Escolar Documentos
Profissional Documentos
Cultura Documentos
Public Appendix 13q
Enviado por
hiyeonDescrição original:
Direitos autorais
Formatos disponíveis
Compartilhar este documento
Compartilhar ou incorporar documento
Você considera este documento útil?
Este conteúdo é inapropriado?
Denunciar este documentoDireitos autorais:
Formatos disponíveis
Public Appendix 13q
Enviado por
hiyeonDireitos autorais:
Formatos disponíveis
WEAVER'S COVE ENERGY
WEAVERS COVE OFFSHORE BERTH PROJECT
Resource Report 13
APPENDIX Q
THERMAL RADIATION AND VAPOR DISPERSION
REPORT
Q-1 CBI Document C01001, Vapor Dispersion, Thermal Radiation
and Sump Sizing Calculations for Offshore J etty Spill
Q-2 Temperature Rise at an LNG Tankers Hull Resulting from a
Fire in the LNG Impoundment, by Pat Convery, PE, Weavers
Cove Energy, Offshore Berth Project
Q-3 LNG Release from an Underwater LNG Transfer Line, by
Phani Raj, Technology & Management Systems, Inc.
WEAVER'S COVE ENERGY
WEAVERS COVE OFFSHORE BERTH PROJECT
Resource Report 13
Q-1 CBI Document C01001, Vapor Dispersion, Thermal Radiation
and Sump Sizing Calculations for Offshore J etty Spill
S
W
A
N
S
E
A
S
O
M
E
R
S
E
T
Thermal Radiation
10,000 BTU/hr-ft2 = 84.82 ft
Thermal Radiation
3,000 BTU/hr-ft2 = 106.02 ft
Thermal Radiation
1,600 BTU/hr-ft2 = 121.47 ft
Vapor Dispersion from Design
Spill Sump (1/2 LFL) = 300.4 ft
Scale 1:3,000
1 inch = 250 feet
125 0 125 250
Feet
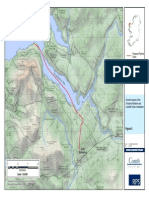
Weaver's Cove Energy, LLC
Figure 11-1
Design Spill - Vapor Dispersion and
Thermal Radiation Distances
G:\Projects\Fall_Riv\79001\alt_berth\Resource_Reports\RR11\Fig11-1_design_spill.mxd
Basemap: 2002-3 RIGIS /
2005 MassGIS Imagery
M
A R
I
Location Within Bay
WEAVER'S COVE ENERGY
WEAVERS COVE OFFSHORE BERTH PROJECT
Resource Report 13
Q-2 Temperature Rise at an LNG Tankers Hull Resulting from a
Fire in the LNG Impoundment, by Pat Convery, PE, Weavers
Cove Energy, Offshore Berth Project
CALCULATI ON
Weavers Cove Energy
Off-Shore Berth Project
Temperature Rise at an LNG Tankers Hull
Resulting from a Fire in the LNG I mpoundment.
Pat Convery, P.E.
1/15/2009
Introduction
The purpose of this calculation is to demonstrate compliance to 33CFR Part 127
paragraph 127.105 which requires that:
Impounding spaces must be located so that the heat flux
froma fire over the impounding spaces does not cause
structural damage to an LNG vessel moored or berthed at
the waterfront facility handling LNG.
The Weavers Cove Energy Offshore Berth will have 2 impounding spaces located at the
offshore berth. One impoundment, the design spill impoundment, is provided and sized
to collect and contain any LNG spilled from a design spill as defined by NFPA 59A
(1994) in paragraph 2-1.2. The spill volume is determined by the requirements set out in
paragraphs 2-2.2.2 for a spill from an accidental leakage source. The accidental leakage
source chosen by WCE was the largest small diameter branch connection producing the
largest flow at operating conditions. (a 3 run-around valve on the unloading arm
isolation valves). This design spill impoundment is located in a segregated area within a
second, larger impounding space.
The large impounding space is designed to contain a spill from a full flow rupture of the
LNG transfer system with the ship pumps running at maximum speed (115% of 12,000
m3/hr) at the offshore berth plus additional space for draining of these lines. The
calculations performed to demonstrate compliance to 33 CFR 127.105 assume a fire over
both impounding spaces.
This scenario was selected in consultation with the US Coast Guard office Sector
Southeast New England. The scenario assumes an LNG vessel, berthed at the Offshore
berth, is unloading normally when a failure occurs somewhere in the LNG transfer
system at the jetty resulting in a full flow spill (115% of 12,000 m3/) for 10 minutes,
filling the impounding spaces. The spill is ignited by an unspecified ignition source and a
fire over the impounding spaces ensues. The tanker then takes 10 minutes to move off
the berth and head toward the navigation channel.
Weavers Cove Energy selected a temperature of 1000 degrees F as a limiting value
above which steel structures may experience a significant loss of strength to cause
structural damage. Damage to paint systems and coatings and localized plate
deformations can be expected to occur at temperatures below this level, however this
level of damage has not been identified as a mechanism to impede the departure of an
LNG vessel or to result in structural damage.
Model
The BREEZE LFG Fire/Risk Version 5.0.3 model published by Trinity Consultants was
used for this analysis. The model incorporates temperature rise formulas developed by
RISC and Arthur D. Little.
1
The various categories of input variables are discussed in the following sections.
Fuel
The composition of the LNG to be received at Weavers Cove is unknown and is
expected to vary from shipment to shipment. For the purpose of this analysis, the key
LNG parameters were selected as follows:
Molecular Weight 16.043 g/g-mole
Boiling Point -258.8 deg. F
This LNG is within the acceptable range for the import terminal. A variation of the LNG
composition within the acceptable range for the terminal has a minor effect on the
temperature rise results but will not change the conclusion. Selection of heavier LNG
compositions results in an increase of less than 1% in radiant flux at the target location
and negligible affect to the results.
Atmospheric Conditions
Two analyses were performed. For the first case the atmospheric conditions were
assumed to be the conditions prescribed in 49CFR Part 193 for calculation of thermal
radiation from LNG pool files. They are based on local worst-case conditions for
radiation flux generation. These are:
Ambient Temp. 12 F
Barometric Pressure 760 mm Hg
Relative Humidity 50%
Wind Speed 26.5 mph
For the second case, the ambient temperature was increased to 100 degrees F. to provide
a more conservative (higher) start temperature for the LNG tanker hull. All other
atmospheric conditions were held the same as in case 1.
1
Hydrocarbon Fires on Offshore Platforms. Topic 3, Final Proprietary Report, submitted to Snamprogetti SPA/SIAF, Italy, by Risk
and Industrial Safety Consultants, Inc., Chicago, IL and Arthur D. Little, Inc., Cambridge, MA, August 10 1984
Geometry
The impoundment sump size was taken to be 65 x 65 to correspond with the latest
design drawings for the Weavers Cove Off-Shore Berth Project impounding spaces.
This impoundment is provided to contain an LNG spill resulting from a full-flow failure
on the jetty platform, resulting in a spill equivalent to the full unloading rate for ten
minutes, plus pump run-out allowance and piping drain-out allowance. This spill is
referred to as the large spill to differentiate it from the design spill as defined in
NFPA 59A and other code documents.
The base of flame was taken to be the top of the concrete impoundment sump wall, 44
MLLW. The actual top of the liquid pool will be at any elevation between 19.7 and
42.4 depending on the actual spill duration and volume.
The distance from the center of the large spill impoundment to the ships hull when the
ship is at berth is 183 feet.
The target height of interest is the height at which the maximum temperature rise is
experienced within the ten-minute event duration. An analysis was performed using the
BREEZE modeling software and determined that the worst-case target height is equal to
the flame base height. The temperature rise at heights below and above the worst-case
height is significantly less.
Target Properties
The target is a typical outer hull of an LNG taker. The material is steel plate, as it is not
insulated on the outer or inner surface. Many types of paints can be expected on either
surface of the outer hull plates. The following input variables were selected based on
industry experience:
Density of steel plate 488.8 lb/cu ft
Thickness 0.6 inches
Specific Heat 465 J /kg-C
Emissivity of plate 0.8
The density and specific heat of the steel is not expected to vary enough between ships to
affect the results of this calculation. The thickness of the outer hull steel does vary
between vessels and with position on the hull structure. 0.6 inches was selected as a
representative thickness at the target height of interest based on consultation with staff
experienced in LNG tanker construction details.
The emissivity of the outer hull does have a material impact on the temperature rise
results, and can vary between vessels based on surface finish of the steel, the outer
coating system, and any surface treatments applied to the steel plate. The common
reference literature indicates emissivity values for steel plate ranging from 0.4 to 0.9
depending on composition, surface conditions, etc. Weavers Cove has assumed a
conservative figure of 0.8 for the purpose of these calculations.
2
Using the worst-case
emissivity of 1.0, the failure temperature of the steel is not reached.
Other Factors
Several factors that could mitigate the effects of a fire in the large spill impoundment on
an LNG tanker have not been accounted for in this analysis. These include:
The use of water curtain fire protection systems on the LNG tanker,
The use of firewater cooling from remote controlled water monitors installed on
the jetty platform,
The use of firewater cooling from fire-fighting tugs that may be present at berth at
the time of the incident,
Reduction on thermal radiation due to the use of Foamglas FPS blocks in the
impoundment sumps,
Wind cooling of the tankers hull.
Results
The results of the simulation are included in Appendix A. A summary of the results is
included in Table 1.
Table 1, Summary of Results
Case
Number
Radiant Flux
at Hull
(BTU/ft
2
-hr)
Ambient
Temperature
Hull
Temperature
After 10 Minutes
Maximum
Hull
Temperature
Time to
Maximum
Hull Temperature
1 8694 12 F 360 F 711 F 42.3 minutes
2 7427 100 F 393 F 689 F 39.6 minutes
Conclusion
A fire over the impounding spaces at the offshore berth is described in these calculations
does not give rise to structural failure of the LNG vessel or be of significant magnitude to
prevent the vessel from departing the berth in the event of a fire in the impounding
spaces for a fire of 10 minutes duration or longer. The calculations show that the
maximum temperature reached at the vessel at berth is 711 degrees F, well below the
identified failure temperature of 1000 degrees F.
2
Using the theoretical worst-case value for emissivity of the steel outer hull, the maximum temperature
occurs after 34 minutes and is 734 degrees F, well below the temperature of concern for structural damage.
APPENDIX A
BREEZE Reports
Appendix B
Formulas Used in BREEZE Model
3
3
BREEZE LFG Fire/Risk Users Guide. Version 5.0 Rev 1.0, Copyright 2004 Trinity Consultants. Used
by permission.
WEAVER'S COVE ENERGY
WEAVERS COVE OFFSHORE BERTH PROJECT
Resource Report 13
Q-3 LNG Release from an Underwater LNG Transfer Line, by
Phani Raj, Technology & Management Systems, Inc.
LNG release from an
Underwater LNG transfer line
A technical report
Submitted to
Mr. Leon Bowdoin
Vice President, Weavers Cove Energy
Fall River, MA 02720
by
Phani Raj
Technology & Management Systems, Inc
Burlington, MA 01803
8
th
January, 2009
Page (i)
Table of Contents
Page #
Executive Summary (iii)
Chapter 1 Introduction
1.1 Background 1
1.2 Objective 2
Chapter 2 Findings from the literature
2.1 Underwater LNG release tests 4
2.1.1 Test by the US Bureau of Mines 4
2.1.2 Maplin Sands tests by Shell 5
2.2 Mixing of hot and cold liquids 5
2.3 Droplet & Bubble formation and Heat Transfer 11
2.4 Summary of findings from the literature 15
Chapter 3 Modeling LNG jet release from a transfer-pipe underwater
3.1 General description of LNG Jet Behavior in Water 17
3.2 Modeling LNG Jet Release, Liquid Droplet Formation and 20
Vapor Bubble Heating
3.2.1 Jet release rate 20
3.2.2 Homogeneous nucleation of a superheated liquid 21
3.2.3 Potential vapor explosion yield 22
3.2.4 Liquid droplet size and vapor bubble size 24
3.2.5 Vapor bubble rise and heating 27
3.3 Example Results 30
Chapter 4 Discussions and Conclusions
4.1 Discussions 33
4.2 Conclusions 37
Nomenclature 38
References 41
Appendix A: Assessment of the results from the U.S. Bureau of Mines 1970 43
underwater LNG release experiment
Page (ii)
List of Tables
Table 1-1 Relevant data on the proposed WCE underwater LNG transfer system 2
Table 3-1 LNG jet release rates for different conditions 21
Table 3-2 Estimated energy release in the LNG jet due to superheat 23
Table 3-3 LNG droplet and its characteristics 26
Table 3-4 Vapor bubble rise in the water column and associated results 31
List of Figures
Figure E-1: Results for vapor bubble rise and heating distances for different (v)
release depths and sizes of holes on the pipe wall
Figure 1-1 Schematic representation of a cross section of the river bottom 3
illustrating the location and back fill on top of the transfer system
Figure 2-1 Sequence of radiographs of a molten woods metal (640
o
C) 9
jet entering heavy water at 14
o
C
Figure 2-2 Cavity formation, water accumulation, rapid evaporation and 10
liquid shattering in a water jet plunging into a molten metal
Figure 3-1 Schematic representation of the different stages in the 18
penetration of LNG jet into water, break up and formation of
evaporating liquid droplets
Figure 3-2: Schematic representation of evaporation of liquid droplets, formation of 18
vapor bubbles, heat transfer from water to bubbles and bubble rise in the
water column
Figure 3-3 Schematic diagram showing the formation, rise and heating up 27
of a bubble of LNG vapor in a water body
Figure 3-4a LNG release at 40 ft depth of water 32
Figure 3-4b LNG release at 30 ft depth of water 32
Figure 3-4c LNG release at 20 ft depth of water 32
Page (iii)
Executive Summary
Weavers Cove Energy, LLC (WCE) is planning the construction of underwater LNG
transfer system to transport LNG from a jetty located off-shore in Mt. Hope Bay, MA to
an on-shore storage tank in the proposed terminal at Fall River, MA. The transfer system
is essentially a pipe-in-pipe (PiP) system with the inner pipe carrying the cryogenic LNG
and the annular space between the inner and outer pipes being filled with a high-grade
insulation material. This pipe system is to be buried 1.5 m (5 ft) below the bottom of the
Taunton River. A deep trench of approximately 2.5 m (8 ft) depth will be excavated at
the bottom of the Taunton River. After placing the transfer system in the trench, it is
proposed to fill the trench with an engineered backfill comprised of mixed gravel.
Because of this type of construction the transfer system is well protected against damage
by any external causes. However, WCE is interested in assessing the consequences of
LNG release underwater from a (very low probability) event that could puncture both the
outer and inner pipes. This report addresses the various phenomena that could occur in
the case of LNG release underwater.
Only two experimental results are cited in the literature on the consequences of release of
LNG underwater. One test was conducted by the US Bureau of Mines in 1970 releasing,
instantaneously, 7.7 kg (17 lb) of LNG at a depth of 4.5 m (15 ft). The other test
conducted by Shell involved the releasing LNG continuously at the rate of 4.1 m
3
/min
(1100 gpm) for about 4 minutes from a 0.2 m (8 inch) diameter pipe whose end was
located just under the surface of water in the sea. In both tests the vapor generated by the
boiling of LNG and emanating from the water surface was hardly visible and was
buoyant indicating clearly that vapors disperse more vertically than horizontally.
Therefore, the potential safety distance for flammable vapor dispersion for LNG release
underwater is far smaller than that for equivalent release of LNG on land or on water
surface. Unfortunately no other quantitative data were gathered in either of these two
tests.
A literature review undertaken in this project indicates that while no underwater LNG jet
release and bulk water interaction studies have been performed (other than the ones cited
above) there are a number of situations have been studied experimentally in other
industries where a cold saturated liquid is injected or comes in intimate contact with a hot
bulk liquid. These industries include the metal casting, nuclear fission and fusion, and
alternative fuel for vehicles. In all of the tests conducted in these industries (with
diversity of tests ranging from liquid nitrogen injected into water to hot molten metal
plunging into water to water jetting into molten metal) it is seen that the jet of the colder
liquid shatters due to very rapid nucleate boiling after getting superheated by the hot
liquid. The shattered jet forms relatively small droplets which evaporate extremely
rapidly forming saturated vapor bubbles. These bubbles are further heated by the bulk
(hot) liquid and attain temperatures close to that of the bulk liquid in distances that are, at
best, an order of magnitude larger than the jet diameters.
The correlations available in the literature from tests conducted with other liquid-liquid
combinations have been utilized in developing a model to describe the interaction of
LNG jet released at considerable depth underwater and the bulk water body, as in a river.
