Escolar Documentos
Profissional Documentos
Cultura Documentos
The Use of GNRH Agonists and Antagonists in Infertility5
Enviado por
hossam626Título original
Direitos autorais
Formatos disponíveis
Compartilhar este documento
Compartilhar ou incorporar documento
Você considera este documento útil?
Este conteúdo é inapropriado?
Denunciar este documentoDireitos autorais:
Formatos disponíveis
The Use of GNRH Agonists and Antagonists in Infertility5
Enviado por
hossam626Direitos autorais:
Formatos disponíveis
The use of GnRH agonists and antagonists in infertility
Fidelis T. Akagbosu
INTRODUCTION
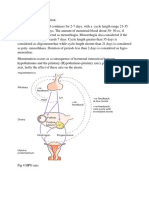
In the early days of in vitro ferrilizarion (IVF), natural cydes were commonly employed1 and classic stimulation prorocols involved the use of clomiphene citrate and gonadotropins. The existence of elevated basal levels of luteinizing hormone (LH) were shown to have a poor correlation with the success of che IVF cycle2. Premacure LH surges, which occurred owing to the positive feedback ofrising estradiol levels induced by the gonadotro-pins, led to premature luteinizarion artd were considered reasons for cancellation of the rreatment cycle. The dis-covery that the continued administration ofgonadotro-pin releasing hormone (GnRH) analogs induced a state ofhypophyseal desensitization provided a welcome solu-tion ro the problems that had caused a large number of IVF cycle cancellations. The first clinical crials of the use of GnRH analogs showed thar they were effective in eliminacing the basal levels of LH3'4 and in reducing the incidence of low responders5'6. The incidence of cycle cancellations dim-inished significantly and the GnRH analogs were soon in routine use. The agonists led to desensitization of the pituitary gonadotropin cells and a reduction in the num-berofGnRH receptors, while the antagonists produced an immediate effect by competitive blockade of the GnRH receptors. Various protocols combining the use ofagonistic ana-logs and gonadotropins in IVF cycles have been des-cribed. Reports in the current literature have associared the use of GnRH agoniscs with high pregnancy rates after IVF and embryo rransfer (ET)7. Debatable facts include the suggesrion that agonist use has a harmful GnRH
effect on oocytes and embryo quality, and possible direct effects on the ovary8. The GnRH antagonists have recently been used in phase III clinical trials and should be available for routine use in IVF cycles in the near fut-ure. Ancagonist use has various advancages, such as short-term and low rnedication exposure during follicular development and immediate suppression ofLH and follicle stimulating hormone (FSH) levels. As there is no 'flare effect', the frequency ofcyst formation would be lower, pacient management would be easier and stress, cost and hyperstimulation may all be reduced. The pitukary response is preserved fol-lowing ovarian stimularion with human menopausal gonadotropin (hMG) and the GnRH ancago-nist cetrorelix9 and it may not be necessary to provide luteal supporc in IVF-ET cycles ifter antagonist use.
.
Gonadotropin releasing hormone is a decapeptide pro-duced in the mediobasal hypothalamus by neurons loc-ated in the arcuate nucleus. It was first postulated by Green and Harris in 194710 and first isolated and characterized by Schally and colleagues in 1971". In narive GnRH, the amino acid sequence in positions 2 and 3 facilitates the release of gonadotropins while the sequence in position 6 is involved in enzymatic cleavage; residues 1, 6 and 10 are important for the three-dimensional structure and thus for receptor binding. GnRH binds to specific transmernbrane receptors in the
83
In Vitro Fertilization aiid Assisted. Reproduction
gonadotropic cells. Knobil12 showed that GnRH givcn continuously causcd a decrease in LH and FSH levels.
rcsult in the arrest of follicular development and cause fall in sex steroid levels to castrate levels. This is the bas for the clinical use ofthe agonists. The pituitary blockad persisrs during the agonist treatment but is completd reversible after therapy. Postmenopausal estradiol leve are commonly reached after 21 days16. In women, normal menstrual cycle is re-established in approx mately 6 weeks.
