Escolar Documentos
Profissional Documentos
Cultura Documentos
Optical Properties of Materials: Issues To Address..
Enviado por
berdog06Descrição original:
Título original
Direitos autorais
Formatos disponíveis
Compartilhar este documento
Compartilhar ou incorporar documento
Você considera este documento útil?
Este conteúdo é inapropriado?
Denunciar este documentoDireitos autorais:
Formatos disponíveis
Optical Properties of Materials: Issues To Address..
Enviado por
berdog06Direitos autorais:
Formatos disponíveis
1
1
ISSUES TO ADDRESS...
What happens when light shines on a material?
Why do materials have characteristic colors?
Optical applications:
luminescence
photoconductivity
solar cell
optical communications fibers
Why are some materials transparent and others not?
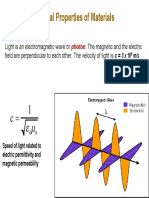
Optical Properties of Materials
2
Optical Properties
Light has both particulate and wavelike properties: Photons
= v = A
hc
h E
m/s) 10 x (3.00 light of speed c
) s J 10 62 . 6 ( constant s Planck'
frequency
wavelength
energy
8
34
=
=
= v
=
= A
x h
E
for visible light: 400 nm (blue) to 800 nm (red)
2
3
Refractive Index, n
Typical glasses ca. 1.5 -1.7
Plastics 1.3 -1.6
PbO (Litharge) 2.67
Diamond 2.41
medium) in light of (velocity
vacuum) in light of (velocity
v
c
Transmitted light distorts electron clouds.
Polarizable ions (e.g., lead) are most effective in decreasing the speed of light.
Light is slower in a material vs vacuum.
n = refractive index
+
no
transmitted
light
transmitted
light
+
electron
cloud
distorts
Note: n is a function of the wavelength of light
4
Total Internal Reflectance
|
|'
=
' sin
sin
n
n
n(low)
n (high)
n > n
1
|
c
|
'
1
|
reflected internally is light for
90 when occurs
c i
i c
| > |
= |' |
angle critical
angle refracted
angle incident
= |
= |'
= |
c
i
i
3
5
Example: Diamond in air
Fiber optic cables are clad in low n material for this reason.
5 . 24
41 . 2
1
sin
sin
1
sin
90 sin
1
41 . 2
sin
sin
= | = |
|
=
|
=
|
|'
=
'
c c
c c
n
n
6
Optical Fibers
prepare preform by extrusion
preform drawn to 125 m or less capillary fibers
plastic cladding applied 60 m
4
7
Optical Fiber Profiles
Step-index Optical Fiber
Graded-index Optical Fiber
8
Incident light is either reflected, absorbed, or
transmitted:
S R A T o
I I I I I + + + =
Light Interaction with Solids
Optical classification of materials:
single
crystal
polycrystalline
dense
polycrystalline
porous
Transparent
Translucent
Opaque
Incident: I
0
Absorbed: I
A
Transmitted: I
T
Scattered: I
S
Reflected: I
R
5
9
Absorption of photons by electron transition:
Metals have a fine succession of energy states.
Near-surface electrons absorb visible light.
Optical Properties of Metals: Absorption
Energy of electron
I
n
c
i
d
e
n
t
p
h
o
t
o
n
Plancks constant
(6.63 x 10
-34
J/s)
freq.
of
incident
light
filled states
unfilled states
AE = hv required!
I
o
o
f
e
n
e
r
g
y
h
v
10
Light Absorption
t
I
I
ln
0
o =
(
t
I
I
o
= e
0
thickness sample
cm ] [ t coefficien absorption linear
1
=
= = o
t
Beers Law: A = lC
6
11
Reflectivity = I
R
/I
o
is between 0.90 and 0.95.
Reflected light is same frequency as incident.
Metals appear reflective (shiny)!