Page (iv)
This model includes the calculation of the jet break-up distance, the maximum size of
liquid droplets formed, their evaporation time and distance of travel, the formation of
bubbles of saturated LNG vapor, heating of the vapor in the bubble by water and the
movement of the vapor bubbles in the water column. Very conservative assumptions are
made on the values of the parameters used (such as the heat transfer coefficients) as well
as in the description of the movement of the droplets and vapor bubbles. For example, it
is assumed that the LNG jet axis is vertical so that all of the material released has a
momentum direction that is vertically up promoting the rise of the liquid droplets. In
addition, the effect of the engineered gravel overburden present on top of the transfer
system is ignored (the gravel will further promote the shattering of LNG released). Also,
the calculations are performed with the largest size droplets that can be formed (smaller
size droplets evaporate faster and the vapor bubbles formed from smaller size droplets
also heat up faster and in smaller travel distance).
The model developed has been applied to determine the depth at which the vapor
produced by the LNG jet released underwater attains the temperature that is close to that
of the surrounding water in the river. Both the depth at which the transfer system exists
(relative to the water surface) and the size of the hole on the pipe wall are varied to see
their effects on the heating of the vapor produced and to determine if the vapor emanating
from the water surface is buoyant or not.
Figure E-1A, Figure E-1B and Figure E-1C, show the results of these calculations. The
figures show the depth at which the largest vapor bubble (generated by the evaporation of
the largest size liquid droplet produced by the shattering of the LNG jet) attains the
temperature equal to that of the surrounding water. Figure E-1A shows the results for the
case of the hole being at a depth of 40 ft below the water surface. The figure includes the
results for different diameter size holes from which the LNG jet is released (from 1 inch
to 6 inches). Figure E-1B and Figure E-1C provide similar results, respectively, for 30 ft
and 20 ft release depths. It is seen that in all cases the vapor reaches the water
temperature (assumed to be 20
o
C or 68
o
F) well below the river water surface. It is noted
that LNG vapor is buoyant at 163 K (-167
o
F) temperature at normal atmospheric
pressure. Heating the vapor to the water temperature makes the vapor very buoyant. This
report also discusses the effect of various assumptions on the final result on vapor
temperature.
It is concluded from both the results of the analysis presented in this report and from the
two limited LNG test data that releasing LNG underwater results in the production of a
vapor cloud that is buoyant at the time of its release from the water surface. To this
extent, LNG release underwater has the same effect on dispersion of vapors as would be
the case when natural gas is released at ambient temperature from, say, a natural gas
pipeline accident.
The report includes an assessment of the findings from the US Bureau of Mines LNG
underwater release test, based on data obtained from a video film of the test.
Page (v)
Figure E-1A: LNG release at 40 ft depth of water
Figure E-1B: LNG release at 30 ft depth of water
Figure E-1C: LNG release at 20 ft depth of water
Figure E-1: Results for vapor bubble rise and heating distances for different
release depths and sizes of holes on the pipe wall
Page 1 of 55
Chapter 1
Introduction
1.1 Background
Weavers Cove Energy, LLC (WCE) is planning the construction of an underwater
LNG transfer system to transport LNG from a jetty located off-shore in Mt. Hope Bay,
MA to an on-shore storage tank in the proposed terminal at Fall River, MA. This
underwater transfer system is to be buried below the soil underneath the Taunton River.
The depth of water along the proposed route of the underwater transfer system varies
approximately between 3 m and 20 m (10 ft and 66 ft). Barges and ships carrying coal to
a power plant routinely traverse this part of the Taunton River. Hence, the transfer system
is proposed to be buried below the dredge depth in the river. To facilitate rapid unloading
of a ship, two transfer lines (of identical design) are to be used, located side by side, in a
trench cut in the soil at the bottom of the river.
The proposed transfer lines will be a double walled, cryogenic duty lines, essentially a
pipe in pipe (PiP) design. The inner, LNG contacting, pipe is to be made of invar (36%
Ni steel) of diameter 70 cm (24 inches) surrounded by high-grade insulation. The outer
pipe is also made of cryogenic stainless steel (317L-SS grade) of diameter 87 cm (30
inches) with an outer corrosion resistant coating. The space between the outer and inner
pipe will be filled with a high grade Aerogel
insulation to prevent heat leak from water
to LNG. The length of the proposed transfer system is approximately 6.8 km (4.25 miles)
from the jetty to the terminal. It is intended to locate the transfer system beneath the soil
in a trench such that the top of the lines are at least 1.4 m to 1.75 m (4 ft to 5 ft) below the
soil depth. The overburden on top of the lines is expected to be filled with
manufactured gravel of nominal size 7.5 cm (3 inch minus). Other relevant details of
this design are indicated in Table 1-1. A schematic representation of transfer system
section is shown in Figure 1-1.
The design of the WCE PiP transfer lines and their location are such that it is well
protected against most types of accidents and mishaps that may result in puncturing the
PiP system. However, an event of extremely low probability could occur resulting in
LNG release if both lines are penetrated.. While it is difficult to visualize an agency or
event that can cause damage to the transfer system of sufficient magnitude to penetrate
both the outer and inner pipes, it is nevertheless important to evaluate the consequences
of LNG release underwater from such an accident or incident. The question of concern is
whether the LNG released from the PiP transfer system would rise to the water surface,
form a boiling and evaporating LNG pool on the water surface and produce cold LNG
vapors that would be dispersed in the prevailing wind and atmospheric condition. This
report addresses this question.
Page 2 of 55
1.2 Objective
The objective of the work indicated in this report was to address and develop a
mathematical model to answer the following questions.
1 What happens when LNG is released as a jet (from a hole in a PiP transfer line (both
the inner and outer pipe walls) into a large water body from a depth of 3 m (10 ft) or
more?
2 What fraction of the liquid released will manifest as a liquid pool on the surface of
water?
3 What is the temperature of the vapor generated in the water body and, which
emanates from the water surface? How does this vapor disperse in the atmosphere (or
what will be the downwind vapor hazard distance)?
This report addresses the above questions. Mathematical models are discussed and
applied to the conditions assumed to exist in the proposed WCE underwater PiP transfer
system.
The report discusses the findings from the literature in Chapter 2. Mathematical analyses
developed or used are discussed in Chapter 3. Discussions on the results, conclusions and
recommendations are indicated in Chapter 4. Additional details of models are provided in
Appendices.
Table 1-1
Relevant data on the proposed WCE LNG PiP transfer system
Parameter magnitude
Parameter Value SI
Unit
Value US Unit
Length of PiP transfer system 6.8 km 4.25 miles
Inner pipe (invar, M93 steel) diameter 61.0 cm 24 inch
Inner pipe thickness 6.35 mm 0.25 Inch
Outer pipe (317 stainless steel) diameter 76.2 cm 30 inch
Outer pipe thickness 11.1 mm 0.438 inch
Avg depth of top of outer pipe below
water level (range 10 ft, to 60 ft)
12 m 40 ft
Depth of engineered gravel (back-fill)
above top of outer pipe
1.4 m 4 ft
Average size of gravel in the back-fill 5 cm 2 inch
Operating pressure in the pipe during
transfer operations
10.34 x
10
5
Pag 150 psig
Avg. LNG temperature in the pipeline
(1)
114.5 K -253.9
o
F
Normal pumping flow rate/ per pipe of
LNG during an unloading operation
4,250 m
3
/hr 18,715 gpm
1
The upstream end pipeline is expected to have LNG at 9.34 x 10
5
Pa (abs) and 114.3 K (135.5 psia and
-254.2
o
F) and the downstream end conditions are expected to be 3.87 x 10
5
Pa (abs) and 114.8 K (56.2
psia and -253.4
o
F). The liquid is not at saturated conditions corresponding to these pressures but is in a
sub-cooled condition.
Page 3 of 55
Figure 1-1: Schematic representation of a cross section of the river bottom
illustrating the location and back fill on top of the transfer
system
The WCE PiP LNG transfer system design includes approximately 2.5 inches of concrete
weight coating over the corrosion protection coating on the 30 nominal diameter OD
casing pipe. It is assumed in this analysis that these two additional layers have no impact
on flow from a hypothetical hole in the inner (and outer) pipe.
Page 4 of 55
Chapter 2
Findings from the literature
As a part of this project a review of the literature was undertaken. This literature review
focused on finding information on the behavior and consequences of LNG release under
water, and the relationship between evaporation, rate of LNG release, depth of release,
temperature of water, etc. In addition, review of data from tests involving similar
situations in other industries was also accomplished. This chapter indicates the results
from these literature reviews.
When LNG is released in the form of a jet underwater into a large pool of ambient
temperature water it is expected that the jet will undergo significant physical and thermal
changes due to both dynamic effects of injection into a liquid (water) and high heat
transfer from water. The result of heat transfer is to boil LNG forming vapor bubbles or
vapor blobs. The cold vapor generated by the evaporation process will be heated by the
surrounding water. The literature review below provides both phenomenological
information on the mixing of water (a relatively hot fluid) and LNG (a cold liquid near its
boiling temperature), and quantitative data on rate controlling parameters.
2.1 Underwater LNG release tests
Only two tests involving the release of LNG under controlled conditions and subsequent
measurements are reported in the literature. These tests were conducted by, (i) the U.S.
Bureau of Mines [Burgess, et al., 1972], and (ii) the Shell Company [Blackmore, et al,
1984].
2.1.1 Test by the US Bureau of Mines
The U.S. Bureau of Mines (Burgess, et al., [1972]) conducted a single test in which LNG
held in a 5-gallon polyethylene container coated with urethane foam to minimize heat
loss through the walls when the container was filled with LNG. The container was
wrapped with 3 feet of 50-grain/ft detonating cord to release LNG as rapidly as possible.
The filled container containing 7.7 kg (17 lbs) of LNG was submerged under water to a
depth of 4.57 m (15 ft). The detonating cord was fired to rupture the container releasing
very rapidly the entire contents of the bottle. The test was conducted in a strip mine lake
near Bruceton, PA
No written reports on the details of the test or the results from it are available. The
description of the test provided in the report by Burgess, et al., [1972] is very limited and
is devoid of results. No physical measurements (such as the temperature of the vapor
emanating from the water surface, vapor concentration downwind, or the down wind
flammable distance) seemed to have been made. However, a film of the test and the
commentary in the film exist. Unfortunately the film shows only the view above water;
none of the physical phenomena occurring underwater upon the LNG release was filmed
(underwater). The results from the test film (and those summarized by the commentator)
indicate the following:
Page 5 of 55
1 The entire mass of LNG released evaporated underwater.
2 No white vapor cloud was observed on the surface of water. It is surmised that the
vapor released was warm and buoyant.
3 The pressure pick ups located under water registered some overpressure. Using this
data the Bureau estimated that the total energy yield by the explosion was
equivalent to that from approximately 0.4536 kg (1 lb) high explosive TNT charge.
4 Measurement made by noting the time stamp in the film indicates that the first sign
of vapor coming out of the water surface is about 8 2 s after the detonator was
charged. The entire vapor is released from the surface in a big bubble within about
2 seconds after the first hiss of vapor emerging from the water surface. It has to be
noted, however, that the time at which the detonator was charged is not indicated
(or commented on) in the film but is guessed by watching the film many times. That
is, the entire 7.7 kg of LNG seems to be evaporated in a matter of about 10 s ( 2 s).
A model has been developed to interpret the results from the US Bureau of Mines test.
The details of this model and the conclusions from it are discussed in Chapter 3.
2.1.2 Maplin Sands tests by Shell
Only one test of releasing LNG continuously underwater is reported by Blackmore, et al.,
[1984]. The test involved the continuous release of LNG (at the rate of 4.1 m
3
/min for
230 seconds in a 4.7 m/s wind speed) from an 8 inch diameter pipe whose end was just
below the surface of water. The authors do not provide any data on the depth of pipe-end
below the water surface; it may be assumed that the pipe-end was less than 0.5 m below
the water surface. The vapor produced was found to be buoyant. No other quantitative
data are provided. It is, however, significant to note that even when LNG was released
underwater from a relatively shallow depth the vapors produced were buoyant.
2.2 Mixing of hot and cold liquids
Other industries where the mixing of a hot liquid and cold liquid are of concern include
metal casting (especially steel and aluminum), nuclear fission, nuclear fusion, and
alternative motor fuel technology. All of these industries are concerned with accidental
mixing of the hot liquid with a cold liquid leading to superheat explosion (of the cold
liquid). It should however be noted that most of the research investigations in these
industries involve laboratory scale tests (of sizes of the order of magnitude of 1 meter
depth or smaller).
The concern in the nuclear fission industry is with the potential meltdown of the uranium
core rods due to loss of coolant followed by the hot molten metal plunging into the
emergency water deluge system. In the case of the nuclear fusion industry the concern is
with water circulating in the jacket to extract heat from the reactor coming in contact
with the liquid helium coolant (at 4 K or -443
o
F) used to cool the super-conducting
magnets. In these situations the sudden and substantial production of superheated vapor
Page 6 of 55
from the evaporation of the colder liquid leads to vapor explosion causing potentially
significant damage to the structure and exposing people to hazards.
The alternative motor fuel industry is designing systems to benefit from the vapor
explosions caused by the mixing of a cryogenic liquid and a warm liquid. Controlled
experiments, mixing liquid nitrogen and water in a cylinder, have been performed to
utilize the effect of vapor explosions to drive a piston engine.
The heat transfer between rising bubbles and liquid baths has been investigated both by
the chemical industry (for liquid agitation and extraction) and the molten metal steel
industry to modify the properties of the casting.
The findings from publications in these various industries on issues of relevance to the
evaluation of LNG release underwater are indicated below.
2.2.1 The experiments of Wen, et al., [2006] involved the injection of liquid nitrogen
(LN
2
), horizontally in the form of a jet, into a water bath (in a closed vessel) and
measuring pressure rise, rate of pressure rise in the vessel as well as the temperature of
nitrogen vapor emanating from the water surface. The mass of water used was
substantially larger than the mass of nitrogen injected. This series of tests comes closest
to simulating (although a closed vessel was used) the LNG release underwater. The
stainless steel vessel was 0.69 L in volume (diameter = 87 mm) and contained 0.2 L of
water. The ratio of the mass of injected LN
2
to mass of water in the vessel was 5.2 x 10
-3
;
that is, the water could be considered to be an infinite bath compared to the LN
2
released.
LN
2
injection velocities in the jet ranged from 0.2 m/s to about 0.85 m/s. The findings
from this series of experiments can be summarized as follows:
1 The rate of pressure rise (dP/dt) increased (linearly) with the injection velocity. This
indicates that the jet undergoes shattering into larger number and smaller average size
LN
2
liquid droplets with higher velocity. Unfortunately, no direct measurements or
visualizations of the shattering of LN
2
were made in these tests, presumably because
of the use of stainless steel vessel.
2 The effective boiling heat transfer coefficient, based on a fictitious surface area of
the jet (surface area of the unbroken liquid jet formed by the diameter of the orifice
and the length of jet injected velocity times time) varies in the range 4 kW/m
2
K to
25 kW/m
2
K. The authors report that (based on other work) the film boiling heat
transfer coefficient for LN
2
boiling on flat surfaces are of the order of magnitude 1
kW/m
2
K and that for nucleate boiling the heat transfer coefficient could be as high as
10 kW/m
2
K.. The authors also present other information (from LN2 flow in pipes)
where the film boiling heat transfer coefficient measured was of the order of 50 W/m
2
K. It is entirely possible that at the level of individual liquid droplets, the heat transfer
coefficient is still in the normal film boiling range (50 W/m
2
K) but that the total
surface area of all droplets combined is significantly greater than the nominal jet
surface area, hence leading to the result in this series where the calculated heat
transfer coefficients were in the 4 to 25 kW/m
2
K based on the jet surface area.
3 The temperature of nitrogen vapor (22.4
o
C) emanating from the water surface was
very close to that of the water bath temperature (22.5
o
C). That is the nitrogen vapor
Page 7 of 55
bubbles are very efficiently heated even though the rise distance in the water bath
from the bottom of the vessel to the top of the water surface was only 34 mm. It
should be noted that at the maximum jet velocity of 0.85 m/s the gas bubble residence
time is, at best, about 40 ms. Therefore, it can be concluded that the heat transfer
between the gas bubble and water is also very high.
