Escolar Documentos
Profissional Documentos
Cultura Documentos
UNIT 2 Organic, Energetics, Kinetics and Equilibrium Part 2
Enviado por
nouran94Direitos autorais
Formatos disponíveis
Compartilhar este documento
Compartilhar ou incorporar documento
Você considera este documento útil?
Este conteúdo é inapropriado?
Denunciar este documentoDireitos autorais:
Formatos disponíveis
UNIT 2 Organic, Energetics, Kinetics and Equilibrium Part 2
Enviado por
nouran94Direitos autorais:
Formatos disponíveis
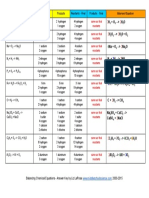
ALKANES
o GENERAL FORMULA: CnH2n+2 o ISOMERISM: All alkanes with 4 or more carbons show structural isomerism o CYCLO-ALKANES: lose two hydrogens
o BOILING POINTS: increase as chain lengths increase due to more Van der Waals dispersion forces holding the molecules together, needing more energy to break. With isomers, the more branched the chain, the lower the boiling point is: Van der Waals dispersion forces are smaller for shorter molecules, and only operate over very short distances between one molecule and its neighbours. o SOLUBILITY: Alkanes are insoluble in water (too much energy needed to break H-bonds in water), but dissolve in organic solvents (only breaking + forming new Van der Waals attractions). o
REACTIONS OF ALKANES:
REACTION WITH OXYGEN (COMBUSTION) Complete combustion (given sufficient oxygen) of any hydrocarbon burns with a blue flame and produces carbon dioxide and water. The hydrocarbons become harder to ignite as the molecules get bigger: bigger molecules have greater Van der Waals attractions which makes it more difficult for them to break away from their neighbours and turn to a gas. REACTION WITH HALOGENS (HALOGENATION) These are photochemical reactions, i.e. take place in the presence of (UV) light, and happen at room temperature. Free radical substitution reactions happen in which hydrogen atoms in the methane are replaced one at a time by chlorine atoms. The original mixture of a colourless and a green gas would produce steamy fumes of hydrogen chloride and a mist of organic liquids
Warren Rieutort-Louis
CRACKING Cracking is the name given to breaking up large hydrocarbon molecules into smaller and more useful bits such as alkenes. This is achieved by using high pressures and temperatures without a catalyst, or lower temperatures and pressures in the presence of a catalyst.
ALKENES
o GENERAL FORMULA: CnH2n o ISOMERISM: All alkenes with 4 or more carbons show structural isomerism: e.g. C4H8.
But, but-2-ene also has geometrical isomerism.
o BOILING POINTS: increase as chain lengths increase due to more Van der Waals dispersion forces holding the molecules together, needing more energy to break. o SOLUBILITY: Alkenes are also insoluble in water (non-polar and too much energy needed to break H-bonds in water), but dissolve in organic solvents (only breaking + forming new Van der Waals attractions). o
REACTIONS OF ALKENES:
The important reactions all centre around the double bond. Typically, the pi bond breaks and the electrons from it are used to join the two carbon atoms to other things. Alkenes undergo addition reactions. The pi bond is open to attack from electrophiles. REACTION WITH HYDROGEN Electrophilic addition Hydrogen adds to an alkene in the presence of a nickel catalyst (heterogeneous) at 150oC and high pressure.
Warren Rieutort-Louis
Hydrogenation can be used to make margarine.
REACTION WITH HALOGENS These are addition reactions. For example, bromine adds to give 1,2-dibromoethane. The reddish-brown bromine is decolourised as it reacts with the alkene.
Room temperature. Reaction speed: Cl2 > Br2 > I2. If you shake an alkene with bromine water (or bubble a gaseous alkene through bromine water), the solution becomes colourless. Alkenes decolourise bromine water.