Optical Properties of Metals: Reflection
Electron transition emits a photon.
re-emitted
photon from
material surface
Energy of electron
filled states
unfilled states
AE
I
R
conducting electron
12
Reflectivity, R
Reflection
Metals reflect almost all light
Copper & gold absorb in blue & green => gold color
ty reflectivi
1
1
2
= |
.
|
\
|
+
=
n
n
R
17 . 0
1 41 . 2
1 41 . 2
2
= |
.
|
\
|
+
= R
reflected is light of % 17
Example: Diamond
7
13
Scattering
Occurs especially in semicrystalline & polycrystalline materials
Semicrystalline mixture of crystalline and amorphous phases
density of crystals higher than amorphous materials
speed of light is lower
causes light to change direction = scatter
can cause significant loss of light during transmission
Common in polymers
Ex: Polyethylene milk bottles cloudy because of crystalline regions
Plexiglass (polymethylmethacrylate) clear essentially no crystals
14
Absorption by electron transition occurs if hv > Egap
If Egap < 1.8 eV, full absorption; color is black (Si, GaAs)
If Egap > 3.1 eV, no absorption; colorless (diamond)
If Egap in between, partial absorption; material has a color.
Selected Absorption: Semiconductors
incident photon
energy hv
Energy of electron
filled states
unfilled states
E
gap
I
o
blue light: hv = 3.1 eV
red light: hv = 1.7 eV
8
15
Wavelength vs. Band Gap
1.85 m = 1850 nm = 18,500 = 5400 cm
-1
= infrared
If donor (or acceptor) states also available, this provides other
absorption frequencies
E
g
= 0.67 eV
e.g.: What is the minimum wavelength absorbed by Ge?
c
=
hc
E
g
=
(6.62 x 10
34
J
s)(3 x 10
8
m/s)
(0.67eV)(1.60 x 10
19
J/eV)
s
1.85
m
note: for Si E
g
=1.1eV
c
s1.13
m
16
Color determined by sum of frequencies of
transmitted light + re-emitted light from electron transitions.
Ex: Cadmium Sulfide (CdS)
Egap = 2.4 eV = 516 nm = 5160 = green
absorbs higher energy visible light (blue, violet),
Red/yellow/orange is transmitted and gives it color.
Color of Nonmetals
Ex: Ruby = Sapphire (Al2O3) + (0.5 to 2) at% Cr2O3
Sapphire is colorless
(i.e., Egap > 3.1eV)
adding Cr2O3 :
alters the band gap
blue light is absorbed
yellow/green is absorbed
red is transmitted
Result: Ruby is deep red in color.
40
60
70
80
50
T
r
a
n
s
m
i
t
t
a
n
c
e
(
%
)
ruby
sapphire
0.3 0.5 0.7 0.9
wavelength, (m)
9
17
Luminescence
Luminescence emission of light by a material
material absorbs light at one frequency & emits at another (lower) frequency.
activator
level
Valence band
Conduction band
trapped
states E
g
E
emission
How stable is the trapped state?
If very stable (long-lived = >10
-8
s) =
phosphorescence
If less stable (<10
-8
s) = fluorescence
Example: glow in the dark toys.
Charge them up by exposing them to
the light. Reemit over time.
phosphorescence
18
Photoluminescence
Arc between electrodes excites mercury in lamp to higher energy level.
Electron falls back emitting UV light (i.e., suntan lamp).
Line inner surface with material that absorbs UV, emits visible
Ca
10
F
2
P
6
O
24
with 20% of F
-
replaced by Cl
-
Adjust color by doping with metal cations
Sb
3+
blue
Mn
2+
orange-red
Hg
uv
electrode electrode
10
19
Cathodoluminescence
Used in T.V. set
Bombard phosphor with electrons
Electrons excite phosphor to high state
Excited state relaxed by emitting photon (visible)
ZnS, doped with Ag
+
+ Cl
-
blue
(Zn,Cd)S, doped with Cu
+
+ Al
3+
green
Y
2
O
2
S, doped with 3% Eu red
Note: light emitted is random in phase & direction
i.e., noncoherent
20
Laser Light
Non-coherent light diverges and cant be tightly collimated
How could we get all the light in phase? (coherent)
LASERS
Light
Amplification by
Stimulated
Emission of
Radiation
Involves a process called population inversion of energy states
11
21
Population Inversion
What if we could increase most species to the excited state?
