Escolar Documentos
Profissional Documentos
Cultura Documentos
Introduction To Interpretation of Infrared Spectra
Enviado por
Benni WewokDescrição original:
Título original
Direitos autorais
Formatos disponíveis
Compartilhar este documento
Compartilhar ou incorporar documento
Você considera este documento útil?
Este conteúdo é inapropriado?
Denunciar este documentoDireitos autorais:
Formatos disponíveis
Introduction To Interpretation of Infrared Spectra
Enviado por
Benni WewokDireitos autorais:
Formatos disponíveis
Introduction to Interpretation of Infrared Spectra
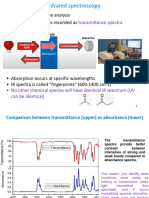
IR Spectroscopy is an extremely effective method for determining the presence or absence of a wide variety of functional groups in a molecule. (For a detailed listing, see the table showing important IR absorptions of various functional groups.) One way to begin analyzing an IR spectrum is to start at the high wavenumber end of the spectrum (typically 4000 cm-1) and look for the presence and absence of characteristic absorptions as you move toward lower wavenumbers. Some of the most common, and distinctive, absorptions are organized into several regions below. This is the type of analysis that you should be able to do without consulting notes. If necessary, a more detailed analysis could then be attempted by consulting a text on IR interpretation.
Important Regions of the IR Spectrum
3600-2700 cm-1 X-H stretch region
The alcohol OH stretch is usually a broad and strong Alcohol O-H absorption near 3400. The NH stretch is typically not as broad Amine or Amide N- or strong as the OH, and in the case of an NH it may appear 2 H as two peaks. The terminal alkyne C-H may be confirmed by a Alkyne C-H weak CC triple bond stretch near 2150 cm-1 Acid O-H This is normally a very broad signal centered near 3000 cm-1. Aromatic (sp2) =C- The aromatic CH's usually appear as a number of weak H absorptions, while the alkene C-H is one or a couple stronger 2 Alkene (sp ) =C-H absorptions. Alkyl (sp3) C-H Almost all organic compounds have alkyl CH's so this is not usually too informative. However, the intensity of these peaks relative to other peaks gives a hint as to the size of the alkyl group. Two medium intensity peaks on the right hand shoulder of the alkyl C-H's. Look for confirming carbonyl C=O peak.
3600-3300 cm-1
3300-2500 cm-1 3200-3000 cm-1
3000-2800 cm-1
2850 and 2750 cm-1 Aldehyde C-H
2300-2100 cm-1
22602210 cm-1 22602100 cm-1
A sharp, medium intensity peak. Carbon Dioxide in the atmosphere may also result in an absorption in this area if not subtracted out. This peak's intensity varies from medium to nothing. Since the intensity is related to the change in dipole moment, symmetrical alkynes will show little or no absorption here!
1850-1500 cm-1 C=X stretch region
Anhydrides have two absorptions, one near 1830-1800 and one near Anhydride C=O 18501775-1740. The absorption frequency increases as the ring size 3-1 decreases. For example: cyclohexanone=1715, 1750 cm 4 membered ring C=O cyclopentanone=1745, cylobutanone=1780, cyclopropanone=1850. Aldehyde C=O 1750Ketone C=O -1 1700 cm Ester C=O Acid C=O 1700Amide C=O -1 1640 cm Conjugated C=O 1680Alkene C=C 1620 cm-1 1600Aromatic C=C 1400 cm-1 This is usually the most intense absorption in the spectrum. Because of the weakening of the C=O due to resonance, amides and conjugated carbonyl's come slightly lower than "normal" C=O. In general, conjugation lowers the absorption by 20-50 cm-1. This absorption is not as intense as that seen for C=O. It is variable and may be fairly small in symmetrical, or nearly symmetrical cases. Look for confirming alkene C-H peaks above 3000. Multiple sharp, medium peaks. The pattern of peaks varies depending upon the substitution pattern. Usually there is one peak around 1600 and several others at lower wavenumbers. Look for confirming aromatic C-H peaks slightly above 3000.
1500-400 cm-1 Fingerprint Region
1300C-O 1000 cm-1 A strong absorption.
Interpretation of peaks in the fingerprint region is complicated by the large number of 1500different vibrations that occur here. These include single bond stretches and a wide Various -1 variety of bending vibrations. This region gets its name since nearly all molecules(even 400 cm very similar ones) have a unique pattern of absorptions in this region.
