Escolar Documentos
Profissional Documentos
Cultura Documentos
The Filarial Life Cycle
Enviado por
a1savedDescrição original:
Direitos autorais
Formatos disponíveis
Compartilhar este documento
Compartilhar ou incorporar documento
Você considera este documento útil?
Este conteúdo é inapropriado?
Denunciar este documentoDireitos autorais:
Formatos disponíveis
The Filarial Life Cycle
Enviado por
a1savedDireitos autorais:
Formatos disponíveis
The filarial life cycle, like that of all nematodes, consists of 5 developmental or larval stages in a vertebral host and
an arthropod intermediate host and vector. Adult female worms produce thousands of first-stage larvae or microfilariae that are ingested by a feeding insect vector. Some microfilariae have a unique daily circadian periodicity in the peripheral circulation. The arthropod vectors, mosquitoes and flies, also have a circadian rhythm in which they obtain blood meals. The highest concentration of microfilariae usually occurs when the local vector is feeding most actively. See the image below.
Filariasis. This figure displays the life cycle of Wuchereria bancrofti in humans and mosquito vectors (ie, Aedes, Anopheles, Culex, Mansonia species). Life cycles of other lymphatic nematodes (ie, Brugia malayi, Brugia timori) are identical, while the life cycles for other filariae differ in the body location of adult worms, the microfilariae present, and the arthropod intermediate hosts and vectors. Microfilariae then undergo two developmental changes in the insect. Third-stage larvae then are inoculated back into the vertebral host during the act of feeding for the final two stages of development. These larvae travel through the dermis and enter regional lymphatic vessels. During the next 9 months, these develop into mature worms (20-100 mm in length). An average parasite can survive for about 5 years. The prepatent period is defined as the interval between a vector bite and the appearance of microfilaria in blood, with an estimated duration of about 12 months. The following factors affect the pathogenesis of filariasis:
The quantity of accumulating adult worm antigen in the lymphatics[5] The duration and level of exposure to infective insect bites[6] The number of secondary bacterial and fungal infections[5] The degree of host immune response[7]
Filarial infection generates significant inflammatory immune responses that participate in the development of symptomatic lymphatic obstruction. Increased levels of immunoglobulin E (IgE) and immunoglobulin G4 (IgG4) secondary to antigenic (from dead worms) stimulation of Th2-type immune response have been demonstrated.
Studies have shown that there is a familial tendency to lymphatic obstruction, providing support for the hypothesis that host genes influence lymphedema susceptibility.[8] Prenatal exposure seems to be an important determinant in conferring greater immune tolerance to parasite antigen.[9] Thus, individuals from endemic areas are often asymptomatic until late in disease when they have high worm burden, whereas nonimmune expatriates tend to have brisk immune responses and more severe early clinical symptoms, even in light infections. Recent studies have shown that lymphatic filarial parasites contain rickettsialikeWolbachia endosymbiotic bacteria. This association has been recognized as contributing to the inflammatory reaction seen in filariasis.[10] Previous Next Secti
Você também pode gostar
- Inclusion and Exclusion Criteria: Experimental Designs: Preliminary InfoDocumento13 páginasInclusion and Exclusion Criteria: Experimental Designs: Preliminary Infoa1savedAinda não há avaliações
- 9 US-Russian Nursing Conference CruiseDocumento2 páginas9 US-Russian Nursing Conference Cruisea1savedAinda não há avaliações
