Escolar Documentos
Profissional Documentos
Cultura Documentos
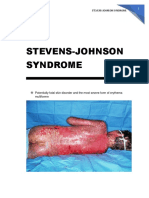
Steven Johnson Syndrome
Enviado por
Khairul AnwarDireitos autorais
Formatos disponíveis
Compartilhar este documento
Compartilhar ou incorporar documento
Você considera este documento útil?
Este conteúdo é inapropriado?
Denunciar este documentoDireitos autorais:
Formatos disponíveis
Steven Johnson Syndrome
Enviado por
Khairul AnwarDireitos autorais:
Formatos disponíveis
CHAPTER 1
Introduction
1.1 Background immune-complexmediated
Stevens-Johnson
Syndrome
(SJS)
is
an
hypersensitivity disorder which typically involves the skin and the mucous membranes.SJS is a serious systemic disorder with the potential for severe morbidity and even death. Significant involvement of oral, nasal, eye, vaginal, urethral, GI, and lower respiratory tract mucous membranes may develop in the course of the illness. The severe variant of SJS showed extensive skin involvement resulting in toxic epidermal necrolysis (TEN). Although SJS/TEN is considered one of the most devastating ocular surface diseases which causes corneal damage and threatens vision, management of ocular involvement may be compromised because more attention is directed to maintaining the vital functions during the acute stage. Furthermore, upon eye examination of patients suffering SJS/TEN at the acute stage it is difficult to recognize hidden conjunctival inflammation and ulceration deep in the fornix and tarsus. Inadequate control of ocular surface inflammation and ulceration at the acute stage will set in a vicious cycle, leading to the chronic stage of scarring (cicatrix), which then contributes greatly to subsequent corneal complications.Although systemic corticosteroids are commonly used and shown to be of some benefit in ameliorating systemic manifestations of the acute phase of SJS and TEN, its therapeutic effect has never been demonstrated in a controlled trial. Furthermore, retrospective studies demonstrate no benefit of corticosteroids or higher rates of morbidity and mortality in corticosteroid-treated patients. Cyclosporin A8 and plasmapheresis have been proposed as alternatives. Recently, IV immunoglobulin has also been advocated for both SJS10 and TEN.11 Despite these measures, specific attention to suppress ocular surface inflammation and to promote early epithelialization has not been considered. A recent retrospective study verified that the extent of lid margin keratinization and tarsal scar (as a result of cicatricial complications) is highly correlated with sight-threatening corneal complications.
1.2 Problem Summation 1. How to diagnose Stevens Johnson Syndrome? 2. What is the treatment for Steven Johnson Syndrome? 1.3 Purpose of Study 1. To understand the diagnosis of Steven Johnson Syndrome
2.
To understand the treatment for Steven Johnson Syndrome
CHAPTER 2 Review of the Literature
2.1 Stevens Johnson Syndrome 2.1.1 Definition Stevens-Johnson Syndrome (SJS) is an immune-complexmediated hypersensitivity disorder which typically involves the skin and the mucous membranes. SJS is a serious systemic disorder with the potential for severe morbidity and even death. Stevens-Johnson Syndrome is a potentially deadly skin disease that usually results from a drug reaction. Another form of the disease is called Toxic Epidermal Necrolysis, and again this usually results from a drug-related reaction. Drugs that have been linked to Stevens-Johnson Syndrome include NSAIDS (non-steroid anti-inflammatory drugs), Allopurinol, Phenytoin, Carbamazepine, barbiturates, anticonvulsants, and sulfa antibiotics. In some cases, the condition is caused by a bacterial infection, and in many cases there is no known cause for the onset of Stevens-Johnson Syndrome or Toxic Epidermal Necrolysis.( French, 2006)(Ginsburg 1982)
2.1.2 Epidemiology SJS occurs with a worldwide distribution similar in etiology and occurrence to that in the United States. However, a study from Germany reported only 1.1 cases per 1 million person-years. In contrast to the drugs most often implicated in western nations, allopurinol is the most common offending agent in Southeast Asian nations, including Malaysia, Singapore, Taiwan, and Hong Kong. Stevens-Johnson syndrome has been described worldwide in all races, although it may be more common in whites. Interestingly, disease is not limited to humans; cases have been reported in dogs, cats, and monkeys. (Chave 2005) The proportion of females has been estimated to be 33-62%. The largest series reports 39.9% of females in a group of 315 patients with Stevens-Johnson syndrome. In a large cohort, the mean age of patients with Stevens-Johnson syndrome was 25 years. In a smaller series, the mean age of patients with Stevens-Johnson syndrome was reported as 47 years. However, cases have been reported in children as young as 3 months. (Chave 2005).