GnRH AGONISTS
The agonists were developed soon after the idcntification ofthe structure ofnative GnRH. The aim was .0 increase GnRH siability and binding affinity to its receptors by modifying the amino acid sequertce13'14. The modifi-cations were mainly at positions 6 and 10 and resuked in increased potency, The agonists have a 100200 times higher binding affinity for the GnRH receptors than does the native molecule. Table 1 shows the amino acid sequences ofGnRH and its analogs currently in use. Analog administrarion initially induces the llberation of high amounis of LH and FSH from the pitultary and an increase in the number ofGnRH receptors (up-regulation). Within 12 h ofadministration, the so-called 'flare effect' leads to a five-fold increase ofFSH, ten-fold rise in LH and four-fold elevation in estradiol15. Contin-uous (nor pulsatile) GnRH analog administration causes opposite effeccs: internalization of the agonist/receptor complex and a decrease in the number of receptors (down-regulatlon). This causes paradoxical suppression of the pituitary gonadotropin synthesis and liberation (desensinzation). The decreased levels of FSH and LH
A. / GnRH Agonists Buserelin Goserelin Leuprorelin Triprorelin Nafarelin AntagomsR Cetrorelix Nal-Glu Antide Ganirelix Azaline B Antarelix D-Nal D-Nal D-Nal D-Nal D-Nal D-Nal D-Phe D-Phe D-Phe D-Phe D-Phe D^Phe D-Pal D-Pal D-Pal D-Pal D-Phe D-Pal 4 4 4 4 4 4 5 Arg NicLys 5 Phe Phe pGlu 2 His 3 Trp 4 Ser mino acid seqiii 5 Tyr
GnRH ANTAGONISTS
The GnRH ancagonists were developed in parallel wii the agonists. Their development hiscory has been pl gued mainly by the high incidence of histamine relea following use ofthe first-generation anragonists. Antag nists competitively bind to pitukary GnRH recepto and do not induce the release of gonadotropins. Rece tor blockade is immediate and prevents the acrion native GnRH. On the basis of the rnechanism ofcor peritive binding, k is possible to modulate the degree hormone suppression by the dose of antagonist that administered. Depending upon rhe indication for th rapy, this permits the physician for instance to mainta defined esrradiol levels, which may have the advantage avoidlng hormone wlthdrawal effects such as vagir dryness or hot flushes17. Aftcr cessarion of treacme with ancagonlsts, recovery of gonadal function to
Table 1 Structural formulae ofnative luteinizing hormone releasing hormone (GnRH) agonistic and antagonistic analogs nces ofGnR}', 6 Gly ! analogs 7 8 Leu Arg
9 Pro
10 Gly-NH;
1 1 1 1 1
2 2 2 2 2
3 3 3 3 3
4 4 4 4 4
5 5 5 5 5
D-Ser D-Ser D-Leu D-Trp D-Nal D-Cit D-Glu D-NicLys D-hArg D-Phe D-Hcit
7 7 7 7 7 7 7 7 7 7 7
8 8 8 8 8 8 8 Lys(iPr) hArg Lys(iPr) Lys(iPr)
9 9 9 9 9 9 9 9 9^ 9r 9
ethylamii AzGly ethylamii Gly-NH: Gly-NH: D-Ala D-Ala D-Ala ',; D-Ala D-Ala D-Ala
The nse ofGnRH agonists and antagonists in infc'^'ility
normal state occurs, depcnding on the duration of the preceding treatmenc. Sincc the antagonists do noc cause pituitary exhaustion, these cells can respond ro an ade-quate stimulus almost immediately. The firsc-generarion antagoniscs were charaaerized by modifications in positions 2 and 3. The in vitro assay systems were effective, but very high doses were requircd. The second-generarion antagonists had modifications in positions 1 and* 6 and; later, the iriyoduction of-D-arginiiic..-in positio;6-:;Allrgic side-cff(fei:swiilT*"the"'' early antagonists included local erythema and in-duption, generalized edema and anap.hylactoid reacrion The observed side-effeccs were caused by the inducrion ofhistamine release, due to the existence ofGnRH rec-eptors on mast cells which degranulace after binding of basic antagonist18 and, to a much lesser excent, also of naciveGnRH19. The rhird- and fourth-generarion antagonisrs are the modern antagonists. These were developed in search for the ideal antagonist with high potency, a long duration of action and no clinically relevant side-effects. They usually have modifications in amino acid positions 1, 2, 3,6and 10 ofGnRH. The invesrigational drug ancarelix has been used in pre-clinical trials while ganirelix and ancide have been used in phase 1 trials. Antide srudies in ovariectomized monkeys showed prolonged suppression ofgonadotropins; similar effeccs have been demonsrraced in humans after subcucaneous injecrion.
female voluiitcers during three cdnsecutive cycles: a ;on-trol cycle, a treatment cycl?and a posc-treatment co-itrol cycle. Wirhin a period of 8 h, LH levels decreased and subsequencly were deeply suppressed; in a pa-aJlel manner, esrradiol reachcd postmenopausal values. Com-pared to baseline values, LH was reduced by l6.1c and FSH by 63.5%. At different time intervals after the ter-mination oftreatment, an LH surge followed by a rost-ovulatory rise in progesterone was found in all women. Th*e posp-treatment-cycl was comparable to tKe ore-treatment cycle.
CLINICAL APPLICATIONS OF GnRH ANALOGS IN ASSISTED REPRODUCTION TECHNIQUES
Premature LH surges have a negacive impact on the quality of oocytes and embryos and subsequentl'.' on pregnancy rate21'22. Pre-treatment with agonists signifi-cantly reduces the occurrence of LH surges. Before rhe agonist era, approximacely 20% ofsrimulated IVF c.'cles were cancelled, owing to premature LH surges. V'.'ith agonist use, this percentage has decreased to 2% anc rer-tilization and implantarion rates have increased23.
