Escolar Documentos
Profissional Documentos
Cultura Documentos
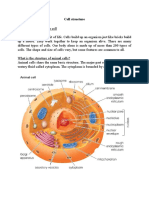
Compund Microscope
Enviado por
bellarosyDireitos autorais
Formatos disponíveis
Compartilhar este documento
Compartilhar ou incorporar documento
Você considera este documento útil?
Este conteúdo é inapropriado?
Denunciar este documentoDireitos autorais:
Formatos disponíveis
Compund Microscope
Enviado por
bellarosyDireitos autorais:
Formatos disponíveis
Copyright 2008 by Department of Integrative Biology, University of California-Berkeley 2.
The Compound Microscope
We will be using microscopes extensively throughout General Biology. This exercise is designed as either an introduction to or a review of microscopy.
Parts of the Microscope and their Function
Requirements It is crucial for you to preview this material before coming to your discussion section. Upon completing this section, you should be able to name the parts of the microscope and know their function. At your desk you will find an Olympus Binocular Microscope (specimen viewed through both eyes). The following is a description of the parts and function of a compound microscope (refer to Fig. 1).
Fig. 1 The Binocular Compound Microscope.
Microscope Tutorial - 1
Copyright 2008 by Department of Integrative Biology, University of California-Berkeley
Stage This is the platform on which material (i.e., the object) is placed to be examined. The object is usually mounted on a glass slide, covered by a cover slip of thin glass (we use disposable plastic cover slips in Biology 1), and placed in the center of the stage over the aperture through which light enters from underneath. Stage clips, or a mechanical stage, are used to hold the slide in place. The specimen can be centered with the mechanical stage adjustment knob. Objectives The objectives contain lenses, which magnify the object being examined. The name objective indicates the position of the lens directly above the object. The binocular microscope has 4x, 10x, 40x and 100x objectives. The 100x objective must only be used with a drop of immersion oil between it and the slide. We rarely use this objective in Biology 1. Revolving Nosepiece The nosepiece, located at the lower end of the body tube, holds the objectives. Turn it gently by means of the objectives. There is a faint click when an objective is in proper alignment. Ocular The ocular, or eyepiece, also contains lenses. It fits into the top of the body tube and gives a secondary magnification of the image formed by the objective. The eyepieces of most microscopes have a magnification of 10. The total magnification is calculated by multiplying the objective and ocular magnifications. In the binocular microscope the eyepiece on the right will in most cases contain a micrometer, i.e., a measuring ruler with 100 subdivisions. In some cases the eyepiece may also contain a small hair, which will appear as a black line and which can be used as a pointer. If the microscope is tilted too much, the eyepiece may fall out and break. Binocular Observation Tube The binocular tube allows adjustment of the two oculars for the particular spacing of your eyes. The left ocular should be set at zero unless your eyes require a correction. The binocular tube can be rotated on the stand allowing the microscope to be used with the stage directly in front of you or with the focusing knobs in front of you. Light Source The light source is built into the base of the microscope. There is an on/off switch and a sliding control which regulates light intensity. Always return the sliding control to zero before turning on the lamp to prevent premature lamp burn-out. Avoiding high lamp intensity will also prolong lamp life.
Microscope Tutorial - 2
Copyright 2008 by Department of Integrative Biology, University of California-Berkeley
Condenser The condenser contains another set of lenses and is located below the stage. It focuses light coming from the lamp onto the material being studied. Notice the adjustment knob by which the condenser may be raised and lowered. The condenser at first should be adjusted so that it is at its uppermost position, very close to the stage. Iris (Aperture) Diaphragm The iris (aperture) diaphragm consists of metal plates and functions much like the iris of your eyes. It is built into the substage condenser. A lever at the side of the condenser regulates the size of the diaphragm opening and thus the amount of light which enters the microscope. Each time you examine a slide, the diaphragm opening should be readjusted to balance contrast, resolution and brightness. Beginning students usually overlook the use of the iris diaphragm and condenser and therefore get poor images.