- Joel ComiskeyDocumento17 páginasJoel Comiskeya1savedAinda não há avaliações
- Drug StudyDocumento16 páginasDrug StudyMadel Gonzales100% (3)
- Lippits Change TheoryDocumento9 páginasLippits Change Theorya1saved100% (1)
- Crohns DiseaseDocumento72 páginasCrohns Diseasea1savedAinda não há avaliações
- Gender Sensitivity RHDocumento1 páginaGender Sensitivity RHa1savedAinda não há avaliações
- Hidden Figures: The American Dream and the Untold Story of the Black Women Mathematicians Who Helped Win the Space RaceNo EverandHidden Figures: The American Dream and the Untold Story of the Black Women Mathematicians Who Helped Win the Space RaceNota: 4 de 5 estrelas4/5 (895)
- The Subtle Art of Not Giving a F*ck: A Counterintuitive Approach to Living a Good LifeNo EverandThe Subtle Art of Not Giving a F*ck: A Counterintuitive Approach to Living a Good LifeNota: 4 de 5 estrelas4/5 (5794)
- The Yellow House: A Memoir (2019 National Book Award Winner)No EverandThe Yellow House: A Memoir (2019 National Book Award Winner)Nota: 4 de 5 estrelas4/5 (98)
- The Little Book of Hygge: Danish Secrets to Happy LivingNo EverandThe Little Book of Hygge: Danish Secrets to Happy LivingNota: 3.5 de 5 estrelas3.5/5 (400)
- Never Split the Difference: Negotiating As If Your Life Depended On ItNo EverandNever Split the Difference: Negotiating As If Your Life Depended On ItNota: 4.5 de 5 estrelas4.5/5 (838)
- Elon Musk: Tesla, SpaceX, and the Quest for a Fantastic FutureNo EverandElon Musk: Tesla, SpaceX, and the Quest for a Fantastic FutureNota: 4.5 de 5 estrelas4.5/5 (474)
- A Heartbreaking Work Of Staggering Genius: A Memoir Based on a True StoryNo EverandA Heartbreaking Work Of Staggering Genius: A Memoir Based on a True StoryNota: 3.5 de 5 estrelas3.5/5 (231)
- The Emperor of All Maladies: A Biography of CancerNo EverandThe Emperor of All Maladies: A Biography of CancerNota: 4.5 de 5 estrelas4.5/5 (271)
- Devil in the Grove: Thurgood Marshall, the Groveland Boys, and the Dawn of a New AmericaNo EverandDevil in the Grove: Thurgood Marshall, the Groveland Boys, and the Dawn of a New AmericaNota: 4.5 de 5 estrelas4.5/5 (266)
- The Hard Thing About Hard Things: Building a Business When There Are No Easy AnswersNo EverandThe Hard Thing About Hard Things: Building a Business When There Are No Easy AnswersNota: 4.5 de 5 estrelas4.5/5 (345)
- The World Is Flat 3.0: A Brief History of the Twenty-first CenturyNo EverandThe World Is Flat 3.0: A Brief History of the Twenty-first CenturyNota: 3.5 de 5 estrelas3.5/5 (2259)
- Team of Rivals: The Political Genius of Abraham LincolnNo EverandTeam of Rivals: The Political Genius of Abraham LincolnNota: 4.5 de 5 estrelas4.5/5 (234)
- The Unwinding: An Inner History of the New AmericaNo EverandThe Unwinding: An Inner History of the New AmericaNota: 4 de 5 estrelas4/5 (45)
- The Gifts of Imperfection: Let Go of Who You Think You're Supposed to Be and Embrace Who You AreNo EverandThe Gifts of Imperfection: Let Go of Who You Think You're Supposed to Be and Embrace Who You AreNota: 4 de 5 estrelas4/5 (1090)
- The Sympathizer: A Novel (Pulitzer Prize for Fiction)No EverandThe Sympathizer: A Novel (Pulitzer Prize for Fiction)Nota: 4.5 de 5 estrelas4.5/5 (121)
- 02 04 22 TG ByneonDocumento112 páginas02 04 22 TG ByneonferranAinda não há avaliações
- Phylum Class Order Genera: Apicomplexa Haematozoa Haemosporida Plasmodium Piroplasmida Coccidia EimeriidaDocumento39 páginasPhylum Class Order Genera: Apicomplexa Haematozoa Haemosporida Plasmodium Piroplasmida Coccidia EimeriidaMegbaruAinda não há avaliações
- Ordinance No. 284 2021 Swab Test Aldo Mco Aldo McoFINAL 051921Documento3 páginasOrdinance No. 284 2021 Swab Test Aldo Mco Aldo McoFINAL 051921Mai MomayAinda não há avaliações
- Basics of TB, TBDocumento24 páginasBasics of TB, TBzinabu tesfayeAinda não há avaliações