The antagonist cetrorelix Cetrorelix is the most advanced of the GnRH antago-nists. It has been well characterized in animal models and has reversible pharmacological action. It is not teraro-genic and is the most porenc GnRH ancagonist in monkeys19. No sysremic side-effeccs have been observed in trials. Local erythema of vary.ing^degrees is.seen.af-ter* cetrorelix injection* (and somerimes also with a^onists!-or native GnRH). Maximum plasma concencration of cecrorelix is reached in 1-2 h after the injecrion with all doses tesred. About 85% protein binding occurs in human albumin and human plasma and no differences in binding have been documented berween males and females16. Klingmuller and colleagues20 scudied mean serum LH levels after subcutaneous injection of 3 mg cerrorelix in
Agonist use in assisted reproduction techniques DifiFerenr treatment schedules using GnRH analogs s.nd gonadotropins are now in use, including the so-csJled 'long' prococol, which aims at complete pitukary sup-pression, and the 'short' and 'ultra-short' protocol;. in which the 'flare-up' effect is urilized. GnRH analog' are used in IVF treacment cycles as well as in frozen embryo transfer cycles when hormone replacement is requ::ed. In the IVF cycles, the 'long' proiocol is generally ihe most effeccive and is most often used ac presenc. How-e'<'eE,.ic has-the-disadvantages-of^a-loi'ig^treatment pe:i6d uiipil"dsensitization is achieved and relatively high i:osts due to an increased requiremenr for hMG24. As a result of the pituirary desensirizatibn, and to allow reco^ ery, luteal phase supporr with progesterone or human chori-onic gonadotropin (hCG) is required after analog us;. Specific effects of GnRH agonist use in IVF have been documented. Hsueh and co-workers25 noced snri- ; gonadotropin actions such as 83% loss ofLH recepcors in rat granulosa cells, decreased aromacase activity and
85
In Vitro Fertilization anii Assisted Reproduction
decrcascd progescerone accumulation. Analog use also leads to the activation ot 20a-OH progesterone dehydro-genase; this stimulates metabolism of progesterone co 20a-OH progescerone with little progestational activity.
Antagonist use in assisted reproduction techniques The simpliciry offered by ancagonists in regulating the menstrual cycle has made them porentially useful in assisted reproducrion cycles. Diedrich and coll-eagues23 were able ro stimulare parients successfully after pre-trearment with cerrorelix by daily administration" scarting on cycle day 7 until induction of ovulacion. The mean number of oocyres retrieved was- 8.1 per patient and the fertillzation rate was 61.5%. In 42% of the embryos, embryo quality was judged as being 'excellent' by morphological criteria. Six pregnancies were achieved in the first 42 parients trearcd by this regimen and three healthy babies were delivered. The mean amount of hMG required per parient was 27 ampules, whereas 40-50 ampules werc required in the 'long' protocol of GnRH agonists in thc center. The amount cff hMG needed after cerrorclix treatment is comparable with the 'ukra-short' prorocol, which up to now has been the least costly rreatmcnt26. Albano and collcagues27 compared differeni doses of thc GnRH antagonist cetrorelix during comrolled ovarinn hyperstimulation (COH). In 69 parients COH v.'as carried out with hMG, starring on day 2 of the menstrual cycle, and cetrorelix was adminiscered from day 6 of the hMG creatment every day up to and includlng the last day ofthe hMG injection. No pre-maturc endogenous LH surge occurred in parients treatcd wkh 0.5 and 0.25 mg ofcecrorelix. The minimal effective dose of cetrorelix able to prevent an LH surge in COH patiencs was 0.25 rng administered daily.
25 p.g ofGnRH was injected. Luteinizing hormone me surcments were made 30 and 80 min after the injectio; As a result ofthe antagonist treatmenc, initial LH leve were very low. After 30 min, LH levels increasc to about 10 mlU/ml in parients who received a do; of 3 mg and to 25 mlU/ml in those given 1 mg < cecrorelix, but fell again thereafter. Since the pituitai response is preserved following the administration i cetrorelix, ovulation may be induced with GnRH (nath or agonist) insread ofhCG. This could be beneficial'i patients with polycystic ovarian disease (PCOD) or ; risk ofovarian hyperstimularionsyndrome (OUSS). To date, no evidence for disturbance of the lure phase has beerr detected witli the use of? antagonists2 Lin and coworkers29 reported that the granulo' cells from women regulated by the GnRH antagoni; cetrorelix showed a higher degree of steroidogenes in all cultures (tescosterone, hCG and cAMP) cha the granulosa cells obtained from the women regulace by the GnRH agonisc buserelin. Furcher clinical scudk will show whether luteal phase support is necessar in IVF-ET cycles where antagonists had been used.