2.1.3 Etiology Stevens-Johnson Syndrome is a serious, potentially life-threatening skin disease. With Stevens-Johnson Syndrome the sufferer can first experience non-specific symptoms, such as headaches, aching body, fever, and a bad cough. Then a rash may develop over the face and trunk of the body, which then spreads to other parts of the body. The rash is patchy and can spread to various areas of the body. Blistering can then appear, usually in places such as the eyes, mouth, nose and genital areas, and the mucous membrane becomes inflamed. With Toxic Epidermal Necrolysis, another variation of the disease, the skin also begins to come away in large amounts. This leaves the sufferer looking as though he or she has burns. The places where the skin has come away can seep fluids quite copiously, and there is also a big risk of infection. (Bianchine, 1968) The most common cause of Stevens-Johnson Syndrome and Toxic Epidermal Necrolysis is through an allergic drug reaction. The drugs that are usually responsible for these reactions include: some NSAIDS (non-steroid anti-inflammatory drugs), Allopurinol, Phenytoin, Carbamazepine, barbiturates, anticonvulsants, and sulfa antibiotics. The onset of symptoms in drug related Stevens-Johnson Syndrome may not appear for one or two weeks after first taking the drug. Reaction to drugs is by far the most common cause of StevensJohnson Syndrome and Toxic Epidermal Necrolysis. Other causes of Stevens-Johnson Syndrome are very few and far between, and other than drug-related reactions there are few known causes. Stevens-Johnson Syndrome has been linked to bacterial infections in the past and also to skin graft problems. In many cases, doctors are unable to determine the cause of the disease in the patient at all. All that is known for certain is that the disease causes a great deal of pain, discomfort, and worry, and ultimately it can lead to death. With Toxic Epidermal Necrolysis, the open wounds left by flaying skin are very susceptible to infection, and this is often what causes death. (Bianchine, 1968) In some East Asian populations studied (Han Chinese and Thai), carbamazepineand phenytoin-induced SJS is strongly associated with HLA-B*1502 (HLA-B75), an HLA-B serotype of the broader serotype HLA-B15. A study in Europe suggested that the gene marker is only relevant for East Asians. (Bianchine, 1968) Based on the Asian findings, similar studies in Europe showed 61% of allopurinolinduced SJS/TEN patients carried the HLA-B58 (phenotype frequency of the B*5801 allele in Europeans is typically 3%). One study concluded: Even when HLA-B alleles behave as
strong risk factors, as for allopurinol, they are neither sufficient nor necessary to explain the disease. (Bianchine, 1968)
2.1.4 Pathophysiology SJS, like toxic epidermal necrolysis and erythema multiforme, are characterized by confluent epidermal necrosis with minimal associated inflammation. The acuity is apparent from the (normal) basket weave-like pattern of the stratum corneum. An idiosyncratic, delayed hypersensitivity reaction has been implicated in the pathophysiology of StevensJohnson syndrome. Certain population groups appear more susceptible to develop StevensJohnson syndrome than the general population. Slow acetylators, patients who are immunocompromised (especially those infected with HIV ), and patients with brain tumors undergoing radiotherapy with concomitant antiepileptics are among those at most risk. (Frieden 1996) Slow acetylators are people whose liver cannot completely detoxify reactive drug metabolites. For example, patients with sulfonamide-induced toxic epidermal necrolysis have been shown to have a slow acetylator genotype that results in increased production of sulfonamide hydroxylamine via the P-450 pathway. These drug metabolites may have direct toxic effects or may act as haptens that interact with host tissues, rendering them antigenic. Antigen presentation and production of tumor necrosis factor (TNF)alpha by the local tissue dendrocytes results in the recruitment and augmentation of T-lymphocyte proliferation and enhances the cytotoxicity of the other immune effector cells. A "killer effector molecule" has been identified that may play a role in the activation of cytotoxic lymphocytes. The activated CD8+ lymphocytes, in turn, can induce epidermal cell apoptosis via several mechanisms, which include the release of granzyme B and perforin. (Frieden 1996) In 1997, Inachi et al demonstrated perforin-mediated apoptosis in patients with Stevens-Johnson syndrome. Perforin, a pore-making monomeric granule released from natural killer cells and cytotoxic T lymphocytes, kills target cells by forming polymers and tubular structures not unlike the membrane attack complex of the complement system. Apoptosis of keratinocytes can also take place as a result of ligation of their surface death receptors with the appropriate molecules. Those can trigger the activation of the caspase system, leading to DNA disorganization and cell death. (Frieden 1996)