2.2.2 Experiments involving the release of water under a pressure ranging from 1 atm
to 2 atm (gage) as a jet over a bath of liquid nitrogen (LN
2
) contained in a pressure vessel
are reported by Archakositt, et al., [2004]. The release point of the jet of water was
above, but close to, the surface of LN
2
bath. The size of the apparatus (1 m high x 101.5
mm diameter) in these tests was larger than in the tests by Wen, et al. The vessel
contained 2 L of LN
2
and the volume of water used varied between 100 cc to 400 cc. The
water jet diameter was 15 mm. As in the previous research above, pressure rise in the
vessel was measured. Unfortunately, no temperature measurements or visual observations
of the interaction between the water jet and LN
2
were made. However, in experiments
involving higher jet velocity fine pieces of ice debris were found at the bottom of the test
vessel; no data are provided on the sizes of these ice particles. The principal finding of
interest to the LNG-water interaction phenomenon is that at higher jet velocities rapid
fragmentation of the injected water occurred and the dimensions of the water drops thus
formed can be assumed to be of the sizes of the ice powder found at the bottom of the
vessel.
2.2.3 Mishima, et al., [1999] have investigated the interaction of a molten metal
plunging into water. The experiments were conducted in a rectangular aluminum (closed)
tank of 200 mm width and 400 mm depth. Molten Woods metal was injected vertically
down into a bath of heavy water. The interaction was filmed both with high speed visual
cameras and neutron absorption radiographs. Molten metal at different temperatures
(range 100
o
C to 650
o
C) was injected into the water bath (10
o
C to 22
o
C) at a velocity of
1.4 m/s in jets of diameter ranging from 1.7 mm to 2.2 mm.
Figure 2-1 shows a sequence of neutron radiograph pictures, 4 ms apart, of the molten
metal jet break-up process. The hot jet penetrates the water and starts to break up at a
depth of about 5 cm below the water surface. The black blotches in the pictures indicate
the molten metal, the white portions are the heavy water and the white jagged regions are
steam bubbles. Atomization of the molten metal occurs into particles as small as 0.05 mm
diameter throughout the jet path. The higher the metal temperature the smaller are the
particles produced. Mishima, et al., also provide the probability density size distribution
of metal fragments produced by the shattering of the hot liquid metal in water. The
maximum pdf (=0.15) occurs at about 100 m and the tail extends up to about 400 m.
This series of tests indicated that the shattering of the penetrating fluid occurs relatively
quickly (in fractions of second) and that the particle size distribution formed is a function
of the superheat.
2.2.4 Tests with a sub-cooled water jet plunging into a pool of molten lead-bismuth alloy
were conducted by Sibamoto, et al., [2002]. The dynamic interaction between the water jet
and the molten metal was visualized and measured using high-frame-rate neutron
radiography. It is shown that the interaction between water and heavy melt in this geometry
differs in several aspects from those in melt injection into water described above. The
Page 8 of 55
maximum depth of jet penetration is limited by the buoyancy and the onset bulk boiling of
water which collects in a cavity created during the initial phases of water jet penetration
of the molten metal. Figure 2-2 shows, at different times after the water jet plunges into the
molten metal. The formation of the cavity in the hot metal and its filling with water, rapid
evaporation of water and the subsequent shattering explosion, are shown. The bulk
boiling starts as the sub-cooled water supply to the cavity becomes limited due to pinching
instabilities in the upper region of the cavity.
These authors provide a graph of the so called Thermal Interaction Zone (TIZ), s a plot in
which the jet liquid temperature is on the Y-axis and the bulk liquid (in this case the molten
metal) temperature is on the X-axis. The graph indicates the regions in which the vapor
explosion is possible. The variables used in the graph include T
MFB
, the minimum film
boiling temperature and T
HN
, the homogeneous nucleation temperature of the jet liquid.
The principal findings from the tests of Sibamoto, et al., [2002] are summarized as follows:
1 As the water jet penetrates into the molten metal, a cavity is formed very quickly and
the cavity expands both vertically and laterally. The
2 The cavity penetration velocity into the molten metal remains constant until it breaks up
at about ten (10) jet diameters. The cavity penetration velocity is dependent upon the
water to metal density ratio. The actual velocity is about 0.69 to 0.77 of the theoretical
velocity given by /[1 ]
m
C J
w
u u
= + where u
C
and u
J
are respectively, the cavity
penetration velocity and jet injection velocity. the densities of molten metal and water,
are, respectively,
m
and
w
.
3 The maximum penetration depth (H
C
) to jet diameter (d
J
) ratio is a function of the
densimetric Froude number (Fr), defined by
J
with R=
J J w
Fr u Rgd = . The
penetration depth is given by 1.45
C
J
H
Fr
d
(
=
(
. It is noted that the water into the molten
metal represents the case of positive buoyancy of the jet and hence this may limit the
penetration depth.
Page 9 of 55
Figure 2-1: Sequence of radiographs of a molten woods metal
(640
o
C) jet entering heavy water at 14
o
C
Source: Mishima, et al., [1999]; Copyright 1999, Nuclear Engineering & Design,
(Permission request for figure reproduction is pending with Elsevier Publishers)
Page 10 of 55
Source: Sibamoto, et al., (2002), Copyright 2002, Nuclear Technology.
Reproduced with permission (2008) from the American Nuclear Society.
Figure 2-2: Cavity formation, water accumulation, rapid evaporation and liquid
shattering in a water jet plunging into a molten metal
4 In the case of entry of jet at high Froude number into another liquid of comparable
density (i.e., the density ratio of the ambient fluid to jet fluid not greater than, say 2) the
jet penetration depth is limited by the dynamic break up of the jet rather than due to
thermal effects discussed above.
5 The maximum volume of cavity formed (V
C
) before shattering occurs is a function of
the Froude number and is given by
3 2
5 / 6
C J
V d Fr ( =
.
6 During the formation and growth of the cavity, a part of the injected water is emitted
out of the cavity as water, a part is vaporized and a part accumulates in the cavity. The
cavity contains evaporating water and part of the vapor formed. The mass of water
accumulated in the cavity is about 94% (at high molten metal temperature) to about
86% (at low molten metal temperature) of the theoretical mass injected up to the time
the cavity vanishes due to super heat explosion.
7 The bulk boiling time (or the time for the superheat explosion) is a function of the ratio
of the temperature of the molten metal to that of water. The higher this ratio the longer
is the super heat explosion time for a given jet velocity. The non dimensional time (t*),
defined by * / where [ =( / ) Characteristic time]
ch ch J J
t t t t d u = = , to super heat
ranges from 180 (at a low bath temperature) to 240 (a high bath temperature).
2.2.4 Perets, et al., [2005] have also experimentally studied the vapor explosion
phenomenon that occurs when a cold liquid (water) jet is injected into a hot liquid surface
(tin). The experiments involved using 1 kg of molten tin (at temperatures ranging from
230
o
C to 700
o
C) in an open geometry and directing a jet of sub cooled water at
temperatures ranging from near 0
o
C to 99
o
C and velocity of 11.3 m/s released from
about 30 cm above the surface of the molten tin. High water velocities were needed for
the water jet to penetrate the molten tin surface. The influence of the injection mass flow
Page 11 of 55
rate and other parameters on the on the likelihood of vapor explosion occurring was
investigated.
The tests indicate that the phenomenon occurs in four distinct stages, namely, (i) first
stage, in which the jet penetrates the hot liquid with a vapor envelope. The jet then
collapses in a time frame of milliseconds (either due to mechanical forces or boiling on
the jet cylinder surface) leading to shattering of the jet , followed by the formation of
large liquid droplets (from the jet), each liquid drop being now surrounded by vapor film
due to film boiling of water, (ii) second stage, in which the vapor film in one or more of
the droplets collapses resulting in the direct hot metal-water contact resulting in very
rapid nucleate boiling, (iii) third stage, in which the droplets with collapsed films explode
(due to extremely rapid nucleate boiling) shattering other droplets due to the shockwave
and thus stripping them of their surrounding vapor film, and (iv) stage four, where the
entire mass of water in the liquid jet is boiling so vigorously that it shatters the molten tin
and sprays the liquid metal all over the laboratory floor. The results indicate that the
collapse of the vapor film surrounding the hot (water) liquid triggers the vapor explosion.
The tests also provide a correlation for the maximum temperature of the bulk liquid, for a
specified water temperature below its saturation temperature (sub-cooled), below which a
vapor explosion occurs. This maximum temperature of the bulk liquid is termed the
quench temperature.
2.3 Droplet & Bubble formation and Heat Transfer
There are no published experimental data on the formation, maximum size of droplets
formed, size distribution of liquid droplets or the maximum size of vapor bubbles formed
and their size distribution, etc for the situation in which a cryogenic liquid enters into a
relatively warm liquid (such as water). Needless to state that data do not exist for
determining the phenomena and effects that occur when a LNG jet released into a large
water body. The release of LNG jet either from the top of the water surface or from a
large depth below the water surface has been studied at all, either experimentally or
theoretically. It is anticipated that when the cold liquid jet enters the warm bulk liquid,
the cold liquid undergoes rapid boiling and shattering producing a spectrum of liquid
droplets. These liquid droplets further evaporate forming a spectrum of vapor bubbles.
Indicated below are the findings from the literature on the formation of liquid droplets
(non evaporating case), formation of bubbles and heat transfer to the bubbles during their
journey in the bulk liquid.
2.3.1 Maximum liquid droplet size produced by a liquid jet into another liquid has
been studied, theoretically, by Okhotskii [1988]. He provides correlations for the
maximum size of droplets formed by the break up, due to mechanical forces, of a liquid
jet injected into a bulk liquid medium. Okhtoskiis theoretical results have been
compared with a data from number of experiments and agreement has been indicated.
These results are summarized below.
The maximum diameter (d
P
) of a liquid droplet resulting from the jet mechanical break
up when the liquid jet is injected into a bulk liquid is given by
Page 12 of 55
2
2
7 3 9
9
2 2
1
2
3
J
,
1 2
3 for Bo
2
J P
J W
J J P J
d
d
We We L
(
| |
( = +
|
(
\
(2-1)
2
2
7 3 9
9
2 2
1
2
3
J
,
2
3 for Bo
2
J P
J W J J J P J
d
d
We Bo We L
(
| |
( = +
|
(
\
(2-2)
Where,
d
J
= A diameter equal to the jet diameter d
J
if
J
d
g
(2-3a)
; ( )
J J J
d if jet diameter d is such that d
g g
=
(2-3b)
The definitions of other parameters are as follows:
Bo = Bond number;
2
( ) '
W J
J
g d
Bo
= (2-4a)
d = Effective diameter of the jet (equations 2-3a and 2-3b) (m)
d
J
= The physical diameter of the jet (or the orifice) (m)
d
P
= Maximum diameter of the liquid droplets in the jet breaks up (m)
g = Acceleration due to gravity = 9.8 (m/s
2
)
Lp
J
= Webber-Reynolds number;
2
2
' Re
J J J
J
J J
d
Lp
We
= = (2-4b)
Re
J
= Jet Reynolds number;
'
Re
J
J
J
U d
= (2-4c)
We
J
= Jet Weber number;
2
'
J J
J
J
U d
We
= (2-4d)
= A constant of value 0.3
= Density deviation = (
W
J
) (kg/m
3
)
J
= Density of jet liquid (kg/m
3
)
W
= Density of water (medium into which the jet is injected) (kg/m
3
)
J
= Surface tension of the jet liquid (N/m)
The correlations of Okhotskii [1988] are applicable to only mechanical break up of a
liquid jet penetrating a bulk liquid medium. It is anticipated that if the jet, in addition, is
shattered to produce liquid droplets due to thermal effects (cold and saturated liquid jet
penetrating a warmer bulk liquid), the maximum size of the droplets formed would be
smaller than that produced by mechanical fragmentation alone. Hence, the result from
equations 2-1 and 2-2 could be considered as the upper bound of droplet size in any
liquid jet shattering when entering another bulk liquid.
Page 13 of 55
2.3.2 Vapor bubble rise characteristics: The phenomenon of the formation of vapor
bubbles from the evaporation of liquid droplets (formed when a jet of cold liquid enters
another warm liquid) has not been visualized for the case of LNG release into water. No
data, photographs or films exist to show how and how fast the LNG droplets are formed
and how fast they boil to form the vapor bubbles. Also, there are no data on the stability
and size distribution of vapor bubbles formed. It is evident that once the LNG vapor
bubbles are formed, they rise up through the water column due to buoyancy. Perry and
Chilton [1973] provide a good discussion of gas bubble shape and rise velocity
characteristics and their dependence on the bubble diameter and other factors for the case
of a gas injected into a liquid. It is seen that for gas bubbles of effective diameter larger
than 4 mm the shape of the bubble changes to that of a helmet and the rise is turbulent
with significant oscillations of the bubble surface and shape. The rise path is not straight
up but may involve a zig-zag and/or helical trajectory. Also, if a swarm of bubbles are
created by the bubble producing process, the rise velocity of swarm will be completely
different from (and generally higher than) that of a single mean diameter bubble. The
swarm rise velocity may be influenced by a chimney effect. Also in a swarm, the
individual bubbles may coalesce or break up due to mechanical interactions and
collisions. In general the rise velocity of bubbles of 4 mm and larger in a water column,
without any wall effect influences, is about 0.2 m/s.
2.3.3 Heat transfer between rising bubbles and a surrounding bulk liquid has been
studied by Iguchi, et al., [1992] releasing helium and air bubbles (at 163 K) at the bottom
of a water bath (at 293 K). A stream of cold gas bubbles from nozzles of diameters 1, 2,
and 5 mm were released at the bottom of the water bath of depth 300 mm. The gas
volumetric flow rates in the nozzle ranged from 41.4 to 179.6 cm
3
/s. That is, the gas
injection velocities at the nozzle ranged from a low of 2 m/s to 180 m/s. The water bath
temperatures were varied between 20
o
C and 50
o
C. The bubble size formed and the
bubble rise velocities were measured using a high speed camera. The temperature
measurements of the bubbles, both on the centerline axis as well as off center line
locations at different depths were made using very sensitive thermocouples. Also
measured were the bath temperatures at different locations.
The results indicate that the temperature of the gases at the time of exit from the surface
of the water bath was very close to that of water. That is the bubbles, though released at
very cold temperatures (at 163 K) were very efficiently heated to the water temperature
(293 K) within the distance of rise of 300 mm. The measured rise velocities of gas
bubbles were in the 0.5 to 0.6 m/s within about 30 mm from a 2 mm diameter nozzle,
with gas volume flow rates of 41.4 and 77.8 cm
3
/s (ie., gas velocity at nozzle exit ranging
from 13 m/s to 25 m/s). The results also indicate that the turbulent terminal velocity of
the bubbles is attained within about 15 diameters from the nozzle even though the exit
velocity of the bubbles at the nozzle is almost 25 to 50 times greater than the terminal
velocity. The data further show heat transfer from the water to the bubble is controlled by
the heat transfer within the gas in the bubble rather than the thermal boundary layer
between the bulk liquid and the bubble surface. The overall heat transfer coefficient is
found to fit a correlation indicated below.
Page 14 of 55
0.7
1.1
1
L
v
Pe
Nu
(
=
(
+
(2-5)
with the following definition of the parameters:
Nu
v
= Nusselt number based on the vapor property;
b
v
v
h d
Nu
K
= (2-6)
Pe
L
= Peclet number based on bulk liquid properties Re Pr
L L L
Pe = (2-7)
Re
L
= Liquid side Reynolds number; Re
b b
L
L
U d
= (2-8)
Pr
L
= Liquid Prandtl number; Pr
L
L
L
= (2-9)
d
b
= Diameter of the gas bubble (m)
h = Overall liquid to gas bubble heat transfer coefficient (W/m
2
K)
K
v
= Vapor (or gas) thermal conductivity (W/m K)
U
b
= Rise velocity of the gas bubble (m/s)
L
= Thermal diffusivity of the liquid (m
2
/s)
= Ratio of viscosity of gas to viscosity of bulk liquid
v
L
=
L
= Dynamic viscosity of the bulk liquid (N s/m
2
)
v
= Dynamic viscosity of the vapor inside the bubble (N s/m
2
)
L
= Kinematic viscosity of the liquid (m
2
/s)
v
= Kinematic viscosity of the vapor inside the bubble (m
2
/s)
It is indicated by Iguchi et al [1992] that the absolute value of the overall heat transfer
between water and the gas (air or helium) was 100 W/m
2
K. The value range of the
parameter [Pe
L
/ (1+)] investigated in this work of lies between 50 and 1000.