Pltuitary responsiveness following cetrorelix use Felberbaum and associates9 demonscrated pituirary responsiveness following cetrorelix use in IVF by applying a GnRH test. Patients in this scudy were srimulated with hMG starring on day 2 ofthe cycle. Cetrorelix was administered daily subcucaneously starting on cycle day 7. Three hours before ovularion induction with hCG,
Polycystic ovarian disease The rreatment of PCOD parients with GnRH agonisi has been well established30'31. Long-term treatmen with agonists has been shown to result in the suppressioi of escradiol and ovarian androgen levels, owing ti the inhibition of gonadotropins. The beneficial effec results from interference with the pathological LH/FSF rario commonly found in these women. Afrer fzill sup pression of the endogenous gonadotropins is reached normal follicular developrnent and synchronizatioi can be established by administration of exogenous LP and FSH or even FSH alone. Pretreatment with an agonist cannot preven the occurrence ofOHSS. This complication is frequen in PCOD patiencs and necesskates careful monitorin; in the treacment cycle. It appears thac the incideno of severe OHSS is higher in hMG-scimulated cycle of 'down-regulared' patients than in those treacec wirh hMG alone. This observation might be explainec by the rapid increase in estradiol levels in the combinec treatment schedule, which results from the fast anc synchronized development ofmulriple follicles16.
86
Thc use ofGnRH agonists and antagonists in infertility Phc cype oft&tcal supporc recomiTicndd'''is important in the presencc ofOHSS. With thc use ofprogesterone, che frequency of complications seems to bc much lower than aftcr mulriple. injectiansQi hCGIn-.compadson* to the agonists, GnRH ancagonist use would have the advantages of immediate suppression of gonadotro-pins and a greater suppression of LH comparcd to FSH secrerion. This difference is clear during-a short-term treatment and might be advantageous with regard to the hig^(;i,LH/F-SH-ratio found in PCOD patients16.
iaaian..h^perstHTHilaiaoisyB'Efe'onie
Ovarian hvperstimulation is a well-known and poten-tially lifethreatening iatrogenic-complication'ofovula-tion induction32. Women of less than 35 years bf age, suffering from PCOD with high estradiol values (> 5000 pg/ml).and multiple early-scage follicles, seem ro be at particularly HigK risk fd th'e development of' OHSS. Ih general during hMG-stimulated cycles, ovulation Is induced by the administration of hCG, which has a high dcgree of homology and synergisric effecrs with LH33 and thus can mimic the normally occurring mid-cycle LH peak. However, in spire of irs high similarity to LH, hCG does not cause idenrical physio-logical reaccions; it has a prolbnged half-life and does not induce the typical mid-cycle increase in FSH 34. These features may be responsible for the occurrence of the complication of OHSS after ovarian stimulation and ovulation induction with hCG. If hCG is not used for ovulation induction, OHSS may be avoided. Successful attempts have been made to in-duce ovulation by a single administration of an agonist, using the flare-up effect, in cases in which an antagonist had been used in the preceding stimulatory phase. In none of the patients treated in this way was severe OHSS observed35. Since the pituitary responsivcness is preserved during the co-administration of an antagonist, the combinarion of both treacment modalities (antagonist and agon-ist) might result in an additional benefit. Under these conditions, luteal phase support may not be necessary.
Endometribsis and'uterihe myorna .'YiilQLigftiAe overalAc^lrsiwictfrIV>p(*iEC-*good-*in end&^ metriosis, the optimal protocol of treacment has not yet been dtermined. Nakamura and' colleagucs36 reported rwo protocols combining GnRH analogs and gonadotro-pins for IVF-ET in patients with various scages ofendo-metriosis who were resiscant to conventional therapies. In theultra-longprotocolgrftup (21^patients), GnRH analog. was administered. for, attleast.60.days prior'co ovarian stimularion along with menotropin. In the long protocol group" (l'l' parierits), the-Gn'R'H an'alog was scarted'at the mid-luteal phase and exogenous gonado-tropin was commenced betweerfth'e' 3rd"and the 7th"day of the mensrrual cvcle after pituirary.suppre.s.sion. The-clinical pregRan6y-rai.orper'transtr \va.s,iound*ro be superiorwitH the ultra-long prococol (67 vs. 27%). Pro-longed GnRH analog suppression of ovarian function before superovularion may thus overcome some.causes of mferCTlt^iw-pauentSAvithendometriosT?'*. GiR^ft analogs aTe efficient' a reducing- ucerine fibroid volume and reversing the related symp-tomarology. The fibroids tend to return to their pre-treatment size about 6 months after creatment is discon-rinued. The beneficial effeccs of pre-surgical GnRH analog use are well established. Romer 37 reported the safety of hysteroscopic myoma resection of submucous myomas with largely intramural components following pre-treatment with rwo or three rnonthly injections of GnRH analogs. At Bourn Hall Clinic, GnRH agonists have been used for 23 months before IVF treacment in women with moderate-sized uterine fibroids. Beneficial efTects of such use include some reduction in fibroid volume and thus easier access ro che ovaries at crans-vaginal oocyte recrieval. It is imporcant to increase the dose of gonadotropins in the stimulatory phase of the IVF cycle to achieve adequate ovarian fbllicular develop-menr. In both endometriosis and urerine fibroids the thera-peutlc use ofGnRH agonists is well established but again the initial 'flare effects' are undesirable. The immediate suppression produced by the administration ofa GnRH-ancagonist would offer advantages in reducing the dura-tion of treatment and leading to a faster improvement of subjective symptoms. Future clinical trials would f'" '.