22
Laser Light Production
pump the lasing material to the excited state
e.g., by flash lamp (non-coherent lamp).
If we let this just decay we get no coherence.
12
23
Laser Cavity
Tuned cavity:
Stimulated Emission
One photon induces the emission
of another photon, in phase with the first.
cascades producing very intense burst
of coherent radiation.
i.e., Pulsed laser
24
Continuous Wave Lasers
Can also use materials such as CO
2
or
yttrium-aluminum-garnet (YAG) for lasers
Set up standing wave in laser cavity
tune frequency by adjusting mirror spacing.
Uses of CW lasers
Welding
Drilling
Cutting laser carved wood, eye surgery
Surface treatment
Scribing ceramics, etc.
Photolithography Excimer laser
13
25
Apply strong forward bias to junction.
Creates excited state by pumping
electrons across the gap,
creating electron-hole pairs.
electron + hole neutral + hv
excited state
ground state
photon of
light
Semiconductor Lasers
26
Types of Lasers
14
27
Uses of Semiconductor Lasers
#1 use = CD & DVD players
Color? - red Blue-Ray? - blue (duh)
Banks of these semiconductor lasers are used
as flash lamps to pump other lasers
Communications
Fibers often turned to a specific frequency (typically in the blue)
only recently was this a attainable
28
Applications of Materials Science
New materials must be developed to make
new & improved optical devices.
Organic Light Emitting Diodes (OLEDs)
White light semiconductor sources
New semiconductors
Materials scientists
(& many others) use lasers as tools.
Solar cells
15
29
Solar Cells
p-n junction: Operation:
incident photon produces hole-elec. pair.
typically 0.5 V potential.
current increases w/light intensity.
Solar powered weather station:
polycrystalline Si
n-type Si
P-type Si
p-n junction
B-doped Si
Si
Si
Si Si B
hole
P
Si
Si
Si Si
conductance
electron
P-doped Si
n-type Si
p-type Si
p-n junction
light
+
-
+ + +
- - -
creation of
hole-electron
pair
30
Spatial Separation of Hole-Electron Pairs
How to prevent recombination of photogenerated electrons and holes?
Usually accomplished by band bending, e.g., p-n heterojunction:
before contact
p-type
n-type
Fermi
level
after contact:
band bending
h
+
e-
after photon:
spatial charge separation
16
31
Grand Challenge: Solar-Power Paint
Solar-Driven Photocatalytic Water Splitting:
glass or
plexiglass
photocatalyst
suspension
H
2
separating
membrane
H
2
H
2
O
2
There remain critical unsolved materials challenges There remain critical unsolved materials challenges
before any practical device. before any practical device.
32
Past History of Photochemical Water Splitting
Expensive. Pt / RuO
2
for H
2
& O
2
production.
Works reasonably well.
Nanocluster catalysts
(post-charge separation)
Long neglected.
S/Se corrosion limited.
Light harvesting problematic.
First proposed by Nozik.
Oxide-Sulfides/Se mostly
(Kamat, Lee, ).
Oxide p-n Heterojunctions
WO
3
or Fe
2
O
3
/ Si multilayer.
Very powerful for light
harvesting.
I
3
/I
relatively robust.
35 years of effort.
Band gap tuning
via soln. potential.
Description Limitations &
Unsolved Problems
Class of Device
Modest Quantum Yield.
Usually requires applied potential. Often
stability issues.
Composite Devices/
Hybrid Photoelectrodes
Dyes die after 10
6
photons.
Very corrosive in practice.
Dye Sensitization
Corrosion limited (even Si).
No robust resolution.