Komaro and Sano [1999] have studied a similar phenomenon using pre-heated gas
bubbles of nitrogen, helium and argon (at 373 K) released at the bottom of liquid baths of
hot water (volatile), ethylene glycol, methyl carbitol, ethylene glycol-glycerin mixture
(non volatile liquids) at room temperature. The rise height of the hot gas bubbles was
0.17 m and the gases were released at the bottom of the liquid baths through a nozzle of
diameter 1.6 mm. The volume flow rates of gases ranged from 40 cm
3
/s to150 cm
3
/s and
the corresponding jet at exit velocities range from 25 m/s to 58.5 m/s. The bubble sizes
observed ranged from 1.8 cm to 3 cm but depended upon the liquid and the gas
characteristics. The bubble rise velocities observed ranged from 0.65 m/s to 0.75 m/s in
water. In the experiments of Komaro and Santo, as in the tests of Iguchi, eta al [1992],
the gas emerging from the liquid surface is essentially at the temperature of the liquid.
That is over a distance of about 0.17 m the gas bubble released almost 100
o
C hotter than
the surrounding water (or other liquid) reaches the liquid bath temperature within a
Page 15 of 55
distance of 0.12 m. The tests range in Reynolds numbers (based on liquid kinematic
viscosity and bubble rise velocity) between 100 and 3000.
The heat transfer coefficient data indicated by Komaro and Sano [1999] can be expressed
as
[ ]
0.5
3
1.8 10
L L
Nu x Pe
=
(2-10)
where,
Nu
L
= Nusselt number based on the bulk liquid property;
b
L
L
h d
Nu
K
= (2-11)
Pe
L
= Peclet number based on bulk liquid properties; see equation 2-7).
It is noticed that the exponent on the Pe
L
in the equations of Iguchi, et al, and that of
Komaro and Sano do not agree, one being 0.5 and the other being 0.7, respectively.
Furthermore, it appears that the Nusselt numbers themselves (compared in consistent
units using the vapor thermal conductivity in the definition of the Nusselt number) do not
agree in magnitudes. In order to demonstrate this discrepancy, we have used the thermal
properties of water (for bulk liquid) and that of saturated LNG vapor at ambient pressure.
We have in addition used Re
L
= 3000 and Pr
L
= 7 (for water). When these values are used
equations 2-5 and 2-11 provide the following results.
From equation 2-5
1,156
v
Nu =
and, from equation 2-11
6.5
v
Nu =
In fact, further examination of the results from Iguchi, et al indicates that their correlation
above does not predict the experimentally measured value (of 100 W/m
2
K) for the heat
transfer coefficient. Their correlation for the condition of the tests gives a result that is at
least 50 times higher than their own measured value. Therefore, it is concluded that the
correlation (in equation 2-10) of Komaro and Sano is a better representation of the heat
transfer process between vapor bubbles and bulk liquid.
2.4 Summary of findings from the literature
The following are the summary findings from the review of the relevant literature related
to the release of a cold, almost saturated liquid as a jet at some depth below the surface of
a bulk liquid (such as water) at normal ambient temperature.
1 In the case of LNG release at a depth of about 4.5 m below the water level in a lake,
all of the released LNG ( 7 kg) evaporated completely within the water column
resulting in no LNG pool formation on the water surface. In addition, the vapor
released from the surface of water was above the dew point temperature of water (and
perhaps at the temperature of water). The latter finding is based on an interpretation
of the observation that no white LNG vapor cloud was observed on the water surface.
2 Even in small scale experiments with liquid nitrogen releases in the form of a jet from
under water, all of the released LN2 is seen to evaporate in a very short distance (of
Page 16 of 55
the order of centimeters to less than a meter) and that the vapor emanating from the
water surface is at the temperature of water. This occurs not withstanding the fact that
the jet velocity is of the order of 10 to 30 m/s.
3 Results from test conducted with liquid metals jetted into water and water jetted into
liquid metals indicate that the shattering of the jet within very short time of jet
penetration into the bulk fluid (within distances of the order of 10 to 15 jet diameters).
The shattering of the jet into droplets is primarily caused by the superheating of the
volatile fluid, followed by a localized vapor explosion. This explosion leads to the
shattering of the jet.
4 In the case of release of a cold or hot vapor bubbles into a bulk liquid at a different
temperature, the vapor bubbles are heated by the surrounding bulk liquid to
essentially the temperature of the bulk liquid within a distance less than one meter,
and many times within 0.3 m. The bubbles, even when released as a stream from a
nozzle at high velocities (of 20 to 50 m/s) slow down to the respective terminal
velocities (consistent with the diameter of the bubbles) in about 15 nozzle diameters.
The rise velocity of bubbles of 5 mm bubbles is in the 0.6 to 0.75 m/s range.
5 The effective heat transfer coefficient correlations for heating vapor or gas bubbles in
a column of water show values close to 100 W/m
2
K.
6 There exist no experimental or theoretical correlations to predict the mean size of
droplets formed or the distribution of droplet sizes when a cold, saturated liquid is
jetted into a warm bulk liquid. The only correlation that exists is for the maximum
diameter of liquid droplets formed when a liquid jet is injected into another liquid at
the same temperature and no evaporation of either of the liquids takes place.
In chapter 3, the above results from the literature are used to develop a model for
describing the processes that occur and determining, quantitatively, the resulting effects
when LNG is released in the form of a jet from a hole in the transfer pipe located at the
bottom of a river at a depth of 10 to 15 m.
Page 17 of 55
Chapter 3
Modeling LNG jet release from a transfer-pipe underwater
3.1 General description of LNG jet behavior in water
It is seen from the review of the literature in Chapter 2 that there are no experiments, save
one, have been conducted of releasing LNG deep under water and measuring the
subsequent phenomena, including the rapid boiling of the liquid, droplet formation and
evaporation, and heating of the rising vapor bubbles. Even in the two experimental series
in which LNG was released under water, the temperature of LNG vapor emanating from
the water surface was not recorded, nor was any photographic measurements made of the
happenings underwater.
Based on the data, film records and other published information (from tests conducted by
other industries) on the behavior of cold (or hot) liquid jets injected into hot (or cold)
bulk liquids, it can be argued that the following phenomena occur, in sequence, when
LNG in a near saturated condition is released in the form of a jet under water. Assuming
that the LNG jet is released vertically from a location, at a specified depth below the
water surface, the possible stages of transformation of the LNG jet are shown
schematically in Figure 3-1 and Figure 3-2.
1 As the LNG jet is released into the bulk liquid (in this case water at ambient
temperature), it penetrates the water as a near smooth rod of liquid, for a very short
distance. Because of the significant temperature difference between water and the
LNG, film boiling in the surface of this rod of LNG. The vapor thus formed
provides a cocoon in which the remainder of the LNG jet will penetrate upward in
the water column. However, the jet velocity at the tip of the penetrating jet is
continually decreasing due to drag forces. This phenomenon is shown in Figure 3-1 as
occurring up to time t
1
.
2 In the second stage, shown as occurring between time t
1
and t
2
in Figure 3-1, a head is
formed at the top end of the jet leading to the formation of a set of double vortices
(the jet at this stage will look like a mushroom). The result of this vortices formation
is the entrainment of water into the rolled up mushroom and the resultant intimate
mixing of water and LNG with the collapse of the film enshrouding the LNG jet. It is
also possible that due to the turbulent instabilities in the jet-vapor film-water interface
on the stem of the jet, the vapor film collapses at different points on the jet stem
leading to nucleate boiling of LNG.
3 The above thermal and mechanical phenomena lead to localized release of droplets of
LNG into water, with the tearing of jet liquid due to the agitation created by the local
nucleate boiling process.
Page 18 of 55
Figure 3-1: Schematic representation of the different stages in the penetration of
LNG jet into water, break up and evaporating liquid droplets formation
Figure 3-2: Schematic representation of evaporation of liquid droplets, formation
of vapor bubbles, heat transfer from water to bubbles and bubble rise
in the water column
Page 19 of 55
The entire phenomena indicated in items 1 through 3 take place in distances of the order
of less than 10 jet diameters (ie., for a jet issuing, say, at 25 m/s, from a 2.5 cm hole, the
time scales of the above sequence of events will be of the order of 10 milli seconds).
4 The liquid droplets formed will be again surrounded by a thin vapor film due to film
boiling of the droplets. In addition, the liquid droplets will be superheated by the
surrounding water as they rise up in the water column. Either due to vapor film
instabilities or due to the fact the bulk liquid in each liquid droplet is heated rapidly to
the superheat limit, there will result localized superheat explosions, initiated in one or
more liquid droplets. These localized explosions result in very rapid release of vapor
bubbles and also create strong but localized shockwaves. These shockwaves will
further shatter the remainder of the coherent liquid jet. It is anticipated that the
phenomenon described above occurs in time scales of extremely short duration of
time (1 to 10 milli seconds). The shattering of the jet will be in all directions and the
formation of the massive number of liquid drops will result in the complete
destruction of the jet momentum (and hence its velocity). The above occurrence is
depicted in Figure 3-2 and is indicated to occur within a total time of t
4
5 The vapor bubbles formed will be at saturation temperature corresponding to the local
total (atmospheric + hydrostatic) pressure. This temperature of the LNG vapor
bubbles will be much less than that of the surrounding water.
6 The vapor bubbles rise at the turbulent terminal velocity corresponding to the bubble
diameter. However, it is possible that the large number of bubbles released will rise as
a swarm at a velocity higher than the individual bubble velocity. Also,
simultaneously, the bubbles will get heated by the water.
7 It is not possible to predict whether the bubbles will coalesce to form a very large
vapor bubble at the time the vapor emanates from the water surface.
It is entirely possible that all of the liquid released in the jet up to the time t
4
will be
shattered in a very short duration of time (of the order of a milli second or less). Such
shattering will lead to the pinching of the jet at the level of the hole. The vapor bubbles
formed by the liquid released up to time t
4
will rise. The entire set of events from 1
through 6 will be repeated for a new batch of liquid issuing from the hole as a jet.
It is noted that in the case of the WCE proposed LNG PiP transfer system, the transfer
system is buried at the bottom of the Taunton River in a trench under an overburden of
engineered gravel of about 1.3 m thick (see Figure 1-1). Therefore, if there is a release of
LNG due to a hole (in both walls of the transfer system)the jet will first encounter a
heavy but porous gravel layer which will promote mechanical shattering of the LNG jet
into droplets sooner than in the case when the jet is released into a quiescent water body.
That is, the presence of the gravel layer will accelerate the jet destruction, formation of
liquid droplets and their evaporation to form vapor bubbles much closer to the bottom of
the River.
Page 20 of 55
To the extent possible, and based on the available data & correlations from the literature,
a model is developed to determine the temperature of the vapor emanating from the water
surface when a LNG jet is released from a specified size hole (in both walls of the
transfer system). This model is formulated and discussed in the next section.
3.2 Modeling LNG Jet Release, Liquid Droplet Formation and Vapor
Bubble Heating
3.2.1 Jet release rate
The velocity of liquid (LNG) release from the jet is given by following equation
( )
2
a W W P
J D
L
P gS P
U C
+
= (3-1)
and the volume flow rate by
2
4
J J J
V d U
(3-2)
where,
C
D
= Coefficient of discharge of the jet orifice (generally 0.6 to 0.7)
d
J
= diameter of the orifice of the jet (or initial jet diameter) (m)
g = Acceleration due to gravity (9.8) (m/s
2
)
P
a
= Atmospheric pressure (N/m
2
)
P
J
= Absolute pressure inside the pipe (N/m
2
)
S
W
= Depth under water of the jet orifice (m)
U
J
= Velocity of liquid at jet exit from the orifice (m/s)
J
V
= Volumetric flow rate of liquid (LNG) our of the orifice (m
3
/s)
Table 3-1 shows the results of flow rate calculations for an assumed 1 diameter for the
hole-size on the pipe wall, and different depths of pipe below the water level. It should
be noted that in modeling the LNG release from the PiP transfer system, it is assumed
that size of punctures in both the inner and outer wall of the transfer system is assumed to
be the same. This is tantamount to assuming that the outer pipe and the insulation
between the inner and outer pipe have no influence on the puncture size. It is further
assumed that the corrosion coating and the approximately 2.5 inch concrete weight
coating on the lines have no influence on the puncture formation or its size. These
assumptions are very conservative.
Page 21 of 55
Table 3-1: LNG jet release rates for different conditions
Depth of jet
release
Volumetric flow
rate
(ft) (m)
P
(N/m
2
)
Jet
velocity
(m/s) (m
3
/s) (gpm)
Remarks
10 3.05 1.004E+06 41.4 1.30E-02 206.3
40 12.2 9.147E+05 39.5 1.24E-02 196.9
60 18.3 8.550E+05 38.2 1.20E-02 190.3
P
J
= 1.034E+06 N/m
2
(150 psig)
d
J
= 2.54 cm (1)
C
D
= 0.62 (Perry & Chilton [1973])
3.2.2 Homogeneous nucleation of a superheated liquid
It is well known in the scientific literature that when a liquid is heated in an environment
devoid of solid nucleation sites the liquid can be heated to beyond its normal boiling
point corresponding to the local pressure. That is, the liquid can be superheated.
However, there is a limit up to which the temperature of the liquid can be increased
above its boiling point. When the liquid temperature reaches (without any boiling) a
critical temperature, termed the homogeneous nucleation temperature or the super
heat limit temperature, (Porteous and Reid [1976], Reid, [1982]). This superheat limit
temperature is generally found to be about 0.89 times the critical temperature of the
liquid undergoing boiling. Superheat explosions of a hydrocarbon liquid in water are
found (in laboratory experiments) to be possible over a temperature range of water given
by
0.89 < (T
W
/T
C
(hydrocarbon) < 0.98
It should be noted that no superheat explosions were observed when liquid methane was
poured, in the laboratory experiments, on to the water surface. However, superheat
explosions (also termed Rapid Phase Transitions- RPTs) have been reported for LNG
spills on water (Burgess, et al., [1972], and other field scale tests reported by Reid
[1982]). The composition of LNG seems to have significant effect on the probability of
an RPT occurring when spilled on a water surface. No systematic tests of LNG releases
underwater have been undertaken.
Assuming that the LNG released underwater superheats up to the superheat limit, the
following analysis is indicated to determine the possible explosive yield of a rapid
superheat energy release.
Atmospheric boiling temperature of LNG
2
= T
B
= 111.6 K
Critical temperature of LNG = T
C
= 190.6 K
Superheat limit temperature = T
SL
= 0.9 * T
C
= 169.6 K
Superheat sensible energy stored = E
SL
= C
P,L
*(T
SL
-T
B
)= 2 x 10
5
J/kg
in a unit mass of LNG
Energy released by the explosion of a unit mass of TNT = 4.7 x 10
6
J/kg
TNT mass equivalence of 1 kg of LNG superheat energy = 4.26 x 10
-2
kgTNT
2
For these calculations the thermodynamic properties of pure methane liquid are used.
Page 22 of 55
3.2.3 Potential vapor explosion yield
In an underwater LNG release test conducted by the US Bureau of Mines (Burgess, et al.,
[1972]), it is reported that an energy equivalent of less than 1 lb (0.45 kg) of TNT release
was realized from the superheat explosion: underwater. A comparison of this data and
that calculated using the above calculations is indicated in Appendix A. It is seen that if
the entire mass of LNG released underwater, 7.71 kg (17 lbs), participated in the
superheat energy release then the equivalent energy released is equal to that from 0.33 kg
(0.73 lbs) of LNG. This comparative result seems to indicate that all of the LNG released
participates in the superheat energy release. The fact that the entire mass of LNG released
participated in the superheat energy release in the test by Burgess, et al., could be
attributed to the relatively small mass of LNG that was used.
According to the findings of laboratory scale experiments of Sibamoto, et al. [2002],
when a cold liquid is injected into a hot liquid, the penetrating liquid jet initially forms a
vapor cavity in which the liquid accumulates until the vapor cavity collapses leading to
the sudden evaporation (of the superheated liquid in the cavity). This release of superheat
energy can be considered as a local explosion. It is uncertain whether such a
phenomenon occurs when a LNG jet enters water, especially from the bottom of the
water body. In the case of Sibamoto, et al., tests colder water was introduced at the top of
a molten metal, thus the jet velocity vector and the upward buoyancy force were in
opposite directions. In the case of LNG entering water at its bottom, the buoyancy force
is in the same direction as the jet velocity vector leading, perhaps to a different
mechanism than the formation of a vapor cavity holding superheated jet liquid.
The following analysis is indicated for the sake of completeness, without being certain
that the results of this analysis will hold for the case of a LNG jet entering a water body.
