Escolar Documentos
Profissional Documentos
Cultura Documentos
Nomenclature - Prob Set
Enviado por
Genny MarantanDireitos autorais
Formatos disponíveis
Compartilhar este documento
Compartilhar ou incorporar documento
Você considera este documento útil?
Este conteúdo é inapropriado?
Denunciar este documentoDireitos autorais:
Formatos disponíveis
Nomenclature - Prob Set
Enviado por
Genny MarantanDireitos autorais:
Formatos disponíveis
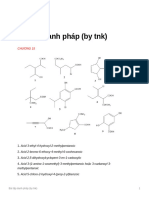
CHEM 31 (Maam Anj) Chemical Nomenclature of Organic Compounds I. ALKANES A. Give the structural (skeletal) formula of: 1.
4-t-butylheptane 2. 6,6-dimethyl-5-(1,2,2-trimethylpropyl)dodecane 3. 3,4,5-trimethyl-4-propyloctane 4. 6-(3-methylbutyl)-undecane 5. 3,4,4,5-tetramethylheptane B. Give the systematic (IUPAC) name of: 1. (CH3)3CCH2C(CH3)3 2. (CH3)3CHCH2CH2CH(C2H5)2 3. (CH3)2CHCH(CH3)CH2C(C2H5)2CH3 4. (CH3CH2)4C
1. 2. 3. 4. 5.
2-dimethylpropane 3-propylhexane 3,5,6,7-tetramethylnonane 3-isopropyl-5,5-dimethyloctane 3,4,5-trimethyl-4-butyloctane
II. CYCLOALKANES A. Give the structural (skeletal) formula of: 1. ethylcyclopentane 2. cis-1,3-dimethylcyclobutane 3. cyclohexylcyclohexane 4. trans-2-chloro-1-isobutylcyclohexane 5. 1,1-dimethyl-4-chlorocycloheptane B. Give the systematic (IUPAC) name of:
1. 5. 2. 3. 6. 4. 7. 5.
8.
6. III. ALKYL HALIDES
9.
A. Draw the structures and provide systematic names for 1-3 by substituting a chlorine for a hydrogen of methylcyclohexane: 1. a primary alkyl halide 2. a tertiary alkyl halide
10. C. Find out why each of the following names is incorrect. Give the correct name:
3. three secondary alkyl halides
1 of 6
B. Name the following compounds, and tell whether each alkyl halide is primary, secondary, or tertiary: 1. 2. 3. 4.
5. 6.
7.
8.
5.
6. IV. ALKENES A. Give the structural (skeletal) formula of: 1. trans-3,4-dimethyl-2-pentene 2. (Z)-2-bromo-2-pentene 3. trans-3,4-dimethylcyclobutane 4. (E)-3-methyl-2-hexene 5. (Z)-3-chloro-4-methyl-3-hexene 6. 3-methylcyclohexene B. Give the IUPAC name of: 1. 2.
9.
10. C. Molly Kule was a lab technician who was asked by her supervisor to add names to the labels on a collection of alkenes that showed only structures on the labels. How many did Molly get right? Correct the incorrect names.
V. ALKYNES A. Give the structural (skeletal) formula of: 1. isopropylacetylene 2. dimethylacetylene
3.
3. cyclopropylacteylene 4. vinylacetylene
4.
5. 3-methyl-1-pentyne
2 of 6
6. 1-chloro-3-hexyne 7. propargyl chloride 8. cyclooctyne 9. dodec-4-ene-2-yne 10. 1,5-diiodopent-3-en-1-yne B. Give the IUPAC name of: 1. 2. 6. 5. 4. 3.
3.
7. VII. ETHERS AND EPOXIDES A. Draw the structural formula for: 1. Isobutyl methyl ether 2. methyl ethynyl ether
4.
