Escolar Documentos
Profissional Documentos
Cultura Documentos
Isoptera (Termite) (Adapted From Inventory and Collection, 2003)
Enviado por
mrusdihDescrição original:
Título original
Direitos autorais
Formatos disponíveis
Compartilhar este documento
Compartilhar ou incorporar documento
Você considera este documento útil?
Este conteúdo é inapropriado?
Denunciar este documentoDireitos autorais:
Formatos disponíveis
Isoptera (Termite) (Adapted From Inventory and Collection, 2003)
Enviado por
mrusdihDireitos autorais:
Formatos disponíveis
ISOPTERA (TERMITE) (ADAPTED FROM INVENTORY AND COLLECTION, 2003)
by Homathevi Rahman and Noel Tawatao
Introduction Termites belong to the insect order Isoptera, and are characterized by their colonial behaviour. They are often referred to as white ants, however, morphologically and phylogenetically they are very different from the ants and the other social Hymenopterans (bees and wasps). The word Isoptera originated from the Greek, in which isos means equal and pteron means wing, and refers to the two pairs of identical wings in the adult (Harris, 1957, Thorne & Carpenter, 1992). Termites are polymorphic, eusocial insects, living in large communities of several hundred to several million individuals, composed of reproductives (winged) forms together with numerous apterous sterile soldiers and workers. Their numerous colonies have great influence in ecosystems. The fossil record indicates that termites evolved about 220 million years ago (Collins, 1988; Thorne & Carpenter, 1992). Several attempts were made to determine the phylogeny of termites which remains unresolved. Evidence indicates that phylogenetically termites are closely related to cockroaches (Kambhampati, 1995). They are said to be derived from a primitive group of wood-dwelling cockroaches, clearly seen in the obligate dependence on mutualistic intestinal protists and, in some higher forms, externally cultivated basidiomycete fungi (Bignell & Eggleton, 1998). There are about 2650 species of termites in 280 genera and seven families that have so far been described worldwide (Kambhampati & Eggleton, 2000). They are widely dispersed throughout the tropics as well as some temperate regions and achieve their highest diversities and abundance in the rain forests of Africa, South America and Southeast Asia (Collins, 1988; Bignell and Eggleton 1998). Of these, about 323 species from 52 genera have been recorded in the Indo-Malayan (Oriental) region (Tho, 1992) and about 104 species (33 genera) have been recorded from Sabah (Thapa, 1981). Termites can either be beneficial or destructive to man, and this is determined very much by which species are present and their feeding and nesting behaviour. Where to look in the forest All termites live in colonies within the confines of excavations within wood above-ground, or in subterranean and epigeal nest systems. They occur wherever there is timber, decaying wood, plant refuse or soil rich in humus on which they can feed (Harris, 1957). Termites nest systems can be classified according to whether they are :
Introductory Course To Entomology
a. Wood nesters. Termites in this group live in or around standing trees or dead logs. Sometimes the dead wood is gradually replaced with wood carton, a woody substance with low nutrient concentrations and high levels of lignin and other undigested components (Collins, 1989). This includes the Kalotermids ( Kalotermes,Glyptotermes ), some Rhinotermids ( Schedorhinotermes, Parrhinotermes, Heterotermes and also Coptotermes ) and some Termitidae members such as Microcerotermes and Termes. b. Hypogeal or subterranean nesters. Termites whose colony centres are below the ground without any indication of their presence (Wood & Johnson, 1986). They use their feces or a mixture of feces and mineral soil in nest construction. The colony centres are often poorly defined and characterless, especially in the soldierless Apicotermitinae. But in some Macrotermitinae, Apicotermes and Homallotermes, a little internal structure or surface holes present together with their complex underground nests. This is to enable the foragers to forage on aboveground vegetation. This group also includes many species that are facultative secondary inhabitants of epigeal mounds, Microcerotermes, Pericapritermes, and soldierless Apicotermitinae (Eggleton et al., 1996). c. Epigeal mound builders. Termites whose colony centres associated with living (free standing or tree buttresses) or dead vegetation above ground (Jones, 1990; Eggleton et al., 1996), commonly known as mound builders. The mounds are usually well characterized, often with very complex structures. Materials used for construction are of three main types: subsoil with relatively low organic content added with salivary secretion (Macrotermes and Cornitermes), wood carton (a mixture of faeces and macerated wood with a high lignin content) (most wood-feeding termites), or a mixture of faeces and organic-rich topsoil (many soil-feeders). Epigeal mound structure can differ widely within genera and also between regions within widely distributed species. Macrotermitinae and Odontotermes are known to build huge mounds of selected clay-rich subsoil (Wood & Johnson, 1986). d. Arboreal nesters. Nests are attached outwardly to trees at different heights. These nests are normally made of wood carton. In most cases the nests are connected to the ground by covered runways. This may assist in distinguishing some arboreal termite nests from those of ants. Nonetheless, some arboreally nesting Nasutitermitinae, (e.g. Hospitalitermes), form open foraging columns without any connecting runways between the nest and foraging sites. Preservation As termites are soft-bodied insects, they are usually preserved in 80% ethanol. All castes from a colony will be preserved in one tube filled with alcohol, together with a small label stating the locality where it was found, collection date and the collectors name. The taxonomic label includes genus or species name (depends on identification level), identification date and determiners name.