87
In Vitro Fertilization and Assisted Refiroduction hopefully address thc place ofantagonisrs in suppressing endoiTietriosis or moderate-sized uterine fibroids before Wf treatment; expeccations would include a shorter per-iod of stimulation, fewer side-effects, reduced require-rnents for gonadotropins and reduced'cost-.
clinics or in the context ofclinical trials. The ancagonist seem to offer many advantages compared to the agonists but their efficacy and safety have to be proved in long term studies. There are ongoing clinical scudies involving cetrorelix and antide in this field. There has been much progress, but the exacc clinica regimens for GnRH antagonists in IVF trcatment cycle; have not yet been established. As with GnRH agonists, ii has to be expected that GnRH anragonisrs, when appliec for complete hormone suppression, would exert side-efFects during long-term treacment based on the pharma-cological action ofthese compounds, such as decrease ir libido or loss ofbone density. Suitable susrained deliver) systems and.the development ofGnRH antagoniscs witl-sufficient oral bioavailability represent present and futur< challenges16.
CONCLUSIONS
It is scill coo early co speculate about.the possible end of the 'agonisr era'16. GnRH agonists are well-tested and easily available pharmaceutical agents in sex steroid-dependent diseases and in the management of IVF cycles. Agonists are still preferred in dinicaL situations where the use of a single monthly injection is required. At presenc, the use of aniagonists is restricted to a few
References
1. Edwards RG, Steptoe PC, Purdy JM. Establishing full term human pregnancies using cleaving embryos grown in vitro. Br JObstet Gynaecol 1980;87:737-56 2. Howlcs CM, Macnamee MC, Edwards RG, et al. Effects of high ronic levelsofluteinisirighormoneonoutcorneofw;tWo fertilisarion. Lancet '986;2:521 3. Porter RN, Smith \- , "raft IL. Inducrion ofovulation for m vitro fertilisation using buserclin and gonadotrophins. Lancet 1984;2:1284 4. Macnamec M, Howles CM, Edwards RG. Pregnancy after IVF when high ronic LH is reduced by long-term treatment with GnRH agoniscs. Hum Reprod 1987;2:569-71 5. Neveu S, Hedon B, BringerJ, et al. Ovarian stimulation by a combination ofgonadotropin releasing hormonc agonist and gonadotropins for in vitro fertilisation. Fertil Steril 1987,47: 639^3 6. Smitz J, Devroey P, Braeckmans P, et al. Managemenr of failed cycles in an IVF/GIFT programme with the combina-tion ofa GnRH analogue and HMG. Hum Refirod 1987;2: 309 7. French IVF Registry FIVNAT 1995 Report. Contraception, Fertilite, Sexualte 1996,24:694-9 8. Remohi J, Pellicer A. Use of GnRH analogs in IVF. In Asch RH, Studd JWW, eds. Annual Progress in Reproductive Medicine. Carnforth, UK: Parthenon Publishing, 1993: 107-25 9. Felberbaum R, Bauer 0, Kupker W, etal. Hormone profiles and pitukary response under ovarian stimulation with HMG and GnRHantagonist (Cetrorelix). Hum Reprod 1994;9(Suppl4):13 10. Green JD, Harris GW. The neurovascular link between the neurohypophysis and adenohypophysis./.fWomno/1947;5: 11. Schally AV, Arimiira A, Baba Y. Isolation and properties ol the FSH and LH-releasing hormone. Biochem Biophys Re: Commun 1971;43:393-9 12. Knobil E. The neuroendocrinecontrolofthe menstrual cycle. Recent Prog Horm Res 1980;36:53-88 13. Koch Y, Baram T, Hazum E, et al. Resisrance ro enzymatic degradation ofLH-RH analogs possessing increased biologi-cal actlviry. Biochem Biophys Res Commun 1997;74:488-92 14. Schally AV, Comaru-Schally AM, Hollander V. Hypotha-lamic and other peptide hormones. In HollanderJR, Frei E, Bast RC, eds. Cancer Medicine, 3rd edn. Philadelphia: Lea & Febiger, 1993:827 15. LemayA, Maheux R, Faure N, etal. Reversible hypogonadism indaced by a luteinizing hormone-releasing hormone (LH-RH) agonist (buserelin) as a new therapeutic approach for