The volume of superheated liquid formed and held in a vapor cavity is given by
'
3
J
SL ch
V V Fr = (3-3)
where,
V
SL
= Volume of superheated liquid in the vapor cavity (m
3
)
V
ch
= A characteristic volume equal to that of a sphere (m
3
)
with diameter the same as that of the jet =
3
6
ch J
V d
= (3-4)
Fr
J
= Modified jet Froude number defined by,
( )
' J
J
J LNG W
U
Fr
d
=
(3-5)
In Table 3-2 are shown the results from an example calculation for the case of LNG
release from a 1 inch diameter hole in the transfer system pipe walls (at 40 ft under
water) and the resulting LNG jet release characteristics. Also indicated in Table 3-2 are
the results of application of the analysis in this section (3.2.3) for the superheat
explosion yield. It is seen that the TNT equivalence of the superheat energy released is
Page 23 of 55
about 2.1 kg. This amount of energy will be sufficient to initiate the shattering of the rest
of the LNG in the jet released op to the time the superheat explosion occurs.
The superheat explosion is assumed to shatter the liquid released (up to the time the
superheat energy is released) into different size droplets of LNG at a temperature
corresponding to the local hydrostatic pressure. These droplets then evaporate by either
film boiling or nucleate boiling and produce vapor bubbles. It is further assumed that the
location of the front end of the jet when this event occurs is about 10 jet diameters. The
sizes of droplets formed and the rise and heating of the vapor bubbles are modeled and
discussed in the next sub-section.
Table 3-2: Estimated energy release in the LNG jet due to superheat
Parameter Symbol Value Unit
Equation #
or Reference
Assumed jet diameter at release (diameter of the
hole on the outer and inner walls of the pipe) =
1inch
d
J
2.54E-02 m
Mean depth in the water column of the transfer
system (top of the outer pipe wall) = 40 ft
h
P
12.2 m
Assumed coefficient of discharge for the hole C
D
0.62
Pipe gage pressure during the transfer operations
= 150 psig
P
P
1.034E+06 N/m
2
Temperature of LNG in the pipe during transfer
operation
T
LNG,P
114.5 K
CALCULATED VALUES
Hydrostatic gage pressure at the top of pipe P
W
1.195E+05 N/m
2
Pressure drop across the hole in the pipe P 9.147E+05 N/m
2
Velocity of jet of LNG issuing from the hole U
J
39.5 m/s 3-1
Volume flow rate of LNG from the jet
1.24E-02 M
3
/s 3-2
Jet Reynolds number Re
J
4.11E+07
Densimetric Froude number Fr
J
64.90 3-5
Characteristic volume (equal to the volume of a
sphere of diameter equal to that of the jet)
V
ch
8.58E-06 M
3
3-4
Maximum volume of LNG cavity in water formed
by the jet before superheat explosion
V
SL
1.08E-01 m
3
Sibamoto, et
al., [2002]
The above volume of LNG is released in a time t
C
8.7 s
Mass of LNG released over time t
c
m
C
48.8 kg
Sensible energy excess (above saturated liquid
enthalpy) in the LNG mass in the cavity at the
time of superheat explosion
E
LNG
9.91E+06 J
TNT energy per unit mass E
TNT
4.7E+06 J/kg
TNT mass equivalent of super heat explosion m*
TNT
2.1 kg
LNG
V
Page 24 of 55
3.2.4 Liquid droplet size and vapor bubble size
As discussed in chapter 2, there are no experimental data which indicate the magnitude or
the distribution of sizes of liquid droplets formed when LNG is released underwater. It
can be anticipated that the sizes of droplets of LNG formed by the dynamic forces of jet
flow in water as well as the thermal interaction will be smaller than that due to just
dynamic effects. Hence, if one models the effects of large size droplets on the formation
and heating of LNG vapor bubbles underwater, the results will be conservative (in that
smaller liquid droplets and vapor bubbles will heat faster).
The correlations of Okhotskii [1988] indicated in equations 2-1 to 2-3 together with the
parameter definitions indicated in equations 2.3a through 2-4d are used to calculate the
size of the maximum diameter of the droplet formed in the LNG jet released underwater,
subject to the assumptions indicated above. The results of these calculations are shown in
Table 3-3. It is seen that for the conditions of release and jet diameter assumed, the
largest diameter of the liquid droplet formed is about 1.6 mm. The laminar rise terminal
velocity of this size droplet is given by
2
(1 / )
; Laminar terminal velocity
18
L W P
d
W
g d
U
= (3-6a)
And the turbulent rise velocity by
(1 / )
4
; Turbulent flow terminal velocity
3
L W p
d
D
g d
U
C
=
`
)
(3-6b)
Where, C
D
is the drag coefficient on a spherical drop (generally assumed to be about
0.44). For the above conditions the droplet terminal velocity is calculated to be 0.7 m/s.
Assuming a water to droplet film boiling heat transfer coefficient (h
FB
) it can be shown
that the rate of decrease of diameter is given by,
( )
FB P W sat P
L
h A T T dV
dt
= (3-7)
Where,
A
P
= Surface area of the droplet for heat transfer = d
P
2
(m
2
)
h
FB
= Film boiling heat transfer coefficient (W/m
2
K)
V
P
= Volume of the droplet at any time = (/6) d
p
3
(m
3
)
L
= Density of LNG (kg/m
3
)
= Heat of vaporization of LNG (J/kg)
Equation 3-6 implies that the droplet diameter decreases linearly with time and all of the
droplet is evaporated in a time (t
evap
) given by
Page 25 of 55
,
2 ( )
L P i
evap
FB sat
d
t
h T T
=
; t
evap
is in seconds (3-8)
Applying equation 3-8 to the conditions of the problem defined above, t
evap
is found to be
5 s.
The total vertical distance travelled by the evaporating droplet, moving at the terminal
velocity (given by equation 3-6) corresponding to its current diameter can be shown to
be,
, ,
1
( ) ( ) ( )
3
d p i evap p i
S U d t d = , with S
d
in meters (3-9)
As indicated in Table 3-3, the vertical distance travelled by the largest liquid droplet
before completely evaporating is about 1.2 m. If this is added to the distance from the jet
release point to the end of the jet when it undergoes shattering, namely about 10
diameters, it is seen that vapor bubbles are formed completely by a vertical distance of
rise of about 1.4 m from the location of the hole in the transfer line lying at the river
bottom. This distance is within the crushed stone overburden on top of the buried line.
Page 26 of 55
Table 3-3: LNG droplet and its characteristics
Parameter Symbol Value Unit
Equation #
or
Reference
Assumed jet diameter at release (diameter of the
hole on the outer and inner walls of the pipe) =
1inch
d
J
2.54E-02 m
Assumed water temperature T
W
293 K
Assumed film boiling heat transfer coefficient
between water and liquid droplet surface
h
d
200 W/m
2
K ABS [2004]
CALCULATED VALUES
Jet Reynolds number Re
J
4.11E+07 Table 3-2
Characteristic diameter d
ch
0.00486769 m 2-3a
Effective jet diameter for droplet size calculations d'
J
0.00486769 m 2-3b
Bond number Bo 9.8696044 2-4a
Weber number We
J
2.65E+05 2-4d
Lp number (Okhotskii [1988]) Lp 1.55E+02 2-4b
Beta (a constant introduced by Okhotskii [1988]) 0.3
Ratio of diameter of the droplet to characteristic
diameter of the jet
d
P
/d'
J
3.23E-01 2-1
Hence, the maximum size of liquid droplet formed d
P
1.573E-03 m
Mass of the largest droplet formed m
P
9.166E-07 kg
Diameter of the saturated vapor bubble formed
by the above droplet
d
Vap
9.871E-03 m
Total heat to vaporize m
P
droplet mass at
saturation conditions.
E
d
4.67E-01 J
Rate of heat input into the droplet of diameter d
P
due to temperature difference between water and
LNG
E 7.048E-02 W
Time to evaporate the largest droplet t
evap
5.0 s 3-8
Laminar (Stokian) terminal velocity of d
P
droplet
size
U
d
0.7 m/s
Reynolds number based on U
d
Re
d
1164.9 Laminar
Vertical distance travelled by the droplet by the
time of complete evaporation [Note: Diameter is
changing with time & terminal velocity is diameter
dependent]
S
d
1.2 m 3-9
Page 27 of 55
3.2.5 Vapor bubble rise and heating
A model is indicated below for the simultaneous rise, heat up and expansion (due to
reduced hydrostatic pressure with rise through the water column) of an initially saturated
condition vapor bubble of LNG rising in a water body. Figure 3-3 shows this process
schematically.
Figure 3-3: Schematic diagram showing the formation, rise and heating up of a
bubble of LNG vapor in a water body
In developing the model described below the following assumptions are made:
1 The vapor contained in the gas bubble is considered to be a perfect gas
2 The conditions inside the vapor bubble are always in thermodynamic equilibrium at
the local conditions of pressure. That is, a quasi steady state is assumed.
3 The initial condition of the vapor in the bubble is represented by the saturated
condition of (LNG vapor) at the total pressure corresponding to the depth at which the
bubble is released, initially.
4 The effect of changes in vapor density on the total buoyancy force on the bubble can
be neglected (because the vapor density is very small compared to that of water).
5 The characteristics of the vapor bubble can be represented by an equivalent volume
sphere. That is, the vapor bubble size is represented by an equivalent diameter sphere,
even though the bubble itself may have significantly different shape than that of a
sphere.
6 The vapor bubble rises at a terminal velocity corresponding to its current diameter
7 The vapor motion is turbulent. That is, the terminal velocity is determined by a
balance of the buoyancy force and turbulent drag on the bubble.
8 The mass of vapor in the bubble is constant during its rise through the water column.
There is no absorption of gas in water.
Page 28 of 55
With the above assumptions, the following equations are written
3
.
1.74 Turbulent flow terminal velocity
dS
U g d
dt
= = = (3-10)
1 "Effective gravity"
g
W
g g
(
= =
(
(3-11)
The total pressure at any depth S is given by
( )
a W
P S P g S = + (3-12)
The equation of state of the vapor inside the rising gas bubble is
g
P
PV mRT or RT
= = (3-13)
The mass of vapor in the bubble is
3
6
i i i
i i
i i
P V P
m d
R T R T
= = (3-14)
The rate of heat transfer to the bubble is represented by
( )
P W
dT
m C h A T T
dt
= (3-15)
The definitions of the parameters in the above equations are as follows:
A = Surface area of the equivalent spherical vapor bubble (m
2
)
C
P
= Specific heat of vapor at constant pressure (J/kg k)
d = Equivalent diameter of the spherical vapor bubble (m)
d
i
= Vapor bubble diameter at the point of generation at depth S
i
(m)
g = Acceleration due to gravity (m/s
2
)
g = Reduced effective gravity due to buoyancy (m/s
2
)
h = Water to vapor bubble overall heat transfer coefficient (W/m
2
K)
m = Mass of vapor in the bubble = Initial mass (kg)
m
i
= Initial mass of vapor in the bubble = (kg)
P = Total pressure at any depth S (N/m
2
)
P
a
= Atmospheric pressure (1.01325E5) (N/m
2
)
P
i
= Total pressure at the initial depth of bubble release (N/m
2
)
R = Gas constant for the vapor (for LNG it is 519.63) (J/kg K)
S = Depth below the water surface. S is positive as depth increases (m)
3
All symbols are defined in the Nomenclature section.
Page 29 of 55
S
i
= Initial depth where the bubble is released (m)
t = Time (s)
t
ch
= Characteristic time in the problem (see equation 3-16 ) (s)
t
ch,T
= Characteristic heating time (See equation 3-17) (s)
T = Temperature of the vapor inside the bubble at any time (K)
Ti
= Initial temperature at release of the vapor in the bubble (K)
U = Terminal velocity of rise of bubble at any location (m/s)
U
i
= Initial terminal velocity of rise (m/s)
g
= Density of gas (vapor) in the bubble at any time (kg/m
3
)
W
= Density of water (kg/m
3
)
Equations 3-10 through 3-15 are transformed to dimensionless form to facilitate ease of
solution. The following characteristic and non dimensional parameters are defined.
i
d
d
= = Dimensionless bubble diameter
i
S
S
= = Dimensionless depth
a
P
p
P
= = Dimensionless total pressure with respect to atmospheric pressure
i
T
T
= = Dimensionless temperature with respect to initial temperature
W
W
i
T
T
= = Dimensionless water temperature
i
U
u
U
= = Dimensionless terminal velocity with respect to initial velocity
i
V
V
= = Dimensionless vapor bubble volume
ch
t
t
= = Dimensionless time
*
W i
a
gS
P
P
= = Dimensionless initial hydrostatic pressure (3-16)
1.74
i i
ch
i i
S S
t
U gd
= =
= Characteristic rise time (3-17)
, 2
i
Initial bubble sensible heat
Heat transfer rate to initial bubble at a temp diff T
i P i
ch T
i i
mC T
t
d hT
= = (3-18)
,
Characteristic rise time
Characteristic heating time
ch
ch T
t
t
= = (3-19)
Based on the above definitions, equation 3-10 through 3-15 become, respectively, the
following
d
u
d
= = (3-20)
*
1 p P = +
(3-21)
Page 30 of 55
3
i i
p p
p p
= =
(3-22)
And ( )
2
W
d
d
= (3-23)
The above set of equations (3-20 through 3-23) has the following initial conditions
At 0; 1, 1, 1, 1, 1 u = = = = = = (3-24)
It should be noted that = 0 represents the water surface.
Substituting equations 3-20 and 3-22 in equation 3-23 we get the following differential
equation
( )
*
1
i
W
p
d
d P
=
( +
(3-25)
The above non linear equation in is solved numerically with the initial condition
indicated in 3-24. Once q is known as a function of , can be obtained easily from
equations 3-22 and hence the bubble volume (
3
= ).
3.3 Example Results
Calculations were performed for different conditions of LNG release from the PiP LNG
transfer system. The two principal parameters varied in the calculations were the depth of
the transfer system underwater and the size of the puncture hole. The calculations
involved the evaluation of the LNG jet characteristics, the length of the jet when it breaks
down into liquid droplets, the maximum size of liquid droplet formed and duration of
time over which the droplet evaporates to produce a vapor bubble. The vapor produced is
assumed to be at a saturated condition corresponding to the pressure at the depth at which
the vapor is generated. The final result of calculations is the depth at which the
temperature of the largest size vapor bubble is close to (within 1%) the temperature of
water in the river. LNG vapor at normal river temperature is positively buoyant in the
atmosphere. The results of these calculations are indicated below. Discussion on these
results and the conclusions therefrom are indicated in Chapter 4.
To illustrate the calculation procedure, a test case involving the release of LNG from a 1
(2.54 x 10
-2
m) diameter hole on the wall of the line located at 40 ft (12.2 m) depth under
water is assumed. Table 3-1, Table 3-2 and Table 3-3 indicate the results on LNG jet
characteristics, the maximum size of LNG droplets formed, the rise velocity of the
droplets and the size of the saturated vapor bubble formed by the evaporation of the
largest size liquid droplet. Table 3-4 below provides the results related to the rise of the
vapor bubble, its heating by water and the depth at which the vapor bubble temperature is
essentially equal to that of the surrounding water.
Page 31 of 55
Table 3-4: Vapor bubble rise in the water column and associated results
Parameter Symbol Value Unit
Equation #
or
Reference
Assumed Values
Temperature of water body (assumed) T
W
293.3 K
Diameter of the vapor bubble formed by largest
liquid droplet
d
i
9.87E-03 m Table 3-3
Volume of vapor bubble V
i
5.04E-07 m
3
Mass of vapor in the bubble m
i
1.72E-06 kg
Effective heat transfer coefficient between vapor
bubble and water body
h 21 W/m
2
K
Iguchi, et al
[1992]
Depth (below the water surface) of vapor release S
i
10.7 m
Table 3-3
results
Calculated parameters
Absolute pressure at bubble release depth P
i
2.153E+05 N/m
2
Saturation temperature of LNG at this pressure T
i
121.7 K
Calculated terminal initial velocity of rise
Ui 0.541 m/s 3-10
Characteristic rise (kinematic) time t
ch
19.79 s 3-17
Characteristic heat transfer time t
ch,T
0.92 s 3-18
Ratio of kinematic to heat transfer characteristic
times
21.6 3-19
Dimensionless water temperature
W
2.41
Dimensionless initial pressure p
i
' 1.95 3-21
Dimensionless maximum hydrostatic pressure P* 0.95 3-16
Estimation for integration step size in h 0.00814327
Dimensionless rise distance at which bubble
temperature is essentially the water temperature
0.171
Solution to
3-25
Rise distance from vapor bubble release point
when the vapor bubble is essentially at the same
temperature as that of water
H
vap,rise
1.83 m
Total distance of rise from jet release point to the
point at which the vapor bubble attains the
temperature of water
H
rise
3.32 m
Depth at which the vapor bubble temperature is
the same as river water temperature
S
min
8.87 m
Calculations similar to the ones indicated in Table 3-2, Table 3-3 and Table 3-4 were
performed for different depths of the PiP transfer system below the water level and for
different diameters of the hole on the pipe wall. These results are shown in Figures 3-4a,
3-4b and 3-4c, respectively for transfer system location depths of 40 ft, 30 ft and 20 ft
below water level. These results are discussed in the chapter 4.