122
Isoptera (Termite)
Collection Usually termites are manually collected using forceps. As for termites that live in mounds and underground, one has to use a trowel to dig out the soil to get the termites. Baits such as tissue paper and softwood can be used to trap termites, especially the pest groups. Classification There are seven extant families: Mastotermitidae, Hodotermitidae, Termopsidae, Serritermitidae, Kalotermitidae, Rhinotermitidae and Termitidae. These families are divided into 14 subfamilies, 270 genera and over 2000 species (Kambhampati et al., 1996). Of these, only three families (Kalotermitidae, Rhinotermitidae and Termitidae) are recorded in Borneo and Peninsula Malaysia (Collins, 1988; Thapa, 1980; Tho, 1992). Based on the composition of the symbiont microbiota in the gut, termites are divided into two groups, lower termites and higher termites, where lower termites house flagellate protozoans and bacteria. Higher termites house a variety of prokaryotic microbes, but no flagellates. Some Termitinae also house cellulolytic amoebae. Lower Termites These are the primitive termites and phylogenetically known to harbour cellulose and /or xylandigesting, flagellate-protozoans and bacteria in their hindgut, to aid in cellulose decomposition (Breznak, 1982; Breznak & Brune, 1994). The protozoans (oxymonad,trichomonad and hypermastigote) are mainly cellulolytic anaerobes and have the ability to degrade cellulose and other polysaccharides (Bignell & Eggleton, 1998). Lower termites are generally found outside forests, or in marginal habitats within forests and mostly all are wood feeders except for Hodotermitidae, which are grass-feeders (Collins, 1989). Kalotermitidae Species in this family often referred to as the dry wood termites (from their nesting habit, in sound wood) and believed to be a sister group to Rhinotermitidae and Termitidae (Kambhampati et al., 1996). This is the largest family of lower termites, with 25 genera and 350 species (Krishna, 1970; Wood & Johnson, 1986). These termites occur in small numbers in rain forests, mainly confined to dead limbs and trunks in the forest canopy (Collins, 1988). Many species in this family are serious pests of forest products and two common species found in Malaysian forest are Cryptotermes cynocephalus and C. domesticus. Soldiers normally have robust, phragmotic heads, which are of particular value in blocking and defending nest galleries (Collins, 1988). Rhinotermitidae Rhinotermids are thought to be evolved from an extinct ancestral Hodotermitidae and there are six subfamilies, Coptotermitinae, Heterotermitinae, Psammotermitinae, Termitogetoninae, Stylotermitinae and Rhinotermitinae. This is the most important family of lower termites in Malaysian forest, and is often refered to as damp wood termites. They are found in standing or fallen trunks and limbs, and can cause severe damage to timber and
123
Introductory Course To Entomology
living trees. Some of the common genera found in Malaysian and Bornean forests are Coptotermes, Heterotermes, Termitogeton, Prorhinotermes, Parrhinotermes and Schedorhinotermes (Collins, 1988; Thapa, 1981; Tho, 1992). Important pest species are Coptotermes curvignathus Holmgren, infesting rubber trees and pine trees and Curvignathus borneensis Oshima (Chey, 1996; Collins, 1988). Higher termites This is the largest group dominating the order with over 80% of the genera and 74% of the species (Krishna, 1969; Wood & Johnson, 1986; Edwards & Mill, 1986). Phylogenectically they form mutualistic relationship with other microorganisms, usually fungi and bacteria, or bacteria alone, despite the presence of endogenous cellulase to digest their food (Slaytor, 1992; Slaytor et al., 1997). They include the most advanced and diverse groups of termites, and exhibit a wide variety of social specializations (Krishna, 1969; Howse, 1970; Breznak, 1982). Termites in this group have a more elaborate external and internal anatomy and social organization compared to lower termites (Breznak & Brune, 1994). Higher termites predominate in tropical forest systems as litter, wood and soil feeders. Termitidae The family Termitidae contains three-quarters of all known species, comprising four subfamilies: Macrotermitinae, Apicotermitinae, Termitinae and Nasutitermitinae (Wood & Johnson, 1986; Collins, 1988). One of the important subfamilies is the Macrotermitinae. Genera in this subfamily are known to cultivate species of the symbiotic basidiomycete fungus Termitomyces on faecal combs within their nests. These termites have high weight-specific consumption rates and a correspondingly greater impact on decomposition processes than other termites. Macrotermitinae are known to originate from Africa, and of the 13 recorded genera, only four genera occur in Malaysian forests: Macrotermes, Odontotermes, Hypotermes and Microtermes. Subfamilies Termitinae and Nasutitermitinae include both wood- and soil- feeding species and they dominate most tropical forest ecosystems. The Termes-group belonging to the subfamily Termitinae are some of the soil-feeders, and they possess snapping mandibles and are widely distributed in Peninsular Malaysia and Borneo. Nasutitermitinae is a highly specialized form of higher termites. Most of these genera found in Malaysian forests are wood-feeders and they are able to consume large quantities of dead wood. Colony structure and life cycle Termites live in large communities and the colony members are of four castes: the reproductives (king and queen), soldiers and workers. In addition, colonies have a large number of young immature forms in all stages and of all castes (Collins, 1984). Each caste varies morphologically and behaviourally but they have to live cooperatively or else the colony will die (Collins, 1984). The number of individuals and ratios of each caste in a colony is very difficult to determine and it varies between species and also depends on the age and size of the colony (Bignell & Eggleton, 1998). Large colonies may include a number
124
Isoptera (Termite)
of supplementary reproductives, producing eggs to augment or replace the founding queen (Bignell & Eggleton, 1998). The parent termites, the king and queen are the functional reproductives. The queens major role is to lay eggs. She develops an enlarged abdomen containing ovariales and associated tissues, a condition known as being physogastric (Collins, 1984). The queen is also involved in pheromonal regulation of controls the production of each caste in a colony (Noirot & Noirot-Timothee, 1969, Moore, 1969). Soldiers and workers are wingless and can be either sterile male or female. Soldiers usually represent one-tenth of the population at most (Harris, 1957). There are also termite genera that lack of this caste, such as Anoplotermes and Protohamitermes. Termites are the only social insects with a true soldier caste whose major role is only to defend the colony (Bignell & Eggleton, 1998). For this purpose, morphologically they are bigger in size and have defensive adaptations such as enlarged mandibles or stopper-like heads (Krishna, 1969). In the highly derived subfamily Nasutitermitinae, the mandibles are reduced and non-functional. Instead the soldiers have a nasus, an elongated projection of the fontanelle and their way of defence is by squirting irritating chemical substances through it (Krishna, 1969; Collins, 1984). Besides having mandibles, and a sclerotized head, soldiers of some genera such as Coptotermes have a frontal gland that discharges a defensive secretion through a frontal pore (Richards & Davies, 1978). This secretion can be toxic or repellent to intruders, such as ants, or tacky and entangle their legs and antennae. The worker caste is the most numerous and plays the major role in the survival of the colony. They collect food, process the digesta, feed other castes and construct the mound or nest (Harris, 1957). All living termites, except the Kalotermitidae, are known to have a true worker caste. In Kalotermitidae, there is no distinct worker caste and the work of the colony is done by immature adults, whose development is stopped temporarily according to the needs of the colony (Harris, 1957). Winged reproductives or alates of both sexes are produced in large numbers in a mature colony. These alates swarm out from mature nests at particular times of the year (often during or just before rains) (Bignell & Eggleton, 1998). They make short, often rather feeble, dispersal flights, and then