endometriosis. Fertil Steril 1984;4l:863-71 16. Reissrnann T, Felberbaum R, Diedrich K, etal. Development and applications of luteinising hormone-releasing hormone anragonists in the trcatment of infertility: an overview. Hum Reprod 1995; 10:1974-81 17. Bouchard P, CaratyA, Medalie D. Mechanism ofaction and clinical uses ofGnRH-antagonists in women. In Bouchard P, Haour F, Franchimont P, Schatz B, eds. Recent Progress on GnRHand Gonadal Peptides. Parls: Elsevier, 1990:209 18. Kiescl L, Runnebaum B. Gonadotrophin-releasing-Hormon und Analoga Physiologic and Pharmakologie. Gynakol Geburtsh Rundsch 1992;32:22-30 19. Weinbauer GF, Behre HM, Nieschlag E. Gonadotrophin re-leasing hormone analogue-induced regulation of cesticular function in monkeys and men. In Bouchard P, Caraty A, Coelingh-Bennink HJT, Palou SN, eds. GnRH, GnRH-Analogs, Gonadotrophins and Gonadal Peptides. Carnforth, UK: Parthenon Publishing, 1993:211
136-46
88
Você também pode gostar
- Physiology of MenstruationDocumento5 páginasPhysiology of Menstruationhossam626Ainda não há avaliações
- Pelvic Organ Prolapse: Prof Hossam HusseinDocumento71 páginasPelvic Organ Prolapse: Prof Hossam Husseinhossam626Ainda não há avaliações
- Investigation of The Infertile Couple For 2Documento14 páginasInvestigation of The Infertile Couple For 2hossam626Ainda não há avaliações
- MCQDocumento58 páginasMCQBethelhem BerhanuAinda não há avaliações
- Maternal Genital Tract Injuries and Obstetric ShockDocumento34 páginasMaternal Genital Tract Injuries and Obstetric Shockhossam626Ainda não há avaliações
- Menopause and Voiding Troubles: Prof - Abdel Karim M. El HemalyDocumento29 páginasMenopause and Voiding Troubles: Prof - Abdel Karim M. El Hemalyhossam6260% (1)
- إدارة الجودة الشاملة لضمان جودة الخدمات الصحية في المستشفيات - د. بن نافلة قدور، د. مزريق عاشورDocumento20 páginasإدارة الجودة الشاملة لضمان جودة الخدمات الصحية في المستشفيات - د. بن نافلة قدور، د. مزريق عاشورhossam626Ainda não há avaliações
- CSDocumento53 páginasCShossam626Ainda não há avaliações
- 3rd Year Medicine Parasitology LabDocumento98 páginas3rd Year Medicine Parasitology LabHassan.shehri100% (3)
- Superovulation Strategies in Assisted6Documento13 páginasSuperovulation Strategies in Assisted6hossam626Ainda não há avaliações
- Cervical Incompetence AFDocumento65 páginasCervical Incompetence AFhossam626Ainda não há avaliações
- Ultrasound in Assisted Conception 10-11Documento29 páginasUltrasound in Assisted Conception 10-11hossam626Ainda não há avaliações
- IntersexDocumento20 páginasIntersexhossam626Ainda não há avaliações
- The Cervix: Prof DR Hossam HusseinDocumento9 páginasThe Cervix: Prof DR Hossam Husseinhossam626Ainda não há avaliações
- HPVDocumento10 páginasHPVhossam626Ainda não há avaliações
- Ahmed Mohamed Abdel Rahim Rammah: Senior Registrar Obstetrics and Gynecology Department Al - Adan Hospital, KuwaitDocumento32 páginasAhmed Mohamed Abdel Rahim Rammah: Senior Registrar Obstetrics and Gynecology Department Al - Adan Hospital, Kuwaithossam626Ainda não há avaliações
- Hormone Replacement Therapy (HRT) Evidence-Based Guidelines: DR Mahdy El-Mazzahy Damietta General HospitalDocumento38 páginasHormone Replacement Therapy (HRT) Evidence-Based Guidelines: DR Mahdy El-Mazzahy Damietta General Hospitalhossam626Ainda não há avaliações
- Abnormal Uterine Bleeding Causes and TreatmentDocumento23 páginasAbnormal Uterine Bleeding Causes and Treatmenthossam626Ainda não há avaliações
- Colposcopy and CytopathologyDocumento43 páginasColposcopy and Cytopathologyhossam626Ainda não há avaliações
- Electronic Fetal MonitoringDocumento70 páginasElectronic Fetal MonitoringHossamaldin Hussein Kamel SalemAinda não há avaliações
- Radiographics US AnomaliesDocumento46 páginasRadiographics US Anomalieshossam626Ainda não há avaliações