Other Helpful Characteristics to Keep in Mind When Interpreting IR Spectra Wavenumber of Absorption
The absorption wavenumber for a stretching vibration is related to both the force constant between the two atoms (k) and the mass of the two atoms (m1 and m2) by the Hooke's Law equation:
From this relationship, two important trends in the wavenumber for stretching vibrations can be deduced. 1. As the bond strength increases, the wavenumber increases. For example:
2. As the mass of one of the two atoms in the bond increases, the wavenumber decreases. (Assuming the change in bond strength is relatively small.) For example:
Intensity of Absorption
The intensity of an absorption in the IR spectrum is related to the change in dipole that occurs during the vibration. Consequently, vibrations that produce a large change in dipole (e.g. C=O stretch) result in a more intense absorption than those that result in a relatively modest change in dipole (e.g. C=C). Vibrations that do not result in a change in dipole moment (e.g., a symmetrical alkyne C triple bond C stretch) will show little or no absorption for this vibration. For additional information on IR spectroscopy check out the Organic Chemistry Online IR Page. This page is maintained by John Hanson. Please e-mail comments to hanson@ups.edu.
Você também pode gostar
- Vocology For The Singing Voice PDFDocumento120 páginasVocology For The Singing Voice PDFNathalia Parra Garza100% (2)
- Bike Share ReportDocumento16 páginasBike Share Reportsanjay975100% (1)
- 6000 Most Common Korean Words - For All TOPIK Levels PDFDocumento232 páginas6000 Most Common Korean Words - For All TOPIK Levels PDFZac67% (3)
- Infrared SpectrosDocumento17 páginasInfrared SpectrosJasper DumalaogAinda não há avaliações
- 4.11 Structure DeterminationDocumento46 páginas4.11 Structure DeterminationJaslinder Kaur Dhillon100% (1)
- Chapter4 RetainingwallDocumento55 páginasChapter4 RetainingwallNur HazwaniAinda não há avaliações
- Introduction to Non-Linear Mechanics. (AM-11), Volume 11No EverandIntroduction to Non-Linear Mechanics. (AM-11), Volume 11Ainda não há avaliações
- Solomons Organic Chemistry Module IR TableDocumento1 páginaSolomons Organic Chemistry Module IR TableBenni WewokAinda não há avaliações
- ISO 20000-1 Gap Analysis QuestionaireDocumento15 páginasISO 20000-1 Gap Analysis QuestionaireUsman Hamid67% (6)
- Analytical Chemistry (CHM111) Laboratory ManualDocumento73 páginasAnalytical Chemistry (CHM111) Laboratory ManualKatrina BucudAinda não há avaliações
- Spec Ir NMR Spectra TablesDocumento15 páginasSpec Ir NMR Spectra TablesMah NovaesAinda não há avaliações
- Introduction To Interpretation of Infrared SpectraDocumento3 páginasIntroduction To Interpretation of Infrared Spectrachinnirao100% (4)
- Raw Material Analysis-IRDocumento58 páginasRaw Material Analysis-IRDilla Wulan NingrumAinda não há avaliações
- ASS Instrumental OrganicDocumento17 páginasASS Instrumental OrganicMohamed SakrAinda não há avaliações
- IR SPECTROSCOPY Notes FullDocumento5 páginasIR SPECTROSCOPY Notes FullKartik KuteAinda não há avaliações
- Lecture 4 IR Spectrum AnalysisDocumento43 páginasLecture 4 IR Spectrum AnalysiskhadijahhannahAinda não há avaliações
- IR Spectroscopy TutorialDocumento36 páginasIR Spectroscopy TutorialreddygrAinda não há avaliações
- Topic 9 NotesDocumento9 páginasTopic 9 NotesRitik YadavAinda não há avaliações
- Organic Compound Identification Using Infrared SpectrosDocumento34 páginasOrganic Compound Identification Using Infrared SpectrosRohit SinghAinda não há avaliações
- 10 03 2006 Biophysics 2Documento30 páginas10 03 2006 Biophysics 2api-3696530Ainda não há avaliações
- FullDocumento10 páginasFullAbdul Wahab KhanAinda não há avaliações