Focusing Mechanism
Coarse Adjustment The stage is moved rapidly up or down by means of the large coarse adjustment knob. THE COARSE ADJUSTMENT SHOULD BE USED ONLY WITH THE LOW AND INTERMEDIATE POWER OBJECTIVES, NEVER WITH THE HIGH POWER OBJECTIVE! An automatic prefocusing lever incorporated into the coarse adjustment prevents running the specimen into the objective. Fine Adjustment Fine adjustments of the level of the stage or body tube can be made with the smaller knob lateral to or beneath the coarse adjustment. After initial focusing with the coarse adjustment, sharp focus is obtained by using the fine adjustment.
Care of the Microscope
The microscope is a delicate and expensive instrument. Always carry the microscope with two hands, one hand on the arm and the other supporting the base. The lenses of the ocular, objectives, and condenser should be cleaned before and after using the microscope. Use only the lens paper provided as other materials will scratch the lenses. If reagents are spilled on any part of the microscope they should be cleaned away immediately. If immersion oil is used, care should be taken to remove all traces of oil from the objectives, using lens paper and xylene. Do not use ethanol to clean the lenses.
Microscope Tutorial - 3
Copyright 2008 by Department of Integrative Biology, University of California-Berkeley
Before putting away the microscope, return the light intensity sliding control to zero and turn off the lamp. Return the low power objective to center position and remove the slide from the stage. Wind up the electrical cord and cover the microscope with a plastic cover. Return the microscope to the correct cabinet (note number on back of microscope).
Setting Up the Microscope
Requirements At the end of this exercise, you should be able to set up the microscope: 1) to obtain optimal illumination (critical illumination), 2) to bring an object into focus at intermediate and high magnifications, and 3) to estimate the size of microscopic objects by use of an eyepiece micrometer which you have calibrated.
Critical Illumination in 5 Steps
1) Your GSI will demonstrate the condenser lens and how the iris diaphragm functions. You are now ready to set up the microscope for critical illumination. First, gently close the iris diaphragm as far as it will go, and turn the condenser knob until the condenser is at its uppermost position. 2) Next, turn the nosepiece to the intermediate (10x) objective and lower the lens to within 1 cm of the stage, watching from the side. 3) Then turn the lamp to the lowest setting at which you can see the light. 4) Center on your stage a slide of a piece of yellow nylon parachute cloth or Kimwipe. Bring the fibers into focus with the coarse and fine adjustment knobs. If the knobs are hard to turn, you may have to release the prefocusing lever which is a safety device to prevent you from mashing the specimen with the lens. Now lower the condenser slowly until you see granularity come into focus superimposed on the nylon. You are seeing the granularity of the ground glass diffuser covering the light source. Now raise the condenser slightly until the granularity of the diffuser just becomes no longer visible. This will be your optimal condenser setting. 5) Setting the iris diaphragm: The last step is to familiarize yourself with the iris diaphragm, which is still at its most closed setting. Open and close the iris slowly, watching the changing quality of the image of the nylon fibers. When the iris is full closed, contrast is maximum and the chance for false lines is the greatest, but the washout of the image takes over, due to imperfections in the condenser. Find a position, slightly opened, where the image is clear, bright, detailed and sharp. This is your optimal iris setting. You will have to change this setting with different magnifications and with the various specimens you view. You have now achieved critical illumination.
Microscope Tutorial - 4
Copyright 2008 by Department of Integrative Biology, University of California-Berkeley
Intermediate and High Magnification
With the fibers in focus with the 10x objective, look at the perimeter of the illuminated area. The circular area seen with a particular objective is called the actual field of view. The size of the actual field will decrease directly in relation to increasing magnification. To determine the diameter of the actual field of view we are using the parachute fibers as a ruler. The distance between the center of one fiber to the center of the next fiber is 100 (micron), which is equal to 0.1 mm. Manipulate the stage such that the center of one parachute fiber aligns with the left edge of the field of view. Now measure the diameter of the actual field of view by counting the number of fibers in view. For example, as in Fig. 2, if there were 6 fibers (center to center) between the left edge and the right edge of the field of view, this would represent 500 (5 x 100 = 500). Repeat the measurement with the 40x objective and complete Table 1.
Fig. 2 Actual field of view.