REACTION WITH HYDROGEN HALIDES All alkenes undergo addition reactions with the hydrogen halides. Symmetrical alkenes: o A hydrogen atom joins to one of the carbon atoms originally in the double bond, and a halogen atom to the other. o e.g. o Conditions are room temperature o Reaction rates increase in the order HF - HCl - HBr - HI. The bond strength falls as you go from HF to HI, and the hydrogen-fluorine bond is particularly strong. o Reaction rates increase as the number of alkyl groups to increases on carbons at either end of the double bond. o This is because alkyl groups push electrons towards the double bond i.e. the pi bond, which attracts others more. Unsymmetrical alkenes: o There is no difference in terms of reaction conditions and factors affecting rate of reaction. o If HCl adds to an unsymmetrical alkene there are several possible ways it could add. But usually, there is only one major product. o Markovnikovs rule states that: When a compound HX is added to an unsymmetrical alkene, the hydrogen Do not quote in becomes attached to the carbon with the most exam hydrogens attached to it already. REACTION WITH POTASSIUM MANGANATE (VII) This is a hydroxylation reaction. Reaction occurs at room temperature. The product is ethan-1,2-diol. If the potassium manganate(VII) solution is acidified with dilute sulphuric acid, the purple solution becomes colourless.
Warren Rieutort-Louis
If the potassium manganate(VII) solution is made alkaline the purple solution first becomes dark green and then produces a dark brown precipitate. Manganate(VII) ions are a strong oxidising agent.
REACTION WITH conc. SULPHURIC ACID + WATER This is a hydrolysis reaction. Ethene is passed into concentrated sulphuric acid (170 C) to make ethyl hydrogensulphate, which is then passed through water. The water reacts with the ethyl hydrogensulphate to produce ethanol, which distils off.
HALOGENOALKANES
o GENERAL FORMULA: R-X where X is a halogen. o DIFFERENT TYPES: Halogenoalkanes fall into different classes depending on how the halogen atom is positioned on the chain of carbon atoms In a primary (1) halogenoalkane, the carbon with the halogen is only attached to one other alkyl group.
In a secondary (2) halogenoalkane, the carbon with the halogen attached is joined directly to two other alkyl groups
In a tertiary (3) halogenoalkane, the carbon atom holding the halogen is attached directly to three alkyl groups
o BOILING POINTS: The increase in boiling point as you go from a chloride to a bromide to an iodide (for a fixed number of carbon atoms) is because of the increase in number of electrons leading to larger dispersion forces (van der Waals). o SOLUBILITY: Not very soluble in water (too much energy required). Soluble in organic solvents. o REACTIVITY: The carbon-halogen bond has got to be broken. That gets easier as you go from fluoride to chloride to bromide to iodide, the compounds get more reactive in that order. o
REACTIONS OF HALOGENOALKANES:
REACTION WITH AQUEOUS KOH Nucleophilic substitution (hydrolysis) Product is an alcohol. Conditions: Heat under reflux in aqueous solution
Warren Rieutort-Louis
REACTION WITH ALCOHOLIC KOH Elimination Product is an alkene. Conditions: Heat under reflux with ethanol as a solvent
CH3CH2Br CH2CH2 + HBr
REACTION WITH ALCOHOLIC KCN Nucleophilic substitution. If a halogenoalkane is heated under reflux with a solution of potassium cyanide in ethanol, a -CN group replaces the halogen and a nitrile is produced.
This is an important reaction as it adds a carbon to the chain.
REACTION WITH EXCESS AMMONIA Nucleophilic substitution. The halogenoalkane is heated with a concentrated solution of ammonia in ethanol. The reaction is carried out in a sealed tube at high temperature and pressure. You couldn't heat this mixture under reflux, because the ammonia would simply escape up the condenser as a gas. Product is an amine. IDENTIFYING HALIDE GROUPS The halogenoalkane is warmed with sodium hydroxide solution in a mixture of ethanol (solvent) and water.