Small Band Gap Semiconductor-
Solution
Heterojunctions
17
33
Pt / Titania Nanocomposites
2H
2
O+ O
2
2H
+
H
2
Pt
e
t
h
t
VB
+
+
CB
4OH
Hydrogen Evolution rates for
various Photocatalysts (l/hr)
Pt/TiO
2
7.7
Pd/TiO
2
6.7
Rh/TiO
2
2.8
Ru/TiO
2
0.2
Sn/TiO
2
0.2
Ni/TiO
2
0.1
TiO
2
<0.1
Toshima, J . Phys. Chem. 1985, 89, 1902
Cf. also: Subramanian, V.; Wolf, E. E.; Kamat, P. V. J . Am. Chem. Soc. 2004, 126, 4943.
34
Existing Systems: Almost completely Metal Sulfides/Selenides
(due to visible light absorption).
Critical Problem: Long term stability of photocatalysts.
Most semiconductors are NOT long-term stable
in water during photolysis.
Potential solution: Oxides are the thermodynamically stable state,
but most oxides do not absorb visible light.
What is needed: Band gap engineering of Oxide-Oxide Interfaces
to create new absorbance mechanisms.
This is an exploratory materials effort, not a device project. This is an exploratory materials effort, not a device project.
Key Issues in
Photochemical Water Splitting
18
35
Heterostructured Photoelectrodes
Type II
Staggered Offset
Heterostructure
Type I
Straddled
Heterostructure
Type III
Broken
Heterostructure
h+
e-
(p n, p i, or n i
Heterojunctions)
Type II inter-junction optical transition: emergent property on nanoscale.
Equivalent to Charge Transfer transitions in molecular systems.
Has an oxides stability BUT ALSO a small effective band gap.
36
Light Absorbance Lessons from MBE:
Engineered Oxide Band Gaps
2H
+
+2e
-
H
2
-0.421
+0.815
2H
2
O+4h
+
4H
+
+O
2
-2
-1
0
1
2
3
4
5
P
o
t
e
n
t
i
a
l
/
V
(
v
s
.
N
H
E
,
p
H
7
)
conduction band too stable,
no water splitting
2.2
F
e
2
O
3
2.1
C
d
O
S
n
O
2
3.5
band gaps too big band gaps too big
inefficient: UV only inefficient: UV only
T
a
2
O
5
4.0
3.1
N
b
2
O
5
Z
n
O
3.2
A
n
a
t
a
s
e
T
i
O
2
3.2
3.4
C
a
T
i
O
3
3.5
K
T
a
O
3
3.2
S
r
T
i
O
3
2.6
I
n
T
a
O
4
I
n
T
a
O
4
A
n
a
t
a
s
e
T
i
O
2
1.7
example of example of
hetero hetero- -oxide oxide
gap gap
C
d
S
2.4
N
O
N
-
O
X
I
D
E
S
19
37
Lateral SL by
MBE step-
flow growth:
50-100 nm
SrTiO
3
LaMnO
3
Goal: Create new visible absorption by staggered Type-2 oxide
heterojunctions. Requires high density of red-ox active interfaces.
SrTiO
3
SrMnO
3
SrCrO
3
SrCoO
3
ABO
3
CDO
3
red-ox
oxide
heterojunction
LaMnO
3
LaScO
3
LaNiO
3
KVO
3
Digital Superlattice for Band Structure Engineering
Vertical SL
by multi-
layer MBE:
New Abs.
New valence-to-conduction band
transition: solid-state analog to
molecular Charge-Transfer
transitions.
Eckstein
38
2 nm
LMO
LMO
LMO
LMO
SMO/STO multilayer
STMO/STO multilayer
LMO/STO multilayer
Z-contrast image
STO = SrTiO
3
SMO = SrMnO
3
LMO = LaMnO
3
Ti
L
23
Mn
L
23
La
M
45
O
K-edge
E
E
L
S
l
i
n
e
s
c
a
n
Electron Energy-loss Spectrum
Complex Interfacial Designer Structures
Eckstein, Zuo with Wen, Petrov (FS-MRL staff)
20
39
Designer Materials: Meta-Materials
Band structure control by nano-scale engineering
New visible absorption in vertical short period superlattices.
