Escolar Documentos
Profissional Documentos
Cultura Documentos
Module 1
Enviado por
jegan172Descrição original:
Direitos autorais
Formatos disponíveis
Compartilhar este documento
Compartilhar ou incorporar documento
Você considera este documento útil?
Este conteúdo é inapropriado?
Denunciar este documentoDireitos autorais:
Formatos disponíveis
Module 1
Enviado por
jegan172Direitos autorais:
Formatos disponíveis
Module1-SICI.
doc 4/09/04
Copyright K. Nasr, 2004 1
Module 1 The Spark Ignition Engine
Problem 1 - Problem Statement
[1]
: We have a 4-cylinder, 2.5 L SI Engine operating on
a 4-stroke cycle at 3000 rpm with a compression ratio, r, of 8.6. It has a stroke to bore
ratio (S/B) of 1.025, running off a fuel with the following properties:
Air to Fuel Ratio = 15:1 (in a 16 kg air-fuel mixture, 15 kg will be air and 1 kg will be fuel).
Heating value = 15000 kJ/kg.
Efficiency of Combustion,
c
=100%
Additionally we are given P
initial
= 100kPa and T
initial
= 60 C.
Objectives: Thermodynamically, model the engine performance via an air-standard Otto
cycle and determine the horsepower produced and the thermal efficiency.
Solution:
Modeling: The solution path gets clearer if it begins with a schematic of the problem
and an actual pressure vs. volume (indicator) diagram illustrating the engine
operation
[2]
. We model the engine performance via an air-standard Otto cycle. The
schematic and p- diagram for an Otto cycle exhibiting the processes involved is
presented. The Otto cycle is the ideal cycle for modeling spark-ignition engines. A
terminology-supported schematic of a piston/cylinder assembly is shown as well
[3]
.
Module1-SICI.doc 4/09/04
Copyright K. Nasr, 2004 2
To accomplish the objectives (power produced and efficiency), a series of questions arise:
Q1. What is the horsepower produced?
One must recall the definition of power. Power is the rate of doing work.
For the Otto cycle, modeling the spark ignition engine, there are two processes that
involve work (i.e. power).
Definition Support:
Work is a form of energy. Work is normally defined as the applied force, F, on an object
times the displacement, s, in the direction of the applied force: W = F
.
s [N
.
m] or [J]
If F is not constant, W = (differential amounts) = F ds [N
.
m] or [J].
Definition Support:
Process: A transformation of a system from one equilibrium state to another.
A constant temperature process is called an isothermal process.
A constant pressure process is called an isobaric or an isopiestic process.
A constant volume process is called an isovolumic or an isochoric process.
Module1-SICI.doc 4/09/04
Copyright K. Nasr, 2004 3
In reference to the Otto cycle, the first process (1 ? 2) involves compression and
therefore consumes work and the other (3 ? 4) involves expansion and it produces
work. The net work is the work of expansion minus the work of compression. That
is to say: W
net
for one cylinder, during one cycle is |
3
W
4
|- |
1
W
2
|.
Power produced, therefore, is the net work output per unit time.
( ) cylinders
cycle rev
RPM W
W
Net
#
/ #
*
,
_
&
We need therefore to find the work of compression and the work of expansion. In
this case, the system is a closed one once modeled via the Otto cycle. The mass is
trapped inside the cylinder and is either doing work (expansion) or work is being
Definition Support:
System, State, and Equilibrium:
Any thermodynamic analysis begins with an identification of a system, a system
boundary, and surroundings.
System A chosen region of space to be the subject of analysis and is bounded by an
arbitrary surface. A system is classified as closed or open.
Surroundings Region outside the system boundary.
Closed system (or control mass): A system for which no mass crosses the boundary. Note
that energy may cross but mass does not.
Open system (or control volume): Any system that is not a closed system; mass and
energy may cross the imaginary boundary.
What describes a system? A system is described in terms of its physical properties.
Examples of properties include pressure, temperature, mass, volume, density, etc.
Knowledge of a systems properties defines its state. A system changes state by
interacting with its surroundings.
Of interest is a state of equilibrium or a state of balance.
Equilibrium is intended to encompass mechanical, thermal, phase, and chemical.
Definition Support:
Dimensions and Units:
Newtons 2
nd
law: Force is proportional to the mass times the acceleration: F m . a
Dimension SI
(Le Systeme
International d
Unites):
EESU
(English Engineering System of
Units); other texts use USCS
(United States Customary System).
Mass kg lbm
Length m ft
Time s s
Force N
If one were to express F = m a/g
c
then g
c
= 1 kgm/N.s
2
lbf
If one were to express F = m a/g
c
then g
c
= 32.174 lbm.ft/lbf.s
2
Module1-SICI.doc 4/09/04
Copyright K. Nasr, 2004 4
done on it (compression). It is worth defining some common properties we will be
dealing with, such as pressure, and temperature.
Q2. How much compression work did it take to go from
1
to
2
?
When a piston pushes on the air-fuel mixture, the piston does work on that system.
This type of work is called Boundary Work, as the boundary of the system has to
move. The system in this case is the gas inside the cylinder. The systems boundary,
in contact with the piston, must move as we put in work to compress the gas and
cause the piston to move.
2
1
Pd P W
Definition Support and The Zeroth Law of Thermodynamics:
Pressure(p): Normal component of a force per unit area F
n
/ A
SI Units: p F
n
/ A [N/m
2
], [Pa] (Pascal)
1 bar = 10
5
Pa, 1 atm = 1.01 bar = 1.01 10
5
Pa 100 kPa.
English Units: p = F
n
/ A [lbf/ft
2
], but typically dealt with and measured
in psi (lbf/in
2
). 1 atm= 14.696 psi
p
abs
= Absolute pressure = actual pressure i.e. relative to zero pressure; p
abs
= p
atm
+ p
gage
A gage reading may be > 0 (gage) or < 0 (vacuum).
Temperature (T): A sense of hotness and coldness. It tells us if two objects in contact
with each other are in thermal equilibrium. Our senses may be misleading at times. T is
measured via a thermometer.
The Celsius scale = The Kelvin scale - 273.15
C= K - 273.15
The Rankine scale = 1.8 ( The Kelvin scale)
F = R - 459.67
Our sense of hotness and coldness may be deceiving, and thus we define temperature via The
Zeroth Law of Thermodynamics which states: Two bodies are in thermal equilibrium if
they both have the same temperature even if they are not in contact. Or if a body A is in
thermal equilibrium with body B, and B is in thermal equilibrium with body C, then A is in
thermal equilibrium with body C.
Pressure versus Volume Diagram
Volume
P
r
e
s
s
u
r
e
Work
Module1-SICI.doc 4/09/04
Copyright K. Nasr, 2004 5
We also know from the indicator diagram that as decreases (as we compress the
air/fuel mixture or what we are calling system), the pressure increases. The volume
and pressure are both changing and a relationship between P and is needed to carry
out the integral.
A good model relationship between the pressure and volume is of the form [P
n
= C]
or P
1
1
n
= P
2
2
n
where n is an exponent (n 1.4 for air). Lets substitute into the
integral for boundary work and integrate:
( )
n n
n n
n
n
n n
P P C
n
C
C
n
V
d C
d
C d
C
W
1 1 2 2
1
1
1
2
1
2 1
1
*
1
2
1
2
1
2
1
2
1
+
We substitute
n
P P
W
1
1 1 2 2
2 1
which will result in a negative quantity,
justifying the thought that work is required to carry out the compression process.
Therefore, the work of compression is negative. Magnitude-wise, the work is the area
under the curve on a pressure vs.volume (P-) diagram.
Furthermore, since air behaves as an ideal gas (as a rule of thumb, air behaves as a
perfect or ideal gas for pressures lower than 20 atm), [p = m R T], the above
expression for work can be written in terms of temperatures, T
1
and T
2
:
Concept Support:
Moving Boundary Work (Known as p d work):
Consider a substance in a piston/cylinder (P/C) device, the piston is allowed to move a
distance (ds) in a quasi-equilibrium manner, the differential work during this process is
quantified as force times displacement. Since force is pressure times area and area times
displacement is volume, the work ends up being pressure times volume. Symbolically,
W
b
= F . ds = p . A . ds = p . d
If the change in volume (d) > 0 (expansion process) W
b
> 0
If the change in volume (d) < 0 (compression process) W
b
< 0.
The displacement is carried out incrementally so that work is the summation of pressure
times a change in volume at every step of the compression process. In engineering, this
summation is an integral that we call boundary work.
The total boundary work done during the entire process as the piston moves:
W
b
= p d p = f () is needed for integration.
Note that the area under the curve on a p- diagram = W
b
Note also, a different process (path) may have been taken to go from the initial state to the
final state and thus is used to indicate that work is a path function. That is to say
that Work depends on the path.
Module1-SICI.doc 4/09/04
Copyright K. Nasr, 2004 6
n
T T mR
n
P P
W
1
) (
1
1 2 1 1 2 2
2 1
(I)
In order to compute a value for the compression work, we need properties at the
beginning and end of the process: volumes and pressures or in this case temperatures.
This leads us therefore into the following question:
Q3. What happens to the pressure, temperature, and volume as compression takes place?
Since weve decided that air behaves as an ideal gas, we can say that:
2 2 2
1 1 1
2 1 2 2
1 2 1 1
P mRT
and
P mRT
or
P T T
r
P T T
r, being the compression ratio and is given for this engine. We are given the compression
ratio (8.6 =
BDC
/
TDC
) and the pressure (100 kPa) and temperature (333 K) at the
beginning of the compression process. Since P is related to for this compression process via
P
n
= C and the gas is ideal, useful relationships between temperatures, pressures, and volumes
can be formulated:
Concept Support:
Equation of State for Gases (Ideal Gas Equation):
Experimental observation of gases at low density p = m R T
Can be written also in various other forms: p v = R T or p = R T
p = pressure, = volume, m = mass, R = specific gas constant, and T = absolute temperature.
Density (): mass per unit volume: = m/ [kg/m
3
] or [lbm/ft
3
].
Specific volume (v): volume per unit mass: v = /m = 1/ [m
3
/kg] or [ft
3
/ lbm].
The specific gas constant, R is related to the universal gas constant, R
u
through the molecular
weight, M of the gas: R = R
u
/M, where R
u
= 8.3145 kJ/kmolK = 1545 ftlbf/lbmolR.
Molecular weights of various substances are listed in Table A-1 of your textbook.
Any gas that complies with [p = m R T] is called an ideal gas and this equation is referred
to as the ideal-gas equation of state good only at low density.
Equations Support:
For a ideal gas, P = m R T, undergoing a process guided by p
n
= C, the following
relationships can be obtained:
) 1 (
1
2
1
2
/ ) 1 (
1
2
1
2
2
1
1
2
; ;
n n n n
T
T
P
P
T
T
P
P
,
_
,
_
,
_
Remember that T must be absolute as it was used from the ideal gas law.
Module1-SICI.doc 4/09/04
Copyright K. Nasr, 2004 7
( ) ( )
C K K r T T
kPa kPa r P P
P
P
n
n
n
5 . 514 49 . 787 ) 6 . 8 )( 333 ( *
75 . 2033 6 . 8 ) 100 (
4 . 1
1 2
4 . 1
1 2
2
1
1
2
,
_
Now that we have determined P
2
and T
2
, we proceed in finding the volumes.