Apoptosis of keratinocytes can be mediated via direct interaction between the celldeath receptor Fas and its ligand. Both can be present on the surfaces of the keratinocytes. Alternatively, activated T-cells can release soluble Fas ligand and interferon-gamma, which induces Fas expression by keratinocytes. Researchers have found increased levels of soluble Fas ligand in the sera of patients with SJS/TEN before skin detachment or onset of mucosal lesions. (Frieden 1996) The death of keratinocytes causes separation of the epidermis from the dermis. Once apoptosis ensues, the dying cells provoke recruitment of more chemokines. This can perpetuate the inflammatory process, which leads to extensive epidermal necrolysis. Higher doses and rapid introduction of allopurinol and lamotrigine may also increase the risk of developing SJS/TEN. Risk is lessened by starting these at the low doses and titrating gradually. There is evidence that systemic lupus is a risk factor as well. (Frieden 1996)
2.1.5 Clinical Manisfestation Stevens-Johnson Syndrome can start with non-specific symptoms such as: Coughing Aching Headaches Feverishness Vomiting Diarrhoea (Bastuji, 1993)
This is usually followed by a red rash across the face and the trunk of the body, which can continue to spread to other parts of the body. (Bastuji, 1993) Blisters then form across the body in places such as the nose, moth, eyes, and genital areas, and the mucous membrane becomes inflamed. With some people the nails and hair begin to come out as well. In the case of Toxic Epidermal Necrolysis patients, the skin can start to come away in sheets leaving exposed flesh that could be likened to serious burning and is very susceptible to infection. (Bastuji, 1993)
Signs of mucosal involvement can include the following: Erythema Edema Sloughing Blistering Ulceration Necrosis
The following ocular signs may be noted on slit-lamp examination: Eyelids: Trichiasis, distichiasis, meibomian gland dysfunction, blepharitis Conjunctiva: Papillae, follicles, keratinization, subepithelial fibrosis, conjunctival shrinkage, foreshortening of fornices, symblepharon, ankyloblepharon Cornea: Superficial punctate keratitis, epithelial defect, stromal ulcer, neovascularization, keratinization, limbitis, conjunctivalization, stromal opacity, perforation. (Bastuji, 1993)
2.1.6 Diagnosis 2.1.6.1 History Taking Typically, the disease process begins with a nonspecific upper respiratory tract infection. This usually is part of a 1- to 14-day prodrome during which fever, sore throat, chills, headache, and malaise may be present. Vomiting and diarrhea occasionally are noted as part of the prodrome. Mucocutaneous lesions develop abruptly. Clusters of outbreaks last from 2-4 weeks. The lesions typically are nonpruritic. A history of fever or localized worsening should suggest a superimposed infection; however, fever has been reported to occur in up to 85% of cases. Involvement of oral and/or mucous membranes may be severe enough that patients may not be able to eat or drink. Patients with genitourinary involvement may complain of dysuria or an inability to void. (Ginsburg 1982) A history of a previous outbreak of SJS or of erythema multiforme may be elicited. Recurrences may occur if the responsible agent is not eliminated or if the patient is reexposed. Typical symptoms are as follows that are cough productive of a thick purulent sputum, headache ,malaise and arthralgia. (Ginsburg 1982)