- Review of Ovulation and Induction Protocolesal2Documento284 páginasReview of Ovulation and Induction Protocolesal2Hossamaldin Hussein Kamel SalemAinda não há avaliações
- Pelvis Canadian Guidlines USDocumento3 páginasPelvis Canadian Guidlines UShossam626Ainda não há avaliações
- Hypertension in PregnancyDocumento34 páginasHypertension in Pregnancyhossam626Ainda não há avaliações
- John Zakour - A Man's Guide To PregnancyDocumento58 páginasJohn Zakour - A Man's Guide To Pregnancyatan_rizal100% (1)
- Contracted Pelvis: Cliquez Pour Modifier Le Style Des Sous-Titres Du MasqueDocumento10 páginasContracted Pelvis: Cliquez Pour Modifier Le Style Des Sous-Titres Du Masquehossam626Ainda não há avaliações
- Physics of UltarasoundDocumento195 páginasPhysics of Ultarasoundhossam62683% (6)
- Infertility Us 15-7Documento46 páginasInfertility Us 15-7hossam626Ainda não há avaliações
- Dysmeno 2Documento15 páginasDysmeno 2hossam626Ainda não há avaliações
- The Subtle Art of Not Giving a F*ck: A Counterintuitive Approach to Living a Good LifeNo EverandThe Subtle Art of Not Giving a F*ck: A Counterintuitive Approach to Living a Good LifeNota: 4 de 5 estrelas4/5 (5794)
- The Little Book of Hygge: Danish Secrets to Happy LivingNo EverandThe Little Book of Hygge: Danish Secrets to Happy LivingNota: 3.5 de 5 estrelas3.5/5 (399)
- A Heartbreaking Work Of Staggering Genius: A Memoir Based on a True StoryNo EverandA Heartbreaking Work Of Staggering Genius: A Memoir Based on a True StoryNota: 3.5 de 5 estrelas3.5/5 (231)
- Hidden Figures: The American Dream and the Untold Story of the Black Women Mathematicians Who Helped Win the Space RaceNo EverandHidden Figures: The American Dream and the Untold Story of the Black Women Mathematicians Who Helped Win the Space RaceNota: 4 de 5 estrelas4/5 (894)
- The Yellow House: A Memoir (2019 National Book Award Winner)No EverandThe Yellow House: A Memoir (2019 National Book Award Winner)Nota: 4 de 5 estrelas4/5 (98)
- Elon Musk: Tesla, SpaceX, and the Quest for a Fantastic FutureNo EverandElon Musk: Tesla, SpaceX, and the Quest for a Fantastic FutureNota: 4.5 de 5 estrelas4.5/5 (474)
- Never Split the Difference: Negotiating As If Your Life Depended On ItNo EverandNever Split the Difference: Negotiating As If Your Life Depended On ItNota: 4.5 de 5 estrelas4.5/5 (838)
- Devil in the Grove: Thurgood Marshall, the Groveland Boys, and the Dawn of a New AmericaNo EverandDevil in the Grove: Thurgood Marshall, the Groveland Boys, and the Dawn of a New AmericaNota: 4.5 de 5 estrelas4.5/5 (265)
- The Emperor of All Maladies: A Biography of CancerNo EverandThe Emperor of All Maladies: A Biography of CancerNota: 4.5 de 5 estrelas4.5/5 (271)
- The Hard Thing About Hard Things: Building a Business When There Are No Easy AnswersNo EverandThe Hard Thing About Hard Things: Building a Business When There Are No Easy AnswersNota: 4.5 de 5 estrelas4.5/5 (344)
- Team of Rivals: The Political Genius of Abraham LincolnNo EverandTeam of Rivals: The Political Genius of Abraham LincolnNota: 4.5 de 5 estrelas4.5/5 (234)
- The Unwinding: An Inner History of the New AmericaNo EverandThe Unwinding: An Inner History of the New AmericaNota: 4 de 5 estrelas4/5 (45)
- The World Is Flat 3.0: A Brief History of the Twenty-first CenturyNo EverandThe World Is Flat 3.0: A Brief History of the Twenty-first CenturyNota: 3.5 de 5 estrelas3.5/5 (2219)
- The Gifts of Imperfection: Let Go of Who You Think You're Supposed to Be and Embrace Who You AreNo EverandThe Gifts of Imperfection: Let Go of Who You Think You're Supposed to Be and Embrace Who You AreNota: 4 de 5 estrelas4/5 (1090)
- The Sympathizer: A Novel (Pulitzer Prize for Fiction)No EverandThe Sympathizer: A Novel (Pulitzer Prize for Fiction)Nota: 4.5 de 5 estrelas4.5/5 (119)
- The Female Reproductive System: 1-1. GENERALDocumento5 páginasThe Female Reproductive System: 1-1. GENERALgladz25Ainda não há avaliações