Acid is used to remove unwanted ions such as the OH-. The test is performed as described on page 22 of module 1
ALCOHOLS
o GENERAL FORMULA: R-OH where OH is a hydroxyl group. o DIFFERENT TYPES: Alcohols follow the same pattern as halogenoalkanes i.e. primary, secondary and tertiary. o BOILING POINT: Boiling point of alcohol is higher than alkane with same number of carbons. This is caused by hydrogen bonding between the slightly positive hydrogen atoms and lone pairs on oxygens in other molecules. Boiling point of alcohols increase as the number of carbons increases. This is
Warren Rieutort-Louis
because as molecules get larger, there are more electrons, hence greater dispersion forces (van der Waals forces) o SOLUBILITY: Small alcohols are soluble. As size increases, they become less soluble. o
REACTIONS OF ALCOHOLS:
REACTION WITH ACIDIFIED POTASSIUM DICHROMATE (VI) These are oxidation reactions. Potassium dichromate (VI) solution is acidified with sulphuric acid. It produces a colour change from orange to blue-green to show that the primary and secondary alcohols have undergone oxidation reactions. FOR PRIMARY ALCOHOLS: Conditions: dilute sulphuric acid and dichromate. Heat and distil.
C2H5OH + [O] CH3CHO + H2O
ALDEHYDE
Conditions: conc. sulphuric acid and dichromate. Heat under reflux.
C2H5OH + 2[O] CH3COOH + H2O
CARBOXYLIC ACID
FOR SECONDARY ALCOHOLS: Conditions: conc. sulphuric acid and dichromate. Heat under reflux. CH3CH(OH)CH3+[O]CH3COCH3+H2O
KETONE
FOR TERTIARY ALCOHOLS: o No reaction REACTION WITH DEHYDRATING AGENTS If ethanol vapour is passed over heated (300 C) aluminium oxide powder catalyst, the ethanol is cracked to give ethene and water vapour.
Ethanol is heated with an excess of concentrated sulphuric acid or concentrated phosphoric (V) acid (H3PO4) at a temperature of 170C. The gases produced are passed through sodium hydroxide solution to remove the carbon dioxide and sulphur dioxide produced from side reactions.
Warren Rieutort-Louis
REACTION WITH HALOGENATING AGENTS WITH PCl5 Phosphorus pentachloride at room temperature produces white steamy fumes of HCl. This is a test for the hydroxyl group. C2H5OH + PCl5 C2H5Cl + POCl3 + HCl
WITH HYDROGEN HALIDES HBr is made in situ from KBr and H2SO4 forms bromoalkanes during heating under reflux. NaBr can also be used instead of the HBr. KBR + H2SO4 HBr + KHSO4. C2H5OH + HBr C2H5Br + H2O With iodine: Iodoalkanes can be made in a reaction phosporus triiodide made in situ from iodine and red phosporus. 2P + 3I2 2PI3 3C2H5OH + PI3 ---> 3C2H5I +H3PO3 The relative reactivities of alcohols in halogenation are tertiary > secondary > primary alcohol.
Single and double bonds: o An alkene is much more reactive than an alkane because it has a double bond. A double bond is weaker that a single bond. o A single bond consists of 1 sigma bond. A double bond consists of a sigma and a pi bond. The pi bond is weaker because it is less under the control of the nuclei involved; hence the pi electrons are more easily accessible i.e. they are open to attack from an electrophile. o In a reaction, alkanes need to break sigma bonds, while alkenes only a pi bond. Hence, this requires less energy and therefore alkenes are more reactive. Bond polarity and enthalpy: o Some bonds like carbon-halogen are polar because there is a difference in E.N. values. This leaves the carbon slightly positive ( +) and open to nucleophilic attack. o A C-I bond is weaker than a C-Cl bond because it is longer due to the size of the halogen atom. Therefore an iodoalkane will react much faster.