pair-up on the ground after the wings have been shed (dealation) (Bignell & Eggleton, 1998). The paired termites will then select a new nesting site and once they are established, mating takes place. The first batch of eggs is produced by the female within a few days. As in cockroaches, termites are also hemimetabolus. The hatched youngs, are translucent white and feeble at first, but very active from the moment they hatch (Edwards & Mill, 1986). These larvae are fed from nutrient-rich salivary secretions produced by the parents. They normally undergo a number of moults until they achieve the mature form as sterile workers or soldiers, depending to the need of the colony (Harris, 1957). These developments are determined by extrinsic factors such as pheromones
125
Introductory Course To Entomology
and hormones (Krishna, 1970). Usually, at the beginning of a colony foundation all the larva become workers and after sometime, an occasional larvae is found with large head and jaws of quite a different shape, and this grows into a soldier (Harris, 1957). The colony grows slowly for many years, accompanied by a continuous increase in the number of individuals, enlargement of the nest and much building activity (Bignell & Eggleton, 1998). Once the colony is well organised, larvae appear with wing buds, which later will become winged termites and the full cycle of development is complete (Harris, 1957). Trophic Groups Almost all species of termite are detritivorous. They consume wide range of freshly dead or decaying plant material including dry grass, leaf litter, decaying wood, dung and humus. Living plant tissues, including lichen and mosses are taken by a few species. Another feeding group that may be common and important in many tropical forests is the soil-feeding termites. Accurate information on the natural history and feeding habits of termites is still scarce for some groups, particularly the subterranean species. Nonetheless, termite species can be categorized into five broad trophic categories according to their food, foraging galleries or columns, colour of the abdomen and known biology (Martius, 1994; De Souza & Brown, 1994; Eggleton et al., 1995; 1996; 1997; Bignell et al., 1997): i. Wood feeders. These primitive wood eating termites feed on wood and woody litter, including dead branches still attached to trees, and they may live in their feeding galleries which in some cases become colony centres (Eggleton et al., 1996; 1997; Wardell, 1987). The condition of wood taken is very important. This may include living trees ( Coptotermes , Schedorhinotermes and Microcerotermes dubius), sound dead wood (Kalotermitidae), and/or fungus-attacked wood (Nasutitermitinae, some Termitinae, and Macrotermitinae) (Wood, 1976; Collins, 1984). Most of these termites are arboreal (attached to trees), subterranean or epigeal nesters (Bignell et al., 1997; Eggleton et al., 1996; 1997). ii. Soil feeders. Termites feeding on the upper mineral soil, with some degree of selection of silt and clay fractions. The vast majority of species in this group ingest topsoil rich in organic matter (Sleaford et al., 1996). They are normally distributed in the soil profile, in the organic litter layer (leaves and twigs) and/or in epigeal mounds (Bignell et al., 1997; Eggleton et al., 1995;1996;1997). This form is found in many Termitinae (the Capritermes-group and Labritermes), several Nasutitermitinae (the Subulitermes-group), and most Apicotermitinae (the Anoplotermes-group) (Wood, 1976; Eggleton et al., 1997). Soil-feeders are very common and abundant in many tropical rain forest (Wood, 1976). In South-east Asian regions, soil-feeders are dominated by the Termitinae with a small number of Nasutitermitinae and Apicotermitinae (Abe, 1987). iii. Soil-wood interface-feeders. Termites in this group feed on highly decayed (friable and soillike) wood, the soil under logs or soil plastered to logs, or soil mixed with leaf litter in stilt-root complexes (Eggleton et al., 1996; 1997). Soil/-wood interface-feeders are only found in the Termitinae, Apicotermitinae and Nasutitermitinae. Most of them nest within dead logs, build epigeal nest or form colony centres in the soil (Eggleton & Bignell, 1995; Eggleton et al., 1996; 1997).