Table 1
Objective Magnification 10x 40x *Total magnification = (ocular magnification) x (objective magnification) Ocular Magnification *Total Magnification Field Diameter
Calibrating the Measuring Eyepiece
Your right or left eyepiece should contain a micrometer, a ruler which you will have to calibrate twice, once for the 10x and once for the 40x objective. With the 10x in place, estimate the distance between the closest black lines by comparing their separation to the known space between fibers of parachute cloth. Since the actual distance between fibers is 0.1mm (100), divide the number of micrometer lines into 0.1mm to obtain the actual distance measured. For example, if 10 marks fit between two fibers, each mark measures 0.01mm (0.1mm/10), or 10 at that particular magnification. Record your measurements for both the 10x objective and the 40x objective in Table 2.
Microscope Tutorial - 5
Copyright 2008 by Department of Integrative Biology, University of California-Berkeley
Table 2
Objective 10x 40x Distance between marks
You have now calibrated your measuring eyepiece for intermediate and high magnification. Use this calibration to measure the size of objects in the field during laboratory exercises. See Figures 3 and 4 for some comparative scales encountered in biology.
x-ray diffraction electron microscope light microscope human eye bacteria, blue-green algae viruses eukaryotic cells
water molecule (1.7 ) amino acids proteins
10
-7
10
-6
10
-5 -3
10
-4
10
-3
0.01
0.1
1.0
Scale in mm (millimeters, 10 meters)
-4 -3
10
10
0.01
Scale in m (micrometers, 10 meters, also called microns)
3 4 5 6
-6
0.1
1.0
10
100
1000
0.1
1.0
10
Scale in nm (nanometers, 10 meters, previously called millimicrons)
3 4 5 6 7
-9
100
10
10
10
10
1.0
10
100
Scale in (Angstrom units, 10 meters, also called Angstroms)
-10
10
10
10
10
10
Fig. 3 Limits of resolution for various instruments. In cell biology, the micrometer and nanometer scales are the most useful. In chemistry, the nanometer and angstrom scales are important. Be familiar with these scales.
Microscope Tutorial - 6
Você também pode gostar
- Microscopy Experiment GuideDocumento5 páginasMicroscopy Experiment GuideMa Juryst Chelsea Albay ArmasAinda não há avaliações
- Lab Exercise 2 Microscope Anph111Documento5 páginasLab Exercise 2 Microscope Anph111Jhon Leonard FatalloAinda não há avaliações
- Microscope Usage and Handling ProceduresDocumento5 páginasMicroscope Usage and Handling Proceduresgrafei pennaAinda não há avaliações
- Microscope Skills ManualDocumento10 páginasMicroscope Skills ManualEdna Lau0% (2)
- Lab Exercise 1 MicrosDocumento7 páginasLab Exercise 1 MicrosaluapAinda não há avaliações
- Onion Root Mitosis LabDocumento6 páginasOnion Root Mitosis Labapi-2469736100% (1)
- Histology of Plant and Animal CellsDocumento4 páginasHistology of Plant and Animal Cellscikaifa25% (4)
- Parts and Function of A MicroscopeDocumento1 páginaParts and Function of A Microscopevanessa81% (16)
- Lab 5 The Cell - Cell StructureDocumento5 páginasLab 5 The Cell - Cell StructureDiegoOrtega8Ainda não há avaliações
- Pratical 1 - MicrosDocumento7 páginasPratical 1 - MicrosanthorAinda não há avaliações
- Microscopes Lab Lesson PlanDocumento3 páginasMicroscopes Lab Lesson Planapi-412438755Ainda não há avaliações
- ManualDocumento6 páginasManualapi-301455039Ainda não há avaliações
- Cell Biology Practical 2 PDFDocumento9 páginasCell Biology Practical 2 PDFEswaran SingamAinda não há avaliações
- 2 Micros PDFDocumento59 páginas2 Micros PDFcloudiest euniceAinda não há avaliações
- Lab Exercise 1Documento4 páginasLab Exercise 1Alessandra WyAinda não há avaliações