Different types of formulae: o Empirical formula: formula which shows the simplest whole number ratio of elements in one molecule
Warren Rieutort-Louis
o Molecular formula: formula which shows the actual number of each element in one molecule o Structural formula: formula, which shows how individual atoms are bonded within the molecule. Theoretical yield + percentage yield: o The percentage yield is always lower than 100% because of side reactions and handling losses such as purification. o The theoretical yield is calculated using the balanced chemical equation. o Percentage yield = 100*actual yield/theoretical yield
Fuels: o Liquid fuels have the advantage of having higher densities than gaseous fuels. More energy may be stored in a liquid fuel than in the same volume of gaseous fuel. o It is easier and safer to pipe liquid fuels than gaseous fuels o The pollution of each fuel must be taken into account. o Example of some fuels and their advantages: Energy Energy Fuel State per per Handling Environment volume mass Hard to contain and pressure is needed to transfer it as a Only very liquid. Can lead to explosive hydrogen gas very low produces high risk. Expensive because must water come from electrolysis of water. methane gas very low high same as hydrogen Forms CO2 and H2O Forms CO2 and H2O Same as above but often produces CO. Can be made from sugar cane (renewable)
butane
liquid moderate (pressure)
high
easy to liquefy and use easy to handle. Easily ignited (i.e. volatile). Larger chains mean larger calorific value). easy to handle
octane
liquid
high
high
ethanol
liquid
moderate moderate
Polymers of simple alkenes: o All these reactions are addition polymerisations
Warren Rieutort-Louis
o An addition reaction is one in which two or more molecules join together to give a single product. During polymerisation of monomers, thousands of these join together to make polymers. Monomer Polymer Properties In LDPE, chains are arranged randomly, so vdW forces are less effective leading to a lower boiling point. This also makes it soft and flexible. Has many lengths of chainsno sharp melting point. In HDPE, chains are arranged less randomly so it is stronger and has a higher melting point +density PP, Resists tension and is tougher than polyethene, Water repellent, Low melting point and density PVC, is hard (because of dipoledipole attractions due to polarity of CCl bond. It is less flexible than polyethene. It is an electrical insulator. PTFE has a high melting point and resists chemical attack (does not corrode) because of strong C-F bond. It is also low-friction and non-stick. Uses Plastic bags, bottles, Flexible sheet material, Pipes, Washing up bowls Ropes, Carpets, Curtains, Crates Guttering Floor tiles, Packaging, Electrical wires, Clothing Cooking+ Garden utensils, Frying pans
Ethene CH2=CH2
Polyethene
Propene CH3CH=CH2
Chloroethene CHCl=CH2
Tetrafluoroethene CF2=CF2
Polymers and compounds of simple alkenes: o Polymers are not biodegradable because of strong C-Halogen bonds. Toxic gases are given off during combustion o DDT is a pesticide used to kill mosquitoes. The strong C-Cl bonds give DDT a long life, however it persists in the environment and builds up in the food chain threatening creatures at the top of the chain (bio-accumulation) o Can be used as refrigerants: CFC. It does not decompose quickly when discarded but does so in the upper atmosphere. The radicals it forms react with ozone. This increases UV radiation reaching the Earth's surface and a increases risks of skin cancers.