We know that for this engine we have four cylinders. From this we can determine the
displacement volume for a single cylinder:
d
=
t
/n where
t
is the total volume (2.5 L) and n is the number of cylinders (4).
d
=
BDC
-
TDC
= 2.5 L/4 = 0.000625 m
3
, where
BDC
=
1
and
TDC
=
2
.
1
=
d
+
c
where
c
is the clearance volume and is equivalent to
2
. So, we need to
find
c
. Recall that the compression ratio is 6 . 8
c
c d
TDC
BDC
r , from which
we can get
c
:
8.6 = (0.000625 m
3
+
c
) /
c
c
= 0.0000822 m
3
=
2
1
=
d
+
c
= 0.000625 + 0.0000822 = 0.0007072 m
3
.
Therefore, for the compression process, the following properties can be tabulated:
Temperature Pressure Volume
State 1: 333 K = 60 C 100 kPa 0.0007072 m
3
State 2: 787.49K =514.5C 2033.75kPa 0.0000822 m
3
Since pressures, volumes, and temperatures are known at the beginning and end of the
compression process, we can plug and chug to get the work of compression via equation (I):
kJ
m kPa m kPa
n
P P
W 2413 . 0
4 . 1 1
0007072 . 0 * 100 0000822 . 0 * 75 . 2033
1
3 3
1 1 2 2
Reconfirm by using:
kJ
K K
K kg
m KN
kg
n
T T mR
W 2413 . 0
4 . 1 1
) 333 49 . 787 (
*
*
287 . 0 * 00074 . 0
1
) (
1 2
It checks .
The answer is negative indicating compression. Note also that R was obtained via:
K kg
kJ
kmol
kg
K kmol
kJ
R
M
R
R
u
*
287 . 0
29
*
3142 . 8
: Air For ,
Are there any other forms of work that the system (gas) experiences during compression?
How about potential energy change? Indeed there is a potential energy change as the
system (gas) has changed elevation from bottom dead center (
1
) to top dead center (
2
).
The potential energy change can be quantified via: [m g z], where m is the mass of the
system, g is acceleration due to gravity, and z is change in elevation.
Module1-SICI.doc 4/09/04
Copyright K. Nasr, 2004 8
To determine the mass of gas in the cylinder, we may assume the fuel-air mixture to
act as an ideal gas. Using given initial conditions:
kg
K
K kg
m kN
m kPa
RT
P
m mRT P 000740 . 0
333 *
*
*
287 . 0
0007072 . 0 * 100
3
This is a constant mass always present in the cylinder (since we are modeling this
system as a closed one) and for the air-standard Otto cycle we will assume that all this
mass is air, although we can get m
air
and m
fuel
from the air/fuel ratio.
kg kg m m
kg kg m m
fuel
air
00004625 . 0 ) 000740 . 0 (
16
1
) (
16
1
00069375 . 0 ) 000740 . 0 (
16
15
) (
16
15
m = m
air
+ m
fuel
= 0.00069375 + 0.00004625 = 0.00074 kg m
air
The change in elevation for this system is the stroke (S) which can be found from
using the cylinder volume,
d
and the stroke to bore ratio (S/B).
) 025 . 1 (
4
*
4
2 2
B B S B
d
yielding B = 0.0919 m = 9.19 cm and S = 9.42 cm.
Lets now compute the potential energy change:
m g z = (0.00074 kg) (9.81 m/s
2
) (9.42 x 10
-2
m) = 0.000684 J = 0.68 x 10
-6
kJ.
Notice that the potential energy change (0.68 x 10
-6
kJ) is small compared to the
boundary work (0.2411kJ) and can therefore be neglected.
Concept Support:
Gravitational Work:
W
g
= Work done by or against a gravitational force field.
In a gravitational field, the force acting on a body is: F = m g
then the work required to raise this body from level z
1
, to level z
2
is:
W
g
= F . dz = mg dz = mg (z
2
- z
1
) [J] or [Btu] Note: 1 Btu = 778 lbf.ft
W
g
is recognized as the change in potential energy, PE.
Module1-SICI.doc 4/09/04
Copyright K. Nasr, 2004 9
Concept Support:
Other Forms of Work:
Shaft Work:
W
sh
= Energy transmission with a rotating shaft
F = applied force
r = moment arm; therefore Torque = = F r
n = # of revolutions
The force acts through a distance (s) which is related to the radius r by: s = (2r)n;
W
sh
= F. s = (/r) . (2 n r) = 2 n [J] or [Btu]
The power transmitted through the shaft is W
sh
.
= 2 [W] or [Btu/s] or [hp]
Where is the rotational speed.
Spring Work:
W
sp
= F dx
to find W
sp
, W
sp
= F dx
We need F= f (x); for linear springs: F = k . x, we get W
sp
= k (x
2
2
- x
1
2
) /2 [J] or [Btu]
Both x
1
and x
2
are measured from an undisturbed position. x
1
= initial displacement, and x
2
= final displacement
Electrical Work:
Electrons crossing the boundary do work on the systems. When N coulombs of electrons move
through a potential difference V,
W
e
electrical work = V N [J] or [Btu]. On a rate basis:
W
e
.
= electrical power [W] or [Btu/s] or [hp] = V I Voltage * Current
I = Current = No. of electrons flowing per unit time.
In general, both V and I vary with time, and W
e
done during a time interval t is
W
e
= V I dt [J] or [Btu].
If V and I are constant W
e
= V I t [J] or [Btu].
Module1-SICI.doc 4/09/04
Copyright K. Nasr, 2004 10
Q4. How much work is developed as a result of the power stroke, process 3 4?
The power stroke, 3 4, is also a boundary-type work where W = ?Pd and P
n
= C.
Similarly to compression, the process of expansion yields:
n
P P
W
1
3 3 4 4
or in terms of temperatures:
n
T T mR
W
1
) (
3 4
We know
3
=
2
,
4
=
1
, and we also know n. The unknown quantities are (T
3
and
T
4
) or (P
3
and P
4
).
From the ideal gas law:
3 4
4 4 4
3 3 3
r
mRT P
mRT P
We know in addition that P
3
3
n
= P
4
4
n
, so that:
) 1 (
3
4
) 1 (
3
4
3
4
3
4
3
3 4
1
n
n
n
n
n
P
P
T
T
r
P P P
,
_
,
_
,
_
,
_
Still, the unknowns remain unknown. Lets go back to the combustion process since
T
3
and P
3
are linked to the combustion process.
Q5. What happens during combustion, process (2 3)?
This is a heat addition process and it happens at a constant volume, therefore the
boundary did not move and the boundary work is zero (no change in volume).
Definition Support:
Heat, Q, denotes an amount of energy transferred across the boundary of a system while
interacting with its surroundings. Heat transfer into a system is added energy and is positive,
while heat transfer from a system is removed energy and is negative.
Heat is a form of energy transfer driven by a temperature difference between the system and
the surroundings. Heat is a form of energy that is transferred across the boundary of a system
at a given temperature to another system (or the surroundings) at a lower temperature. Heat
flows from a high temperature environment to a low temperature environment [J] or [Btu].
Comparison of Heat and Work:
1. Heat and work are both transient phenomena crossing the boundary of a system.
2. Both heat and work are boundary phenomena.
3. Both heat and work are path functions and inexact differentials.
Sign Convention: Heat to a system (+) Work done by a system > 0
Heat from a system (-) Work done on a system < 0.
Module1-SICI.doc 4/09/04
Copyright K. Nasr, 2004 11
Now, we know that the fuel has a heating value of 15000 kJ/kg. This means that for
each 1 kg of fuel we burn, 15000 kJ of heat is released. Recall that since m
fuel
<< m
air
,
we can assume the entire mass to be air (m
air
= m).
We know the mass of the fuel m
fuel
, so now we can determine how much heat is
released during combustion by burning it. Since we have 100% combustion
efficiency,
f f c in
m HV Q * * to determine the added heat (Q
in
).
Substitute, we get:
Q
in
= (1) (15000 kJ/kg of fuel) (0.00004625 kg of fuel) = 693.8 J
The sign on the heat added is positive, indicating that the heat was added to the
system.
During combustion (heat addition), the volume stays constant as the piston stays at
TDC or at
2
.
3
2
3 3
2 2
mRT
mRT
P
P
/
/
We know P
2
,
2
, and T
2,
We dont know P
3
and T
3
.
We know, however that P
3
& T
3
depend on the heat of combustion. We need to relate
the heat released to properties such as temperature and pressure.
At the beginning of combustion, the air, made up of matter (molecules), had an
energy associated with it and that is called Internal Energy, U.
The total energy, E, of a system is the sum of its internal energy (U), its kinetic
energy (mV
2
), and its potential energy (m g z). The total energy, E, of the system
changes as a result of its interaction with the outside (surroundings) in terms of heat
and work.
Concept Support:
Accelerational Work:
W
a
= work associated with a change in velocity, V, of a system. W
a
required to accelerate a
body of mass m from V
1
to V
2
. It is obtained via help from Newtons 2nd Law: F = m a
a = dV/dt Definition of acceleration
V = ds/dt Differential displacement ds
W
a
= F
.
ds = (m dV/dt)
.
(Vdt) = m V . dV = m (V
2
2
- V
1
2
)/2 [J] or [Btu]
W
a
is known as change in kinetic energy, KE.
Concept Support:
Internal Energy (U):
This energy represents the rotation, translation, and vibration, of these molecules.
The Internal Energy increases as the temperature increases.
Internal Energy, U [J] or [Btu]
Specific Internal Energy: u = U/m [J/kg] or [Btu/lbm]
Module1-SICI.doc 4/09/04
Copyright K. Nasr, 2004 12
This is to say that energy is conserved; it can be converted, it can change form, it can
be stored, but it cannot be created nor destroyed. This concept is referred to as the
Conservation of Energy, and is the First Law of Thermodynamics. Symbolically it is
written as:
E PE KE U W Q + + . (II)
) (
2
) (
) (
1 2
2
1
2
2
1 2
z z mg
V V m
u u m W Q +
+
Module1-SICI.doc 4/09/04
Copyright K. Nasr, 2004 13
Concept Support:
1st Law of Thermodynamics: Law of Conservation of Energy.
The 1st Law is based on experimental observations.
Energy is conserved, cant be created nor destroyed. It can only change form.
Closed System: Q - W = U + KE + PE
Consider a control mass (closed system) undergoing a change of state from (1) to (2); the 1st law
states that the net amount of energy transfer across the boundary as heat and work equals the net
change of the energy of the control mass. In equation form:
Q - W = E = E
2
- E
1
= (U
2
- U
1
) + (KE
2
- KE
1
) + (PE
2
- PE
1
)
) (
2
) (
) (
1 2
2
1
2
2
1 2
z z mg
V V m
u u m W Q +
+
i.e. If I transfer energy to the system, this energy has to show up inside the system and cause a
change of the systems energy. Energy is conserved.
1 and 2 are initial and final states of the system.
Q - W = Net energy transfer to (or from) the system as heat and work [J] or [Btu]
U + KE + PE = Net increase (or decrease) in the total systems energy
W = Boundary work plus any other form of work
Boundary Work: W
b
= Pd or: w
b
= Pdv
Q = Energy transfer due to temperature difference between system and
surroundings. [J] or [Btu]
W = Energy transfer between system and surroundings other than heat.
