic Integration (1967)Documento272 páginasSymbolic Integration (1967)uhouhfohvnouneunAinda não há avaliações
- GEG 402 Slides of Numerical Analysis of Ordinary Differential Equations 3Documento54 páginasGEG 402 Slides of Numerical Analysis of Ordinary Differential Equations 3Benedict HounsinouAinda não há avaliações
- Govt. College Women University Faisalabad: Raghisa KhalidDocumento9 páginasGovt. College Women University Faisalabad: Raghisa KhalidraghisaAinda não há avaliações
- Medida e Integração - The Elements of Integration and Lebesgue Measure - BartleDocumento193 páginasMedida e Integração - The Elements of Integration and Lebesgue Measure - BartleAlexandre FernandesAinda não há avaliações
- 1988 - Fronts Propagating With Curvature - Dependent Speed Algorithms Based On Hamilton-Jacobi Formulations - Osher, Sethian PDFDocumento38 páginas1988 - Fronts Propagating With Curvature - Dependent Speed Algorithms Based On Hamilton-Jacobi Formulations - Osher, Sethian PDFRodrigo AbdoAinda não há avaliações
- Sdepde PDFDocumento202 páginasSdepde PDFGustaf TegnérAinda não há avaliações
- Courant-Friedrichs-Lewy ConditionDocumento3 páginasCourant-Friedrichs-Lewy ConditionpoliskarmaAinda não há avaliações
- 85409Documento110 páginas85409arunAinda não há avaliações
- Provisional Module Results For Mathematics Students 2017-18 PDFDocumento110 páginasProvisional Module Results For Mathematics Students 2017-18 PDFTomas VaiciusAinda não há avaliações
- Section 9.4 Lagrange Multipliers Problems 1-15 Odd, 19-25 OddDocumento4 páginasSection 9.4 Lagrange Multipliers Problems 1-15 Odd, 19-25 OddxxambertaimexxAinda não há avaliações
- The Logarithmic FunctionDocumento3 páginasThe Logarithmic FunctionCharalampidis DimitrisAinda não há avaliações
- Department of Statistical and Actuarial Sciences SS 2864b Jan-Apr 2010 Instructor: D. Murdoch Final Exam, April 27, 1-4 PMDocumento13 páginasDepartment of Statistical and Actuarial Sciences SS 2864b Jan-Apr 2010 Instructor: D. Murdoch Final Exam, April 27, 1-4 PMArth PatelAinda não há avaliações
- Solved - (A) Design The Optimal Conical Container (Fig. P16.2) T...Documento3 páginasSolved - (A) Design The Optimal Conical Container (Fig. P16.2) T...Alexander Estrada OrtizAinda não há avaliações
- Econometrics Chapter 4Documento5 páginasEconometrics Chapter 4Jade NguyenAinda não há avaliações
- Assignment Analysis 1Documento2 páginasAssignment Analysis 1yashAinda não há avaliações
- TECSON - THEOREMS ON LIMITS - MAT060 - CcUuDocumento18 páginasTECSON - THEOREMS ON LIMITS - MAT060 - CcUuChris John TecsonAinda não há avaliações
- Curvature of Digital CurvesDocumento12 páginasCurvature of Digital CurvesMichael HeydtAinda não há avaliações
- Department of Mathematics TUTORIAL 3Documento2 páginasDepartment of Mathematics TUTORIAL 3Neethesh kumarAinda não há avaliações
- Math 124A - November 10, 2011 Viktor Grigoryan: TT 2 XX TDocumento4 páginasMath 124A - November 10, 2011 Viktor Grigoryan: TT 2 XX Tarpan mukherjeeAinda não há avaliações
- Roots of PolynomialsDocumento14 páginasRoots of PolynomialsJRAinda não há avaliações
- Classification of 2nd Order PDEDocumento7 páginasClassification of 2nd Order PDEMani AgarwalAinda não há avaliações
- Accelerated Numerical Method For Singularly Perturbed Differential Difference EquationsDocumento6 páginasAccelerated Numerical Method For Singularly Perturbed Differential Difference EquationsSultan GodanaAinda não há avaliações
- CH 7.1 Area of A Region Between 2 Curves PDFDocumento10 páginasCH 7.1 Area of A Region Between 2 Curves PDFAtin Fifa100% (1)