2.1.6.2 Physical Examination The rash can begin as macules that develop into papules, vesicles, bullae, urticarial plaques, or confluent erythema. The center of these lesions may be vesicular, purpuric, or necrotic. The typical lesion has the appearance of a target. The target is considered pathognomonic. Lesions may become bullous and later rupture, leaving denuded skin. The skin becomes susceptible to secondary infection. Urticarial lesions typically are not pruritic. Infection may be responsible for the scarring associated with morbidity. Although lesions may occur anywhere, the palms, soles, dorsum of hands, and extensor surfaces are most commonly affected. The rash may be confined to any one area of the body, most often the trunk. Mucosal involvement may include erythema, edema, sloughing, blistering, ulceration, and necrosis. (Hurwits 1990) The following signs may be noted on examination: Fever Orthostasis Tachycardia Hypotension Altered level of consciousness Epistaxis Conjunctivitis Corneal ulcerations Erosive vulvovaginitis or balanitis Seizures Coma (Ginsburg 1982)
2.1.6.3 Laboratory No laboratory studies (other than biopsy) exist that can aid the physician in establishing the diagnosis. A complete blood count (CBC) may reveal a normal white blood cell (WBC) count or a nonspecific leukocytosis. A severely elevated WBC count indicates the possibility of a superimposed bacterial infection. Determine renal function and evaluate urine for blood. Electrolytes and other chemistries may be needed to help manage related problems. Cultures of blood, urine, and wounds are indicated when an infection is clinically suspected. (Hurwits 1990)
2.1.6.4 Radiology Chest radiograph may indicate the existence of a pneumonitis when clinically suspected. Otherwise, routine plain films are not indicated. (Hurwits 1990) 2.1.6.5 Other Tests Skin biopsy is the definitive diagnostic study but is not an ED procedure. Skin biopsy demonstrates that the bullae are subepidermal. Epidermal cell necrosis may be noted. Perivascular areas are infiltrated with lymphocytes. (Hurwits 1990) 2.1.7 Differential Diagnosis The gravity of the diagnosis must be recognized. Because patients with StevensJohnson syndrome (SJS) who present early in the development of the disease may not yet be critically ill, the clinician may misdiagnose the condition and discharge the patient. SJS should be considered in all patients with target lesions and mucous membrane involvement. (Hurwits 1990) Other problems to be considered in the differential diagnosis include the following: Staphylococcal scalded skin syndrome Irradiation Trauma Progressive systemic sclerosis (scleroderma) Erythroderma ichthyosiform congenita Porphyria cutanea tarda Epidermolysis bullosa acquisita Linear immunoglobulin A bullous disease Paraneoplastic pemphigus Bullous systemic lupus erythematosus Corynebacterium diphtheriae conjunctivitis Sebaceous cell carcinoma Adenoviral conjunctivitis Intraepithelial epithelioma Acute generalized exanthematic pustulosis Chemical Burns in Emergency Medicine Exfoliative Dermatitis Keratoconjunctivitis, Atopic
Ocular Burns Sjogren Syndrome Thermal Burns in Emergency Medicine Toxic Shock Syndrome Trachoma (Hurwits 1990)
2.1.8 Management and Treatment At Prehospital Care doctors should recognize the presence of severe fluid loss and should treat patients with SJS as they would patients with thermal burns. In Emergency Department Care most cases present early and prior to obvious signs of hemodynamic compromise. Perhaps the single most important role for the ED physician is to detect SJS early and initiate the appropriate ED and inpatient management. Care in the ED must be directed to fluid replacement and electrolyte correction. Skin lesions are treated as burns. Patients with SJS then should be treated with special attention to airway and hemodynamic stability, fluid status, wound/burn care, and pain control. (Fromowritz 2007) Treatment of SJS is primarily supportive and symptomatic. Manage oral lesions with mouthwashes. Topical anesthetics are useful in reducing pain and allowing the patient to take in fluids. Areas of denuded skin must be covered with compresses of saline or Burow solution. Underlying diseases and secondary infections must be identified and treated. Offending drugs must be stopped. The use of systemic steroids is controversial. Some authors believe that they are contraindicated. Treatment with systemic steroids has been associated with an increased incidence of complications. Address tetanus prophylaxis. (Fromowritz 2007)
Specific treatment SJS is life threatening conditions. The success of treatment depends on early recognition of the condition, prompt removal of the causative medications and intensive supportive care in a well-equipped hospital. Several agents with anti-inflammatory or immunosuppressive properties have been tried to alter the course of the disease but no single agent has their efficacy clearly proven by clinical trials. (Fromowritz 2007) 1. Intravenous Immunoglobulin (IVIG)