- Viral Meningitis: David R. ChadwickDocumento14 páginasViral Meningitis: David R. ChadwickVina HardiantiAinda não há avaliações
- Bli 225Documento4 páginasBli 225nkAinda não há avaliações
- NCM 109 LEC Alterations With Infectious Inflammatory and Immunologic Responses PDFDocumento18 páginasNCM 109 LEC Alterations With Infectious Inflammatory and Immunologic Responses PDFcalliemozartAinda não há avaliações
- Dengue TriviaDocumento2 páginasDengue TriviaSamuel Luz Barquia, Jr.100% (1)
- EFSA Journal - 2022 - The European Union One Health 2021 Zoonoses ReportDocumento273 páginasEFSA Journal - 2022 - The European Union One Health 2021 Zoonoses ReportJulio San MartinAinda não há avaliações
- Reactii Severe Transf RevDocumento26 páginasReactii Severe Transf RevCapdemai OvidiuAinda não há avaliações
- (Full Last Name, First Name, Middle Name) : Lrd-Div-Spe-Fm-005Documento2 páginas(Full Last Name, First Name, Middle Name) : Lrd-Div-Spe-Fm-005RGC Calamba FacilityAinda não há avaliações
- 2015 Leishmania and Leishmaniasis HabilDocumento82 páginas2015 Leishmania and Leishmaniasis Habilesammm350Ainda não há avaliações
- Certificate For COVID-19 Vaccination: Beneficiary DetailsDocumento1 páginaCertificate For COVID-19 Vaccination: Beneficiary Detailsyenduri sampathAinda não há avaliações
- Covid-19 Protocol On Crew Change and Repatriation of SeafarersDocumento19 páginasCovid-19 Protocol On Crew Change and Repatriation of SeafarersPaingAinda não há avaliações
- Final AppendicesDocumento8 páginasFinal AppendicesHei Nah MontanaAinda não há avaliações
- Reading and Writing Skills Module 39999Documento2 páginasReading and Writing Skills Module 39999Ericka Rivera SantosAinda não há avaliações
- T Rich in Ella Testing WildboarDocumento14 páginasT Rich in Ella Testing WildboarFilon Ioan AlexandruAinda não há avaliações
- COVID-19 Vaccine Booster Doses and Third Doses - COVID-19Documento6 páginasCOVID-19 Vaccine Booster Doses and Third Doses - COVID-19Reynan MaurícioAinda não há avaliações
- Diarrhea Decision TreeDocumento1 páginaDiarrhea Decision TreeMatthew BrandAinda não há avaliações
- New Case Study Booklet-1Documento13 páginasNew Case Study Booklet-1Dr Sumant SharmaAinda não há avaliações
- TB DiagnosisDocumento3 páginasTB Diagnosisمصطفى سهيلAinda não há avaliações
- Propuesta A The Dakota Building: Idioma Extranjero: Inglés. 2015/2016Documento4 páginasPropuesta A The Dakota Building: Idioma Extranjero: Inglés. 2015/2016Monika PerezAinda não há avaliações
- Chicken PoxDocumento4 páginasChicken PoxFloyd SevillaAinda não há avaliações
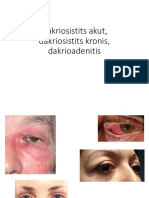
- Dakriosistits Akut, Dakriosistits Kronis, DakrioadenitisDocumento37 páginasDakriosistits Akut, Dakriosistits Kronis, DakrioadenitisyuliasmindeAinda não há avaliações
- Common Illnesses: 1. Write The Correct Illness After Reading The Clues. Each Illness Has Two CluesDocumento4 páginasCommon Illnesses: 1. Write The Correct Illness After Reading The Clues. Each Illness Has Two Cluesmcarmendgj74Ainda não há avaliações
- Prevalensi Strain Avian Pathogenic Escherichia Coli (APEC) Penyebab Kolibasilosis Pada Burung PuyuhDocumento11 páginasPrevalensi Strain Avian Pathogenic Escherichia Coli (APEC) Penyebab Kolibasilosis Pada Burung PuyuhMonik TanAinda não há avaliações
- 05 2019 Pediatric Infectious Diseases LAYOUT R3Documento58 páginas05 2019 Pediatric Infectious Diseases LAYOUT R3khalid alharbiAinda não há avaliações
- Prevention of Nosocomial Infections in Medical Intensive Care UnitDocumento11 páginasPrevention of Nosocomial Infections in Medical Intensive Care UnitGrant Wynn ArnucoAinda não há avaliações
- Sahya Part 1Documento69 páginasSahya Part 1Homeopathy Torrents100% (2)
- MumpsDocumento1 páginaMumpsshailesh284Ainda não há avaliações