E = Systems overall energy [KE (kinetic energy) + PE (potential energy)
+ U (translational, rotational, and vibrational)]
U = Change in internal energy of the system [J] or [Btu]
KE = Change of kinetic energy of the system [J] or [Btu]
PE = Change of potential energy of the system. [J] or [Btu]
PE and KE are associated with the coordinate frame chosen in the analysis.
KE = work required to accelerate a body from rest to a certain velocity: KE = m V
2
PE = work needed to raise a weight a distance z = z
2
-z
1
: PE = m g z
U includes all other forms of energy (translation, rotation, and vibration or molecules) and is
associated with the thermodynamic state of the system.
Sign Convention: Q to a system is > 0 W done by a system is > 0
Q from a system is < 0 W done on a system is < 0
Module1-SICI.doc 4/09/04
Copyright K. Nasr, 2004 14
So, for the combustion (Heat addition) process (2 3) having no work, no kinetic energy
change, and no potential energy change, the 1
st
Law (
2
Q
3
2
W
3
= E, Eq. II) becomes
2
Q
3
= U.
Now we need to express the internal energy change in terms of properties such as the
temperature.
2
Q
3
= U is saying that the heat added to the gas causes a change in the internal
energy as a result of an increase in temperature. Therefore, it takes a finite amount of
energy to raise the temperature of a substance. So, we define c
v
, the constant volume
specific heat, as the amount of heat it takes to raise a unit mass one degree.
Concept Support:
Other Forms of the 1
st
Law of Thermodynamics:
Recall, 1st Law on a total basis: Q - W = E [J] or [Btu]
) (
2
) (
) (
1 2
2
1
2
2
1 2
z z mg
V V m
u u m W Q +
+
u = U/m Specific Internal Energy
1st Law on a unit-mass basis (or specific basis): q - w = e [kJ/kg] or [Btu/lbm]
q w u u V V g z z + + ( ) ( / )( ) ( )
2 1 2
2
1
2
2 1
1 2
q = Q/m, w = W/m, and e = E/m = (U + KE + PE)/m
1st Law on a rate basis : Q W
dE
dt
. .
[W] or [Btu/s] or [hp]
Q
.
Net heat transfer rate
W
.
Power
dE/dt Rate of change of total energy
1st Law on a differential basis:
Q - W = dE [J] or [Btu]
q - w = de [kJ/kg] or [Btu/lbm]
is used for Q and W to indicate that Q and W are not properties. Q and W are
path functions. There is no such thing as Q or W.
d is used for E because E is a property E = E
2
- E
1
Module1-SICI.doc 4/09/04
Copyright K. Nasr, 2004 15
c
v
is a measurable property for a substance and is normally tabulated as a function of
temperature. c
v
for air is 0.718 kJ/kg . K at 300 K (Table A-20 of your textbook).
Thus,
2
Q
3
= U becomes
2
Q
3
= U = m c
v
T = m c
v
(T
3
- T
2
) when c
V
is a weak function
of temperature (Note: U = m c
v
T is true for all ideal gases of constant specific heats).
Knowing the heat added, we get:
K K
K kg
kJ
kg
J
T
mc
Q
T
V
20 . 2093 49 . 787
*
718 . 0 * 00074 . 0
8 . 693
2
3 2
3
+ + .
Now P
3
can be calculated from the Ideal Gas Law:
2
3
3
3
3
mRT mRT
P (since
3
=
2
)
or recognizing that 2 3 is a constant volume process for an ideal gas:
( ) C K T r
T
T
kPa kPa
r
P P P
kPa
K
K
kPa
T
T
P P
T
T
P
P
n
n
n
n
612 14 . 885 ) 6 . 8 (
81 . 265
6 . 8
1
86 . 5405
1
86 . 5405
49 . 787
20 . 2093
* 75 . 2033
4
4 . 0 1
1
3
4
3
4
4 . 1
3
4
3
3 4
2
3
2 3
2
3
2
3
,
_
,
_
,
_
,
_
,
_
,
_
.
Concept Support:
Specific Heats:
Definition: Specific heat Amount of heat required per unit mass to raise the temperature by
one degree.
Specific heat is a property related to the internal energy.
Constant-Volume Specific Heat: c
v
= (Q/T)
v
/ m
1st Law : Q = dU + W in the absence of dKE and dPE.
Assuming a quasi-equilibrium process W = p d
Since it is a constant process p d 0 and Q = dU
c
v
= (Q/T)
v
/ m= (U/T)
/ m = (u/T)
v
, Thus:
c
= (u/T)
and has the units [kJ/kgK], [kJ/kg C] or [Btu/lbm R].
Constant-Pressure Specific Heat: c
p
= (Q/T)
p
/ m
Specific Heat values of some gases can be found in Table A-20 of your textbook.
c
p
has the units [kJ/kgK], [kJ/kg C] or [Btu/lbm R].
Module1-SICI.doc 4/09/04
Copyright K. Nasr, 2004 16
Therefore, for the expansion process, the following properties can be tabulated:
Temperature Pressure Volume
State 3: 2093.20K = 1820.05C 5405.86kPa 0.0000822m
3
State 4: 885.14K = 612C 265.81kPa 0.0007072m
3
The work of expansion can now be obtained:
J
m kPa m Pa
n
P P
W 4 . 641
4 . 1 1
0000822 . 0 * 86 . 5405 0007072 . 0 * 81 . 265
1
3 3
3 3 4 4
Reconfirm with:
J
K K
K kg
kJ
kg
n
T T mR
W 4 . 641
4 . 1 1
) 20 . 2093 14 . 885 ( *
*
287 . 0 * 000740 . 0
1
) (
3 4
It Checks.
This is expansion work and is therefore a positive quantity. This is the work produced
for one cylinder during one cycle.
Q6. Now that weve determined the work needed for compression and the work
produced during expansion, can we determine the net work of the cycle?
For one cycle of one cylinder the net work is:
kJ J J J W W W
Net
4 . 0 1 . 400 3 . 241 4 . 641
2 1 4 3
To determine the total power output, we need to recall that there are four cylinders.
We also need to remember that there are two revolutions per cycle.
Recalling this and that power is the rate of doing work, we use the following equation:
( ) ( )
hp kW W
cylinders
cycle rev
RPM J
cylinders
cycle rev
RPM W
W
Net
66 . 53 01 . 40
4
sec 60
min 1
/ 2
3000 * 1 . 400
#
/ #
*
,
_
,
_
,
_
&
&
[Conversion factors are available on the inside front cover of the textbook].
Definition Support:
Net Work is the total of all work performed on or by a system for all processes.
Module1-SICI.doc 4/09/04
Copyright K. Nasr, 2004 17
Q7. What is the thermal efficiency of this engine?
The thermal efficiency is the net work produced (what we finally get) relative to the
net heat input (what we pay for).
With the above calculated net work, and the heat input, we can determine the thermal
efficiency of this engine. The thermal efficiency of this engine based on the Otto
cycle model is:
% 67 . 57
8 . 693
1 . 400
J
J
Q
W
In
Net
Practical Note: The actual efficiency of an engine is roughly 50% of
Otto
.
One sees therefore that an estimation of the heat input was required for the evaluation of
thermal efficiency of the engine (cycle). In this application, the heat input was given.
Q8. How much heat is rejected (lost or wasted) for the process from 4 1?
This process is similar to the heat added in 2 3 by the fact that it happens at a
constant volume;
4
Q
1
= U and
4
W
1
= 0, therefore:
J K K
K kg
kJ
kg T T mc U U Q
V
4 . 293 ) 14 . 885 333 (
*
718 . 0 * 00074 . 0 ) (
4 1 4 1 1 4
Note this is a negative quantity indicating that it is heat out of the system.
Q9. Check if energy is conserved for the whole cycle.
Using the 1
st
Law applied to the whole cycle: 1 2 2 3 4 1?
Q
Net
- W
Net
= U = U
1
- U
1
= 0?
(Q
In
- |Q
Out
|) - (W
Exp
- |W
Comp
|) = (
2
Q
3
- |
4
Q
1
|) - (
3
W
4
- |
1
W
2
|) = 0 ?
(693.8 J 293.4 J) - (641.4 J 241.3 J) = -0.3 J
This is not exactly zero because of significant figures used in the computation of heat
and work. It is close enough to zero and therefore energy is conserved.
It is often helpful to summarize the heat and work terms for a cycle. For this Otto cycle:
Process W (kJ) Q (kJ) Comment
1 2 -0.2413 0 Work done on the system, Adiabatic process.
2 3 0 0.6938 No Work, Heat into system
3 4 0.6414 0 Work done by the system, Adiabatic process
4 1 0 -0.2934 No Work, Heat out of the system
Net 400.1 400.4 W
net
=Q
Net
Concept Support:
Thermal Efficiency of a Heat Engine: An engine that produces power is called a heat engine.
It uses heat to produce power or work. The thermal efficiency of a heat engine can be defined as:
in
net
Q
W
Module1-SICI.doc 4/09/04
Copyright K. Nasr, 2004 18
Since energy is conserved for the heat engine operating in a cycle so that Q
net =
W
net
,
the thermal efficiency can be written as:
in
out
in
out in
in
net
net
net
thermal
Q
Q
Q
Q Q
Q
Q
Q
W | |
1
| |
Lets reconfirm our computed value for the efficiency:
% 71 . 57
8 . 693
4 . 293
1 1
J
J
Q
Q
in
out
thermal
It checks with the previously calculated value.
It can also be shown that for an ideal gas of constant specific heats,
Otto
is a function
of the compression ratio, r, only:
V
P k
Otto
c
c
k r
; 1
1
= specific heat ratio. (III)
Using Equation III, we get 57.71% 6 . 8 1
4 . 1 1
Otto
which checks.
Often various engines are compared based on the mean effective pressure, mep,
where:
TDC BDC
cycle net
W
mep
,
In words it is the pressure that would produce the same work output of one cycle for
the displaced volume. For this Otto cycle:
kPa
m
N
x
m
J
MEP 19 . 640 10 19 . 640
000625 . 0
1 . 400
2
3
3
To summarize the solution presented here for this problem on spark ignition engines as
modeled via the Otto cycle, an Excel worksheet has been generated and is presented as
follows:
Module1-SICI.doc 4/09/04
Copyright K. Nasr, 2004 19
V1=V4= 0.000707237 m^3
V2=V3= 8.22368E-05 m^3
k= 1.4
P1= 100 kPa
P2= 2.34E+02 kPa
P3= 5.41E+03 kPa
P4= 265.81 kPa
T1= 60 C= 333 K
T2= 514.5 C= 787.5 K
T3= 1820.05 C= 2093.2 K
T4= 612 C= 885.15 K
r= 8.6
k= 1.4
cv= 0.718 kJ/kg*k Cold Air-Standard
cp= 1.004 kJ/kg*k Eval @ 300K
R= 0.287 kJ/kg*k
1->2
Q= No Heat Transfer 0.0000 kJ
W= m(u1-u2)->W/m = cv(T1-T2) -0.2413 kJ
2->3
Q= HV*m 0.6938 kJ
W= Constant Volume 0.0000 kJ
3->4
Q= No Heat Transfer 0.0000 kJ
W= mcv(T3-T4) 0.6414 kJ
4->1
Q= mcv(T1-T4) -0.2934 kJ
W= Constant Volume 0.0000 kJ
Wnet= 0.4001 kJ
Qnet= 0.4004 kJ
E Balance 0.0003 difference 0.074981% Error
Efficiency Wnet/Qin 57.67%
r model 57.71% =1-r^(1-k)
0.079235% Error
This completes the first section of the Otto cycle. To practice further with applications of
the principles and equations learned earlier, lets work out thermodynamically another
important problem: the Compression Ignition Engine. The Diesel cycle is the ideal cycle
which models compression ignition engines.