Page 32 of 55
Figure 3-4a: LNG release at 40 ft depth of water
Figure 3-4b: LNG release at 30 ft depth of water
Figure 3-4c: LNG release at 20 ft depth of water
Figure 3-4: Results for vapor bubble rise and heating distances for different
pipe depths and hole sizes on the pipe wall
Page 33 of 55
Chapter 4
Discussions and Conclusions
4.1 Discussions
In the scenario of release of LNG, a cryogenic liquid, underwater it was anticipated that
some portion of the released volume would evaporate due to thermal interaction with
water and the remaining part would float to the water surface and spread as a boiling pool
generating cold, heavier-than-air vapors. It was also anticipated that these cold vapors
would disperse in the atmosphere as a heavy gas, and result in large ground level
dispersion distances (compared to that from an equivalent mass of buoyant vapor). The
results presented in Figures 3-4a, 3-4b and 3-4c seem to contradict the above scenario in
that they indicate that for most probable size releases (with hole diameters ranging from
1inch to 6 inches) and depth of water above the transfer system ranging from 10 ft to 40
ft, the vapor emanating from the water surface is at the temperature of water and
therefore buoyant. Furthermore, the results indicate that no liquid pool formation occurs
on the water surface; that is, all of the released liquid evaporates well within the water
body, even in the case of release from as shallow a depth as 20 ft. These results are
significant in that LNG release underwater may not pose a ground level vapor dispersion
problem.
There is only one test, conducted by the US Bureau of Mines in 1970 (with LNG release
underwater), that supports the results developed in this report. Unfortunately, no other
tests, of either the laboratory scale or field size with LNG releases underwater have been
conducted. Therefore, the details of the phenomena that occur with LNG release
underwater and the dependence of the various parameters (jet break up distance,
intermittency of jet break-up, superheat caused spontaneous vaporization and their
associated shockwave production, sizes of liquid droplets formed, vapor bubbles and
their heating and movement in water, etc) on the circumstances and details of the release
are not available. In this report an attempt has been made, therefore, to look at test results
from similar release situations in other industries and settings, where interactions between
a cold (and vaporizable) liquid and a hot bulk liquid occur and have been studied. The
results and correlations from these laboratory and prototype scale tests in other related
industries have been used in developing the model for LNG release underwater. The
findings from the literature on these correlations were discussed in Chapter 2.
The model developed to describe the happenings when LNG is released underwater in
the form of jet from a hole on the transfer line wall was indicated in Chapter 3. A number
of assumptions were made in the development of this model. The effects of these
assumptions are discussed below.
1 Initial penetration distance of the cold liquid jet: It was evident from the literature
findings that when a jet of cold (hot), vaporizable liquid penetrates another bulk
liquid that is hotter (or colder) than the penetrating liquid the jet breaks down and
shatters to form liquid droplets of various sizes. While there are correlations for the
length of penetration of one liquid jet into another liquid when both liquids are at the
Page 34 of 55
same temperature, there are no correlations to predict the jet penetration length when
there is significant thermal interaction between the liquids, especially when such
interaction leads to superheat caused, spontaneous shattering of the jet liquid. It is
however, known, empirically from laboratory scale tests that this distance of
penetration is about 10 jet nozzle diameters for the case of a colder liquid jet of
density lower than that of the bulk liquid entering from the top of the bulk liquid. In
this case the buoyancy of the jet liquid, perhaps, assists in limiting the penetration
depth. Unfortunately, no direct photographic evidence is available for determining the
jet penetration distance before completely shattering when a buoyant, vaporizable
liquid is introduced at the bottom of a heavier and hotter bulk liquid.
This empirical result above has been used in the model presented in Chapter 3 to
estimate the LNG jet penetration (vertically up) distance. It is not possible to state
whether this simple factor formula is sufficient for different release rates, jet
diameters and release depth underwater. Proper scaling laws may have to be
developed in the future, based on physics and carefully conducted experiments with
LNG releases underwater. In any such future tests the values of the independent
variables (such as the depth of release, the rate of release, the initial diameter of the
LNG jet and water temperature) should be varied over realistic range of values and
the jet break down phenomenon should be carefully measured. Until such detailed
data or correlations therefrom are developed, it is reasonable to assume that the jet
breakup distance is 10 times the jet diameter, since there is experimental evidence for
such a factor from other liquid jet-bulk liquid situations.
2 Sizes of liquid droplets: The conclusion from the literature findings indicated in
Chapter 2 is that no data or correlations are available for the maximum droplet size or
the distribution of liquid droplets formed when a LNG jet penetrates a warm water
body and their dependence on the release conditions. In the model described in
Chapter 3 the correlation developed by Okhotskii [1988] was used to calculate the
maximum liquid droplet size formed. Okhotskiis correlations are based on
theoretical analyses and experimental results applicable to the penetration of a liquid
jet into another bulk liquid when there are no thermal or chemical interactions
between the liquids. That is the breakup of the jet liquid is solely due to mechanical
effects and the development of shear stress induced waves in the liquids and
turbulence caused tearing.
It can be argued that using Okhotskii correlation results in a very conservative
calculation. This is because, in the case of the thermal interaction of LNG with water,
the superheat induced rapid boiling and the generation of shock waves will result in
the formation of the largest droplet size which will be smaller than that obtained from
Okhotskii correlation based purely on mechanical shattering of a jet. Assuming a
larger droplet size leads to larger size of vapor bubbles formed and less efficient
heating of the bubbles by water. However, as we see from the results in Figure 3-4a,
3-4b and 3-4c, even with this large droplet assumption, the vapor is heated to water
temperature at considerable depth below the water surface.
Page 35 of 55
3 Droplet movement and vaporization distance: It is implicitly assumed that the jet
momentum, at the time the droplets are formed, is completely consumed and that the
droplets at the time of their formation are released vertically with no vertical velocity.
This may or may not be an accurate representation of what actually happens during
the LNG-water interaction. If the dominant mechanism for droplet formation is the
superheat explosion generated shockwave, then it is conceivable that the droplets are
eject with considerable momentum in all directions.
The assumption made in the model presented in Chapter 3 that all droplets are
released vertically and rise at their terminal rise velocity (consistent with the balance
of the drag force and the buoyancy force o the droplet) is a conservative. This is
because, since the droplet sizes are small (of the order of one millimeter) even if they
are ejected with a relatively high velocity, this velocity will be dissipated over a
length of about 10 diameters
4
due to the drag (note that the drag is proportional to the
square of the velocity if the droplet motion is turbulent). Secondly, the assumption
that all liquid drops produced rise in the vertical direction is also conservative, noting
that droplets can be formed and ejected (by the violence of the shock induced birth) in
all directions.
The heating rate of the droplets is based on the assumption that the droplets are
surrounded by a vapor film and heat transfer is dominated by film boiling heat
transfer process. Again, this is a conservative assumption in that the film boiling heat
transfer coefficient is lower than that in the case of nucleate boiling. The latter type of
boiling occurs when the vapor film breaks down and there is intimate contact between
water and LNG. Such a phenomenon has been observed in the case of other cold jet
liquid penetrating a hot bulk liquid. The use of a reduced value for the water-to-LNG
droplet heat transfer coefficient is conservative in that it results in a longer duration of
presence of the droplet in water and, therefore, a longer rise path for the droplet.
The model in Chapter 3 does not take into account any coalescence of the droplets
formed during their rise and evaporation. There exists no data on this phenomenon on
which to predict the rate of coalescence nor is there any theoretical assessment of the
parameters on which this phenomenon may depend. Also not included in the model is
the rise rate of a partially evaporated droplet attached to the vapor formed. It is
anticipated that this intermediate stage entity of an attached vapor bubble and droplet
combination would rise faster than a droplet alone. Again, absent experimental data
on the behavior of such an entity and its rise and vaporization characteristics, it is
prudent to consider the rise of a droplet alone and then the heating and the rise rate of
a vapor bubble formed by total evaporation of the droplet.
4
For a motion of the droplet in another liquid, it can be shown that the ratio of the distance
over which velocity reduces to 1% of the initial velocity to that of the diameter is
4
ln for turbulent motion
3
L
i
D W
S
U
U
d C
| |
=
|
\
and 1 for laminar motion
18
i L
i
W W
U d S
U
U
d
| |
=
|
\
Page 36 of 55
4 Vapor bubble rise and heating: The model proposed assumes that a single vapor
bubble is formed by the evaporation of the liquid droplet. The volume of this bubble
will be about 200 times the volume of the droplet. It is entirely possible that the
bubble formed will undergo additional break-up due to mechanical forces during its
rise through the water column. The assumption made in the model (of a single bubble
formation and no further breakup) is conservative. This is because for the heating of a
gas bubble, the smaller the bubble the faster is the heating for specified water
temperature and heat transfer coefficient. A small bubble has larger surface area to
volume ratio.
The assumption in the model is that the characteristics of the ensemble of the rising
vapor bubbles (especially their rate of heating) can be described by focusing on single
vapor bubble. This may not always be correct. There is some evidence in chemical
engineering literature which suggests that an ensemble or swarm of gas bubbles
rises at a faster rate than the terminal velocity of rise of a single bubble. On the other
hand, the heat transfer rate to the swarm of bubbles may be much higher than that for
a single bubble because of the increased turbulence created by the motion of the
swarm. Unfortunately, there are no correlations that indicate which effect
dominates and relate the various dependent quantities (rise rate, heat transfer rate, etc)
with the sizes and size distribution of bubbles, number of bubbles per unit volume in
the swarm and local water conditions. It is therefore felt that the assumptions made in
the model are justified. At the very least, the model results that vapor is heated to near
water temperature within a relatively short distance of release underwater is
consistent with the data from a single underwater LNG release test conducted by the
US Bureau of Mines (Burgess, et al., [1972]). Similar observations on the heating of
the vapor bubbles generated by liquid nitrogen release into water have been made in
laboratory scale tests (Wen, et al., [2006]).
A very low value for the overall coefficient of heat transfer between water and the
vapor inside the vapor bubble is used in the model described in Chapter 3. We have
used the correlation developed by Komaro and Sano [1998] which predicts a lower
value for the heat transfer coefficient than the measured value. The value obtained
from the correlation is of the order of magnitude 25 kW/m
2
K. This is a conservative
assumption leading to a lower heat transfer to the bubble and larger rise distance
before the vapor bubble temperature is close to that of water. Some literature data
indicate that the heat transfer coefficient value could be higher by factors of about 5
compared to the above assumed value.
In summary, therefore, we are confident that the calculation procedure indicated in
Chapter 3 is conservative, not withstanding the assumptions made in the model. The
predicted rise distance by which the temperature of the vapor released by the LNG-water
interaction attains a value close to that of the water temperature is well within the water
column, even for the case of relatively shallow location of the transfer line (20 ft depth)
and release of LNG from a large (6 inch) diameter hole.
Page 37 of 55
4.2 Conclusions
Based on the model proposed, the discussion on the effects of assumptions in the model,
and results obtained, the following conclusions are made.
1 The model indicated in this report for evaluating the interaction between a LNG jet
released underwater and the surrounding bulk water body is a reasonable
representation of the physical phenomena that occur.
2 The model results are consistent with observations in a single test involving the
release of LNG, underwater, in a lake.
3 Even though data and correlations on the interaction between underwater LNG jet
release and water are not available from controlled tests (with independent variables
varied over the range of interest), the results from experiments simulating similar
situations in other industries are relevant and are applicable to LNG-water
interaction modeling. These results have been used in the model indicated in this
report.
4 For the scenarios of LNG release from the transfer system evaluated with the
model, it is seen that the vapor emanating from the water surface will be at the
temperature of water and, hence, buoyant.
5 There is no difference between the dispersion of natural gas released from a gas
pipeline underwater and the vapor emanating from the water surface caused by a
PiP LNG transfer system leak underwater.
Page 38 of 55
Nomenclature
A = Surface area of the equivalent spherical vapor bubble (m
2
)
Bo = Bond number;
2
( ) '
W J
J
g d
Bo
=
C
P
= Specific heat of vapor at constant pressure (J/kg k)
d = Equivalent diameter of the spherical vapor bubble (m)
d = Effective diameter of the jet (equations 2-3a and 2-3b) (m)
d
b
= Diameter of the gas bubble (m)
d
i
= Vapor bubble diameter at the point of generation at depth S
i
(m)
d
J
= The physical diameter of the jet (or the orifice) (m)
d
P
= Maximum diameter of the liquid droplets in the jet breaks up (m)
g = Acceleration due to gravity = 9.8 (m/s
2
)
g = Reduced effective gravity due to buoyancy (m/s
2
)
h = Overall liquid to gas bubble heat transfer coefficient (W/m
2
K)
K
v
= Vapor (or gas) thermal conductivity (W/m K)
Lp
J
= Webber-Reynolds number;
2
2
' Re
J J J
J
J J
d
Lp
We
= =
m = Mass of vapor in the bubble = Initial mass (kg)
m
i
= Initial mass of vapor in the bubble = (kg)
Nu
v
= Nusselt number based on the vapor property;
b
v
v
h d
Nu
K
=
P = Total pressure at any depth S (N/m
2
)
*
W i
a
gS
P
P
= = Dimensionless initial hydrostatic pressure
a
P
p
P
= = Dimensionless total pressure with respect to atmospheric pressure
P
a
= Atmospheric pressure (1.01325E5) (N/m
2
)
P
i
= Total pressure at the initial depth of bubble release (N/m
2
)
Pe
L
= Peclet number based on bulk liquid properties Re Pr
L L L
Pe =
Pr
L
= Liquid Prandtl number; Pr
L
L
L
=
R = Gas constant for the vapor (for LNG it is 519.63) (J/kg K)
Re
J
= Jet Reynolds number;
'
Re
J
J
J
U d
=
Re
L
= Liquid side Reynolds number; Re
b b
L
L
U d
=
S = Depth below the water surface. S is positive as depth increases (m)
S
i
= Initial depth where the bubble is released (m)
t = Time (s)
t
ch
= Characteristic vapor bubble rise time
1.74
i i
ch
i i
S S
t
U gd
= =
(s)
Page 39 of 55
t
ch,T
= Characteristic heating time (See equation 3-17) (s)
, 2
i
Initial bubble sensible heat
Heat transfer rate to initial bubble at a temp diff T
i P i
ch T
i i
mC T
t
d hT
= =
T = Temperature of the vapor inside the bubble at any time (K)
T
i
= Initial temperature at release of the vapor in the bubble (K)
i
U
u
U
= = Dimensionless terminal velocity with respect to initial velocity
U = Terminal velocity of rise of bubble at any location (m/s)
U
b
= Rise velocity of the gas bubble (m/s)
U
i
= Initial terminal velocity of rise (m/s)
V = Volume of the droplet or vapor bubble (m
3
)
i
V
V
= = Dimensionless vapor bubble volume
We
J
= Jet Weber number;
2
'
J J
J
J
U d
We
=
Greek letters
L
= Thermal diffusivity of the liquid (m
2
/s)
= A constant of value 0.3
i
d
d
= = Dimensionless bubble diameter
i
S
S
= = Dimensionless depth
,
Characteristic rise time
Characteristic heating time
ch
ch T
t
t
= =
= Ratio of viscosity of gas to viscosity of bulk liquid
v
L
L
= Dynamic viscosity of the bulk liquid (N s/m
2
)
v
= Dynamic viscosity of the vapor inside the bubble (N s/m
2
)
L
= Kinematic viscosity of the liquid (m
2
/s)
v
= Kinematic viscosity of the vapor inside the bubble (m
2
/s)
g
= Density of gas (vapor) in the bubble at any time (kg/m
3
)
J
= Density of jet liquid (kg/m
3
)
W
= Density of water (medium into which the jet is injected) (kg/m
3
)
= Density deviation = (
W
J
) (kg/m
3
)
J
= Surface tension of the jet liquid (N/m)
i
T
T
= = Dimensionless temperature with respect to initial temperature
Page 40 of 55
W
W
i
T
T
= = Dimensionless water temperature
ch
t
t
= = Dimensionless time
Subscripts
b = Property of the bulk liquid
i = Initial condition
J = Jet properties
L = Liquid injected into the bulk liquid (LNG)
v = Property of vapor
W = Property of water
Page 41 of 55
References
ABS, Consequence Assessment Methods for Incidents Involving Releases from
Liquefied Natural Gas Carriers, ABS Consulting Inc., report to Federal Energy
Regulatory Commission under contract number FERC04C40196, Washington, DC.,
May 2004.