5. VI. ALCOHOLS A. Draw the structural formula for: 1. neopentyl alcohol 2. ethane-1,2-diol 3. 2-ethyl-5-methylcyclohexanol 4. 6-bromo-4-ethyl-2-heptanol 5. 2-chloro-3-pentanol 6. benzyl alcohol 7. propane-1,2,3-triol 8. isoamyl alcohol B. Give each of the following compounds a systematic name, and indicate whether each is a primary, secondary, or tertiary alcohol: 1. 4. 2. 3 of 6
3. 4-t-butyl-3-methoxyheptane 4. diallyl ether 5. 3-isopropoxypenta-1,4-diyne 6. 2,2,3,3-tetramethyloxirane 7. 2,3-epoxy-2-methylpentane B. Give each of the following compounds a systematic name, and indicate whether each is a primary, secondary, or tertiary alcohol: 1. 2.
3.
IX. THIOLS (MERCAPTANS) AND SULFIDES (THIOETHERS) 5. A. Draw the structure of each of the following compounds: 1. ethyl neopentyl sulfide 2. 2-methylthiocyclopentanone 3. cyclohexanethiol 4. 2-methyl-2-propanethiol 5. 1,3-propenedithiol B. Give the systematic and/or common name of: 2. methyldipropylamine 3. N-ethylethanamine 2. 3. 1. (CH3CH2CH2)2S
6. VIII. AMINES A. Draw the structure of: 1. 2-methyl-N-propyl-1-propanamine
4. N,N-dimethylpentan-3-amine 5. 5-methylhexan-1-amine 6. Cyclohexylethylmethylamine B. For each of the following compounds, give the systematic name and the common name (for those that have common names), and indicate whether the amines are primary, secondary, tertiary or quaternary: 1.
4. 5. X. AROMATIC COMPOUNDS A. Draw the structure of: 1. m-divinylbenzene 2. o-diisopropylbenzene 3. 2,4-dinitrotoluene
2. 3.
4. 3-chlorotoluene 5. p-isobutylphenol
4.
6. m-chlorobenzonitrile 7. 2-bromo-4-iodophenol 8. o-nitroaniline
5.
9. phenylacetonitrile 10. ortho-chlorobenzenesulfonic acid B. Give the systematic name of:
6.
7.
1.
2. 4 of 6
1. -methoxyvaleric acid 2. p-toluic acid 3. 3. cyclopentanecarboxylic acid 4. propenoic acid 5. 2-hydroxypropane- 1,2,3-tricarboxylic acid 4. B. Give the systematic name of:
5. XI. ALDEHYDES AND KETONES A. Draw the structure and give the systematic name of: 1. acetone 2. methyl isobutyl ketone 3. formaldehyde 4. valeraldehyde 5. -bromopropionaldehyde 6. 2,4-pentanedione 7. 2-(3-oxopentyl)-cyclohexanone 8. Butyrophenone 9. 4-hexen-2-one 10. benzophenone B. Give the systematic name of:
1.
2. 3. 4.
5. XIII. CARBOXYLIC ACID DERIVATIVES A. Draw the structure of: 1. N,3-diethylhexanamide 2. cyclohexanecarboxamide 3. acetic formic anhydride 4. potassium methylbutyrate 5. N,N-dimethylformamide
1.
6. cyclohexanecarbonyl chloride 7. benzoic anhydride
2. 3.
8. 3-methylbutanenitrile 9. N-benzylethanamide 10. 2-azacyclobutanone B. Give the systematic name of:
4.
5. XII. CARBOXYLIC COMPOUNDS A. Draw the structure of:
1.
2. 5 of 6
3. 4.