- Infrared SpectrosDocumento9 páginasInfrared SpectrosMakeedaAinda não há avaliações
- 6-IR Spectroscopy of Alkane, Alkene and Carbonyl CompoundsDocumento8 páginas6-IR Spectroscopy of Alkane, Alkene and Carbonyl Compoundsbloodhound13042005Ainda não há avaliações
- Interpretation of Spectra of Different CompoundsDocumento15 páginasInterpretation of Spectra of Different Compoundsmariam nawabAinda não há avaliações
- Kuliah Infra Merah-LengkapDocumento63 páginasKuliah Infra Merah-LengkapM Nur M. MahmudAinda não há avaliações
- Infrared SpectrosDocumento9 páginasInfrared Spectrosbac_nobita7657Ainda não há avaliações
- Interpreting of 1 HexeneDocumento1 páginaInterpreting of 1 HexeneShamsheer KhanAinda não há avaliações
- Spectroscopy Ir NMR and UvDocumento26 páginasSpectroscopy Ir NMR and UvHaider JalalAinda não há avaliações
- Publication 3 2208 1587Documento63 páginasPublication 3 2208 1587Tharakeshwari MuraliAinda não há avaliações
- Infrared SpectrosDocumento63 páginasInfrared SpectrosAsim Alaa Al SalehiAinda não há avaliações
- IR SpectraDocumento5 páginasIR SpectraXyphrus EmeritusAinda não há avaliações
- Infrared Spectroscopy: Conformational IsomersDocumento7 páginasInfrared Spectroscopy: Conformational IsomersRiyan NazarudinAinda não há avaliações
- Infrared SpectrosDocumento4 páginasInfrared Spectrosjellybean07100% (1)
- Lecture22A IR 112805Documento45 páginasLecture22A IR 112805Mohammad RehanAinda não há avaliações
- B.Tech Spectroscopy Module 5.4: Applications of IR SpectrosDocumento16 páginasB.Tech Spectroscopy Module 5.4: Applications of IR SpectrosKAMALAAinda não há avaliações
- Infra RedDocumento7 páginasInfra RedDennianasAinda não há avaliações
- Ftir SpectrosDocumento36 páginasFtir SpectrosHafiz Mudaser AhmadAinda não há avaliações
- PHR410 Chapter 2Documento36 páginasPHR410 Chapter 2pulock.paulAinda não há avaliações
- Chem 212: Physical Analytical ChemistryDocumento22 páginasChem 212: Physical Analytical ChemistrysamuelAinda não há avaliações
- Infrared SpectroscopyDocumento12 páginasInfrared Spectroscopysanjay sAinda não há avaliações
- How Do I Interpret An Infrared SpectrumDocumento2 páginasHow Do I Interpret An Infrared SpectrumJuan Francisco Olivares GonzalesAinda não há avaliações
- 0302 紅外線光譜儀 孫仲銘老師Documento47 páginas0302 紅外線光譜儀 孫仲銘老師周浩銘Ainda não há avaliações
- Interpretation of IR Compounds BY KhanDocumento7 páginasInterpretation of IR Compounds BY KhanShamsheer KhanAinda não há avaliações
- IR SpectrosDocumento33 páginasIR SpectrosKikiMariaAinda não há avaliações
- Session 7 (IR)Documento27 páginasSession 7 (IR)ashenafiAinda não há avaliações
- IR SpectrosDocumento44 páginasIR SpectrosVansh YadavAinda não há avaliações
- When You Analyze An Infrared Spectrum, It Is Easier If You Follow A Series of StepsDocumento1 páginaWhen You Analyze An Infrared Spectrum, It Is Easier If You Follow A Series of StepsSean McNamaraAinda não há avaliações
- IR Spectroscopy Problem Set 4 Answer KeyDocumento6 páginasIR Spectroscopy Problem Set 4 Answer KeyJules BrunoAinda não há avaliações
- IR and NMR SpectrosDocumento13 páginasIR and NMR SpectrosAnand BarapatreAinda não há avaliações
- Organic 7Documento41 páginasOrganic 7sm737744Ainda não há avaliações
- Bpo C Chapter 10Documento55 páginasBpo C Chapter 10DewiSugiartiAinda não há avaliações
- Infrared SpectrosDocumento14 páginasInfrared SpectrosV G Viju KumarAinda não há avaliações
- Chm557 Chapter 3Documento70 páginasChm557 Chapter 3Nurul izzatiAinda não há avaliações
- Characteristic IR Absorption Frequencies of Organic Functional GroupsDocumento19 páginasCharacteristic IR Absorption Frequencies of Organic Functional GroupsChandra Reddy100% (1)
- Interpreting IRDocumento5 páginasInterpreting IRzebasiltAinda não há avaliações