Module1-SICI.doc 4/09/04
Copyright K. Nasr, 2004 20
Module 1 (Continued) The Compression Ignition Engine Diesel Cycle
Problem 2 - Problem Statement: Consider an engine operating on the ideal Diesel cycle.
The volume of one cylinder is 1200 cm
3
at the beginning of the compression process, 75 cm
3
at the end, and 150 cm
3
after the heat addition process. Air is at 17 C and 100 kPa at the
beginning of the compression process. Determine the net work per cycle, in kJ and the mean
effective pressure.
Objectives: Formulate the solution to a diesel cycle problem based on concepts learned from
the Otto cycle of spark-ignition engines.
The Air-Standard Diesel Cycle: Is the ideal cycle for modeling Compression-Ignition (CI)
Engines.
It differs from the Otto cycle in the manner combustion is done. Combustion occurs at a
constant pressure for CIs. No external spark is needed; the diesel fuel is injected into the
cylinder and combustion occurs because of the high temperature of the compressed air.
Compression ratio: r =
1
/
2
, also we define a cutoff ratio: r
c
=
3
/
2
The main value of such this analysis is obtaining qualitative performance of this engine.
This means that if a variable is changed and the change resulted in an increase in thermal
efficiency, the actual engine will experience an increase in thermal efficiency but the increase
will be smaller that that obtained from the model.
1st Law (Closed System): Q - W = U + KE + PE
) (
2
) (
) (
1 2
2
1
2
2
1 2
z z mg
V V m
u u m W Q +
+
Module1-SICI.doc 4/09/04
Copyright K. Nasr, 2004 21
Process 1st Law For Constant Specific Heats
12: Isentropic Compression
process of air from BDC to TDC
W
comp
= - U = m (u
1
- u
2
) W
comp
= - U = m c
v
(T
1
- T
2
)
23: Constant-pressure
combustion increasing the volume
(simulated by a quasi-equilibrium
heat addition process)
Q
in
= H = m (h
3
- h
2
) Q
in
= H = m c
p
(T
3
- T
2
)
34: Isentropic expansion of the
air
W
3-4
= - U = m (u
3
- u
4
) W
3-4
= - U = m c
v
(T
3
- T
4
)
41: Heat transfer to surroundings
completing the cycle
Q
out
= U = m (u
1
- u
4
) Q
out
= U = m c
v
(T
1
- T
4
)
Efficiency (Diesel Cycle)
Diesel
= W
net
. / Q
in
.
= 1 - Q
out
/Q
in
Diesel
= 1 - r
(1-k)
* (r
c
k
- 1) / [k (r
c
- 1)]
Note the introduction of a property called enthalpy, H. H = U + p . It is a convenient property
for now. It has resulted from having a constant pressure heat addition so that:
2
W
3
= P(
3
-
2
).
Enthalpy is a function of three properties: the internal energy, pressure, and volume. If we assume
constant specific heats, then the change in enthalpy (H) becomes (m c
p
T), where c
p
is the
constant pressure specific heat.
Assuming constant specific heats, evaluated at 300 K (cold air-standard analysis), the following
presents a skeleton of the solution to the problem above.
Determination of Compression and Cut Off Ratios
2
75
150
16
75
1200
3
3
2
3
3
3
2
1
cm
cm
r
cm
cm
r
c
From the 1
st
Law, ignoring potential and kinetic energy changes, we know that:
Concept Support:
Constant Pressure Specific Heat, c
P:
c
p
= (Q/T)
p
/ m
1st Law: Q = dU + p d = dH, Substitute:
c
p
= (Q/T)
p
/ m = (H/T)
p
/m = (h/T)
p
; h specific enthalpy [kJ/kg] or [Btu/lbm]
Specific Heat values of some gases can be found in Table A-20 of your textbook.
c
p
has the units [kJ/kgK], [kJ/kg C] or [Btu/lbm R].
Module1-SICI.doc 4/09/04
Copyright K. Nasr, 2004 22
0 0 + + + + U PE KE U W Q
1 2, Compression Stroke
) ( ) (
0
2 1 2 1
T T mc u u m W
Q
V
2 3, Heat Addition, Q
in
U W Q but P = constant and therefore W = P(?
3
- ?
2
)
) (
) ( ) (
) ( ) (
2 3 2 3
2 2 3 3
2 3 2 3
T T mc H H
U P U P
U U P U W Q
P
+ +
+ +
3 4, Expansion Stroke
) ( ) (
0
4 3 4 3
T T mc u u m W
Q
V
4 1, Heat Rejection, Q
out
) ( ) (
4 1 4 1 4 1
T T mc u u m U U Q
V
Net work for the cycle:
| |
2 1 4 3 3 2
W W W W
Net
+ = | |
1 4 3 2
Q Q Q
Net
Thermal Efficiency:
in
Net
Q
W
Assuming constant specific heats, all four temperatures are needed. We will use values at
300 K (Cold Air-Standard Analysis).
c
V
= 0.718 kJ/kg . K, c
P
= 1 kJ/kg . K, 4 . 1
V
P
c
c
k
Lets analyze the processes assuming constant specific heats and performing cold-air
standard analysis:
Process 1 2
Since 1 2 is an isentropic process, we can say:
k
k
k
k
k
k
k
P
P
T T
r
T T T
P
P
T
T
1
1
2
1 2
1
1
1
1
2
1 2
1
1
2
1
1
2
1
2
* or
1
* *
,
_
,
_
,
_
,
_
,
_
Process 2 3
Module1-SICI.doc 4/09/04
Copyright K. Nasr, 2004 23
) (
2 3 3 2
T T mc Q
P
and we know T
2
but we still need T
3
to solve this.
Using the Ideal Gas Equation, we can relate P
2
,
2
, and T
2
, and P
3
,
3
, and T
3
.
3
3 3
2
2 2
T
P
mR
T
P
Since we know that P
2
= P
3
for the Diesel cycle, we can find T
3
2
2
2 3
2 2
2 3 3
3
T r
T
P
T P
T
c
/
/
Now we can solve
2
Q
3
:
) (
2 3 3 2
T T mc Q
P
Process 3 4
3 4 is also an isentropic process, using the same relationship as in 1 2, we get:
k k
k
k
k
T T T T
P
P
T
T
,
_
,
_
,
_
,
_
1
3
1
3 4
1
3
4
3 4
1
3
4
1
3
4
3
4
* *
Now that we have all four temperature values, we can go back to our 1
st
Law and apply it
to each process to get work and heat for each process.
Applying the 1
st
law to the whole cycle, we can obtain the net energy terms:
1 4 3 2
2 1 4 3 3 2
Q Q Q
W W W W
Net
Net
+
For the efficiency, first well use Net Work and Heat Input to determine the thermal
efficiency.
in
Net
Q
W
Reconfirm using:
1
]
1
) 1 (
1 1
1
1
c
k
c
k
r k
r
r
valid only if the specific heats are constant.
Calculating all of the above using an Excel spread sheet, we get the following results:
Module1-SICI.doc 4/09/04
Copyright K. Nasr, 2004 24
V1=V4= 1200 cm^3 1.20E-03 m^3
V2= 75 cm^3 7.50E-05 m^3
V3= 150 cm^3 1.50E-04 m^3
k= 1.4
P1= 100 kPa
P2=P3= 4.85E+03 kPa
P4= 263.9016 kPa
T1= 17 C= 290.15 K
T2= 606.4203 C= 879.57032 K
T3= 1485.991 C= 1.76E+03 K
T4= 492.5604 C= 765.71044 K
r= 16
rc= 2
k= 1.4
cv= 0.718 kJ/kg*k Cold Air-Standard
cp= 1.004 kJ/kg*k Eval @ 300K
R= 0.287 kJ/kg*k
1->2
Q= No Heat Transfer 0.00 kJ/kg
W= m(u1-u2)->W/m = cv(T1-T2) -423.20 kJ/kg
2->3
Q= H3-H2=mcp(T3-T2) 883.09 kJ/kg
W= P2(V3-V2)=mR(T3-T2) 252.44 kJ/kg
3->4
Q= No Heat Transfer 0.00 kJ/kg
W= mcv(T3-T4) 713.28 kJ/kg
4->1
Q= mcv(T1-T4) -341.45 kJ/kg
W= Constant Volume 0.00 kJ/kg
Wnet= 542.5158 kJ/kg
Qnet= 541.6362 kJ/kg
E Balance 0.87957 difference 0.162391% Error
Efficiency Wnet/Qin 61.43%
r, rc model 61.38% =1-|cv(T1-T4)|/cp(T3-T2)
0.087068% Error
Module1-SICI.doc 4/09/04
Copyright K. Nasr, 2004 25
Notes:
The power stroke of a Diesel cycle involves two contributions: The first contribution occurs
during the heat addition process (2 3) and a second contribution during the expansion
process (3 4).
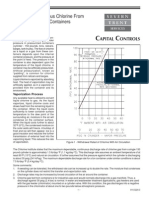
For a given compression ratio r,
Diesel
<
Otto
. For example, for r
c
= 2 and at r = 10,
Diesel
= 53% while
Otto
= 60%.
However at operating compression ratios (r = 9 for Otto and r = 20 for Diesel), the Diesel
cycle yields higher efficiencies. As an example,
Diesel
=
65% at r = 20 and
Otto
= 58.5% at r
= 9. . To illustrate this comparison, recall that for constant specific heats:
,
_
) 1 (
1
1
1
1
1
c
k
c k
Diesel
k
Otto
r k
r
r
r
For k=1.4, plotting the thermal efficiencies of the Otto and Diesel cycles vs. r, we get:
We also note that as r
c
increases,
Diesel
decreases.
Note that the above analyses and numerical values of all quantities involved using c
v
and c
p
, specific heats. In addition, it assumes the specific heats to have constant
values, independent of temperature. This is called the constant specific heat model.
In fact, the analyses presented above for the Otto and Diesel cycle is called the cold
air-standard analysis since it uses constant c
v
and c
p
values at 300 K. We will come
back to this point later and hope to find a way to perform an analysis which accounts
for the variation of specific heats with temperature.
Efficiency Vs. Compression Ratio
0%
10%
20%
30%
40%
50%
60%
70%
80%
90%
0 5 10 15 20 25
Compression Ratio
E
f
f
i
c
i
e
n
c
y
Otto Cycle Diesel Cycle, rc=2 Diesel Cycle, rc=3 Diesel Cycle, rc=4
Module1-SICI.doc 4/09/04
Copyright K. Nasr, 2004 26
Q7. Reflecting back on the solution of these applications, one questions the goodness
of using P
n
= C for the compression and expansion processes. If appropriate, where
did this relationship come from and what are the limitations on its use?
P
n
= C describes what is called a polytropic process in Thermodynamics. It is a
quasi-equilibrium process linking pressure to volume as a power function.