VI
VII
5. VIII 6. 7. XI
8. 9. 10. XIV. Look at the structures of some organic compounds. Name the functional group present in each molecule and encircle the part of the molecule that you refer to. What is the IHD of each compound? Support your answer with calculations
II
III
IV
6 of 6
7 of 6
Você também pode gostar
- Moncyclic Azepines: The Syntheses and Chemical Properties of the Monocyclic AzepinesNo EverandMoncyclic Azepines: The Syntheses and Chemical Properties of the Monocyclic AzepinesGeorge R. ProctorAinda não há avaliações
- Organic WorksheetsDocumento16 páginasOrganic WorksheetsjcdanezAinda não há avaliações
- Chapter Test Cyclic Hydrocarbon Nomenclature 1Documento4 páginasChapter Test Cyclic Hydrocarbon Nomenclature 1Allyza Mikaella LabradorAinda não há avaliações
- Chapter Test Alcohol and Ether Nomenclature 1Documento5 páginasChapter Test Alcohol and Ether Nomenclature 1Allyza Mikaella LabradorAinda não há avaliações
- Kish Salas Bschem 2H2 Activity No. 1 Nomenclature of Saturated HydrocarbonsDocumento2 páginasKish Salas Bschem 2H2 Activity No. 1 Nomenclature of Saturated HydrocarbonsKish Floran SalasAinda não há avaliações
- Organic ChemistryDocumento94 páginasOrganic Chemistrybj primeAinda não há avaliações
- WS SL NamingPracticeDocumento1 páginaWS SL NamingPracticeGüşta İrem SakızAinda não há avaliações
- IUPACDocumento2 páginasIUPACAnubrata SarkarAinda não há avaliações
- Key Quiz Organic CompoundsDocumento3 páginasKey Quiz Organic CompoundsMA. ESTHER FAUSTA TIAMZONAinda não há avaliações
- Q2 GC Summative W 5-6Documento2 páginasQ2 GC Summative W 5-6Shane RickzelleAinda não há avaliações
- Hydrocarbons WorksheetDocumento2 páginasHydrocarbons WorksheetCatherine ChartzeenAinda não há avaliações
- Oc NomenclaturepracticeDocumento10 páginasOc Nomenclaturepracticekrishiv vyas :- 1022Ainda não há avaliações
- Document 1Documento9 páginasDocument 1Nishi tomarAinda não há avaliações
- Bài Tập Danh PhápDocumento9 páginasBài Tập Danh PhápTrần Thị Kiều TrangAinda não há avaliações
- Quiz 01: Systematic NomenclatureDocumento2 páginasQuiz 01: Systematic NomenclatureErica WuAinda não há avaliações
- NotesDocumento28 páginasNotesYbynybybyhAinda não há avaliações
- GENCHEMMID1Documento10 páginasGENCHEMMID1Metran Arvic Earl JustinAinda não há avaliações
- Organic Chemistry QuizDocumento7 páginasOrganic Chemistry QuizCarlo Jay BasulAinda não há avaliações
- NomenclatureDocumento3 páginasNomenclaturecrystalgal9462Ainda não há avaliações
- Latihan Soal Kimia Organik I: Kelas CaDocumento21 páginasLatihan Soal Kimia Organik I: Kelas CarestyAinda não há avaliações
- 2016 Worksheet For Medicine StudentsDocumento2 páginas2016 Worksheet For Medicine StudentsbedadadenbalprosperityAinda não há avaliações
- 15 Naming and Drawing Functional Groups Practice WorksheetDocumento4 páginas15 Naming and Drawing Functional Groups Practice WorksheetCorey Becker33% (6)
- Arjuna JEE 2.0 (2024) : IUPAC NomenclatureDocumento3 páginasArjuna JEE 2.0 (2024) : IUPAC NomenclatureDEV SHARMAAinda não há avaliações