126
Isoptera (Termite)
iv. Litter-foragers. Termites that forage for leaf litter and small woody items litter in various stages of decay. Food sources are often taken back and stored temporarily in the nest. This group includes some subterranean and other mound-building Macrotermitinae (with fungal association), as well as certain Nasutitermitinae that forage on the surface of the ground or litter layers (Bignell et al., 1997; Eggleton et al., 1996; 1997; Collins, 1984). Genera such as Laccessititermes and Longipeditermes are also known as arboreal forages. v. Micro-epiphyte-feeders. Termites of this group forage for moss, algae, lichens and fungi on tree barks. In South -east Asia, Hospitalitermes hospitalis is known to feed on lichen (Jones & GathorneHardy, 1995; Eggleton et al., 1997; Collins, 1981). Grass-feeders are another important feeding group. Grass feeders will also take dung and may sometimes scavenge vertebrate corpses. Grass-feeders are mainly of the family Hodotermitidae, found only in savanna and deserts (Krishna, 1970). As for South-east Asian tropical rain forest, the common functional groups are, wood-, soil-, soil/-wood-interface, litter and micro-epiphyte feeders (Eggleton et al., 1998; Jones, 1998; Collins, 1989).
Termites Role In The Ecosystem The dominance of termites in tropical ecosystems is mainly related to their ability of utilizing dead plant material rich in cellulose (the most abundant organic matter on the earth) (Abe, 1995). With this they become important in processes such as decomposition of organic matter (Peakins & Josens, 1978; Wood & Johnson, 1986), supplying material for many food chains, soil engineering (translocating and altering soils physically and chemically and maintaining soil fertility) (Lee & Wood, 1971; Wood, 1988), providing a possible input of nitrogen through symbiont fixation (Wood & Sands, 1978; Collins, 1984), methane gas release, and carbon-flux.
127
Introductory Course To Entomology
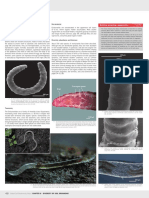
Important Morphological Characters of Soldier Caste Used for Identification
la
fr d a fo
f e h g
Dorsal and profile view of soldier heads, postmentum and pronotum: a = head length to lateral base of mandibles, b = maximum head width, c = mandible length to lateral base, d = height of head including postmentum, e = length of postmentum, f = maximum width of postmentum, g = length of pronotum, h = width of pronotum, fr = frons, fo = fontanelle, la = labrum.
128
Isoptera (Termite)
Some Termite Species (Photography by Richard Davies Natural History Musem of London, and Martubat Jamlan, Universiti Malaysia Sabah).
Pericapritermes sp
Hirtitermes spinocephalus
Hospitalitermes sp
Havilanditermes sp
Prohamitermes mirabilis
Microcerotermes serrula
129
Introductory Course To Entomology
References Abe, T. 1987. Evolution of life types in termites. In: Kawano, S., Connell, J.H. & Hidda, T. (eds) Evolution and coadaption in biotic communities. University of Tokyo Press, Tokyo: 125-148. Abe, T. 1995. The termite-symbionts system: How does it work and has it evolved as a superefficient decomposer in tropical terrestrial ecosystems? Center for Ecological Research, Kyoto University, Kyoto. Ahmad, M. 1950. The phylogeny of termites genera based on imago-worker mandibles. Bull. Of American Museum of Natural History. 95: 43-85. Ahmad, M. & Akhtar, M.S. 1981. New termite genera of the Capritermes complex from Malaysia, with a note on the status of Pseudocapritermes (Isoptera : Termitidae). Pakistan Journal of Zoology, 13 (1&2): 1-21. Bignell, D.E. & Eggleton, P. 1998. Termites. In: Calow, P. (ed) Encyclopedia of ecology and environmental management. Blackwell scientific, Oxford: 744-746. Bignell, D.E., Eggleton, P., Nunes, L. & Thomas, K.L. 1997. Termites as mediators of carbon fluxes in tropical forest: budgets for carbon dioxide and methane emissions. In: Watt, A.D., Stork, N.E. & Hunter, M.D. (eds). Forest and Insects. Chapman and Hall, London: 109-134. Breznak, J.A. 1982. Intestinal microbiota of termites and other xylophagous insects. Annual Review of Microbiology, 36: 323-343. Breznak, J.A. & Brune, A. 1994. Role of microorganisms in the digestion of lignocellulose by termites. Annual Review of Entomology. 39: 453-487. Chey, V.K. 1996. Forest pest insects in Sabah. Sabah Forest Record. 15: 1-178. Collins, N.M. 1981. The role of termites in the decomposition of wood and leaf litter in the Southern Guinea Savanna of Nigeria. Oceologia 51: 389-399. Collins, N.M. 1984. The termites (Isoptera) of the Gunung Mulu National Park, with a key to the genera known from Sarawak. Sarawak Mus. J. 30 : 65-87. Collins, N.M. 1988. Termites. In: Cranbrook, E (ed) Key Environments Malaysia. Oxford: Pergamon Press.