Warren Rieutort-Louis
Você também pode gostar
- The Subtle Art of Not Giving a F*ck: A Counterintuitive Approach to Living a Good LifeNo EverandThe Subtle Art of Not Giving a F*ck: A Counterintuitive Approach to Living a Good LifeNota: 4 de 5 estrelas4/5 (5794)
- 0580 s04 MsDocumento20 páginas0580 s04 Msnouran94Ainda não há avaliações
- The Yellow House: A Memoir (2019 National Book Award Winner)No EverandThe Yellow House: A Memoir (2019 National Book Award Winner)Nota: 4 de 5 estrelas4/5 (98)
- Periodic TableDocumento1 páginaPeriodic Tablenouran94Ainda não há avaliações
- A Heartbreaking Work Of Staggering Genius: A Memoir Based on a True StoryNo EverandA Heartbreaking Work Of Staggering Genius: A Memoir Based on a True StoryNota: 3.5 de 5 estrelas3.5/5 (231)
- 0580 s04 MsDocumento20 páginas0580 s04 Msnouran94Ainda não há avaliações
- Hidden Figures: The American Dream and the Untold Story of the Black Women Mathematicians Who Helped Win the Space RaceNo EverandHidden Figures: The American Dream and the Untold Story of the Black Women Mathematicians Who Helped Win the Space RaceNota: 4 de 5 estrelas4/5 (895)
- 0580 s04 MsDocumento20 páginas0580 s04 Msnouran94Ainda não há avaliações
- The Little Book of Hygge: Danish Secrets to Happy LivingNo EverandThe Little Book of Hygge: Danish Secrets to Happy LivingNota: 3.5 de 5 estrelas3.5/5 (400)
- Bonding and ElectonegativityDocumento6 páginasBonding and Electonegativitynouran94Ainda não há avaliações
- Synoptic Revision Topic 1Documento9 páginasSynoptic Revision Topic 1nouran94Ainda não há avaliações
- Never Split the Difference: Negotiating As If Your Life Depended On ItNo EverandNever Split the Difference: Negotiating As If Your Life Depended On ItNota: 4.5 de 5 estrelas4.5/5 (838)
- 4.1 - Energetics IIDocumento3 páginas4.1 - Energetics IInouran94Ainda não há avaliações
- Elon Musk: Tesla, SpaceX, and the Quest for a Fantastic FutureNo EverandElon Musk: Tesla, SpaceX, and the Quest for a Fantastic FutureNota: 4.5 de 5 estrelas4.5/5 (474)
- Bio Processes Unit 5Documento13 páginasBio Processes Unit 5Thaminah ThassimAinda não há avaliações
- 6BI04 01 Que 20100616Documento24 páginas6BI04 01 Que 20100616nouran94Ainda não há avaliações
- Revison Notes U2 in 8 PagesDocumento9 páginasRevison Notes U2 in 8 Pagesnouran94Ainda não há avaliações
- The Emperor of All Maladies: A Biography of CancerNo EverandThe Emperor of All Maladies: A Biography of CancerNota: 4.5 de 5 estrelas4.5/5 (271)
- 12 May S1 RADocumento10 páginas12 May S1 RAnouran94Ainda não há avaliações
- 9713 Applied Information Technology and Communication TechnologyDocumento7 páginas9713 Applied Information Technology and Communication Technologynouran94100% (1)
- Team of Rivals: The Political Genius of Abraham LincolnNo EverandTeam of Rivals: The Political Genius of Abraham LincolnNota: 4.5 de 5 estrelas4.5/5 (234)
- Reactions, Mechanism and ReagentDocumento2 páginasReactions, Mechanism and Reagentnouran94Ainda não há avaliações
- Devil in the Grove: Thurgood Marshall, the Groveland Boys, and the Dawn of a New AmericaNo EverandDevil in the Grove: Thurgood Marshall, the Groveland Boys, and the Dawn of a New AmericaNota: 4.5 de 5 estrelas4.5/5 (266)
- Questions On Respiration: Water, Energy, Glucose, Food, Carbon Dioxide, Cells, OxygenDocumento1 páginaQuestions On Respiration: Water, Energy, Glucose, Food, Carbon Dioxide, Cells, Oxygennouran94Ainda não há avaliações