- Naming Organic MoleculesDocumento47 páginasNaming Organic MoleculesSandeep BadarlaAinda não há avaliações
- Organic Chemistry Naming ExaminationDocumento6 páginasOrganic Chemistry Naming ExaminationHaa Kksak100% (1)
- Work Sheet Alkanes Sbi 09Documento6 páginasWork Sheet Alkanes Sbi 09neneng rohayatiAinda não há avaliações
- Chem Test 1Documento5 páginasChem Test 1dishaali110Ainda não há avaliações
- 61c3f345fc2e1700f41ec8be - ## - IUPAC Nomenclature: Homework PYQDocumento29 páginas61c3f345fc2e1700f41ec8be - ## - IUPAC Nomenclature: Homework PYQHhsjsAinda não há avaliações
- CONS Orgchem WorksheetsDocumento18 páginasCONS Orgchem Worksheetssan_cdkeyAinda não há avaliações
- Nomenclature (Exercise 1)Documento3 páginasNomenclature (Exercise 1)kristiane93Ainda não há avaliações
- 2023 - 03 - 25 - Oc Mka 10 Hours Maha One ShotDocumento197 páginas2023 - 03 - 25 - Oc Mka 10 Hours Maha One ShotgvygtvcAinda não há avaliações
- Organic Chemistry QuizDocumento7 páginasOrganic Chemistry QuizCarlo Jay BasulAinda não há avaliações
- Ch-3-Organic ChemDocumento3 páginasCh-3-Organic Chemzia khanAinda não há avaliações
- Exercise - Nomenclature of Organic Compounds FinalDocumento10 páginasExercise - Nomenclature of Organic Compounds FinalVina YuAinda não há avaliações
- Home Work 1 Answers3Documento6 páginasHome Work 1 Answers3John Fil PabloAinda não há avaliações
- Alcohol and EtherDocumento30 páginasAlcohol and EtherSamratAinda não há avaliações
- Unit 2 HydrocarbonDocumento41 páginasUnit 2 Hydrocarbonzila maskamAinda não há avaliações
- PROBSET-Drawing-And-Nomenclature Grp10 PT14 Sanchez, Sarmiento, ThettathDocumento4 páginasPROBSET-Drawing-And-Nomenclature Grp10 PT14 Sanchez, Sarmiento, ThettathDale Miko SanchezAinda não há avaliações
- Q2 2022 OrgchemDocumento2 páginasQ2 2022 OrgchemHazeljoyce AlcantaraAinda não há avaliações
- Cbse Test Paper-01 CLASS - XII CHEMISTRY (Haloalkanes and Haloarenes) Topic:-NomenclatureDocumento4 páginasCbse Test Paper-01 CLASS - XII CHEMISTRY (Haloalkanes and Haloarenes) Topic:-NomenclatureSumit SharmaAinda não há avaliações
- Cbse Test Paper-01 CLASS - XII CHEMISTRY (Haloalkanes and Haloarenes) Topic:-NomenclatureDocumento4 páginasCbse Test Paper-01 CLASS - XII CHEMISTRY (Haloalkanes and Haloarenes) Topic:-NomenclatureSumit SharmaAinda não há avaliações
- Module 5 OrgchemDocumento9 páginasModule 5 OrgchemJHUNNTY LOZANOAinda não há avaliações
- He Following Are The List of Aliphatic Compounds, According To The Number of Carbon Atoms They Contain. Complete The Table BelowDocumento17 páginasHe Following Are The List of Aliphatic Compounds, According To The Number of Carbon Atoms They Contain. Complete The Table BelowJohn Rafael DiazAinda não há avaliações
- Easy and Selective Method For The Synthesis of Various Mono-O-Functionalized Calix (4) Arenes: De-O-Functionalization Using TiclDocumento9 páginasEasy and Selective Method For The Synthesis of Various Mono-O-Functionalized Calix (4) Arenes: De-O-Functionalization Using TiclDiogomussumAinda não há avaliações
- Work Sheet Alkanes Sbi 09Documento5 páginasWork Sheet Alkanes Sbi 09Elfi Susanti VHAinda não há avaliações