130
Isoptera (Termite)
Collins, N.M. 1989. Termites. In: Lieth, H. & Werger, M.J.A. (eds). Tropical Rain Forest Ecosystems. Elsevier Science Publishers, Amsterdam: 455-471. de Souza, O.F.F. & Brown, V.K. 1994. Effects of habitat fragmentation on Amazonian termite communities. Journal of Tropical Ecology. 10: 197-206. Edwards, R. & Mill, A.E. 1986. Termites in buildings- their biology & control. Rentokil Ltd. Eggleton, P.E., Bignell, D.E., Sands, W.A., Waite B., Wood, T.G., & Lawton, J.H. 1995. The species richness of termites (Isoptera) under differing levels of forest disturbance in the Mbalmayo Forest Reserve, southern Cameroon. Journal of Tropical Ecology. 11: 85-98. Eggleton, P., Bignell, D.E., Sands, W.A., Mawdsley, N.A., Lawton J.H., Wood, T.G. and Bignell, N.C. 1996. The diversity, abundance and biomass of termites under differing levels of disturbance in the Mbalmayo Forest Reserve, southern Cameroon. Phil. Trans. R. Soc. London. B. 351: 51-68. Eggleton, P., Homathevi, R., Jeeva, D., Jones, D.T., Davies, R.G. & Maryati, M. 1997. The species richness and composition of termites (Isoptera) in primary and regenerating lowland dipterocarp forest in Sabah, East Malaysia. Ecotropica. 3: 119-128. Gathorne-Hardy, F. 2001. A review of the South-east Asian Nasutitermitinae (Isoptera: Termitidae), with descriptions of one new genus and a new species and including a key to the genera. Journal of Natural History. 35: 1485-1506. Grasse, P.P. & Noirot, C. 1954. Apicotermes arquieri (Isoptera): ses constructions, sa biologe. Considerations generales sur la sous famile des Apicotermitinae nov. Annales des Sciences Naturelles Zoologique. 16: 345-388. Harris, W.V. 1957. An introduction to Malayan termites. Malay. Nat. J. 12: 20-32. Homathevi, R. 1999. Diversity and ecology of forest termite (Isoptera) populations in Sabah, East Malaysia, with special reference to the Termes-Capritermes clade. PhD. thesis. Kota Kinabalu, Sabah, Malaysia: School of Science and Technology, Universiti Malaysia Sabah. Howse, P.E. 1970. Termites: A Study in Social Behaviour. 1-144. Jones, J.A. 1990. Termites, soil fertility and carbon cycling in dry tropical Africa: a hypothesis. Journal of Tropical Ecology 6: 291-305
131
Introductory Course To Entomology