Prepared from pooled plasma, IVIG contains immune antibodies that interfere with the apoptotic pathway mediated by the Fas ligand and receptor. Theoretically it is best to give IVIG early (within 24-72 hours from first appearance of bullae)4,5 before Fas ligand and receptor binding has occurred, although it may still be effective if new bullae are still appearing. Sucrose-depleted IVIG is preferred since it has lower possibility of renal toxicity. Patient with IgA deficiency will develop anaphylaxis to IVIG. It is best to obtain a patient's IgA level before administering but awaiting the report might delay treatment. History of recurrent sinopulmonary infection and gastrointestinal infection may help to identify those with IgA deficiency which is very rare. Results of studies of IVIG on SJS and TEN has been conflicting, and IVIG should not be considered as a routine treatment. Some studies have suggested the higher dose of 3g/kg total dose given over 3 days has better effects over the lower dose of 2g/kg total dose. (Brett,2001) 2. Systemic Corticosteroid Some studies have advocated the use of systemic corticosteroids in the early stage of SJS.. Other studies failed to prove the effect of the agent and have demonstrated an increase in the chance of sepsis and other complications. Its use in SJS is still controversial but should not be recommended when extensive skin loss has already occurred. (Patterson,1994) 3. Cyclosporin A Supported by favourable outcomes6 in several case reports and series, which used cyclosporin A at a dose of 3-4mg/kg/day in short term, thus avoiding its side effects which commonly occur in long term use, this agent seems promising but more comprehensive studies are needed. (Brett,2001) 4. Other Agents Theoretically removal of the offending medication, its metabolites or cytokines by plasmapheresis or haemodialysis could help the improvement of SJS. However the lack of good clinical evidence and the risk of sepsis associated with in-dwelling catheter does not support these as recommendable treatments. Thalidomide based on its anti-TNF effect has been tried but the study was prematurely terminated since excess mortality was reported. (Brett,2001)
2.1.9 Complications
Ophthalmologic: Corneal ulceration, anterior uveitis, panophthalmitis, blindness Gastroenterologic: Esophageal strictures Genitourinary: Renal tubular necrosis, renal failure, penile scarring, vaginal stenosis Pulmonary:Tracheobronchial shedding with resultant respiratory failure Cutaneous: Scarring and cosmetic deformity, recurrences of infection through slowhealing ulcerations.
2.1.10 Prognosis Individual lesions typically should heal within 1-2 weeks, unless secondary infection occurs. The majority of patients recover without sequelae. Development of serious sequelae, such as respiratory failure, renal failure, and blindness, determines prognosis in those affected. Up to 15% of all patients with SJS die as a result of the condition.
References
Bastuji-Garin S et al: A clinical classification of cases of toxic epidermal necrolysis, StevensJohnson syndrome and erythema multiforme. Arch Dermatol 1993;129:92-96 Bianchine JR, Macaraeg PV Jr, Lasagna L, et al: Drugs as etiologic factors in the StevensJohnson syndrome. Am J Med 1968 Mar; 44(3): 390-405[Medline]. Brett AS, Philips D, Lynn AW: Intravenous immunoglobulin therapy for Stevens-Johnson syndrome. South Med J 2001 Mar; 94(3): 342-3[Medline]. Chave TA, Mortimer NJ et al: Toxic epidermal necrolysis: current evidence, practical management and future directions. Br J Dermatol 2005;153:241-253 French LE: Toxic epidermal necrolysis and Stevens Johnsonsyndrome: our current understanding. Allergol Int 2006 Mar; 55(1): 9-16 Fromowitz J, Ramos-Caro et al: Practical guidelines for the management of toxic epidermal necrolysis and Stevens-Johnson Syndrome. Int J Dermatol 2007;46:1092-1094 Frieden IJ: Hypersensitivity reactions. In Rudolph AM et al. Rudolph's Pediatrics. 20th ed. Stamford, Conn: Appleton & Lange; 1996:906-8. Ginsburg CM: Stevens-Johnson syndrome in children. Pediatr Infect Dis 1982 May-Jun; 1(3): 155-8[Medline]. Hurwitz S: Erythema multiforme: a review of its characteristics, diagnostic criteria, and management. Pediatr Rev 1990 Jan; 11(7): 217-22[Medline]. Patterson R, Miller M, Kaplan M, et al. Effectiveness of early therapy with corticosteroids in Stevens-Johnson syndrome: experience with 41 cases and a hypothesis regarding pathogenesis. Ann Allergy 1994;73:2734.