- 08 - Infrared Spectroscopy ManualDocumento5 páginas08 - Infrared Spectroscopy ManualShubham BobadeAinda não há avaliações
- Infrared SpectrosDocumento110 páginasInfrared SpectrosBHARTI GAURAinda não há avaliações
- 424 Spectra TablesDocumento19 páginas424 Spectra TablespradeepiitdAinda não há avaliações
- Interpreting An InfraDocumento4 páginasInterpreting An InfraАружан ЖарылқапAinda não há avaliações
- Chap 12 - IRDocumento30 páginasChap 12 - IRTanvir AhmedAinda não há avaliações
- IR Spectroscopy (Solutions)Documento4 páginasIR Spectroscopy (Solutions)LakshayAinda não há avaliações
- What Are IR Radiations?: Basic PrincipleDocumento8 páginasWhat Are IR Radiations?: Basic PrincipleHania KhanAinda não há avaliações
- Infrared Tutorial 2Documento71 páginasInfrared Tutorial 2Hammo Ez AldienAinda não há avaliações
- Fourth International Conference on Non-Aqueous Solutions: Vienna 1974No EverandFourth International Conference on Non-Aqueous Solutions: Vienna 1974V. GutmannAinda não há avaliações
- IR Absorption TableDocumento2 páginasIR Absorption TablefikrifazAinda não há avaliações
- InteractionDocumento4 páginasInteractionValentino ArisAinda não há avaliações
- Electrocatalysts For Oxygen ElectrodesDocumento68 páginasElectrocatalysts For Oxygen ElectrodesBenni WewokAinda não há avaliações
- Practical Guide For Calculating ZakatDocumento4 páginasPractical Guide For Calculating ZakatBenni WewokAinda não há avaliações
- Interpretation of Infrared Spectra, A Practical ApproachDocumento24 páginasInterpretation of Infrared Spectra, A Practical ApproachLucas TimmerAinda não há avaliações
- Ionic ConductorsDocumento20 páginasIonic ConductorsGregorio GuzmanAinda não há avaliações
- What Is CivilizationDocumento1 páginaWhat Is CivilizationBenni WewokAinda não há avaliações
- An Approach To Polymer Degradation Through MicrobesDocumento4 páginasAn Approach To Polymer Degradation Through MicrobesBenni WewokAinda não há avaliações
- Polymer BiodegradationDocumento12 páginasPolymer BiodegradationBenni WewokAinda não há avaliações
- Ageing and Degradation in Microstructured Polymer Optical FiberDocumento11 páginasAgeing and Degradation in Microstructured Polymer Optical FiberBenni WewokAinda não há avaliações
- AB2 - 5 Surfaces and Surface IntegralsDocumento17 páginasAB2 - 5 Surfaces and Surface IntegralsnooktabletAinda não há avaliações
- Index NotationDocumento10 páginasIndex Notationnavy12347777Ainda não há avaliações
- Polymer BiodegradationDocumento12 páginasPolymer BiodegradationBenni WewokAinda não há avaliações
- Taylor Series and PolynomialsDocumento19 páginasTaylor Series and PolynomialsBenni WewokAinda não há avaliações
- Polymer ScaffoldDocumento6 páginasPolymer ScaffoldBenni WewokAinda não há avaliações
- Characterization of A Novel Polymeric ScaffoldDocumento9 páginasCharacterization of A Novel Polymeric ScaffoldBenni WewokAinda não há avaliações
- Taylor SeriesDocumento7 páginasTaylor SeriesnottherAinda não há avaliações
- Marine HydrodynamicsDocumento10 páginasMarine HydrodynamicsBenni WewokAinda não há avaliações
- Pyrolysis - Wikipedia, The Free EncyclopediaDocumento9 páginasPyrolysis - Wikipedia, The Free EncyclopediaBenni WewokAinda não há avaliações
- Taylor SeriesDocumento7 páginasTaylor SeriesnottherAinda não há avaliações
- Examples of Stokes' Theorem and Gauss' Divergence TheoremDocumento6 páginasExamples of Stokes' Theorem and Gauss' Divergence TheoremBenni WewokAinda não há avaliações
- The Material DerivativeDocumento8 páginasThe Material DerivativeBenni WewokAinda não há avaliações
- P12 (01) - Fabrication of Polymeric Scaffolds With A Controlled Distribution of PoresDocumento7 páginasP12 (01) - Fabrication of Polymeric Scaffolds With A Controlled Distribution of PoresBenni WewokAinda não há avaliações