Archakositt, U., S. Nilsuwankosit and T. Sumitra, Effect of Volumetric Ratio and
Injection Pressure on WaterLiquid Nitrogen Interaction, J. Nuclear Science and
Technology, v. 41, n 4, p 432439, April, 2004.
Blackmore D.R.; Colenbrander G.W.; Puttock J.S., Maplin Sands Experiments 1980:
Dispersion results from continuous releases of refrigerated liquid propane and LNG,
NATO, Challenges of Modern Society - Air Pollution Modelling and its Application
3, Volume 5, Pages 353-373, Editor, Wispelaere C. de, Published by Plenum, New
York, USA, 1984.
Burgess, D.S., J.N. Murphy, and M.G. Zabetakis, Hazards of LNG Spillage into
Water, Final report to US Coast Guard, PMSRC Report No. S-4177, US Bureau of
Mines, Dept. of the Interior, Pittsburgh, PA, NTIS # AD754-498, September, 1972.
Iguchi, M., Z. Morita, H. Tokunaga and H. Tatemichi, Heat transfer between
bubbles and liquid during cold gas injection, ISIJ International, v 32, n 7, p 865-872,
1992.
Komaro, S. V. and M. Sano, Bubble Behavior and Heat Transfer in Preheated Gas
Injection into Liquid Bath, ISIJ International, v. 38, n 10, p 1045-1052, 1998.
Mishima, K., T. Hibiki, Y. Saito, J. Sugimoto, K. Moriyama, Visualization study of
molten metalwater interaction by using neutron radiography, Nuclear Engineering
and Design, v 189, p 391403, 1999.
Okhotskii, V. B., Droplet formation from a jet of one liquid into another, Journal of
Engineering Physics and Thermophysics (Translated from Inzhenerno-Fizicheskii
Zhurnal), v 54, n 2, February, 1988.
Perets, Y., Harari, and E. Sher, Vapor Explosion of Coolant Jet When Penetrating a
Hot Molten Metal, Nuclear Science and Engineering v 150, p 237244 , 2005.
Perry, R.H. and C.H. Chilton, Chemical Engineers Handbook, 5
th
Edition,
McGraw Hill Book Co., New York, 18, p70-71, 1973.
Porteus, W.M, and R.C. Reid, Light hydrocarbon vapor explosions, Chemical
Engineering Progress, p83-89, May 1976.
Reid, R.C. (editor), Rapid-Phase Transitions, Proceedings of the MIT-GRI LNG
Safety and Research Workshop, GRI Report # 82-0019.1, Gas Research Institute,
Chicago, IL, August 1982.
Sibamoto, T., Y. Kukita, H. Nakamura, Visualization and measurement of sub-
cooled water jet injection into high-temperature melt by using high-frame-rate
neutron radiography, Nuclear Technology, v. 139, p 205-220, September, 2002.
Page 42 of 55
Wen, D.S., H.S. Chen, Y.L. Ding, P. Dearman, Liquid nitrogen injection into water:
Pressure build-up and heat transfer, Cryogenics, v 46, p 740-748, 2006.
Wen, D., Y. Ding, and G. Lin, Phase change heat transfer of liquid nitrogen upon
injection into aqueous based TiO2 nanofluids, J. Nanopart Res, v 10, p 987-996,
2008.
Page 43 of 55
APPENDIX A
Assessment of the results from the
U.S. Bureau of Mines 1970 underwater LNG release experiment
In 1970 the U.S. Bureau of Mines conducted a test in which LNG held in a bottle was
released en masse underwater in a lake near Pittsburg, PA. The details of the tests are
indicated in a video of vintage 1970.
Total mass of LNG released = 17 lbs = 7.71 kg
Depth of water at which release occurred = 15 ft = 4.57 m
The commentary in the film indicates that
5 All of the LNG release seemed to have evaporated underwater.
6 No white vapor was found on the surface of water. It is surmised that the vapor
released was warm and buoyant.
7 The pressure pick ups located under water registered some overpressure. Using this
data the Bureau estimated that the total energy yield by the explosion was
equivalent to that from a 1 lb (0.4536 kg) high explosive charge (or TNT).
A.1 Some calculations
The heating value of TNT = 4.69 MJ/kg
Bureaus estimate the energy yield from the superheat explosion = 0.4536 kg of TNT
= 2.13 MJ
The superheat explosion yield is the same as the total sensible heat stored in the liquid
(due to superheating up to the superheat limit temperature) and released when the liquid
attains the saturated temperature corresponding to the pressure at the given location.
A.2 Simple heat transfer model
Consider the situation shown schematically in Figure A-1. It is assumed that the
instantaneously released LNG (of mass 7.7 kg) has initially a form of a sphere of volume
equal to the released volume.
Released volume of LNG V
LNG
= (7.71/450) = 17.13 x 10
-3
m
3
The equivalent diameter of this sphere is D = [(6/) V
LNG
]
(1/3)
= 0.3209 m.
Page 44 of 55
Figure A-1: Schematic representation of the heating of LNG by water
It is assumed that this liquid is initially at the saturation temperature corresponding to the
local pressure (assumed for the purposes of calculation to be the normal atmospheric
pressure). This spherical blob of liquid is heated by the surrounding water. When the
surface temperature of the blob reaches the super heat critical temperature, we assume
that there will be spontaneous boiling (super heat explosion) resulting in the shattering
of the spherical liquid into a spectrum of droplets. The energy released in this
explosion will be the excess sensible heat above the saturation enthalpy stored in the
liquid blob at the time of the explosion. This excess energy is calculated below.
A.2.1 Assumptions.
1 The heat transfer analysis is conducted with a 1-D model for heat penetration from
the surface of the blob.
2 The temperature distribution radially is linear
3 The convective heat transfer coefficient is equal to the film boiling heat transfer
coefficient
4 Quasi steady state is assumed.
5 There are circulations within the blob and the heat transfer into the body of the blob is
only through a conduction mechanism.
The sensible excess energy over the saturated liquid state, stored in the blob, at any time t
is given by
2
( )
( ) ( )
2
S Sat
LNG Sat
T T
E D b t C T
+ (
=
(
(1a)
Page 45 of 55
2
( )
( ) ( )
2
S Sat
LNG
T T
E D b t C
(
=
(
(1b)
The thermal boundary layer penetration distance (t) at any time t is given by
( ) 2 ( ) 2
LNG LNG
K
b t t t
C
= = = Thermal boundary layer (2)
where
T
S
= Surface temperature
T
C
= Critical temperature of liquid
T
SL
= Superheat Limit temperature = 0.9 T
C
T
Sat
= Saturation temperature of liquid
We need to determine the penetration depth of the boundary layer at the time the surface
temperature is equal to the superheat limit temperature.
It can be shown from one dimensional heat transfer analysis that
" ( )
W S
dE
q h T T
dt
= = (3)
Substituting equation 1b in 3 we get
[ ( ) ( ( ) )] ( )
2
S Sat W S
C d
b t T t T h T T
dt
= (4)
We define the following characteristic parameters and non-dimensional parameters.
L
ch
= Characteristic length scale (set equal to the blob radius) [m] (5a)
t
ch
= Characteristic time scale in the problem =
2
ch
ch
C L
t
h
= [s] (5b)
2
( )
2
ch ch W Sat
C
E D L T T
= = Blob characteristic energy excess [J] (5c)
(3/ 2) ( )
ch W Sat
E MC T T =
/
ch
t t = = Dimensionless time ; (5d)
( )
( )
ch
b t
L
= = Dimensionless boundary layer thickness at any time (5e)
B = = Modified dimensionless thermal boundary layer thickness (5f)
( )
( )
( )
S Sat
S
W Sat
T T
T T
= =
Dimensionless surface temperature (5g)
Page 46 of 55
2
ch
LNG ch
L
B
t
= = A characteristic constant (5h)
( ) = Dimensionless excess energy
S
ch
E
e
E
= = (5i)
In view of the parameters defined in equations 5a through 5h equation 4 is written in
dimensionless form as follows
(1 ) @ 0, 0 0
S
S S
d
with and
d
= = = = (6)
Also from equation 2 and equations 5 we can show that
2
2
B
| |
=
|
\
(7)
And by differentiating equation 7 with we get,
1
d
B
d
= (8)
Dividing equation 6 by equation 8 results in
( )
(1 ) @ 0, 0
S
S S
d
B with
d
= = = (9a)
Or with B = (9b)
( )
(1 ) @ 0, 0
S
S S
d
with
d
= = = (9c)
It can be shown that the above equation in becomes
1
(1 ) 1
S
S
d
d
+ + = (9d)
The solution this equation with the initial conditions indicated is
1 1
( 1) integration constant ;
S
C e e C
= + = (10)
The value of the constant C1 can be shown from the initial conditions to be 1 (by
properly considering the limits as tends to zero). Therefore, the solution is
[ ( 1)]
S
e
+
= (11)
and ( )
S
e
B
= (12)
Page 47 of 55
Equation 11 gives the relationship between the blob surface temperature and the
thickness of the thermal boundary layer. Equation 12 gives the total sensible energy
excess stored in the system when the surface temperature is T
S
.
If we set the limit of the surface temperature to the superheat limit, then it is possible to
obtain the thermal boundary layer thickness at that instant. From this, utilizing equation
2, the time at which the surface temperature reaches the superheat limit can be
determined. Also, from equation 1b, the energy excess sensible heat in the blob can be
determined. If this energy is assumed to be liberated instantaneously by a superheat
explosion then the TNT equivalence of the explosion can be evaluated. These are
indicated in the numerical example below for the conditions used in the US Bureau of
Mines underwater LNG release test.
A.2.2 LNG and other environmental properties
Density of LNG liquid
LNG
= 450 kg/m
3
Thermal conductivity of LNG liquid K = 0.186 W/m K
Specific heat of liquid LNG C = 3500 J/kg K
Thermal diffusivity of liquid LNG = 1.181 x 10
-7
m
2
/s
LNG critical temperature (using that of Methane) T
C
= 190.6 K
Superheat limit (per Reid theory) 0.9 T
C
= 176.4 K
Assumed film heat transfer coefficient h = 60 W/m
2
K
A.2.3 Numerical Example:
Total mass of LNG released M = 7.71 kg
Volume of LNG released V = 1.713 x 10
-2
m
3
Radius of a spherical blob of liquid R = L
ch
= 0.16 m
Characteristic parameters
Time t
ch
= 32.8 s
Parameter B = 1.61
Sensible heat excess energy E
ch
= 7.326 x 10
6
J
Characteristic total excess energy
TNT
ch
M = (7.326/4.69) = 1.562 kg TNT
Dimensionless surface superheat limit temperature
S
* = 0.3558
Using a numerical solution to equation 11, we get:
Dimensionless thermal boundary layer thickness at * = 0.955
(at superheat explosion limit temperature for the surface)
Dimensional thermal boundary layer thickness b* = 1.48 mm
Superheat limit explosion dimensionless time * =
2
/(2B) = 0.283
Page 48 of 55
Superheat limit explosion time t* = 9.3 s
Total excess energy released E*
max
= 1.503 x 10
6
J
Super heat energy released in TNT mass units M
TNT
= 0.33 kg
TNT mass equivalence if all mass of the LNG blob
participated in the superheat explosion = 7.71x3500x(176.4-112)/4.69E6 = 0.371 kg
A.3 Discussions
The observation in the experiments that no LNG surfaced to the water level but only
vapors were emitted is indicative that all LNG was evaporated underwater. It was also
calculated from the acoustic signals that the energy released from the superheat
explosion or RPT was about 1 lb of TMNT equivalent (or 0.45 kg).
If one assumes that the superheat explosion is entirely due to the heating of the surface
area of the unbroken blob of the liquid released, then it is hard to explain the 0.45 kg
TNT equivalent yield. The calculation, based on 1-D heat transfer, indicates that the
energy of explosion from the release of all superheat energy (when the surface
temperature reaches the superheat critical temperature for LNG) is of the order of 0.01 kg
as opposed to 0.45 kg that was measured. If, on the other hand, one assumes that all of
the liquid participated in the superheat explosion, then it is seen that the energy released
is about 0.37 kg, very close to the back calculated value of about 1 lb (0.45 kg).
The above finding implies that any initial explosion would result in the fragmentation of
the blob into a spectrum of LNG droplets which then further superheat explode and
rapidly evaporate. The vapor thus produced is heated by the water before it rises to the
surface, thereby reaching a temperature that is above the dew point of water. This is the
reason that no white cloud was noticed in the tests.