LaMnO
3
(LMO) / SrTiO
3
(STO) superlattices: N X N, N = 1, 2, 4, 8 ML.
Absorption spectrum different from simple sum of components.
1 X 1 is most different; spectra approach simple sum for large N.
New properties, not found in parent phases, arise at heterojunctions.
absorption of N x N vertical superlattices absorption of SrTiO
3
Photon Energy (eV)
A
b
s
o
r
b
a
n
c
e
0
2
4
6
8
10
1 2 3 4 5
620nm 413nm 310nm
Solar Spectral Range
0
5000
1 10
4
1.5 10
4
2 10
4
2.5 10
4
3 10
4
3.5 10
4
4 10
4
0
5000
1 10
4
1.5 10
4
2 10
4
2.5 10
4
3 10
4
3.5 10
4
0.5 1 1.5 2 2.5 3 3.5
p
s
u
p
e
r
l
a
t
t
i
c
e
o
p
t
i
c
a
l
c
o
n
d
u
c
t
i
v
i
t
y
m
o
d
e
l
o
p
t
i
c
a
l
c
o
n
d
u
c
t
i
v
i
t
y
energy (eV)
1x1
2x2
3x3
8x8
dielectric model
New absorption
feature
Shifted
absorption
edge
Eckstein
40
620nm 413nm 310nm 620nm 413nm 310nm
Lets compare optical absorption spectra engineered in
superlattices made from LaMnO
3
(LMO) and SrTiO
3
(STO).
Lateral Superlattices vs. Vertical Superlattices
3-D nanostructure of superlattice changes the optical absorption!
Designer Materials: Meta-Materials
Band structure control by nano-scale engineering
Eckstein
21
41
When light (radiation) shines on a material, it may be:
reflected, absorbed and/or transmitted.
Optical classification:
transparent, translucent, opaque
Metals:
fine succession of energy states causes absorption
and reflection.
Non-Metals:
may have full (Egap < 1.8eV) , no (Egap > 3.1eV), or
partial absorption (1.8eV < Egap = 3.1eV).
color is determined by light wavelengths that are
transmitted or re-emitted from electron transitions.
color may be changed by adding impurities which
change the band gap magnitude (e.g., Ruby)
Refraction:
speed of transmitted light varies among materials.
SUMMARY
Você também pode gostar
- Sinopec Engineering PresentationDocumento83 páginasSinopec Engineering Presentationstavros7Ainda não há avaliações
- JOB SAFETY ANALYSIS FOR WELDINGDocumento2 páginasJOB SAFETY ANALYSIS FOR WELDINGSravan Dasari100% (3)
- General Physics 2 Light as an Electromagnetic WaveDocumento9 páginasGeneral Physics 2 Light as an Electromagnetic WaveDaniella Tupas100% (1)
- Geometric shape imperfections types and causes reviewDocumento18 páginasGeometric shape imperfections types and causes reviewamit4709Ainda não há avaliações
- Optical Sources, Detectors, and Systems: Fundamentals and ApplicationsNo EverandOptical Sources, Detectors, and Systems: Fundamentals and ApplicationsAinda não há avaliações
- Piping Engineering Man-Hour EstimateDocumento16 páginasPiping Engineering Man-Hour EstimatekamlAinda não há avaliações
- LaserDocumento72 páginasLaserVinay PrakashAinda não há avaliações
- Introduction to Optical Properties of MaterialsDocumento61 páginasIntroduction to Optical Properties of MaterialsSurendra PandaAinda não há avaliações
- Optical Properties of Materials: Reflection, Refraction, Absorption & MoreDocumento18 páginasOptical Properties of Materials: Reflection, Refraction, Absorption & MoreAshish Manatosh BarikAinda não há avaliações
- Optical PropertiesDocumento36 páginasOptical PropertiesJoseph GabuyaAinda não há avaliações
- Optical Properties of MaterialsDocumento36 páginasOptical Properties of MaterialsZhenhua HuangAinda não há avaliações