We also note that the work of compression
2
1
Pd obtained by using P
n
= C going
from 1 2 is the same for that going from 2 1, following the same path,
backwards. This is called a reversible process.
If in addition the process happens without heat transfer across the boundary (adiabatic
i.e. Q =0), then the polytropic process becomes labeled an isentropic process and
the exponent n becomes equivalent to
V
P
c
c
k , the specific heat ratio of the gas. In
other words, a polytropic process of an ideal gas with constant specific heats is an
isentropic process with
V
P
c
c
k n .
Incidentally, a process involving no heat transfer is defined as an adiabatic process.
And therefore a process that is reversible and adiabatic is an isentropic process.
So now the question becomes: "Are the compression and expansion processes for the
Otto and Diesel cycles isentropic?"
The real compression and expansion strokes are not truly reversible as there is friction
between the piston rings and the cylinder, and friction between the gas and the solid
surfaces inside the cylinder, but both contributions of friction are small and minimal.
The processes are therefore modeled as reversible.
Also, for one stroke, heat transfer will be present but can be neglected since the
compression/expansion process is happening rapidly. There is little time for heat
transfer to take place and therefore is modeled as an adiabatic process.
In summary, we model the expansion and compression processes for the Otto and
Diesel cycles as isentropic processes (reversible and adiabatic) where
V
P
c
c
k n .
Concept Support:
Reversible Processes:
A process is called internally reversible if no irreversibilities occur within the boundaries of
the system during the process. That is, the paths of the forward and reverse processes
coincide for an internally reversible process.
A process is said to be totally reversible if both the system and the surroundings can be
restored to their original conditions.
A reversible process is a hypothetical ideal process to which all real (irreversible) processes
are compared.
Module1-SICI.doc 4/09/04
Copyright K. Nasr, 2004 27
We then summarize the isentropic process of an ideal gas having constant specific
heats between any two states, state (1) and state (2):
The table below summarizes the Otto cycle. All processes are assumed reversible.
Process 1st Law For Constant Specific Heats
12: Isentropic Compression process
of air from BDC to TDC
W
comp
= - U = m (u
1
- u
2
) W
comp
= - U = m c
v
(T
1
- T
2
)
23: Constant-volume combustion
elevating the pressure (simulated by a
quasi-equilibrium heat addition process)
Q
in
= U = m (u
3
- u
2
) Q
in
= U = m c
v
(T
3
- T
2
)
34: Isentropic Power Stroke or
expansion of the air (simulating the
work by combustion products)
W
exp
= - U = m (u
3
- u
4
) W
exp
= - U = m c
v
(T
3
- T
4
)
41: Heat transfer to surroundings
completing the cycle
Q
out
= U = m (u
1
- u
4
) Q
out
= U = m c
v
(T
1
- T
4
)
Efficiency (Otto Cycle)
Otto
= W
net
. / Q
in
.
= 1 - |Q
out
| / |Q
in
|, Also,
W
net
=
W
exp
- |W
comp
|
Otto
= 1 - T
1
/T
2
= 1 - (
2
/
1
)
(k-1)
= 1 - r
(1-k)
; k = c
p
/c
v
The table below summarizes the Diesel cycle. All processes are assumed reversible.
Process 1st Law For Constant Specific Heats
12: Isentropic Compression process
of air from BDC to TDC
W
comp
= - U = m (u
1
- u
2
) W
comp
= - U = m c
v
(T
1
- T
2
)
23: Constant-pressure combustion
increasing the volume (simulated by a
quasi-equilibrium heat addition
process)
Q
in
= H = m (h
3
- h
2
) Q
in
= H = m c
p
(T
3
- T
2
)
34: Isentropic expansion of the air W
3-4
= - U = m (u
3
- u
4
) W
3-4
= - U = m c
v
(T
3
- T
4
)
41: Heat transfer to surroundings
completing the cycle
Q
out
= U = m (u
1
- u
4
) Q
out
= U = m c
v
(T
1
- T
4
)
Efficiency (Diesel Cycle)
Diesel
= W
net
. / Q
in
.
= 1 - Q
out
/Q
in
Diesel
= 1 - r
(1-k)
* (r
c
k
- 1) / [k (r
c
- 1)]
Concept Support:
Isentropic process of an Ideal Gas with Constant Specific Heats: P
k
=C
k k
k
k
V
P
P
P
P
P
T
T
c
c
k
k
P P
W
,
_
,
_
,
_
1
2
1
2
1
1
2
1
1
2
1
2
1 1 2 2
,
,
1
Valid if: 1) Process is isentropic, 2) gas is ideal, 3) specific heats are constant.
Module1-SICI.doc 4/09/04
Copyright K. Nasr, 2004 28
It is worth summarizing the most common features when modeling spark ignition and
compression ignition engines:
Common Features for Modeling SI and CI Engines:
1. Air is the working fluid. The injected amount of fuel is neglected.
2. No inlet or exhaust process (Closed System).
3. The actual combustion process is replaced by an external heat addition process.
4. The exhaust process is replaced by a heat rejection process.
5. All processes are assumed to be quasi-equilibrium and reversible.
6. The air is assumed to behave as an ideal gas.
7. One additional assumption is often made considering specific heats to be constant. That is
the specific heats are weak function of temperature.
Q8. Now that we know how to compute the horsepower for an engine, and obtain the
thermal efficiency, natural questions arise:
How can we improve the efficiency and generate more power?
Can efficiency be improved to 100%?
What is the maximum efficiency?
The answers to those questions are tied to the terms in the thermal efficiency.
Recall that:
in
Net
Q
W
=
in
Out
Q
Q
1 and therefore in order to increase the efficiency, Q
out
needs to be made smaller. The efficiency would be 100% if Q
out
= 0. We know,
however, that Q
out
cannot be equal to zero as there exists heat rejection. This brings up
the Second Law of Thermodynamics in a statement known as the Kelvin-Planck
Statement.
Concept Support:
Kelvin-Planck Statement of the Second Law of Thermodynamics:
It is impossible for a device to operate in a thermodynamic cycle and deliver a net amount
of work to its surroundings while receiving energy by heat transfer from a single reservoir.
The Kelvin-Planck Statement applies directly to heat engines, such as the Otto and Diesel cycles.
The Kelvin-Planck Statement says that there is no cyclic heat engine with 100% efficiency.
Therefore, what is the maximum efficiency?
The Carnot Cycle and Principles for a heat engine (such as car engine) gives the maximum
value for the efficiency. It is a hypothetical cycle that could not be achieved under practical
situations but sets the upper limit on the efficiency.
Concept Support:
Carnot Principles:
1. The efficiency of an irreversible heat engine is always less than the efficiency of a reversible
one operating between the two heat reservoirs.
Module1-SICI.doc 4/09/04
Copyright K. Nasr, 2004 29
2. The efficiencies of all reversible heat engines operating between the same two heat reservoirs
are the same.
Thermal (Heat) Reservoir: A body of large capacity; i.e. (mass specific heat) that can
absorb or supply a finite amounts of heat without undergoing any change in temperature.
Examples: Atmosphere does not change in temperature as a result of dumping heat (heat
losses) from residential buildings in winter time. Other examples: Ocean, river, etc...
A thermal reservoir does not really have to be very large, it just needs to be large relative to
the amount of energy it supplies or absorbs. For example the air in the room is a reservoir
relative to the T.V. A reservoir maybe a source or a sink.
For a heat engine operating between a high temperature, T
H
, and a low temperature, T
L
, the
maximum efficiency is represented by the equation:
H
L
H
L H
Carnot
T
T
T
T T
1
max
Lets go back to the Otto cycle problem and assuming a high temperature in the Otto cycle
of
,
_
2
3 2
T T
T
H
(= 1440.35 K in this case) and the low temperature as the ambient or
environmental temperature (= 290 K in this case). Thus,
th, max
=
Carnot
= % 87 . 79
35 . 1440
290
1
K
K
.
The maximum efficiency is 80% for this Otto cycle and therefore there is room to improve the
efficiency from its Otto-modeled value of 57.7% to that of Carnot, 80%. Recall also, that the
actual thermal efficiency of an SI is roughly half that obtained via the Otto cycle model.
We have seen the Kelvin-Planck statement as a statement of the 2
nd
Law of Thermodynamics.
However there is a need for an equation which we can call the Second Law of Thermodynamics.
This equation can be applied to a process to examine whether this process can occur or not. To
obtain such an equation, we proceed in discussing the isentropic process further.
Recall that an isentropic process is a reversible and adiabatic process. The isentropic process
gives a notion of maximum work output/minimum work input for an adiabatic system. Applying
this idea to the Otto and Diesel cycles, since the compression and expansion processes were
modeled as isentropic, one concludes that more work would be actually required during
compression and less work would be actually produced during expansion.
An isentropic process is a process of constant property called entropy, S. Entropy is a measure of
the molecular disorder level (chaos) of a system. We are normally interested in the entropy
change of a system rather that its specific value at a given state. For example, did the level of
chaos increase, decrease, or remain constant as a gas is compressed?
It would be necessary then to compare actual processes to isentropic processes and perhaps find
ways to quantify and minimize the differences so that an actual process approaches an isentropic
process.
Module1-SICI.doc 4/09/04
Copyright K. Nasr, 2004 30
Concept Support:
Entropy:
The 1st Law dealt with a property called ENERGY. Energy is a conserved property.
The 2nd Law will deal with a property called ENTROPY. Entropy is not a conserved
property, and there is no such thing as conservation of entropy.
Lets introduce this property called specific entropy, s = S/m:
To introduce entropy, we employ Gibbs Relation:
T ds = du + p dv the specific entropy, s is calculated from measured quantities.
Integrate:
1 2
s =
1
2
du/T +
1
2
(p/T)dv
We also note another Tds equation in terms of the Specific Enthalpy, h:
h = u + p v
dh = du + p dv + v dp
Thus: T ds = dh - v dp
We notice the resemblance of (T ds = du + p dv) to the 1st law (q - w = du), where
w = p dv for an internally reversible process
q = du + p dv, Therefore: q = T ds , where q = Q/m and w=W/m
1 2
s =
1
2
q/T or S =
1
2
Q/T; for an internally reversible process
and Q is the area under the curve on a temperature-entropy (T-s) diagram.
We then generalize for any process and state:
(S
2
- S
1
)
1
2
Q/T Known as the 2nd Law Corollary
where;
= for an internally reversible process, and
> for an internally irreversible process.
T is the temperature of the systems boundary where heat transfer is taking place.
(S
2
- S
1
) Entropy change of the system. [kJ/K] or [Btu/R]
Note that Q and T are measurable quantities, and thus S can be measured.
The best performance is obtained for reversible processes. Reversible processes are
used as a guide for comparison to the processes of practical irreversible devices.
Module1-SICI.doc 4/09/04
Copyright K. Nasr, 2004 31
In real life, there are no processes that are exactly isentropic. In other words, there
are no processes that are completely reversible. Irreversibilities are always present.
Through the 2
nd
Law corollary, (S
2
- S
1
)
1
2
Q/T, we can obtain a relationship between the
entropy change (change in level of chaos) of a system and entropy generation that is due to
irreversibilities. The 2
nd
Law corollary can be made an equality for any process.