- Tutorial 5Documento4 páginasTutorial 5Eqieyn JerrAinda não há avaliações
- PRACTICE SHEET - 01 (Chemistry) : Basic Concept of Organic (IUPAC, Isomerism)Documento6 páginasPRACTICE SHEET - 01 (Chemistry) : Basic Concept of Organic (IUPAC, Isomerism)ABD 17Ainda não há avaliações
- Tutorial Problems, 2013/2014: Che 153 Organic Chemistry For EngineersDocumento21 páginasTutorial Problems, 2013/2014: Che 153 Organic Chemistry For EngineersIng Rashid BawahAinda não há avaliações
- RA Sir JEE PYQ Organic ChemistryDocumento191 páginasRA Sir JEE PYQ Organic Chemistrydash gupta100% (4)
- Carboxylic Acids Worksheet1 Key 2Documento2 páginasCarboxylic Acids Worksheet1 Key 2Ana Pamela MejiaAinda não há avaliações
- DPP NomenclatureDocumento7 páginasDPP Nomenclaturegamishtag18Ainda não há avaliações
- Nomenclature - DPP 03 - Arjuna NEET 2024Documento3 páginasNomenclature - DPP 03 - Arjuna NEET 2024mdjakaullah847226Ainda não há avaliações
- Hydrocarbons Worksheet AnswersDocumento2 páginasHydrocarbons Worksheet AnswersNorman Vryne Cadua100% (1)
- Nomenclature - DPP 02 (Of Lec 04) - (Arjuna JEE 2023)Documento2 páginasNomenclature - DPP 02 (Of Lec 04) - (Arjuna JEE 2023)jeemainsmaterial97Ainda não há avaliações
- Pervious Year Question (PYQ) - 2022Documento212 páginasPervious Year Question (PYQ) - 2022Vasu PantAinda não há avaliações
- DPPONIUPACSUPERSIXER4Documento5 páginasDPPONIUPACSUPERSIXER4Kartik YadavAinda não há avaliações
- Problem Set 2 Solution PDFDocumento2 páginasProblem Set 2 Solution PDFLouisAinda não há avaliações
- Nomenclature of Polyfunctional Organic CompoundsDocumento6 páginasNomenclature of Polyfunctional Organic CompoundsEman Jamil El-AgroudyAinda não há avaliações
- Pharm D POC QuestionsDocumento16 páginasPharm D POC Questionspradeep36Ainda não há avaliações
- Documentation Sheet Sterilization EnglishDocumento2 páginasDocumentation Sheet Sterilization EnglishBanita CarmenAinda não há avaliações
- NT PotentialMiningDevelopmentsDocumento13 páginasNT PotentialMiningDevelopmentsho100hoAinda não há avaliações
- Unit 3 - Chemistry - WWW - Rgpvnotes.inDocumento16 páginasUnit 3 - Chemistry - WWW - Rgpvnotes.inN S PatidarAinda não há avaliações
- Manuf Lec Finals PDFDocumento35 páginasManuf Lec Finals PDFRaizane Sky PalecAinda não há avaliações
- Exp 7 (Solved)Documento9 páginasExp 7 (Solved)mahmudul100% (1)
- #Tutorial 3 - Fossil Fule Steam Generators TroubleshootingDocumento5 páginas#Tutorial 3 - Fossil Fule Steam Generators Troubleshootingmohamed EldesokyAinda não há avaliações
- Chemistry 2 Year Ch-08 & 10 Marks.50 Timing: 1:30 HourDocumento2 páginasChemistry 2 Year Ch-08 & 10 Marks.50 Timing: 1:30 HourMusaddiq AzizAinda não há avaliações
- Corrosion Inhibitor Advice - Ready To Buy Online ChemicalsDocumento3 páginasCorrosion Inhibitor Advice - Ready To Buy Online ChemicalsAhmed TaherAinda não há avaliações
- Materials and Design: Da Li, Ligang Liu, Yunkun Zhang, Chunlei Ye, Xuejun Ren, Yulin Yang, Qingxiang YangDocumento6 páginasMaterials and Design: Da Li, Ligang Liu, Yunkun Zhang, Chunlei Ye, Xuejun Ren, Yulin Yang, Qingxiang YangjoeljAinda não há avaliações