Jones, D.T. & Gathrone-Hardy, F. 1995. Foraging activity of the processional termite Hospitalitermes hospitalis (Termitidae: Nasutitermitinae) in the rain forest of Brunei, north-west Borneo. Ins. Soc. 42: 359-369 Kambhampati, S. & Eggleton, P. 2000. Phylogenetics and Taxonomy. In: Abe, T., Bignell, D.E. & Higashi, M. (eds.). Termites: Evolution, Sociality, Symbiosis, Ecology. Kluwer Academic Publishers. Pp 1-23. Kambhampati, S., Kjer, K.M. & Thorne, B.L. 1996. Phylogentic relationship among termite families based on DNA sequence of mitochondrial 16S ribosomal RNA gene. Insect Molecular Biology, 5(4): 229-238. Krishna, K. 1970. Taxonomy, phylogeny and distribution of termite. In: Krishna, K. & Weesner, F.M. (eds). Biology of termites. 2. New York and London: Academic Press: 643 pg Lee, K.E. & Wood, T.G. 1971. Termites and soil. London: Academic Press, New York. Martius, C. 1994. Diversity and ecology of termites in Amazonian forests. Pedobiologia. 38: 407-428. Noirot, C. & Noirot-Timothee, C. 1969. The digestive system. In: Krishna, K. & Weesner, F.M. (eds). Biology of termites. Academic Press, New York and London. Peakins, G.J. & Josens, G. 1978. Respiration and energy flow. In : Brian, M.V. (ed). Production ecology of ants and termites: Cambridge University Press: 111-163. Richards, O.W. & Davies, R.G. 1978. IMMS outlines of entomology. Chapman and Hall, London. Slaytor, M. 1992. Cellulose digestion in termites and cockroaches: what role do symbionts play? Comparative Biochemistry and Physiology 103B: 775-784. Slaytor, M.,Veivers, P.C. & Lo, N. 1997. Aerobic and anaerobic metabolism in the higher termite Nasutitermes walkeri (Hill). Insect Biochemistry and Molecular Biology 27: 291-303. Sleaford, F., Bignell, D.E. & Eggleton, P. 1996. A pilot analysis of gut contents in termites from the Mbalmayo forest reserve, Cameroon. Ecological Entomology 21: 57-73. Thapa, R.S. 1981. Termites of Sabah. Sabah Forest Record 12: 1-374.
132
Isoptera (Termite)
Tho, Y.P. 1992. Termites of Peninsular Malaysia. Malayan Forest Record No. 36. Forest Research Institute Malaysia: 224 Thorne, B.L. & Carpenter, J.M. 1992. Phylogeny of the Dictyoptera. Systematic Entomology 17. 253-268. Wardle D.A. 1987. Control of termites in nurseries and young plantations in Africa: Established practices and alternative courses of action. Commonw, For Rev. 66 (1):77-89. Wood, T.G. 1976. The role of termites (Isoptera) in decomposition processes. In: Anderson, J.M. & MacFadyen, A. (eds) The Role of Terresterial and Aquatic Organisms in Decomposition Processes. Blackwell Scientific Publications, Oxford: 145-168. Wood, T.G. 1988 Termites and the soil environment. Biol. Fertl. Soils. 6:228-236. Wood, T.G. & Sands, W.A. 1978. The role of termites in ecosystems. In: Brian, M.V. (ed). Production Ecology of Ants and Termites, Cambridge University Press, Cambridge: 55-80. Wood, T.G. & Johnson, R.A. 1986. The biology, physiology and ecology of termites. In: Vinson, S.B. (ed.) The economic impact and control of social insects Praeger publications: 1-68.