Mathematically, this is written as:
T
Q
S S
) (
1 2
(IV)
[entropy change of system] = [entropy transfer associated with heat] + [entropy generation]
is like heat and work, is a path function, not a property. It is entropy generation
(production) due to irreversibilities and has the units of [kJ/K] or [Btu/R].
= 0 for internally reversible processes, and
> 0 for internally irreversible processes.
Note that if (S
2
- S
1
) < Q/T, then the process is impossible.
Eq. (I V) is normally called the 2
nd
Law of Thermodynamics for a closed system.
We note that this equation, in addition to presenting an entropy balance, i.e. telling us how
the entropy of a system changes, it tells us whether a process can occur or not. This is
extremely important as for a process to occur (for a process to be possible), it must satisfy
both the 1
st
and 2
nd
Laws of Thermodynamics.
Satisfying the 2
nd
Law of Thermodynamics means that for a real process is always a
positive quantity. If
for a process is computed and it turns out that it is a negative
quantity, then you can be sure the process is impossible, even if it had satisfied the 1
st
Law.
Do not confuse S with , S is
entropy change of a system while
is a measure of
irreversibilities and is called entropy generation.
Now Looking back at the isentropic compression and expansion process of the Otto cycle,
we see that S = 0 because we had assumed an adiabatic process ( )
0
T
Q
and a reversible
process ( = 0). So, the next question is then how did the entropy change of this ideal gas for
the other two processes (heat addition and heat rejection)?
Definition Support:
Irreversibilites:
A number of factors render processes irreversible. Listed here are some:
1. Friction
2. Unrestrained expansion (fast process)
3. Mixing of two gases
4. Q across T (a can of soda in a warm room)
5. Electric Resistance
6. Inelastic deformation of solids
7. Chemical Reactions
Module1-SICI.doc 4/09/04
Copyright K. Nasr, 2004 32
Concept Support:
Entropy Change of an Ideal Gas: (P v = R T)
Lets begin with the Tds equations:
Tds = du + p dv Tds = dh - v dp
Recall: du = c
v
(T) dT dh = c
p
(T) dT
ds = [c
v
(T) / T] dT + (p/T) dv ds = [c
p
(T) / T] dT - (v/T) dp
ds = [c
v
(T) / T]dT + (R/v) dv ds = [c
p
(T) / T] dT - (R/p) dp
Integrate;
s(T
2
, v
2
) - s(T
1
, v
1
) =
T1
T2
[c
v
(T) / T] dT + R ln(v
2
/v
1
) (V)
s(T
2
, p
2
) - s(T
1
, p
1
) =
T1
T2
[c
p
(T) / T] dT - R ln(p
2
/p
1
) (VI)
In order to tabulate s values for ideal gases we introduce a reference state o where:
s
T
o
=
To
T
[c
p
(T) / T]
dT
We can express
T1
T2
[c
p
(T) / T] dT =
T1
To
[c
p
(T) / T] dT +
To
T2
[c
p
(T) / T] dT
or
T1
T2
[c
p
(T) / T]dT = s
T
o
2
- s
T
o
1
Therefore, Eq. (VI) becomes: s(T
2
, p
2
) - s(T
1
, p
1
) = s
T
o
2
- s
T
o
1
- R ln (p
2
/p
1
)
(VII)
The values of s
T
o
are tabulated versus temperature for ideal gases (Table A-22 for Air).
We note that Eq. (VII) accounts for the variation of specific heats with temperature. This
means that Eq. (VII) should be used for the Variable Specific Heats Model.
Special Case: Constant Specific Heats Model
If the specific heats are considered weak functions of temperature, Equations (V) and
(VI) yield directly:
s(T
2
, v
2
) - s(T
1
, v
1
) = c
v
ln(T
2
/T
1
) + R ln(v
2
/v
1
) (VIII)
s(T
2
, p
2
) - s(T
1
, p
1
) = c
p
ln(T
2
/T
1
) - R ln(p
2
/p
1
) (IX)
Equations (VIII) and (IX) give the entropy change of an ideal gas using the Constant Specific
Heats Model.
Module1-SICI.doc 4/09/04
Copyright K. Nasr, 2004 33
For the Otto cycle problem (based on constant specific heat values), we had:
Temperature Pressure Volume
State 1: 333 K = 60 C 100 kPa 0.0007072 m
3
State 2: 787.49K =514.5C 2033.75kPa 0.0000822 m
3
State 3: 2093.20K = 1820.05C 5405.86kPa 0.0000822m
3
State 4: 885.14K = 612C 265.81kPa 0.0007072m
3
Using equation (VIII), we get the change in entropy for the heat addition process of air:
( )
K kg
J
K kg
kJ
R
T
T
c s s
V
*
92 . 701
*
1 ln * 287 . 0
49 . 787
20 . 2093
ln * 718 . 0 ln ln
2
3
2
3
2 3
1
]
1
+
,
_
,
_
,
_
or by knowing the temperatures and pressures, using Eq. (IX):
K kg
J
K kg
kJ
P
P
R
T
T
c s s
P
*
94 . 700
* 75 . 2033
86 . 5405
ln * 287 . 0
49 . 787
20 . 2093
ln * 004 . 1 ln ln
2
3
2
3
2 3
1
]
1
,
_
,
_
,
_
,
_
Therefore, during the heat addition process, the entropy level of air increased by 0.7 kJ/kg K.
The same can be done for the heat rejection process:
( )
K kg
J
K kg
kJ
R
T
T
c s s
V
*
92 . 701
*
1 ln * 287 . 0
14 . 885
333
ln * 718 . 0 ln ln
4
1
4
1
4 1
1
]
1
+
,
_
,
_
,
_
or by knowing the temperatures and pressures:
K kg
J
K kg
kJ
P
P
R
T
T
c s s
P
*
94 . 700
* 81 . 265
100
ln * 287 . 0
14 . 885
333
ln * 004 . 1 ln ln
4
1
4
1
4 1
1
]
1
,
_
,
_
,
_
,
_
The entropy level for the heat rejection process decreased by the same amount, 0.7 kJ/kg K.
This is expected since processes (1) (2) and (3) (4) are isentropic.
Lets visualize the above by plotting the process on a Temperature-Entropy (T-s) Diagram:
Module1-SICI.doc 4/09/04
Copyright K. Nasr, 2004 34
Now that we have computed the entropy change of air during the heat addition and heat
rejection processes and knowing that the four processes are reversible ( = 0), we can use
this information to estimate the temperature of the boundary (separating the system from the
surroundings) for both the heat addition and heat rejection processes. Starting with:
T
Q
S S
1 2
Where is the entropy production. Assuming for uniform heat addition occurs across a
boundary of temperature T
b,in
and having computed (s
3
- s
2
), we get:
K
kgK kJ kg
kJ
s s m Q T
in b
1340
) / 7 . 0 ( * ) 00074 . 0 (
6938 . 0
) ( /
2 3 ,
Note that T
3
= 2093 K and T
2
= 788 K.
Doing the same for the heat rejection process and assuming a uniform heat rejection at a
boundary temperature of T
b,out
, we can compute that temperature:
K
kgK kJ kg
kJ
s s m Q T
out b
598
) / 7 . 0 ( * ) 00074 . 0 (
293 . 0
) ( /
4 1 ,
Note that T
4
= 885 K and T
1
= 333 K. These are theoretical boundary temperatures as the
processes were assumed to be reversible.
Module1-SICI.doc 4/09/04
Copyright K. Nasr, 2004 35
Getting More Accurate Results for Otto and Diesel Cycles: Variable vs.
Constant Specific Heats Model
We have hinted at the fact that better results would be obtained if the specific heats, c
v
and c
p
, dependence on temperature was accounted for. Here we re-solve the Otto cycle
problem using the variable specific heat model. Indeed, c
v
and c
p
depend heavily on
temperature and especially for the temperatures encountered for the spark ignition engine.
The variation of specific heats with temperature is shown below for air. As examples:
K. 3000 at 1.29 and K 300 at 1.4 k
K 3000 at 291 . 1 and K 300 at 004 . 1
K 3000 at 004 . 1 and K 300 at 718 . 0
* * p
* * v
K kg
kJ
K kg
kJ
K kg
kJ
K kg
kJ
c
c
Specific Heats of Air
[4]
Therefore, a better and more accurate analysis would be to account for the variation
of specific heats with temperature and use the fact that for an ideal gas the internal
energy and enthalpy are functions of temperature only.
Specific Heats as a Function of Temperature
0.7
0.8
0.9
1
1.1
1.2
1.3
1.4
0 500 1000 1500 2000 2500 3000 3500
Temperature
V
a
l
u
e
Constant Pressure Specific Heat (kJ/kg*K) Constant Volume Specific Heat (kJ/kg*K) Specific Heat Ratio
Concept Support:
Internal Energy, Enthalpy, and Specific Heats of Ideal Gases: u=u(T), h=h(T)
u and h for an ideal gas are a function of T only u = u(T), h= h(T) only
c
v
(T) = du/dT and c
p
(T) = dh/dT
h(T) = u(T) + Pv = u(T) + RT, thus dh = du + d(Pv) = du + d(RT) = du + RdT, and
dh - du = RdT or c
p
- c
v
= R. Also, c
p
/c
v
= k (ratio of specific heats)
Specific Heat Values of some gases are tabulated in Table A-20, pp. 838.
For air as an ideal gas, h and u are given in Table A-22, pp. 840.
Module1-SICI.doc 4/09/04
Copyright K. Nasr, 2004 36
The internal energy and enthalpy can then be tabulated across temperature directly rather than
relying on the specific heats being constant. This modeling enhancement accounting for variation of
the specific heat with temperature and is called the variable specific heat model. Lets examine
what it means to have an isentropic process of an ideal gas accounting for the variation of specific
heats with temperature.
Concept Support:
Isentropic Process for an Ideal Gas:
Recall that for an ideal gas: s(T
2
, p
2
) - s(T
1
, p
1
) = s
T
o
2
- s
T
o
1
- R ln (p
2
/p
1
)
Isentropic process s = 0 yielding: s
T
o
2
- s
T
o
1
= R ln (p
2
/p
1
)
Therefore; (p
2
/p
1
)
s = const
=
Exp
s
R
Exp
s
R
func T
func T
T
T
( )
( )
( )
( )
2
1
2
1
o
o
For convenience, define the Relative Pressure, p
r
: p
r
(T) = Exp(
s
R
)
T
o
(
p
2
p
1
)
s const
p
r
p
r
2
1
(X)
The relative pressure is tabulated as a function of T, as shown in Table A-22 for air.
Using p v = R T; v
2
/v
1
= ]
) (T p
RT
[ / ]
) (T p
RT
[ =
p
RT
p
RT
1 r
1
2 r
2
1
1
2
2
= (T
2
) / (T
1
)
Thus, we can also introduce a Relative Volume, v
r
, as a function of T only:
v
r
=
RT
p (T)
r
; we get: (v
2
/v
1
)
s = const
=
v
v
r2
r1
(XI)
The relative volume is tabulated as a function of T, as shown in Table A-22 for air.
Use equations (X) and (XI) for an ideal gas undergoing an isentropic process.