- X-Ray Photoelectron Spectroscopy (XPS) : Electron Spectroscopy For Chemical Analysis (ESCA)Documento24 páginasX-Ray Photoelectron Spectroscopy (XPS) : Electron Spectroscopy For Chemical Analysis (ESCA)Jatin DarveAinda não há avaliações
- Catalysis of A Reaction Between Sodium Thiosulfate and Iron (III) Nitrate SolutionsDocumento3 páginasCatalysis of A Reaction Between Sodium Thiosulfate and Iron (III) Nitrate Solutionssachin0002Ainda não há avaliações
- Industrial Insulation Applications: Green Engineering-1 Insulation Spreadsheets - ContentDocumento55 páginasIndustrial Insulation Applications: Green Engineering-1 Insulation Spreadsheets - ContentRashel HasanAinda não há avaliações
- IWS Sample QandA ExaminationsDocumento23 páginasIWS Sample QandA ExaminationsRohit Malhotra71% (7)
- Introduction To Optoelectronics: Ronette S. Garcia Bsee-IvDocumento37 páginasIntroduction To Optoelectronics: Ronette S. Garcia Bsee-IvRv Abejo AngaganAinda não há avaliações
- FLSmidth Pfister For Slideshare PDFDocumento29 páginasFLSmidth Pfister For Slideshare PDFMalik Israr HussainAinda não há avaliações
- PhotoconductivityDocumento33 páginasPhotoconductivitySarathy Kannan42% (12)
- Chapter 20: Optical Properties: Issues To Address..Documento30 páginasChapter 20: Optical Properties: Issues To Address..dreamgurl9011Ainda não há avaliações
- Optical Properties of MaterialsDocumento79 páginasOptical Properties of Materialspicassa4Ainda não há avaliações
- Materials Sciences Optical PropertiesDocumento22 páginasMaterials Sciences Optical PropertiesSangetha ChelladoraiAinda não há avaliações
- Physical Elec NotesDocumento22 páginasPhysical Elec NotesSimion OngoriAinda não há avaliações
- Light Interaction With Small Structures: Molecules NanoparticlesDocumento22 páginasLight Interaction With Small Structures: Molecules NanoparticlesmileivandaAinda não há avaliações
- Mse 255 Lecture 5Documento28 páginasMse 255 Lecture 5percydziksAinda não há avaliações
- EENG 292 Lec1Documento6 páginasEENG 292 Lec1Simion OngoriAinda não há avaliações
- LaserDocumento29 páginasLaserGaurav YadavAinda não há avaliações
- Optical Properties of Metals: Absorption, Reflection and ColorDocumento12 páginasOptical Properties of Metals: Absorption, Reflection and ColorraytanuiAinda não há avaliações
- Optical Properties of Solids ExplainedDocumento14 páginasOptical Properties of Solids Explainedcont1122100% (2)
- Introduction To Optical PropertiesDocumento25 páginasIntroduction To Optical Propertiespham minhAinda não há avaliações
- Phy475 NotesDocumento81 páginasPhy475 NotesProfAndré GazotoAinda não há avaliações
- Notes - OptoelectronicsDocumento7 páginasNotes - Optoelectronicsshivangg100% (1)
- EENG 292 NotesDocumento30 páginasEENG 292 NotesSimion OngoriAinda não há avaliações
- Optical Properties of MaterialsDocumento24 páginasOptical Properties of Materialsgwenny_castle100% (1)
- Chapter 21 AviDocumento13 páginasChapter 21 Avianton_deocampoAinda não há avaliações
- Chap 32Documento48 páginasChap 32Grand chemistryAinda não há avaliações
- Solid State Chemistry-4: Dr. Shaista Ali CHEM-3207Documento45 páginasSolid State Chemistry-4: Dr. Shaista Ali CHEM-3207Muhammad Shehzad HaiderAinda não há avaliações