Você também pode gostar
- Stevens Johnson SyndromeDocumento6 páginasStevens Johnson SyndromeAudrey LeonarAinda não há avaliações
- Stevens Johnson DiseaseDocumento5 páginasStevens Johnson DiseaseShammy RNAinda não há avaliações
- Stevens-Johnson Syndrome and Toxic Epidermal Necrolysis - Pathogenesis, Clinical Manifestations, and Diagnosis - UpToDateDocumento41 páginasStevens-Johnson Syndrome and Toxic Epidermal Necrolysis - Pathogenesis, Clinical Manifestations, and Diagnosis - UpToDateDicky SangadjiAinda não há avaliações
- Stevens-Johnson SyndromeDocumento6 páginasStevens-Johnson SyndromeLau ColastreAinda não há avaliações
- Rabies: Ragina AguilaDocumento55 páginasRabies: Ragina AguilaCharles Lester AdalimAinda não há avaliações
- Sickle Cell DiseaseDocumento23 páginasSickle Cell Diseasealejandrino_leoaugusto100% (1)
- Chronic Myeloid LeukemiaDocumento7 páginasChronic Myeloid LeukemiahemendreAinda não há avaliações
- Leptospirosis: Causes, Incidence, and Risk FactorsDocumento6 páginasLeptospirosis: Causes, Incidence, and Risk FactorsJackii DoronilaAinda não há avaliações
- Hirschprungs DiseaseDocumento26 páginasHirschprungs DiseaseAndrian PramanaAinda não há avaliações
- Assignment 4Documento8 páginasAssignment 4api-328441669100% (1)
- Amoebiasis in Wild Mammals: Ayesha Ahmed M Phil. Parasitology 1 Semester 2013-Ag-2712Documento25 páginasAmoebiasis in Wild Mammals: Ayesha Ahmed M Phil. Parasitology 1 Semester 2013-Ag-2712Abdullah AzeemAinda não há avaliações
- Pneumococcal PneumoniaDocumento13 páginasPneumococcal PneumoniaRae Marie AquinoAinda não há avaliações
- Case Study PneumoniaDocumento14 páginasCase Study PneumoniaJester GalayAinda não há avaliações
- Thyroid Papillary Carcinoma CaseDocumento6 páginasThyroid Papillary Carcinoma CaseRandy F BabaoAinda não há avaliações
- RabiesDocumento10 páginasRabiesWinda LiraAinda não há avaliações
- Group 8: Parik Rabasto Patel, D Patel, J Raghuwanshi Regis Moleta Moreno NaromalDocumento35 páginasGroup 8: Parik Rabasto Patel, D Patel, J Raghuwanshi Regis Moleta Moreno NaromalDominique RabastoAinda não há avaliações
- Anatomy and Phsyiology of MeningococcemiaDocumento2 páginasAnatomy and Phsyiology of MeningococcemiaKevin Comahig100% (1)
- Tuberculosis: Dr. Kaem Shir AliDocumento52 páginasTuberculosis: Dr. Kaem Shir AliMwanja MosesAinda não há avaliações
- Cerebrovascular Accident: TypesDocumento10 páginasCerebrovascular Accident: TypesJulia SalvioAinda não há avaliações
- Ulcerative ColitisDocumento18 páginasUlcerative ColitisHoussein EL HajjAinda não há avaliações
- NCP 1Documento3 páginasNCP 1kat2111993Ainda não há avaliações
- MyocarditisDocumento29 páginasMyocarditispanvilai0% (1)
- Nar SingDocumento14 páginasNar Singbasinang_jangilAinda não há avaliações
- Hypertension: Department of Internal MedicineDocumento58 páginasHypertension: Department of Internal MedicineLouije MombzAinda não há avaliações
- Anatomy and Physiology DengueDocumento5 páginasAnatomy and Physiology Denguenva226Ainda não há avaliações
- Hyperosmolar HyperglycemicDocumento8 páginasHyperosmolar HyperglycemicCésar Augusto Sánchez SolisAinda não há avaliações
- CRFDocumento50 páginasCRFKevin MontoyaAinda não há avaliações
- Introduction CASE STUDYDocumento3 páginasIntroduction CASE STUDYDavid CalaloAinda não há avaliações
- POTTs Disease PathoDocumento3 páginasPOTTs Disease PathoEdgel QuidolesAinda não há avaliações