Special Case: Lets find out how properties are related for isentropic processes of an ideal gas
when the specific heats are considered to be constant: Recall that for constant specific heats:
s(T
2
, v
2
) - s(T
1
, v
1
) = c
v
ln(T
2
/T
1
) + R ln(v
2
/v
1
)
s(T
2
, p
2
) - s(T
1
, p
1
) = c
p
ln(T
2
/T
1
) - R ln(p
2
/p
1
)
Now, for an isentropic process: s = 0 c
v
ln(T
2
/T
1
) = - R ln(v
2
/v
1
)
c
p
ln(T
2
/T
1
) = R ln(p
2
/p
1
)
This is further simplified by letting k = c
p
/ c
v
, and recalling that c
p
- c
v
= R, we get:
k k
k
k
p
p
T
T
p
p
T
T
)
v
v
( )
v
v
( ) (
2
1
1
2 ) 1 (
2
1
1
2
) 1 (
1
2
1
2
These relationships are valid only for: Ideal Gas, Isentropic Process & Constant Specific Heats.
Module1-SICI.doc 4/09/04
Copyright K. Nasr, 2004 37
'
K kg
kJ o
T r
kg
kJ
TableA
r r
s p
K T u
v r v
* 4
4 4
22
3 4
86060 . 2 , 59 . 78
76 . 909 , 73 . 682
27 . 33 *
4
Here is a flow chart for the solution of the Otto cycle problem using the variable
specific heat model:
The 1
st
Law of Thermodynamics (Q W = U + KE + PE) gives:
o
T r r
TableA
s p , v u T
u u m Q u u m W
u u m Q u u m W
1 1 1 1
22
1
1 4 1 4 4 3 4 3
2 3 3 2 2 1 2 1
, ,
Energies) Internal (Specific s u' the all Need
) ( ), (
) ( ), (
(1) (2) is isentropic
o
T r
TableA
r
r
r
s p T u v
v
v
v
v
2 2 2 2
22
2
2
1
2
1
, , ,
o
T r r
TableA
s T p v u u
m
Q
u
3 3 3 3
22
3 2
3 2
3
, , , ; +
(3) (4) is isentropic
o
T r
TableA
r
r
r
r
r
s p T u v
v
v
r
v
v
v
v
4 4 4 4
22
4
3
4
3
4
3
4
, , ,
Lets apply numerical values to the above equations and use linear interpolation:
81 . 478 70 . 238 333
1 1
22
1
r kg
kJ
TableA
, v u K T
(1) (2) is isentropic
'
K kg
kJ o
T r
kg
kJ
TableA r
r
s p
K T u
r
v
v
* 2
2 2
22 1
2
66176 . 2 , 27 . 39
760 , 560
676 . 55
2
'
K kg
kJ o
T
r r
TableA
kg
kJ
kg
kJ
s K T
p v
kg
kJ
u
* 3
3 3
22
3
6758 . 3 , 1811
3 . 1346 , 869 . 3
57 . 1497 560
00074 . 0
6938 . 0
3
(3) (4) is isentropic
While we are finding the specific internal energies and the relative volumes, we can also
determine the temperatures, reference entropies, and relative pressures for the Variable
Specific Heat Model as tabulated below:
State T u s
o
p
r
v
r
(1) 333K
(Given)
238.70 kJ/kg 1.8175 kJ/kg*K 1.999 478.81
(2) 760K 560 kJ/kg 2.66176 kJ/kg*K 39.27 55.54
(3) 1811K 1497.57 kJ/kg 3.6758 kJ/kg*K 1346.3 3.869
(4) 909.76K 682.73 kJ/kg 2.86060 kJ/kg*K 78.59 33.27
Module1-SICI.doc 4/09/04
Copyright K. Nasr, 2004 38
Knowing all internal energy values, we can compute the quantities of interest:
1
W
2
,
3
W
4
,
2
Q
3
, and
4
Q
1
.
( )
( )
( )
( )
conserved. is Energy ,
3652 . 0
3652 . 0
3286 . 0 70 . 238 73 . 682 * 00074 . 0 ) (
6030 . 0 73 . 682 57 . 1497 * 00074 . 0 ) (
6938 . 0 560 57 . 1497 * 00074 . 0 ) (
2378 . 0 560 70 . 238 * 00074 . 0 ) (
1 4 1 4
4 3 4 3
2 3 3 2
2 1 2 1
Net Net
Net
Net
kg
kJ
kg
kJ
kg
kJ
kg
kJ
Q W
kJ Q
kJ W
kJ kg u u m Q
kJ kg u u m W
kJ kg u u m Q
kJ kg u u m W
To continue our application of the Variable Specific Heat Model, We would like to
determine the entropy change during the heat addition and heat rejection processes and
compare to these obtained via the Constant Specific Heat Model. Note that for the Otto
cycle, all processes were assumed to be reversible and therefore entropy production () is
zero. To do this we need to recalculate the pressures using our results from the Variable
Specific Heat Model.
K kg
kJ
o
T
o
T
K kg
kJ
o
T
o
T
r
r
r
r
P
P
R s s s s s
P
P
R s s s s s
kPa P
p
p
P
kPa P
T
T
P
kPa P
p
p
P
*
4
1
4 1 4 1 ion HeatReject
*
2
3
2 3 2 3 on HeatAdditi
3
3
4
4
2
2
3
3
1
1
2
2
7546 . 0 ln
7648 . 0 ln
22 . 273 *
36 . 4680 *
15 . 1964 *
,
_
,
_
Essentially the same value for the heat addition process. This is validated by the temperature-
entropy (T-s) diagram. They differ slightly because of rounding significant figures when
calculating reference entropy values and pressures. Recall also that the change in entropy during
the heat addition process using the Constant Specific Heat model (CSHM) was calculated as 0.7
kJ/kg K. A difference of 7.23 % between the two models. So now for the Variable Specific
Heat Model (VSHM), we get the following table of values:
Process W (kJ) Q (kJ) s (kJ/kgK)
gen
(kJ/K)
(1) (2) -0.2378 0 0 (Isentropic) 0 (Isentropic)
(2) (3) 0 0.6938 0.7648 0 (Reversible)
(3) (4) 0.6030 0 0 (Isentropic) 0 (Isentropic)
(4) (1) 0 -0.3286 -0.7546 0 (Reversible)
Net 0.3652 0.3652 0 (practically)
((2) (3) is a constant volume process.)
Module1-SICI.doc 4/09/04
Copyright K. Nasr, 2004 39
Now comparing the Constant Specific Heat Model (CSHM) results to the Variable
Specific Heat Model (VSHM) results, we present the following table:
Process W
CSHM
(kJ)
W
VSHM
(kJ)
Difference Q
CSHM
(kJ)
Q
VSHM
(kJ)
Difference
(1) (2) -0.2413 -0.2378 1.47% 0 0
(2) (3) 0 0 0.6938 0.6938 0%
(3) (4) 0.6414 0.6030 6.37% 0 0
(4) (1) 0 0 -0.2934 -0.3286 10.71%
Net 0.4001 0.3652 9.56% 0.4004 0.3652 9.64%
This allows us to make the following conclusions about the two models:
1. For low temperatures (process (1) (2)), the Constant Specific Heat Model can
be applied reliably and U
ideal gas
= m*c
V
*T and H
ideal gas
=m*c
P
*T.
2. For high temperatures (processes (2) (3), (3) (4), & (4) (1)), the variation
of specific heats with temperature should be accounted for to obtain better results.
Errors as high as 10% are not uncommon between the two models.
3. The variable specific heat model is more accurate than the constant specific heat
model because it accounts for the variation of specific heats with temperature.
4. The Variable Specific Heat Model is still a model and reflects a qualitative
analysis of the systems performance.
Module1-SICI.doc 4/09/04
Copyright K. Nasr, 2004 40
Learning Objectives:
Upon completion of Module 1 on Internal Combustion Engines, Spark Ignition and
Compression Ignition Engines, you should be able to accomplish the following learning
objectives:
1. Apply the ideal gas laws for perfect gases.
2. Recite the four processes of the Otto and Diesel cycles.
3. Know the difference between the Otto and Diesel cycles.
4. Quantify boundary work by or on a system for any relationship between pressure and
volume.
5. Apply the 1
st
Law of Thermodynamics for any process.
6. Match processes with the signs obtained for work and heat.
7. Define and use the thermal efficiency concept.
8. Define properties such as internal energy and enthalpy.
9. Make use of specific heats for the constant specific heat model.
10. Evaluate the internal energy change, enthalpy change, and entropy change for
constant specific heats.
11. Compute a value for the mean effective pressure.
12. Define and explain an isentropic process.
13. Utilize the variable specific heat model.
14. Evaluate the internal energy change, enthalpy change, and entropy change for
variable specific heats.
15. State the Kelvin-Planck Statement of the 2
nd
Law.
16. State the Carnot Principles.
17. Apply an understanding of processes such as reversible, adiabatic, polytropic,
isentropic, isothermal, etc.
18. Apply the 2
nd
Law of Thermodynamics.
19. Make use of relative pressures and relative volumes of analyzing isentropic processes.
20. Explain and compute entropy production for a closed system.
Through coverage of Module 1, we have covered the corresponding items in your textbook:
1. Chapter 1. Introducing concepts and definitions
2. Chapter 2. Energy and the 1
st
Law of Thermodynamics.
3. Chapter 3. Page 116120 = Sections 3.5 & 3.6: Ideal gas model, Internal Energy,
Enthalpy, and Specific Heats of Ideal Gases, Page 130 = Section 3.8:
Polytropic Processes of an Ideal Gas.
4. Chapter 5. Page 206217 = Section 5.25.4: Statements of the 2
nd
Law of
Thermodynamics.
5. Chapter 6. Page 246251, Page 254257, Page 274280: Entropy.
6. Chapter 9. Page 425440: Gas Power Systems, Otto & Diesel Cycles.
Note: we have modeled gas power cycles first with constant specific heats.
Later, we accounted for the variation of specific heats with temperature
through reference entropies and relative pressure and relative volumes. This
is called the variable specific heat model. Your Chapter 9 examples are solved
with the variable specific heats model.
Module1-SICI.doc 4/09/04
Copyright K. Nasr, 2004 41
[1]
Adapted from Engineering Fundamentals of the Internal Combustion Engine, W.W.
Pulkrabek, Prentice Hall, 1997, Example 3-1, Pg. 77.
[2]
From Thermodynamics: An Enigineering Approach, Y. A. Cengel & M. A. Boles,
4
th
. Ed., McGraw-Hill, 2002, Figure 8-13, Pg. 458.
[3]
From Fundamentals of Engineering Thermodynamics, M. J. Moran & H. N.
Shapiro, 4
th
. Ed., Wiley, 2000, Figure 9.1, Pg. 426.
[4]
From Engineering Fundamentals of the Internal Combustion Engine, W. W.
Pulkrabek, Prentice Hall, 1997, Table A-1, Pg. 379.