- Microscope UseDocumento7 páginasMicroscope UsebegAinda não há avaliações
- 4 - Cheek and Onion Cell LabDocumento2 páginas4 - Cheek and Onion Cell LabBrian NeisesAinda não há avaliações
- Light MicroscopeDocumento35 páginasLight MicroscopeDR VARSHA A SINGHAinda não há avaliações
- Animal Cell Vs Plant CellDocumento4 páginasAnimal Cell Vs Plant CellRizza Mae Samalca100% (2)
- Microscope CareDocumento5 páginasMicroscope Care3ciaGarcia20Ainda não há avaliações
- Microscope Handouts G7Documento3 páginasMicroscope Handouts G7Gloriefe QuitongAinda não há avaliações
- Botany Chapter 1 Notes PDFDocumento2 páginasBotany Chapter 1 Notes PDFSUDHIR MISHRAAinda não há avaliações
- The Compund Microscope (Hardcopy)Documento11 páginasThe Compund Microscope (Hardcopy)JheanAlphonsineT.MeansAinda não há avaliações
- PLANT and ANIMAL CELLSDocumento16 páginasPLANT and ANIMAL CELLSjoan ruby bautista100% (1)
- How to Count Cells Using a Neubauer ChamberDocumento3 páginasHow to Count Cells Using a Neubauer ChamberAthanasios S. ArampatzisAinda não há avaliações
- Genbio2 12-Q3-SLM15Documento15 páginasGenbio2 12-Q3-SLM15Jordan Dingayan100% (1)
- Chemistry of Life Elements and CompoundsDocumento31 páginasChemistry of Life Elements and CompoundsHapsah MuhammadAinda não há avaliações
- Virtual MicroscopeDocumento9 páginasVirtual MicroscopeMark PugongAinda não há avaliações
- Pond WaterDocumento4 páginasPond Waterapi-264011999Ainda não há avaliações
- 3.1 MicroscopeBasicsDocumento22 páginas3.1 MicroscopeBasicsJamaica Salas SuacilloAinda não há avaliações
- FCB 7-GeneticsDocumento15 páginasFCB 7-GeneticsYuri PaderesAinda não há avaliações
- Grasshopper DissectionDocumento13 páginasGrasshopper DissectionMuhammad AzrieAinda não há avaliações
- Microscopes: Compiled by Guided byDocumento136 páginasMicroscopes: Compiled by Guided byAmeena100% (3)
- Microscope LabDocumento3 páginasMicroscope Labpepotera100% (1)
- S1 Topic 7: Using A MicroscopeDocumento10 páginasS1 Topic 7: Using A MicroscopeLaura May TandocAinda não há avaliações
- MICROSCOPEDocumento47 páginasMICROSCOPEAubrey Anne Torralba100% (1)
- Dyi Dichotomous Key Guided Inquiry PaperworkDocumento7 páginasDyi Dichotomous Key Guided Inquiry Paperworkapi-250608665Ainda não há avaliações
- Osmosis in Plant CellDocumento8 páginasOsmosis in Plant CellsandalailaAinda não há avaliações
- Understanding Microscopy: A Guide to Proper Compound Microscope Use and MaintenanceDocumento4 páginasUnderstanding Microscopy: A Guide to Proper Compound Microscope Use and Maintenancecharles mepaniaAinda não há avaliações
- Parts of A Compound MicroscopeDocumento5 páginasParts of A Compound MicroscopeKim Ashley BallesterosAinda não há avaliações
- Jessica Le - Virtual Microscope Lab WorksheetDocumento5 páginasJessica Le - Virtual Microscope Lab Worksheetapi-523306558Ainda não há avaliações
- School of Chemical & Life Sciences Diploma in Food Science & Nutrition Module CLF105: Food Microbiology I Laboratory ManualDocumento12 páginasSchool of Chemical & Life Sciences Diploma in Food Science & Nutrition Module CLF105: Food Microbiology I Laboratory ManualTommy LeungAinda não há avaliações
- The Compound MicroscopeDocumento15 páginasThe Compound MicroscopeAlrei D Mea0% (1)
- Plant CellDocumento112 páginasPlant CellAlfonso PlantillaAinda não há avaliações
- Magnifying Microscopes: How They WorkDocumento15 páginasMagnifying Microscopes: How They WorkMark Arnold MalaluanAinda não há avaliações
- Meiosis Lab 211Documento8 páginasMeiosis Lab 211Tracy LykaAinda não há avaliações