- The Hard Thing About Hard Things: Building a Business When There Are No Easy AnswersNo EverandThe Hard Thing About Hard Things: Building a Business When There Are No Easy AnswersNota: 4.5 de 5 estrelas4.5/5 (344)
- 6BI04 01 Que 20120307Documento24 páginas6BI04 01 Que 20120307nouran94Ainda não há avaliações
- S1 June 2004Documento5 páginasS1 June 2004Jonathan BlackAinda não há avaliações
- The World Is Flat 3.0: A Brief History of the Twenty-first CenturyNo EverandThe World Is Flat 3.0: A Brief History of the Twenty-first CenturyNota: 3.5 de 5 estrelas3.5/5 (2259)
- 05a C3 January 2012Documento5 páginas05a C3 January 2012nouran94Ainda não há avaliações
- 9713 Y08 SM 1Documento7 páginas9713 Y08 SM 1nouran94Ainda não há avaliações
- The Gifts of Imperfection: Let Go of Who You Think You're Supposed to Be and Embrace Who You AreNo EverandThe Gifts of Imperfection: Let Go of Who You Think You're Supposed to Be and Embrace Who You AreNota: 4 de 5 estrelas4/5 (1090)
- Unit 4 Notes Edexcel BiologyDocumento19 páginasUnit 4 Notes Edexcel BiologyAe Banpong69% (13)
- 6BI02 01 Que 20120307Documento24 páginas6BI02 01 Que 20120307nouran94Ainda não há avaliações
- 9713 Y08 SM 1Documento7 páginas9713 Y08 SM 1nouran94Ainda não há avaliações
- The Unwinding: An Inner History of the New AmericaNo EverandThe Unwinding: An Inner History of the New AmericaNota: 4 de 5 estrelas4/5 (45)
- January 2012 Mark Scheme Unit 1Documento24 páginasJanuary 2012 Mark Scheme Unit 1Shadman Sakib ChoudhuryAinda não há avaliações
- 6BI04 01 Que 20100616Documento24 páginas6BI04 01 Que 20100616nouran94Ainda não há avaliações
- Edexcel - Core 3 and 4 Revision SheetDocumento6 páginasEdexcel - Core 3 and 4 Revision SheetAshraf Uz ZamanAinda não há avaliações
- Mark Scheme (Results) January 2008: GCE Mathematics (6683/01)Documento8 páginasMark Scheme (Results) January 2008: GCE Mathematics (6683/01)yauchunlokAinda não há avaliações
- January 2012 Mark Scheme Unit 1Documento24 páginasJanuary 2012 Mark Scheme Unit 1Shadman Sakib ChoudhuryAinda não há avaliações
- 6BI02 01 Que 20120307Documento24 páginas6BI02 01 Que 20120307nouran94Ainda não há avaliações
- Abhijay Test Series Test 1 Revision NotesDocumento11 páginasAbhijay Test Series Test 1 Revision Noteskanangupta123Ainda não há avaliações
- The Sympathizer: A Novel (Pulitzer Prize for Fiction)No EverandThe Sympathizer: A Novel (Pulitzer Prize for Fiction)Nota: 4.5 de 5 estrelas4.5/5 (121)
- Binder 2 EdexcelDocumento9 páginasBinder 2 EdexcelahmedAinda não há avaliações
- Chemistry of ChloritesDocumento11 páginasChemistry of ChloritesangelofgloryAinda não há avaliações
- 2 Complex Ions Intro and ShapeDocumento10 páginas2 Complex Ions Intro and ShapeSilvia WulandariAinda não há avaliações
- Abrir Nitrification Progress of Nitrogen-Rich HeterocyclDocumento20 páginasAbrir Nitrification Progress of Nitrogen-Rich Heterocyclenzo.s.berAinda não há avaliações
- Organometallic Compounds Questions and Answers - SanfoundryDocumento8 páginasOrganometallic Compounds Questions and Answers - Sanfoundryمحمد احمد مجیبAinda não há avaliações
- Lipids Are A Large and Diverse Group of Natural Occurring Organic Compounds That AreDocumento8 páginasLipids Are A Large and Diverse Group of Natural Occurring Organic Compounds That AregymnasrischerAinda não há avaliações
- IUPAC Nomenclature of Organic Chemistry: Basic PrinciplesDocumento17 páginasIUPAC Nomenclature of Organic Chemistry: Basic PrinciplesSUBHENDU5174124Ainda não há avaliações
- Quinones-Vii': UK 8 Nom&r W77: Accepted For PmbkatioDocumento3 páginasQuinones-Vii': UK 8 Nom&r W77: Accepted For PmbkatioprashantAinda não há avaliações