- Question Paper Unit A161 02 Modules b1 b2 b3 Higher TierDocumento16 páginasQuestion Paper Unit A161 02 Modules b1 b2 b3 Higher TierAnonymous QFkCS837bAinda não há avaliações
- Sci8 q4 Mod2 v4Documento31 páginasSci8 q4 Mod2 v4Jay-ar RiosAinda não há avaliações
- Understanding the Process of Cell Division and GeneticsDocumento12 páginasUnderstanding the Process of Cell Division and GeneticsAnonymous klhru5EAinda não há avaliações
- Mitosis and MeiosisDocumento3 páginasMitosis and Meiosisfghvfhf50% (2)
- Hermaphroditism, The Condition of Having Both MaleDocumento11 páginasHermaphroditism, The Condition of Having Both Maleaimee mallariAinda não há avaliações
- CAESAR, Lael O. (1991) - Character in Job. Tesis Doctoral. University of Wisconsin-Madison.Documento373 páginasCAESAR, Lael O. (1991) - Character in Job. Tesis Doctoral. University of Wisconsin-Madison.Abel Muñoz PérezAinda não há avaliações
- MCN LectureDocumento8 páginasMCN LectureEmily BernatAinda não há avaliações
- CalotesDocumento7 páginasCalotesRaj Kumar JayapalAinda não há avaliações
- BIOLOGY SS2 3RD TERM E-NOTES (Reviewed)Documento89 páginasBIOLOGY SS2 3RD TERM E-NOTES (Reviewed)kanajoseph2009Ainda não há avaliações
- Nature of Disposition of Sperm and Human EmbryosDocumento52 páginasNature of Disposition of Sperm and Human Embryosفارس بن بارسAinda não há avaliações
- Jurnal Pendampingan Kesejahteraan JaninDocumento6 páginasJurnal Pendampingan Kesejahteraan JaninRita ChatrinAinda não há avaliações
- Basic of Human Embryology PDFDocumento28 páginasBasic of Human Embryology PDFaureliatanasalAinda não há avaliações
- LESSON 8 – UNDERSTANDING MEIOSISDocumento7 páginasLESSON 8 – UNDERSTANDING MEIOSISKEITHLYN EIZEL RAMITERREAinda não há avaliações
- Nagpur Gynecologist ListDocumento46 páginasNagpur Gynecologist ListsreevidyaAinda não há avaliações
- Revision Notes Class 8 Science Chapter 10 - Reaching The Age of AdolescenceDocumento2 páginasRevision Notes Class 8 Science Chapter 10 - Reaching The Age of AdolescenceArpit SharmaAinda não há avaliações
- C. J. Van Vliet - The Coiled Serpent (1939)Documento470 páginasC. J. Van Vliet - The Coiled Serpent (1939)muruganaviator100% (1)
- The Miracle of Life, Images of Gestation of Baby in The Womb, in Utero Photos of An Embryo With Development From Conception To Birth.Documento17 páginasThe Miracle of Life, Images of Gestation of Baby in The Womb, in Utero Photos of An Embryo With Development From Conception To Birth.Lily of the ValleysAinda não há avaliações
- DNA copying essential for reproductionDocumento10 páginasDNA copying essential for reproductionLizannAinda não há avaliações
- Natural Selection Quiz (5.2Documento2 páginasNatural Selection Quiz (5.2laura pongutaAinda não há avaliações
- General-Biology-2-PRELIM-EXAM - MAGBANUA STEM 11BDocumento4 páginasGeneral-Biology-2-PRELIM-EXAM - MAGBANUA STEM 11BSophia MagbanuaAinda não há avaliações
- Biology II: Continuity of Life Macromolecules and MicromoleculesDocumento23 páginasBiology II: Continuity of Life Macromolecules and MicromoleculesLester Randy RubintinusAinda não há avaliações
- Case Study of Cesarean SectionDocumento9 páginasCase Study of Cesarean Sectionzarian wu72% (18)
- Chapter 1 Reproduction NotesDocumento10 páginasChapter 1 Reproduction NotesPoornimaAinda não há avaliações
- Week 5 Worksheet AnswersDocumento5 páginasWeek 5 Worksheet AnswersaskldhfdasjkAinda não há avaliações
- CH 23 Lecture OutlineDocumento6 páginasCH 23 Lecture OutlineZohal Ghulam-JelaniAinda não há avaliações
- Vog QDocumento62 páginasVog QDeep PatelAinda não há avaliações
- ContraceptionDocumento35 páginasContraceptionRhomizal MazaliAinda não há avaliações
- Botany Lab Manual: Bsc.-Ii MedicalDocumento49 páginasBotany Lab Manual: Bsc.-Ii MedicalRajesh KalalAinda não há avaliações
- PterisDocumento4 páginasPterisSreeja RajAinda não há avaliações