Page 49 of 55
A.4 List of symbols
b(t) = Time dependent thickness of the thermal boundary layer (m)
2
ch
LNG ch
L
B
t
= = A characteristic constant
C = Specific heat of the liquid (J/kg K)
D = Diameter of the equivalent spherical liquid blob (m)
E(t) = Sensible heat energy excess above the saturation condition (J)
2
( )
2
ch ch W Sat
C
E D L T T
= = Characteristic energy excess in the blob (J)
h = Heat transfer coefficient between water and liquid surface (W/m
2
K)
K = Thermal conductivity of the liquid (W/m K)
L
ch
= Characteristic length scale (set equal to the blob radius) (m)
q = Heat flux between water and LNG droplet (W/m
2
)
t = time (s)
t
ch
= Characteristic time scale in the problem =
2
ch
ch
C L
t
h
= (s)
T
C
= Critical temperature of liquid (K)
T
SL
= Superheat Limit temperature = 0.9 T
C
(K)
T
S
= Temperature of the liquid drop surface at any time (K)
T
Sat
= Saturated temperature of LNG at local absolute pressure (K)
T
W
= Water temperature (K)
Greek letters
LNG
= Thermal diffusivity of LNG (m
2
/s)
( )
( )
ch
b t
L
= = Dimensionless boundary layer thickness at any time
( ) = Dimensionless excess energy
S
ch
E
e
E
= =
( )
( )
( )
S Sat
S
W Sat
T T
T T
= =
Dimensionless surface temperature
= Density of LNG liquid (kg/m
3
)
B = = Modified dimensionless thermal boundary layer thickness
/
ch
t t = = Dimensionless time
Você também pode gostar
- HP Relief Systems in LNG Receiving TerminalsDocumento16 páginasHP Relief Systems in LNG Receiving TerminalsHangga Ruky WarmiajiAinda não há avaliações
- Water Deluge Protection of LPGDocumento20 páginasWater Deluge Protection of LPGVivi OktaviantiAinda não há avaliações
- Natural Gas Liquefaction Technology For Floating LNG FacilitiesDocumento12 páginasNatural Gas Liquefaction Technology For Floating LNG FacilitieshortalemosAinda não há avaliações
- Ventilation PDFDocumento7 páginasVentilation PDFErick Becker Lino SantosAinda não há avaliações
- BoiloffDocumento8 páginasBoiloffJetul PatelAinda não há avaliações
- The Role of Consequence Modeling in LNG Facility SitingDocumento10 páginasThe Role of Consequence Modeling in LNG Facility Siting고병석Ainda não há avaliações
- Is9 2008Documento5 páginasIs9 2008eng.tomasoniAinda não há avaliações
- Appendix 1.athermal-Hydraulics Analysis of Spent Fuel Pool HeatupDocumento275 páginasAppendix 1.athermal-Hydraulics Analysis of Spent Fuel Pool HeatupEnformableAinda não há avaliações
- ES-20.14 Waste Heat PTT Public Co., LTD Recovery Units Engineering Standard REV: 02Documento10 páginasES-20.14 Waste Heat PTT Public Co., LTD Recovery Units Engineering Standard REV: 02Nikki RobertsAinda não há avaliações
- API - STD - 521 Fire Gas ExpansionDocumento3 páginasAPI - STD - 521 Fire Gas Expansioneliealtawil100% (1)
- Paper Gastech 09Documento15 páginasPaper Gastech 09JimmyJarufeAinda não há avaliações
- Unu GTP 2015 28Documento34 páginasUnu GTP 2015 28Brian ChristiantoroAinda não há avaliações
- Lightning Rim Seal FireDocumento9 páginasLightning Rim Seal FireTanishq samaiyaAinda não há avaliações
- RCC Dams Thermal Induced Cracking Performance of RCC DamsDocumento16 páginasRCC Dams Thermal Induced Cracking Performance of RCC DamsCarlos L. Oyuela100% (1)
- ML111310236Documento8 páginasML111310236Pammy JainAinda não há avaliações
- Ageing Assessment of RBMK-1500 Fuel Channel in Case of Delayed Hydride CrackingDocumento7 páginasAgeing Assessment of RBMK-1500 Fuel Channel in Case of Delayed Hydride CrackingRoman KrautschneiderAinda não há avaliações
- Local Application Water Fire Protection Systems: Montreal A in TheDocumento11 páginasLocal Application Water Fire Protection Systems: Montreal A in TheأبومحمدالزياتAinda não há avaliações
- Aboveground Fuel Oil Storage TankDocumento77 páginasAboveground Fuel Oil Storage Tankaswin8bojongmania100% (1)
- Use of Glass Reinforced Epoxy (GRE) Piping For Fire Water Applications - OffshoreDocumento10 páginasUse of Glass Reinforced Epoxy (GRE) Piping For Fire Water Applications - OffshoreMubeenAinda não há avaliações
- Design of A Chimney With GRP Liner For Low and High Temperature OperationDocumento5 páginasDesign of A Chimney With GRP Liner For Low and High Temperature OperationhozipekAinda não há avaliações
- Cryogenic GuidelinesDocumento31 páginasCryogenic Guidelineszvola100Ainda não há avaliações
- 12 PDFDocumento12 páginas12 PDFAndi SungAinda não há avaliações
- Panel O-28 Ships Mat - Thermal Insulation R.dec.1963.T-RDocumento151 páginasPanel O-28 Ships Mat - Thermal Insulation R.dec.1963.T-Rmaria_bustelo_2Ainda não há avaliações
- Conceptual Design of Salt Cavern Based LNG TerminalDocumento25 páginasConceptual Design of Salt Cavern Based LNG TerminalNurcahyo Djati W100% (3)
- Design EquationsDocumento7 páginasDesign EquationsThiagarajanAinda não há avaliações
- Rack PDFDocumento8 páginasRack PDFWan Norain Awang LongAinda não há avaliações
- AIChE Design LNG Facilities To Minimize Risks From Cryogenic Exposure, 2009Documento17 páginasAIChE Design LNG Facilities To Minimize Risks From Cryogenic Exposure, 2009jeremyg998100% (1)
- How To Boost HRSG Performance and Increase Your Plant's Bottom LineDocumento28 páginasHow To Boost HRSG Performance and Increase Your Plant's Bottom Lineabdulyunus_amir100% (1)
- Vess FireDocumento21 páginasVess FirecsAinda não há avaliações
- Simulation of Rapid Depressurization and PDFDocumento13 páginasSimulation of Rapid Depressurization and PDFhoangvubui4632100% (1)
- Benson Boiler AccidentbDocumento6 páginasBenson Boiler AccidentbarunyogAinda não há avaliações
- Refractory Dry Out RDO ProcedureDocumento10 páginasRefractory Dry Out RDO ProcedureDangol63% (8)
- Hot BoxDocumento5 páginasHot Boxsv27Ainda não há avaliações
- Analysis of Temperature and Pressure Changes in Liquefied NaturalDocumento9 páginasAnalysis of Temperature and Pressure Changes in Liquefied Naturaldaimon_pAinda não há avaliações
- Lessons Learned From An Incident at A Cryogenic Gas Processing FacilityDocumento6 páginasLessons Learned From An Incident at A Cryogenic Gas Processing FacilityTareqAinda não há avaliações
- Results of The Gas Carrier Reliquefaction Plant Trial: TransportDocumento5 páginasResults of The Gas Carrier Reliquefaction Plant Trial: TransportishaqAinda não há avaliações
- Coroziune CorozivDocumento45 páginasCoroziune CorozivDianaIcleanuAinda não há avaliações
- Thermal Analysis of Exposed Pipeline Under Natural ConvectionDocumento6 páginasThermal Analysis of Exposed Pipeline Under Natural ConvectionSyahrizalYusoff100% (1)
- Encyclopaedia Britannica, 11th Edition, Volume 8, Slice 3 "Destructors" to "Diameter"No EverandEncyclopaedia Britannica, 11th Edition, Volume 8, Slice 3 "Destructors" to "Diameter"Ainda não há avaliações
- Interview Question.Documento7 páginasInterview Question.PankajDhobleAinda não há avaliações
- Cracks in Propeller HubDocumento2 páginasCracks in Propeller Hublitos_92Ainda não há avaliações
- Calculation of Boil-Off Rate of Liquefied Natural Gas in Mark III Tanks of Ship Carriers by Numerical AnalysisDocumento42 páginasCalculation of Boil-Off Rate of Liquefied Natural Gas in Mark III Tanks of Ship Carriers by Numerical AnalysisBenjamin OncinaAinda não há avaliações
- Analsis of Wire Drawing MachineDocumento15 páginasAnalsis of Wire Drawing MachineCallistus Agu100% (1)
- Film Cooling in Rocket Thrust ChamberDocumento13 páginasFilm Cooling in Rocket Thrust ChamberNitish KrishnanAinda não há avaliações
- Comparisons Between LNG TerminalsDocumento26 páginasComparisons Between LNG TerminalsNurcahyo Djati W100% (5)
- Appendix 13L-Storage TanksDocumento103 páginasAppendix 13L-Storage TanksNitzOOAinda não há avaliações
- Fire Engulfment of LPG Storage TanksDocumento20 páginasFire Engulfment of LPG Storage TanksKhan Lala100% (1)
- OTC 16086 Conceptual Comparison of On-Platform Versus Onshore Gas DehydrationDocumento6 páginasOTC 16086 Conceptual Comparison of On-Platform Versus Onshore Gas DehydrationDaveAinda não há avaliações
- High Integrity Protective SystemsDocumento9 páginasHigh Integrity Protective SystemsAbhiyan Anala ArvindAinda não há avaliações
- Flare Sweep GasDocumento5 páginasFlare Sweep GasChem.EnggAinda não há avaliações
- Calculation and Analysis of Critical Heat Flux atDocumento16 páginasCalculation and Analysis of Critical Heat Flux atAmairani Fuentes AndradeAinda não há avaliações
- Air-Cooling Influence On Wire Arc Additive Manufactured SurfacesDocumento7 páginasAir-Cooling Influence On Wire Arc Additive Manufactured SurfacesFlavio Emanuel de Lima SilvaAinda não há avaliações
- SPE 63079 Well Testing With A Permanent Monitoring SystemDocumento9 páginasSPE 63079 Well Testing With A Permanent Monitoring SystemyeralhAinda não há avaliações
- BForbes ANCOLD 1998Documento13 páginasBForbes ANCOLD 1998LTE002Ainda não há avaliações
- Ship Oil Pollution Emergency Plan (SOP EP) : Length OverallDocumento5 páginasShip Oil Pollution Emergency Plan (SOP EP) : Length OverallVijay RaghavanAinda não há avaliações
- Upgrade Your Furnace For Clean FuelsDocumento4 páginasUpgrade Your Furnace For Clean Fuelssagar1503Ainda não há avaliações
- Oregon LNG Tank SpecDocumento16 páginasOregon LNG Tank Specdr61gt100% (3)
- Pipeline Rules of Thumb Handbook: A Manual of Quick, Accurate Solutions to Everyday Pipeline Engineering ProblemsNo EverandPipeline Rules of Thumb Handbook: A Manual of Quick, Accurate Solutions to Everyday Pipeline Engineering ProblemsAinda não há avaliações
- Process Heat Transfer: Principles, Applications and Rules of ThumbNo EverandProcess Heat Transfer: Principles, Applications and Rules of ThumbNota: 4.5 de 5 estrelas4.5/5 (11)
- D.F.I. Helical Presentation 7Documento68 páginasD.F.I. Helical Presentation 7hiyeonAinda não há avaliações
- Compressors IntroductionDocumento12 páginasCompressors Introductionhiyeon100% (1)
- Perko-Helical Piles FlierDocumento2 páginasPerko-Helical Piles FlierhiyeonAinda não há avaliações
- Lateral Capacity of Helix PiersDocumento8 páginasLateral Capacity of Helix PiershiyeonAinda não há avaliações
- Chemical Engineering - Plant Engineering 2 Ideal Gas - RevisionDocumento4 páginasChemical Engineering - Plant Engineering 2 Ideal Gas - RevisionhiyeonAinda não há avaliações
- Storage Tanks and SilosDocumento9 páginasStorage Tanks and SiloshiyeonAinda não há avaliações
- Corrosion of Helix PiersDocumento7 páginasCorrosion of Helix PiershiyeonAinda não há avaliações
- Chemical Engineering - Plant Engineering 2 Power RequirementDocumento2 páginasChemical Engineering - Plant Engineering 2 Power RequirementhiyeonAinda não há avaliações
- The Design of Natural Gas PipelinesDocumento7 páginasThe Design of Natural Gas PipelineshiyeonAinda não há avaliações
- 8042306Documento99 páginas8042306Darshan KashiAinda não há avaliações
- IS 4995 (Part 2) 1974Documento25 páginasIS 4995 (Part 2) 1974Nagaraju ChintaAinda não há avaliações
- PT Concrete Storage StructuresDocumento51 páginasPT Concrete Storage StructurescavnqnAinda não há avaliações
- NG. Compressor StationDocumento12 páginasNG. Compressor StationBrian MayAinda não há avaliações
- EC201 (Rev) Tcm92 22934kompressDocumento12 páginasEC201 (Rev) Tcm92 22934kompressJoe GoparAinda não há avaliações
- 11gas LiftDocumento18 páginas11gas LifthiyeonAinda não há avaliações
- 10 Transient Two-Phase Flow: Objectives: Quantitative Insights Into Transient Mechanisms and PhenomenaDocumento6 páginas10 Transient Two-Phase Flow: Objectives: Quantitative Insights Into Transient Mechanisms and PhenomenahiyeonAinda não há avaliações
- Calculations in Natural Gas CourseDocumento6 páginasCalculations in Natural Gas CourseHilmi ScpgzAinda não há avaliações
- Gas Composition Examples TransportDocumento1 páginaGas Composition Examples TransporthiyeonAinda não há avaliações
- Gas Composition ExamplesDocumento1 páginaGas Composition ExampleshiyeonAinda não há avaliações
- Cd5796 Emma OlgaDocumento26 páginasCd5796 Emma OlgahiyeonAinda não há avaliações
- Appendix T Route Selection MatrixDocumento9 páginasAppendix T Route Selection MatrixhiyeonAinda não há avaliações
- 9 MultiphaseDocumento26 páginas9 MultiphaseAshrafNamamuTeratasAinda não há avaliações
- 8.1 Physical Properties of The Gas: 8.1.1 Pressure and VolumeDocumento16 páginas8.1 Physical Properties of The Gas: 8.1.1 Pressure and VolumehiyeonAinda não há avaliações
- Addendum Part 3Documento16 páginasAddendum Part 3hiyeonAinda não há avaliações
- Beregninger Gass Z-FaktorDocumento8 páginasBeregninger Gass Z-FaktorhiyeonAinda não há avaliações
- Vol2 Appendix QDocumento314 páginasVol2 Appendix QhiyeonAinda não há avaliações
- 03 COR 25 1MDR0470 Route Selection C Butler FINALDocumento17 páginas03 COR 25 1MDR0470 Route Selection C Butler FINALhiyeonAinda não há avaliações
- Landfall Valve Installation: Sru Wad Dac Onb AyDocumento1 páginaLandfall Valve Installation: Sru Wad Dac Onb AyhiyeonAinda não há avaliações
- Conversions UnitsDocumento1 páginaConversions UnitshiyeonAinda não há avaliações
- Calculations in Natural Gas CourseDocumento6 páginasCalculations in Natural Gas CourseHilmi ScpgzAinda não há avaliações
- Attachment Catalogue Hilti HUD-1Documento5 páginasAttachment Catalogue Hilti HUD-1penghzAinda não há avaliações
- Numerical Analysis of Moisture-Induced Strains and Stresses in Glued-Laminated TimberDocumento13 páginasNumerical Analysis of Moisture-Induced Strains and Stresses in Glued-Laminated TimberXXAinda não há avaliações
- Mixture Suspension QuizDocumento9 páginasMixture Suspension QuizCatherine RenanteAinda não há avaliações
- ASTM Standards For Erosion and Sediment ControlDocumento3 páginasASTM Standards For Erosion and Sediment ControlChuquiure L. Angel100% (1)
- 1 - Management Plan (Draft)Documento18 páginas1 - Management Plan (Draft)Sahil SharmaAinda não há avaliações
- Technical Info Librel RMX 26Documento4 páginasTechnical Info Librel RMX 26Rijalul AuthonAinda não há avaliações
- Staying Ahead of The CurveDocumento8 páginasStaying Ahead of The CurvehimanshuAinda não há avaliações
- Extrusion Die DesignDocumento67 páginasExtrusion Die DesignShubham Chaudhary100% (1)
- PQR Amp WPQ Standard Testing Parameter WorksheetDocumento4 páginasPQR Amp WPQ Standard Testing Parameter WorksheetvinodAinda não há avaliações
- STP of Etratab BolusDocumento5 páginasSTP of Etratab BolusBejoy KarimAinda não há avaliações
- 21 - Corrosion Monitoring in Oil & Gas IndustryDocumento5 páginas21 - Corrosion Monitoring in Oil & Gas IndustryMohamed F. OmarAinda não há avaliações
- SL Somos WaterClear Ultra Material Specifications PDFDocumento2 páginasSL Somos WaterClear Ultra Material Specifications PDFTushar Prakash ChaudhariAinda não há avaliações
- Product & Service Datasheet: Medium Voltage (6kV To 36kV) Subsea Power Cable Repair JointDocumento2 páginasProduct & Service Datasheet: Medium Voltage (6kV To 36kV) Subsea Power Cable Repair Jointnader mahfoudhiAinda não há avaliações
- Combined Science Paper 6 Summer 02Documento16 páginasCombined Science Paper 6 Summer 02igcsepapersAinda não há avaliações
- Device Technology For Nanoscale III-V Compound Semiconductor Field Effect TransistorsDocumento161 páginasDevice Technology For Nanoscale III-V Compound Semiconductor Field Effect TransistorsRaghu Vamsi ChavaliAinda não há avaliações
- Sahraoui2004 PDFDocumento13 páginasSahraoui2004 PDFAzril DahariAinda não há avaliações
- SYNOCURE886S70Documento2 páginasSYNOCURE886S70Samuel AgusAinda não há avaliações
- 2016 - Thermal Performance Calculation and Analysis of Heat Transfer Tube in Super Open Rack VaporizerDocumento10 páginas2016 - Thermal Performance Calculation and Analysis of Heat Transfer Tube in Super Open Rack VaporizerLong Nguyễn HoàngAinda não há avaliações
- Model: 1 22 (1/4" NPT) Replacement Parts ListDocumento1 páginaModel: 1 22 (1/4" NPT) Replacement Parts ListAldrin HernandezAinda não há avaliações
- Bom of Studs & Nuts For Balance SystemDocumento4 páginasBom of Studs & Nuts For Balance SystemmishtinilAinda não há avaliações
- Zoznam NoriemDocumento4 páginasZoznam NoriemPeter TvardzíkAinda não há avaliações
- Photodiode Investigatory ProjectDocumento25 páginasPhotodiode Investigatory ProjectNishant KumarAinda não há avaliações
- Activity 1 - Chemical Safety (GHS-SDS)Documento11 páginasActivity 1 - Chemical Safety (GHS-SDS)John Joshua Latras ManticahonAinda não há avaliações
- Quarter3 Exam Ict 9 ANSWER KEYDocumento4 páginasQuarter3 Exam Ict 9 ANSWER KEYJenelyn RusianaAinda não há avaliações
- Press Release PHBYC - Flood Prone LGU Builds Flood BoatsDocumento4 páginasPress Release PHBYC - Flood Prone LGU Builds Flood BoatsRoy EspirituAinda não há avaliações
- Excel First Review and Training Center, Inc.: Cebu: Davao: Manila: BaguioDocumento3 páginasExcel First Review and Training Center, Inc.: Cebu: Davao: Manila: BaguioJohn Anthony YumulAinda não há avaliações
- BS en 1593-1999 NDT - Leak Testing - Bubble EmissionDocumento14 páginasBS en 1593-1999 NDT - Leak Testing - Bubble Emissionrinshad100% (1)
- 2008 04 PDFDocumento98 páginas2008 04 PDFValeria100% (1)
- 02 Pfe ReportDocumento129 páginas02 Pfe ReportMaAinda não há avaliações
- GMW14668 - Minimum Performance Requirements For Decorative Chromium Plated PlasticsDocumento20 páginasGMW14668 - Minimum Performance Requirements For Decorative Chromium Plated Plastics廖健翔Ainda não há avaliações