- Opto-Electronic Devices: A Study of The Devices and Their CharacteristicsDocumento26 páginasOpto-Electronic Devices: A Study of The Devices and Their CharacteristicsdebabratalogonAinda não há avaliações
- Fiber OpticsDocumento79 páginasFiber OpticsSonakshi GuptaAinda não há avaliações
- EE-403 Optical SourceDocumento115 páginasEE-403 Optical SourceAyaz AhmadAinda não há avaliações
- Fox NotesDocumento81 páginasFox NotesIamThomasJohnsonAinda não há avaliações
- Week 7 Optical Communication SystemDocumento56 páginasWeek 7 Optical Communication SystemNaEem KhanAinda não há avaliações
- Materials Lecture - XpsDocumento30 páginasMaterials Lecture - XpsHamza IslamAinda não há avaliações
- Lasers-Simplified NotesDocumento4 páginasLasers-Simplified Noteskrish_cvr2937Ainda não há avaliações
- Lec-PH301 18 Optical Sources-3 15.09.2023Documento32 páginasLec-PH301 18 Optical Sources-3 15.09.2023Latu BharaliAinda não há avaliações
- Plasm On IcsDocumento15 páginasPlasm On Icschouht98477714Ainda não há avaliações
- Light Emitting Diode - Design Principles: EBB 424EDocumento32 páginasLight Emitting Diode - Design Principles: EBB 424Euog12Ainda não há avaliações
- 1st Year Chemistry Chapter No. 5-6 - SQs - NOTESPKDocumento14 páginas1st Year Chemistry Chapter No. 5-6 - SQs - NOTESPKZeeshan ahmedAinda não há avaliações
- Introduction To Optical PropertiesDocumento77 páginasIntroduction To Optical PropertiesUmar HamidAinda não há avaliações
- Tech LedDocumento26 páginasTech LedSumit ThakurAinda não há avaliações
- Leacture Notes Unit 2 1 37Documento37 páginasLeacture Notes Unit 2 1 37keregiAinda não há avaliações
- Optical PropertiesDocumento10 páginasOptical PropertiesMark Anthony Asañez BrianAinda não há avaliações
- Basic Spectroscopy LectureDocumento134 páginasBasic Spectroscopy LectureMa Rian CombineAinda não há avaliações
- Introduction To LASERsDocumento15 páginasIntroduction To LASERsBushra SaleemAinda não há avaliações
- Chap 7 Components of Optical Instruments - IIIDocumento12 páginasChap 7 Components of Optical Instruments - IIISkriikkAinda não há avaliações
- Photon InteractionDocumento6 páginasPhoton InteractionSaravjeet SinghAinda não há avaliações
- Sriharsha KarumuriDocumento12 páginasSriharsha KarumuriAn DangAinda não há avaliações
- L13 Optical CharacterizationDocumento35 páginasL13 Optical CharacterizationVivek BelaAinda não há avaliações
- LED3Documento23 páginasLED3kundannnAinda não há avaliações
- Permittivity and Transmission of MetalsDocumento3 páginasPermittivity and Transmission of MetalsmaxAinda não há avaliações
- Mechanism behind photon emission in LEDsDocumento4 páginasMechanism behind photon emission in LEDsCelebral AssasinAinda não há avaliações
- Lect2-Light ReactionDocumento62 páginasLect2-Light ReactionGilang Satya ArdhanaAinda não há avaliações
- Chapter - Three - 1Documento71 páginasChapter - Three - 1Alene tesfawAinda não há avaliações
- Mos Mosfet OperationDocumento98 páginasMos Mosfet OperationMaria AllenAinda não há avaliações
- FSE Solar Cell - 2022Documento51 páginasFSE Solar Cell - 2022Jignesh GadhwalAinda não há avaliações
- Sensor Devices Sensor Devices Radiation SensorsDocumento53 páginasSensor Devices Sensor Devices Radiation SensorssantoshpbkAinda não há avaliações