- Study Material - Engineering Chemistry MODULE 5-MODULE - 550Documento12 páginasStudy Material - Engineering Chemistry MODULE 5-MODULE - 550ChanduAinda não há avaliações
- CHAP4 - Magmatic Sulfide DepositsDocumento11 páginasCHAP4 - Magmatic Sulfide DepositsJaime CuyaAinda não há avaliações
- Acticide MBSDocumento4 páginasActicide MBSNRunixAinda não há avaliações
- Epa 9081 Cation-Exchange Capacity of Soils (Sodium Acetate)Documento4 páginasEpa 9081 Cation-Exchange Capacity of Soils (Sodium Acetate)ErickAinda não há avaliações
- Histopathologic TechniquesDocumento23 páginasHistopathologic TechniquesElla Sales83% (6)
- Hirayama HVE 50 PDFDocumento40 páginasHirayama HVE 50 PDFtyanto101150% (2)
- New Solvent For Polyamide 66 and Its Use For Preparing Single-Polymer Composite Coated-FabricDocumento24 páginasNew Solvent For Polyamide 66 and Its Use For Preparing Single-Polymer Composite Coated-FabricSSAinda não há avaliações
- Fire and Fire ExtinguishmentDocumento28 páginasFire and Fire ExtinguishmentRahul RamachandranAinda não há avaliações
- Acids and BasesDocumento18 páginasAcids and BasesSunnyMoon21Ainda não há avaliações
- Molecular Geometry and CrystallineDocumento102 páginasMolecular Geometry and CrystallineRenAinda não há avaliações
- J.vibspec.2013.02.001 Ftir Batio3Documento6 páginasJ.vibspec.2013.02.001 Ftir Batio3Seni Ramadhanti SAinda não há avaliações
- Section A: CLASS X (2020-21) Science (Code 086) Sample Paper-1Documento8 páginasSection A: CLASS X (2020-21) Science (Code 086) Sample Paper-1Anime FeelsAinda não há avaliações
- Final Exam BCH 3023: Multiple ChoiceDocumento13 páginasFinal Exam BCH 3023: Multiple ChoicecwodAinda não há avaliações
- Cold Process Soap Swirling Tips: Temperatures WaterDocumento8 páginasCold Process Soap Swirling Tips: Temperatures WaterGua DaAinda não há avaliações
- 5 Ways To Reduce COD in WastewaterDocumento2 páginas5 Ways To Reduce COD in WastewaterSo MriAinda não há avaliações
- Feasibility of Modifying Existing Chemistry Demonstrations by Using Substitute MaterialsDocumento18 páginasFeasibility of Modifying Existing Chemistry Demonstrations by Using Substitute MaterialsRosalyn TercianoAinda não há avaliações
- Cambridge International Advanced Subsidiary and Advanced LevelDocumento12 páginasCambridge International Advanced Subsidiary and Advanced LevelJeremiah HopeAinda não há avaliações
- Shell Morlina S4 B 150: Performance, Features & Benefits Main ApplicationsDocumento4 páginasShell Morlina S4 B 150: Performance, Features & Benefits Main ApplicationsMustafa KhanAinda não há avaliações
- AMP16609P61Documento1 páginaAMP16609P61Gutha Giribabu NaiduAinda não há avaliações
- Chapter 11 - Surfacing and Shape Welding PDFDocumento15 páginasChapter 11 - Surfacing and Shape Welding PDFCarlos GarcíaAinda não há avaliações
- Liquid - Liquid Extraction in A Packed Bed: Experiment No: 2Documento23 páginasLiquid - Liquid Extraction in A Packed Bed: Experiment No: 2Sameep JainAinda não há avaliações