- Portal Vein Thrombus ChildrenDocumento11 páginasPortal Vein Thrombus ChildrenViswas ChhapolaAinda não há avaliações
- OsteoarthritisDocumento15 páginasOsteoarthritisMichael BanksAinda não há avaliações
- Meningitis Pathophysiology PDFDocumento59 páginasMeningitis Pathophysiology PDFpaswordnyalupa100% (1)
- Case Study CvaDocumento33 páginasCase Study CvaArmand Bong Santiago100% (1)
- What Is A Communicable DiseaseDocumento3 páginasWhat Is A Communicable DiseaseChrisel D. SamaniegoAinda não há avaliações
- STDDocumento78 páginasSTDKrupa KarnikAinda não há avaliações
- DrowningDocumento7 páginasDrowningmedhatsabriAinda não há avaliações
- 5 Hematologic-ExaminationsDocumento12 páginas5 Hematologic-ExaminationsMark Jireck AndresAinda não há avaliações
- What Is CellulitisDocumento3 páginasWhat Is CellulitisMarichu TiendaAinda não há avaliações
- Assessment of Immune FunctionDocumento3 páginasAssessment of Immune Functionhalloween candyAinda não há avaliações
- Pyogenic Liver AbscessDocumento10 páginasPyogenic Liver AbscessErnesto Sebastian GarcíaAinda não há avaliações
- Tumor MarkersDocumento14 páginasTumor MarkersPatrick LizarondoAinda não há avaliações
- UTI (Urinary Tract Infection)Documento9 páginasUTI (Urinary Tract Infection)Carson BirthAinda não há avaliações
- Hiv Case StudyDocumento2 páginasHiv Case Studyapi-485814878Ainda não há avaliações
- CyclosporineDocumento3 páginasCyclosporineraki9999Ainda não há avaliações
- Addison's Disease: Adrenal Insufficiency and Adrenal CrisisDocumento15 páginasAddison's Disease: Adrenal Insufficiency and Adrenal CrisisMaryOAinda não há avaliações
- Drug Study CovidDocumento5 páginasDrug Study CovidR Hornilla Arcega0% (1)
- On The Inviolability of Human LifeDocumento6 páginasOn The Inviolability of Human LifeEunbit YiAinda não há avaliações
- Approach To Pleural EffusionDocumento46 páginasApproach To Pleural EffusionBaskoro Tri LaksonoAinda não há avaliações
- Leptospirosis: Pauline Teo Siew ChinDocumento18 páginasLeptospirosis: Pauline Teo Siew Chinfarmasi_hmAinda não há avaliações
- S.I.R.S & M.O.DDocumento36 páginasS.I.R.S & M.O.Dshrikanth007Ainda não há avaliações
- Ebola Virus DiseaseDocumento19 páginasEbola Virus DiseaseAuliani Annisa FebriAinda não há avaliações
- Report KAP COVID-19 Among The Community of Shah AlamDocumento45 páginasReport KAP COVID-19 Among The Community of Shah AlamAdriana AfiqahAinda não há avaliações
- Nootropil: Qualitative and Quantitative CompositionDocumento12 páginasNootropil: Qualitative and Quantitative CompositionMuhammad TalhaAinda não há avaliações
- LA Myxoma Case PresentationDocumento34 páginasLA Myxoma Case PresentationWiwik Puji LestariAinda não há avaliações
- (MED1) 3.04 Approach To Hypertension (Dr. Bago-Azares)Documento11 páginas(MED1) 3.04 Approach To Hypertension (Dr. Bago-Azares)NoreenAinda não há avaliações
- PTBDocumento46 páginasPTBJai AdoraAinda não há avaliações
- Multiple Organ Dysfunction Syndrome: Contemporary Insights On The Clinicopathological SpectrumDocumento16 páginasMultiple Organ Dysfunction Syndrome: Contemporary Insights On The Clinicopathological SpectrumNurul Kamilah SadliAinda não há avaliações
- Management of Tuberculosis: A guide for clinicians (eBook edition)No EverandManagement of Tuberculosis: A guide for clinicians (eBook edition)Ainda não há avaliações