- Onion MitosisDocumento4 páginasOnion MitosisMohd AdibAinda não há avaliações
- To Prepare Stained Temporary Mounts of Onion Peel and To Record Observations and Draw LabelledDocumento2 páginasTo Prepare Stained Temporary Mounts of Onion Peel and To Record Observations and Draw LabelledAnirudhAinda não há avaliações
- Onion Cell NotesDocumento7 páginasOnion Cell NotesAnjehyn Elle100% (1)
- Root Tip Mitosis LabDocumento2 páginasRoot Tip Mitosis LabJohn OsborneAinda não há avaliações
- Compound Microscope Parts and Functions GuideDocumento2 páginasCompound Microscope Parts and Functions Guidemydiamondstar17Ainda não há avaliações
- Microscope Care and Handling TechniqueDocumento12 páginasMicroscope Care and Handling TechniqueCabdicasiis xiisAinda não há avaliações
- 5e Flower Dissection Investigation - Spring 2016Documento3 páginas5e Flower Dissection Investigation - Spring 2016api-309653899Ainda não há avaliações
- Lab Report 1Documento5 páginasLab Report 1yosaAinda não há avaliações
- Lab Report Cells Seen Under The MicroscopeDocumento5 páginasLab Report Cells Seen Under The Microscopeorlandoe2560% (1)
- Setting Up A Microscope For Use Putting Away A MicroscopeDocumento4 páginasSetting Up A Microscope For Use Putting Away A MicroscopeNia MelladoAinda não há avaliações
- 04 Microscope UseDocumento3 páginas04 Microscope Usepixiewinx20Ainda não há avaliações
- Lab 1: Learn Microscope Use and CareDocumento8 páginasLab 1: Learn Microscope Use and Carerashmi_harryAinda não há avaliações
- MICROSCOPE NOTES - 2018 by LN PDFDocumento7 páginasMICROSCOPE NOTES - 2018 by LN PDFLuyando NzalaAinda não há avaliações
- The MicroscopeDocumento4 páginasThe MicroscopeRyz DoctoraAinda não há avaliações
- SpermDocumento9 páginasSpermbellarosyAinda não há avaliações
- For Hundreds of YearsDocumento3 páginasFor Hundreds of YearsbellarosyAinda não há avaliações
- Practical 2 Tests 251Documento33 páginasPractical 2 Tests 251bellarosyAinda não há avaliações
- AS Level Electricity - CircuitsDocumento32 páginasAS Level Electricity - CircuitsbellarosyAinda não há avaliações
- Abortion EssayDocumento2 páginasAbortion Essaybellarosy100% (1)
- RespirationDocumento43 páginasRespirationDevender ThakurAinda não há avaliações
- Note Chapter2 StudentDocumento14 páginasNote Chapter2 StudentbellarosyAinda não há avaliações
- f4 Chapter 2 HomeostasisDocumento6 páginasf4 Chapter 2 HomeostasisSalina Sayang Kamal ArifinAinda não há avaliações
- 1.1 1.2 IntroductionDocumento24 páginas1.1 1.2 IntroductionbellarosyAinda não há avaliações
- 2 0 Forces and Motion1Documento35 páginas2 0 Forces and Motion1Klorin MinAinda não há avaliações
- Ad Pat P and Cellular RespirationDocumento31 páginasAd Pat P and Cellular RespirationbellarosyAinda não há avaliações
- Weida Water TankDocumento1 páginaWeida Water TankbellarosyAinda não há avaliações
- SPM EssaysDocumento6 páginasSPM Essaysbellarosy0% (1)
- Environmental IssuesDocumento1 páginaEnvironmental IssuesbellarosyAinda não há avaliações
- Drugs AbuseDocumento29 páginasDrugs AbusebellarosyAinda não há avaliações
- Calendar 2016 PlannerDocumento12 páginasCalendar 2016 PlanneraggelikikaAinda não há avaliações
- Quiz.1 Chap 1 PhyDocumento2 páginasQuiz.1 Chap 1 PhybellarosyAinda não há avaliações
- Oxidation and Reduction ReactionsDocumento4 páginasOxidation and Reduction ReactionsbellarosyAinda não há avaliações
- Quiz Chap 1 PhyDocumento4 páginasQuiz Chap 1 PhybellarosyAinda não há avaliações
- Rate of ReactionDocumento2 páginasRate of Reactionnatasha_muslim93Ainda não há avaliações