- DivergenceDocumento10 páginasDivergenceBenni WewokAinda não há avaliações
- The Material DerivativeDocumento8 páginasThe Material DerivativeBenni WewokAinda não há avaliações
- Fluid Mechanics EquationsDocumento6 páginasFluid Mechanics EquationsratnacfdAinda não há avaliações
- Conservation of MassDocumento18 páginasConservation of MassBenni WewokAinda não há avaliações
- Euler's Equation For Fluid FlowDocumento6 páginasEuler's Equation For Fluid FlowBenni WewokAinda não há avaliações
- Accommodating Expansion of Brickwork: Technical Notes 18ADocumento13 páginasAccommodating Expansion of Brickwork: Technical Notes 18AWissam AlameddineAinda não há avaliações
- Literature Review On Parking SpaceDocumento6 páginasLiterature Review On Parking Spacefvgy6fn3100% (1)
- Research 9: Activity 4: Background of The StudyDocumento7 páginasResearch 9: Activity 4: Background of The StudyPhilip AmelingAinda não há avaliações
- TCS3400 DS000411 4-00Documento34 páginasTCS3400 DS000411 4-00Miguel_Angel92Ainda não há avaliações
- Chapter 6 SBLDocumento4 páginasChapter 6 SBLbrave manAinda não há avaliações
- G20 SolutionDocumento11 páginasG20 SolutionAbidemi Benjamen AttehAinda não há avaliações
- Term Test Pointers For Review - 1st TermDocumento2 páginasTerm Test Pointers For Review - 1st Termjessica holgadoAinda não há avaliações
- Da Insem AllDocumento217 páginasDa Insem AllTECOA136TejasJadhavAinda não há avaliações
- Pradeep Kshetrapal - Genius Physics (Class 12) - For IIT-JEE and CBSE 2 - Libgen - LiDocumento338 páginasPradeep Kshetrapal - Genius Physics (Class 12) - For IIT-JEE and CBSE 2 - Libgen - Lisujan subediAinda não há avaliações
- Chilere - Unitatile de Racire - Technical - Brochures - NX - 0152P - 0812P - EN PDFDocumento72 páginasChilere - Unitatile de Racire - Technical - Brochures - NX - 0152P - 0812P - EN PDFDaniel MilosevskiAinda não há avaliações
- Enterpreneurship Assignment 2Documento8 páginasEnterpreneurship Assignment 2Khusbu JaiswalAinda não há avaliações
- Ideal Vs Real OttoDocumento5 páginasIdeal Vs Real Ottoa7med SoulimanAinda não há avaliações
- THE REFUND - Frigyes Karinthy: AuthorDocumento6 páginasTHE REFUND - Frigyes Karinthy: AuthorMilind JamnekarAinda não há avaliações
- Nokia 2690 RM-635 Service ManualDocumento18 páginasNokia 2690 RM-635 Service ManualEdgar Jose Aranguibel MorilloAinda não há avaliações
- Functional Materials For Sustainable Energy TechnologiesDocumento15 páginasFunctional Materials For Sustainable Energy TechnologiesChristhy Vanessa Ruiz MadroñeroAinda não há avaliações
- Bhaktavatsalam Memorial College For Women: Hand Book 2020 - 21Documento37 páginasBhaktavatsalam Memorial College For Women: Hand Book 2020 - 21Anu RsAinda não há avaliações
- VSR Trans. PPT3Documento16 páginasVSR Trans. PPT3VSR TRANSAinda não há avaliações
- UntitledDocumento37 páginasUntitledUnknown UserAinda não há avaliações
- صيانة المولدات و المحولات الكهربائيهDocumento15 páginasصيانة المولدات و المحولات الكهربائيهMostafa AllamAinda não há avaliações
- CHAPTER 2 - ALGEBRA (Latest)Documento41 páginasCHAPTER 2 - ALGEBRA (Latest)FirdausAinda não há avaliações
- 141-203 Solar 660 60 - 40 - 30 - 225 Amp Fleet Battery ChargerDocumento10 páginas141-203 Solar 660 60 - 40 - 30 - 225 Amp Fleet Battery Chargerjose alberto alvarezAinda não há avaliações
- NeedScope On TechnologyDocumento22 páginasNeedScope On TechnologyNguyen Ngo Dinh PhuongAinda não há avaliações
- Inglês - Degrees of ComparisonDocumento4 páginasInglês - Degrees of ComparisonVersehgi IIAinda não há avaliações
- 1.rle Rubrics For Case PresentationDocumento2 páginas1.rle Rubrics For Case PresentationKhristine EstosoAinda não há avaliações