Você também pode gostar
- A Heartbreaking Work Of Staggering Genius: A Memoir Based on a True StoryNo EverandA Heartbreaking Work Of Staggering Genius: A Memoir Based on a True StoryNota: 3.5 de 5 estrelas3.5/5 (231)
- The Sympathizer: A Novel (Pulitzer Prize for Fiction)No EverandThe Sympathizer: A Novel (Pulitzer Prize for Fiction)Nota: 4.5 de 5 estrelas4.5/5 (121)
- Never Split the Difference: Negotiating As If Your Life Depended On ItNo EverandNever Split the Difference: Negotiating As If Your Life Depended On ItNota: 4.5 de 5 estrelas4.5/5 (838)
- The Little Book of Hygge: Danish Secrets to Happy LivingNo EverandThe Little Book of Hygge: Danish Secrets to Happy LivingNota: 3.5 de 5 estrelas3.5/5 (400)
- Devil in the Grove: Thurgood Marshall, the Groveland Boys, and the Dawn of a New AmericaNo EverandDevil in the Grove: Thurgood Marshall, the Groveland Boys, and the Dawn of a New AmericaNota: 4.5 de 5 estrelas4.5/5 (266)
- The Subtle Art of Not Giving a F*ck: A Counterintuitive Approach to Living a Good LifeNo EverandThe Subtle Art of Not Giving a F*ck: A Counterintuitive Approach to Living a Good LifeNota: 4 de 5 estrelas4/5 (5795)
- The Gifts of Imperfection: Let Go of Who You Think You're Supposed to Be and Embrace Who You AreNo EverandThe Gifts of Imperfection: Let Go of Who You Think You're Supposed to Be and Embrace Who You AreNota: 4 de 5 estrelas4/5 (1090)
- The World Is Flat 3.0: A Brief History of the Twenty-first CenturyNo EverandThe World Is Flat 3.0: A Brief History of the Twenty-first CenturyNota: 3.5 de 5 estrelas3.5/5 (2259)
- The Hard Thing About Hard Things: Building a Business When There Are No Easy AnswersNo EverandThe Hard Thing About Hard Things: Building a Business When There Are No Easy AnswersNota: 4.5 de 5 estrelas4.5/5 (345)
- The Emperor of All Maladies: A Biography of CancerNo EverandThe Emperor of All Maladies: A Biography of CancerNota: 4.5 de 5 estrelas4.5/5 (271)
- Team of Rivals: The Political Genius of Abraham LincolnNo EverandTeam of Rivals: The Political Genius of Abraham LincolnNota: 4.5 de 5 estrelas4.5/5 (234)
- Hidden Figures: The American Dream and the Untold Story of the Black Women Mathematicians Who Helped Win the Space RaceNo EverandHidden Figures: The American Dream and the Untold Story of the Black Women Mathematicians Who Helped Win the Space RaceNota: 4 de 5 estrelas4/5 (895)
- Elon Musk: Tesla, SpaceX, and the Quest for a Fantastic FutureNo EverandElon Musk: Tesla, SpaceX, and the Quest for a Fantastic FutureNota: 4.5 de 5 estrelas4.5/5 (474)
- The Yellow House: A Memoir (2019 National Book Award Winner)No EverandThe Yellow House: A Memoir (2019 National Book Award Winner)Nota: 4 de 5 estrelas4/5 (98)
- The Unwinding: An Inner History of the New AmericaNo EverandThe Unwinding: An Inner History of the New AmericaNota: 4 de 5 estrelas4/5 (45)
- Study Plan For The Mechanical PE ExamDocumento2 páginasStudy Plan For The Mechanical PE ExamMatthew Leaper100% (2)
- Anti-Dandruff Shampoo With Zinc Pyrithione: INCI Name, Trade Name Weight % FunctionDocumento2 páginasAnti-Dandruff Shampoo With Zinc Pyrithione: INCI Name, Trade Name Weight % FunctionandreeaAinda não há avaliações
- Solar and Stellar Magnetic Activity - ISBN0521582865 PDFDocumento402 páginasSolar and Stellar Magnetic Activity - ISBN0521582865 PDFOmar Musalem100% (1)
- GEMSS-M-15 Rev 02 - Desalination Plant and Desalinated Water TanksDocumento20 páginasGEMSS-M-15 Rev 02 - Desalination Plant and Desalinated Water TanksKaramSobhyAinda não há avaliações
- (S. Ueno) Biological Effects of Magnetic and Elect PDFDocumento243 páginas(S. Ueno) Biological Effects of Magnetic and Elect PDFmeloszAinda não há avaliações
- Tpl-Kipl Jvkarnalstp-20cdd003 Design Docoments Part 1 Client 28-11-18Documento1.057 páginasTpl-Kipl Jvkarnalstp-20cdd003 Design Docoments Part 1 Client 28-11-18navneet3bawa100% (1)
- A Sustainable Bioplastic Obtained From Rice StrawDocumento12 páginasA Sustainable Bioplastic Obtained From Rice StrawNovrynda Eko SatriawanAinda não há avaliações
- PTC's Mechanical Engineering: Product DesignDocumento2 páginasPTC's Mechanical Engineering: Product Designjegan172Ainda não há avaliações
- English German French Italian Spanish Japanese Korean Chinese Simplified Chinese TraditionalDocumento1 páginaEnglish German French Italian Spanish Japanese Korean Chinese Simplified Chinese Traditionaljegan172Ainda não há avaliações
- English German French Italian Spanish Japanese Korean Chinese Simplified Chinese TraditionalDocumento1 páginaEnglish German French Italian Spanish Japanese Korean Chinese Simplified Chinese Traditionaljegan172Ainda não há avaliações
- Product Design: 3D Cad Parametric ModelingDocumento1 páginaProduct Design: 3D Cad Parametric Modelingjegan172Ainda não há avaliações
- Solutions Windchill Product Lifecycle Management (PLM) MathcadDocumento1 páginaSolutions Windchill Product Lifecycle Management (PLM) Mathcadjegan172Ainda não há avaliações
- Creo Is A Family or Suite Of: CocreateDocumento1 páginaCreo Is A Family or Suite Of: Cocreatejegan172Ainda não há avaliações
- Welcome To CNC Training ProgrammeDocumento4 páginasWelcome To CNC Training Programmejegan172Ainda não há avaliações
- Basic Components of NC Machine SystemDocumento1 páginaBasic Components of NC Machine Systemjegan172Ainda não há avaliações
- Basic Components of NC Machine SystemDocumento1 páginaBasic Components of NC Machine Systemjegan172Ainda não há avaliações
- Ubpro Lling M TH Lu TH B Me THR Uti T TH ND ND TH Uti The Lling #1 LlingDocumento1 páginaUbpro Lling M TH Lu TH B Me THR Uti T TH ND ND TH Uti The Lling #1 Llingjegan172Ainda não há avaliações
- A Basic Example: Setup and DefinitionsDocumento1 páginaA Basic Example: Setup and Definitionsjegan172Ainda não há avaliações
- Branding Client Server Collaborative Software Platform: BM Notes (Formerly Lotus Notes SeeDocumento1 páginaBranding Client Server Collaborative Software Platform: BM Notes (Formerly Lotus Notes Seejegan172Ainda não há avaliações
- This MacroDocumento1 páginaThis Macrojegan172Ainda não há avaliações
- Autocad 1Documento1 páginaAutocad 1jegan172Ainda não há avaliações
- Auto CadDocumento1 páginaAuto Cadjegan172Ainda não há avaliações
- January 2005 ProgrammingDocumento1 páginaJanuary 2005 Programmingjegan172Ainda não há avaliações
- Polar Osnap Osnap PolarDocumento2 páginasPolar Osnap Osnap Polarjegan172Ainda não há avaliações
- Autocad ProDocumento1 páginaAutocad Projegan172Ainda não há avaliações
- Autocad TutDocumento1 páginaAutocad Tutjegan172Ainda não há avaliações
- G Code Example LatheDocumento1 páginaG Code Example Lathejegan172Ainda não há avaliações
- A Basic Example: Setup and DefinitionsDocumento1 páginaA Basic Example: Setup and Definitionsjegan172Ainda não há avaliações
- Program Structure: January 2005 ProgrammingDocumento1 páginaProgram Structure: January 2005 Programmingjegan172Ainda não há avaliações
- G-Code Program: A Basic ExampleDocumento1 páginaG-Code Program: A Basic Examplejegan172Ainda não há avaliações
- A Basic Example: Setup and DefinitionsDocumento1 páginaA Basic Example: Setup and Definitionsjegan172Ainda não há avaliações
- Phar 1205L Activity 2Documento6 páginasPhar 1205L Activity 2Draco PhoenixAinda não há avaliações
- HILTI ExBar Rebar Design Soft WareDocumento30 páginasHILTI ExBar Rebar Design Soft WareAline Tabet Abi SaadAinda não há avaliações
- Hexcel Sandwich DesignDocumento28 páginasHexcel Sandwich DesignAdnan AnsariAinda não há avaliações
- Indion 850 Resin Engg Data SheetDocumento6 páginasIndion 850 Resin Engg Data SheetsoumitrabanAinda não há avaliações
- Bonding Theories of Coordination CompoundsDocumento6 páginasBonding Theories of Coordination Compoundsmark carlo SanorjoAinda não há avaliações
- Removal of Gaseous Chlorine From Cylinders and Ton ContainersDocumento4 páginasRemoval of Gaseous Chlorine From Cylinders and Ton Containersmailmaverick8167Ainda não há avaliações
- Density of KOH SolutionsDocumento1 páginaDensity of KOH SolutionsjohnihaasAinda não há avaliações
- Dental Materials Reviewer Pt. 10Documento5 páginasDental Materials Reviewer Pt. 10Ryo MiyataAinda não há avaliações
- Chemguard c333 Ar-Afff 3% MsdsDocumento7 páginasChemguard c333 Ar-Afff 3% MsdsrendydunAinda não há avaliações
- Wheatstone BridgeDocumento4 páginasWheatstone BridgeTasnim RahmanAinda não há avaliações
- PG Brochure 2014Documento59 páginasPG Brochure 2014ParinitaSahooAinda não há avaliações
- Herb-Modle Peptide in Anaerobic Titration and Reduce-05032013Documento4 páginasHerb-Modle Peptide in Anaerobic Titration and Reduce-05032013陳育孝Ainda não há avaliações
- 2.Pv Semisolid FDADocumento37 páginas2.Pv Semisolid FDARahayu Maulida RAinda não há avaliações
- Chapter 5 - NewDocumento25 páginasChapter 5 - NewJW MAinda não há avaliações
- Pearson's Classification of Lewis Acids and Lewis Bases Into Hard and Soft - Acids and BasesDocumento5 páginasPearson's Classification of Lewis Acids and Lewis Bases Into Hard and Soft - Acids and BasesThantea ChhakchhuakAinda não há avaliações
- Unit 4 Study Guide Solutions - Kinetics & ThermoDocumento3 páginasUnit 4 Study Guide Solutions - Kinetics & ThermoPenguin/CatAinda não há avaliações
- Iit Model Paper PDFDocumento14 páginasIit Model Paper PDFstudysteps.in100% (2)
- FaienceDocumento15 páginasFaienceRenata TatomirAinda não há avaliações
- HW3 QM2 Fall2014Documento1 páginaHW3 QM2 Fall2014Mena Awan100% (1)
- Metalcraft 4Documento90 páginasMetalcraft 4Ana DuranAinda não há avaliações
- Autolab Brochure 2013 en LRDocumento40 páginasAutolab Brochure 2013 en LRdanidondoniAinda não há avaliações
- Section A: Multiple Choice Questions (Compulsory)Documento19 páginasSection A: Multiple Choice Questions (Compulsory)ivyAinda não há avaliações
- The Latent Heat of Fusion of IceDocumento4 páginasThe Latent Heat of Fusion of Iceeid elsayedAinda não há avaliações