- Supplementary Notes On "CHAPTER: 2 (Section - Forces) ": Normal Contact ForceDocumento5 páginasSupplementary Notes On "CHAPTER: 2 (Section - Forces) ": Normal Contact ForcebellarosyAinda não há avaliações
- Microscope Lab Pro Vs EukDocumento5 páginasMicroscope Lab Pro Vs Eukapi-304326920Ainda não há avaliações
- Guide to Using a Phase Contrast MicroscopeDocumento13 páginasGuide to Using a Phase Contrast MicroscopeCOCORICORICOAinda não há avaliações
- SOP Zooplankton Analysis GuideDocumento23 páginasSOP Zooplankton Analysis GuideAnggun SaputriAinda não há avaliações
- Lab Report #1 BiologyDocumento3 páginasLab Report #1 BiologyCharles Maclean100% (1)
- Experiment9 PlastidDocumento4 páginasExperiment9 PlastidRahaizuddin RaniAinda não há avaliações
- CAPE Bio Mark SchemeDocumento4 páginasCAPE Bio Mark Schemeron97150% (2)
- BX61 Instr ManualDocumento37 páginasBX61 Instr Manualdavierang8362Ainda não há avaliações
- Varney - Wet Smear SlideDocumento2 páginasVarney - Wet Smear SlideevillanuevaAinda não há avaliações
- Handsout Pract 16 Lactophenol Cotton Blue StainDocumento2 páginasHandsout Pract 16 Lactophenol Cotton Blue StainEka PujiastutiAinda não há avaliações
- PVA-DABCO Coverslipping Solution For ImmunofluorescenceDocumento2 páginasPVA-DABCO Coverslipping Solution For ImmunofluorescenceDgek LondonAinda não há avaliações
- Microscope Lab ReportDocumento3 páginasMicroscope Lab ReportKarina Gonzalez70% (10)
- Microscope 2-Wet and Dry Mounting ExptDocumento5 páginasMicroscope 2-Wet and Dry Mounting ExptRalc RamsAinda não há avaliações
- Microscopic Examination of CellsDocumento16 páginasMicroscopic Examination of CellsShania MAinda não há avaliações
- Laboratory Objectives: LAB EXERCISE: Microscopy and The CellDocumento17 páginasLaboratory Objectives: LAB EXERCISE: Microscopy and The CellJasper LeysonAinda não há avaliações
- Exercise The MicroscopeDocumento15 páginasExercise The Microscopemydiamondstar17Ainda não há avaliações
- Cytopreparation For Pathologist PDFDocumento32 páginasCytopreparation For Pathologist PDFNurul Laili NahliaAinda não há avaliações
- Observe Animal Cells Under the MicroscopeDocumento3 páginasObserve Animal Cells Under the MicroscopebrittneyAinda não há avaliações
- Olympus Cx31Documento23 páginasOlympus Cx31Yudi FirmatiaAinda não há avaliações
- Interactive Notebook 3 Plant Cells and Animal CellsDocumento11 páginasInteractive Notebook 3 Plant Cells and Animal Cellsapi-241062194100% (1)
- The Microtomist's Formulary and Guide (1954) by GrayDocumento816 páginasThe Microtomist's Formulary and Guide (1954) by GrayDan JohnsonAinda não há avaliações
- Biomedx Microscope ManualDocumento63 páginasBiomedx Microscope ManualbiomedxAinda não há avaliações
- Microscope Instructon ManualDocumento141 páginasMicroscope Instructon ManualVe SeptianaAinda não há avaliações
- Examine Plant and Animal CellsDocumento2 páginasExamine Plant and Animal CellsCikgu Eyda53% (17)
- Cell Microscope Lab Activity RegularDocumento4 páginasCell Microscope Lab Activity RegularGay DelgadoAinda não há avaliações
- Crystal Violet Staining of Teeth BacteriaDocumento2 páginasCrystal Violet Staining of Teeth Bacteriaapi-234025117Ainda não há avaliações
- Science Supplies Catalogue - 2015/2016Documento24 páginasScience Supplies Catalogue - 2015/2016JayAinda não há avaliações
- 152593Documento158 páginas152593Augustine DharmarajAinda não há avaliações
- Consul PDF 25777Documento2 páginasConsul PDF 25777Eben OforiAinda não há avaliações
- BS en Iso 00137-2015Documento22 páginasBS en Iso 00137-2015huychungng100% (1)
- Olympus CX - 21 Manual de Usuario PDFDocumento28 páginasOlympus CX - 21 Manual de Usuario PDFApocalipsis2072Ainda não há avaliações