- Organic ChemDocumento4 páginasOrganic ChemKarren MakamureAinda não há avaliações
- Organic Pathways For Unit 4Documento2 páginasOrganic Pathways For Unit 4rajhiniAinda não há avaliações
- Cosmetic ADocumento3 páginasCosmetic AKatieleBritoAinda não há avaliações
- 20 DQ Halo and HaloDocumento15 páginas20 DQ Halo and HaloAyushi KapoorAinda não há avaliações
- Organic Reactions Volume 26Documento183 páginasOrganic Reactions Volume 26lerayerAinda não há avaliações
- OzonolysisDocumento2 páginasOzonolysisKamaraj NaiduAinda não há avaliações
- June 2016 (IAL) QP - Unit 2 Edexcel ChemistryDocumento24 páginasJune 2016 (IAL) QP - Unit 2 Edexcel ChemistryKithnula KitulagodaAinda não há avaliações
- SP-220 DataSheet Rev.7Documento4 páginasSP-220 DataSheet Rev.7Rika RahmayantiAinda não há avaliações
- Heterocyclic ChemistryDocumento49 páginasHeterocyclic ChemistryVã RãAinda não há avaliações
- Biomolecules Crash Course NotesDocumento22 páginasBiomolecules Crash Course NotesAayush sainiAinda não há avaliações
- Final-Laboratory-Reportexperiment-no.1-inting FinalDocumento5 páginasFinal-Laboratory-Reportexperiment-no.1-inting FinalChanie Baguio Pitogo100% (1)
- Objective 1. To Compare The Reaction of Aliphatic and Aromatic Hydrocarbon 2. To Determine The Identity of Unknown CompoundDocumento7 páginasObjective 1. To Compare The Reaction of Aliphatic and Aromatic Hydrocarbon 2. To Determine The Identity of Unknown CompoundAHLA AMANI AHMAD SYAYUTHIAinda não há avaliações
- Nucleic Acids QuestionsDocumento4 páginasNucleic Acids QuestionsShubham GhoshAinda não há avaliações
- Notes Organic Chemistry and AlkanesDocumento17 páginasNotes Organic Chemistry and Alkanessrk78Ainda não há avaliações
- Balancing Chemical Equations AnswersDocumento1 páginaBalancing Chemical Equations AnswersSeema ChaturvediAinda não há avaliações
- Class X - Micro Plan 2019-2020Documento5 páginasClass X - Micro Plan 2019-2020sooraj nikamAinda não há avaliações
- Acid Base Tiltrations ExperimentDocumento1 páginaAcid Base Tiltrations ExperimentJethro Exequiel Sibayan100% (1)
- 1 - SALT - 01 (PB (NO3) 2)Documento2 páginas1 - SALT - 01 (PB (NO3) 2)SahanaAinda não há avaliações
- Improvements in The Denighs Colorimetric Method For Phosphorus and Arsenic'Documento4 páginasImprovements in The Denighs Colorimetric Method For Phosphorus and Arsenic'Marcelino Putra PerdanaAinda não há avaliações
- Synthesis of Pyrimidine DerivativesDocumento32 páginasSynthesis of Pyrimidine DerivativesJ-Paul DétoAinda não há avaliações
- Metalated Hetero Cycles and Their Applications in Synthetic Organic ChemistryDocumento56 páginasMetalated Hetero Cycles and Their Applications in Synthetic Organic Chemistrygokay05Ainda não há avaliações
- Sodium Bicarbonate: Nature's Unique First Aid RemedyNo EverandSodium Bicarbonate: Nature's Unique First Aid RemedyNota: 5 de 5 estrelas5/5 (21)
- Process Plant Equipment: Operation, Control, and ReliabilityNo EverandProcess Plant Equipment: Operation, Control, and ReliabilityNota: 5 de 5 estrelas5/5 (1)
- A New Approach to HAZOP of Complex Chemical ProcessesNo EverandA New Approach to HAZOP of Complex Chemical ProcessesAinda não há avaliações
- Guidelines for Chemical Process Quantitative Risk AnalysisNo EverandGuidelines for Chemical Process Quantitative Risk AnalysisNota: 5 de 5 estrelas5/5 (1)
- Functional Safety from Scratch: A Practical Guide to Process Industry ApplicationsNo EverandFunctional Safety from Scratch: A Practical Guide to Process Industry ApplicationsAinda não há avaliações