- Atomic Absorption Spectroscopy - University NotesDocumento29 páginasAtomic Absorption Spectroscopy - University NotesLilac44Ainda não há avaliações
- Optical Properties PDFDocumento27 páginasOptical Properties PDFaljhon100% (1)
- Zintek® 300 HP + Techseal® Glossy Black SLDocumento9 páginasZintek® 300 HP + Techseal® Glossy Black SLSyedMazharAliShahAinda não há avaliações
- STT Pipe Welding Reduces Spatter & SmokeDocumento3 páginasSTT Pipe Welding Reduces Spatter & SmokeahmedAinda não há avaliações
- SRA166 210 01A BrochureDocumento2 páginasSRA166 210 01A BrochureXanti Zabala Da RosaAinda não há avaliações
- Solid Waste Management 1Documento558 páginasSolid Waste Management 1Daisy100% (2)
- Introduction To HydraulicDocumento28 páginasIntroduction To HydraulicMohamed ElmakkyAinda não há avaliações
- Reduction of Mouldboard Plough Share Wear by A Combination Technique of HardfacingDocumento5 páginasReduction of Mouldboard Plough Share Wear by A Combination Technique of HardfacingJuan Ambrosio MartinezAinda não há avaliações
- ECP Spares Catalogue PDFDocumento68 páginasECP Spares Catalogue PDFgoogle manAinda não há avaliações
- Environmental Guidelines For The Textile Dyeing and Finishing IndustryDocumento43 páginasEnvironmental Guidelines For The Textile Dyeing and Finishing IndustryMohita VadrevuAinda não há avaliações
- Report On Visvesvaraya Iron and Steel Plant, Bhadravathi.Documento11 páginasReport On Visvesvaraya Iron and Steel Plant, Bhadravathi.Ramesh Kavitha Sanjit 18BME0677Ainda não há avaliações
- As 5056-2006 Metallic Coatings - Powder Metal (And Composites) Applied by Mechanical Means at Ambient TemperaDocumento7 páginasAs 5056-2006 Metallic Coatings - Powder Metal (And Composites) Applied by Mechanical Means at Ambient TemperaSAI Global - APACAinda não há avaliações
- Synthesis and Characterization of Co2FeAl Heusler Alloy NanoparticleDocumento5 páginasSynthesis and Characterization of Co2FeAl Heusler Alloy Nanoparticlekarthik kaonAinda não há avaliações
- 1.2210 Datasheet, 1.2210 Property, 1.2210 Standard Specification, 1Documento3 páginas1.2210 Datasheet, 1.2210 Property, 1.2210 Standard Specification, 1adaAinda não há avaliações
- Supply and Fabrication Steel Structures SpecificationDocumento18 páginasSupply and Fabrication Steel Structures Specificationgowtham_venkat_4Ainda não há avaliações
- PDFDocumento123 páginasPDFPrakash WarrierAinda não há avaliações
- Lift Truck Gear From DTAUTO - CADocumento32 páginasLift Truck Gear From DTAUTO - CAGregGGHAinda não há avaliações
- Shell Morlina s2 B 320Documento4 páginasShell Morlina s2 B 320Aep HidayatAinda não há avaliações
- Hexion Starting Formulation 8014Documento4 páginasHexion Starting Formulation 8014uzzy2Ainda não há avaliações
- Pa 180010Documento6 páginasPa 180010Trịnh Đức HạnhAinda não há avaliações
- Astm A192 PDFDocumento2 páginasAstm A192 PDFgaminAinda não há avaliações
- rk86 enDocumento2 páginasrk86 enMohd Farhan ZulkepliAinda não há avaliações
- Cnveyors ComponentsDocumento16 páginasCnveyors ComponentsLizbeth AtamariAinda não há avaliações
- Introduction To Powder Metallurgy A ReviDocumento7 páginasIntroduction To Powder Metallurgy A ReviFerry SetiawanAinda não há avaliações