- Amanita Muscaria - Wikipedia, The Free Encyclopedia PDFDocumento17 páginasAmanita Muscaria - Wikipedia, The Free Encyclopedia PDFAmeli ApuyAinda não há avaliações
- Circulatory SystemDocumento5 páginasCirculatory SystemTan XiangAinda não há avaliações
- 5 Disease Transmission and Outbreak Investigation - 1Documento10 páginas5 Disease Transmission and Outbreak Investigation - 1RoniAnasoka100% (1)
- Essay Chicago StyleDocumento6 páginasEssay Chicago Stylefesehos0tej2100% (2)
- Abbas Basic Immunology 3eDocumento7 páginasAbbas Basic Immunology 3evudka100% (1)
- Interview DrPalevskyDocumento20 páginasInterview DrPalevskyzapperindiaAinda não há avaliações
- Trisomy 21Documento32 páginasTrisomy 21Alexandru EsteraAinda não há avaliações
- ANP 202 Principles of Animal Production PDFDocumento207 páginasANP 202 Principles of Animal Production PDFBELKYSAinda não há avaliações
- Fur TierDocumento22 páginasFur Tierhodijat2009Ainda não há avaliações
- Noise Induced Hearing LossDocumento4 páginasNoise Induced Hearing LossrrrawAinda não há avaliações
- Keto Resource GuideDocumento11 páginasKeto Resource GuidejoseAinda não há avaliações
- Immunoglobulins/Antibody: Presented By: Shashi Regd. No. 11006142 Roll No. B15 Section RP8003Documento17 páginasImmunoglobulins/Antibody: Presented By: Shashi Regd. No. 11006142 Roll No. B15 Section RP8003Shashi SharmaAinda não há avaliações
- Bio12 Cheat Sheet RubricDocumento2 páginasBio12 Cheat Sheet Rubricapi-310678040Ainda não há avaliações
- Chromosomal MutationDocumento3 páginasChromosomal MutationChristian jayr BalcitaAinda não há avaliações
- Recombinant DNA Applications: Food IndustryDocumento2 páginasRecombinant DNA Applications: Food IndustryetayhailuAinda não há avaliações
- Vectors For Gene DeliveryDocumento6 páginasVectors For Gene DeliveryMuhammad Ikram RabbaniAinda não há avaliações
- Health HistoryDocumento4 páginasHealth Historyapi-454903860Ainda não há avaliações
- My Assignment On NeurotransmitterDocumento8 páginasMy Assignment On NeurotransmitterSiti Fairuz HassanAinda não há avaliações
- Veterinary Epidemiology (VEP-411)Documento15 páginasVeterinary Epidemiology (VEP-411)Vijay Kumar Anumolu100% (1)
- Lec 7proteus SPPDocumento22 páginasLec 7proteus SPPbujalkanAinda não há avaliações
- STS Module-Gene TherapyDocumento6 páginasSTS Module-Gene TherapyWinter SummerAinda não há avaliações
- Concept Map PsoriasisDocumento1 páginaConcept Map PsoriasisEran Mark RojasAinda não há avaliações
- Atopic DermatitisDocumento30 páginasAtopic DermatitislcaldezAinda não há avaliações
- BiologyDocumento4 páginasBiologyAbanggi k sangmaAinda não há avaliações
- IFA Sports Nutrition Certification Test Answer Form: Tester: Date: NameDocumento8 páginasIFA Sports Nutrition Certification Test Answer Form: Tester: Date: NameSimbarashe MabambeAinda não há avaliações
- 1Documento10 páginas1shishirchemAinda não há avaliações
- HURDCO International School: Subject-Biology Chapter-2 Cell Structure and OrganisationDocumento20 páginasHURDCO International School: Subject-Biology Chapter-2 Cell Structure and OrganisationMahin IslamAinda não há avaliações
- MHC Class II DeficiencyDocumento2 páginasMHC Class II DeficiencyBre GlynnAinda não há avaliações
- Tutorial On ANS PharmacologyDocumento23 páginasTutorial On ANS PharmacologyRomaine-Ricardo FrancisAinda não há avaliações
- MyxomaDocumento36 páginasMyxomasgoeldoc_550661200Ainda não há avaliações