133
Você também pode gostar
- Bio Cheat Sheet MasterDocumento7 páginasBio Cheat Sheet MasterChris_Barber0986% (7)
- Test Bank For Evolution 2nd Edition Media Update by Carl T Bergstrom Lee Alan Dugatkin Full DownloadDocumento14 páginasTest Bank For Evolution 2nd Edition Media Update by Carl T Bergstrom Lee Alan Dugatkin Full Downloadaaronwarrenpgozeainyq100% (25)
- Beetle: Beetle Beetles Are A Group of Insects That Form The Order Coleoptera, in TheDocumento38 páginasBeetle: Beetle Beetles Are A Group of Insects That Form The Order Coleoptera, in Theenzo abrahamAinda não há avaliações
- Bioengineering - A Conceptual ApproachDocumento314 páginasBioengineering - A Conceptual ApproachNay Minn KhantAinda não há avaliações
- STB 211 Pest and Pest ControlDocumento57 páginasSTB 211 Pest and Pest ControlDaniel50% (2)
- Study Guide For Entomology 10Documento9 páginasStudy Guide For Entomology 10Alex TunqueAinda não há avaliações
- Basics of Animal Communication - Interaction, Signalling and Sensemaking in The Animal KingdomDocumento160 páginasBasics of Animal Communication - Interaction, Signalling and Sensemaking in The Animal KingdommatijahajekAinda não há avaliações
- The Effect of Lights On The Mung Bean GrowthDocumento4 páginasThe Effect of Lights On The Mung Bean GrowthNadya Awaliah30% (10)
- Culture Techniques of Moina The Ideal Daphnia For Feeding Freshwater Fish FryDocumento7 páginasCulture Techniques of Moina The Ideal Daphnia For Feeding Freshwater Fish FrymrusdihAinda não há avaliações
- Butterfly-Farming The Flying Gems by Labay PIFGEX 2009Documento30 páginasButterfly-Farming The Flying Gems by Labay PIFGEX 2009Anonymous HXLczq375% (4)
- Reflections On The Heart of BorneoDocumento234 páginasReflections On The Heart of BorneomrusdihAinda não há avaliações
- Freeze Dryers-eBook LM 2017-FINALDocumento7 páginasFreeze Dryers-eBook LM 2017-FINALmrusdihAinda não há avaliações
- Original PDF Psychology Themes and Variations 5th Canadian Edition PDFDocumento41 páginasOriginal PDF Psychology Themes and Variations 5th Canadian Edition PDFkyle.lentz942100% (34)
- Behaviour and Ecological Impacts of Termites: Fecundity Investigations in MoundsDocumento10 páginasBehaviour and Ecological Impacts of Termites: Fecundity Investigations in MoundsCompaore IssahAinda não há avaliações
- TR 1Documento1 páginaTR 1test1245 testAinda não há avaliações
- Appraisal of The Economic Activities of Termites: A Review: Received: October 2011 Accepted: April 2012Documento6 páginasAppraisal of The Economic Activities of Termites: A Review: Received: October 2011 Accepted: April 2012Reem Alaa AldinAinda não há avaliações
- LepidepteraDocumento41 páginasLepidepteraSaid H AbdirahmanAinda não há avaliações
- Etymology: Taxonomy and EvolutionDocumento6 páginasEtymology: Taxonomy and EvolutionMithileshsingh94Ainda não há avaliações
- RRLDocumento4 páginasRRLJohnMichaelBatulaAinda não há avaliações
- Weinbeer - Kalko. 2004. Morphological Characterictics Predict Alternate Foraging Strategy and Microhabitat Selection in The Orange-Bellied BatDocumento8 páginasWeinbeer - Kalko. 2004. Morphological Characterictics Predict Alternate Foraging Strategy and Microhabitat Selection in The Orange-Bellied BatJ E Fernando CernaAinda não há avaliações
- Butterfly PDFDocumento24 páginasButterfly PDFzakir hussainAinda não há avaliações
- Biological Control: Luc Barbaro, Andrea BattistiDocumento8 páginasBiological Control: Luc Barbaro, Andrea Battistinima2020Ainda não há avaliações
- Donavan 2001 PDFDocumento11 páginasDonavan 2001 PDFthunder61992Ainda não há avaliações
- Literature ReviewDocumento7 páginasLiterature ReviewDeepa Chitralekha RanaAinda não há avaliações
- Hojun Song 2018Documento25 páginasHojun Song 2018johanna abrilAinda não há avaliações
- Liz's Essay: Compare and Contrast The Community Structure of Tropical Rain Forest and Temperate Deciduous ForestsDocumento8 páginasLiz's Essay: Compare and Contrast The Community Structure of Tropical Rain Forest and Temperate Deciduous ForestsBrunei essays100% (1)
- Reticulitermes Flavipes: Geographic RangeDocumento8 páginasReticulitermes Flavipes: Geographic RangeHanifah HerliniAinda não há avaliações
- Management of TermiteDocumento32 páginasManagement of TermiteCosmas Naibaho100% (1)
- Soil Ants: Solenopsis Made Up Only 1,4 - 3,9% of The Ant Biomass. in Spite of The Biology of These Tiny SpeciesDocumento7 páginasSoil Ants: Solenopsis Made Up Only 1,4 - 3,9% of The Ant Biomass. in Spite of The Biology of These Tiny SpeciesmvbgarciaAinda não há avaliações
- CRPT1 Insect OrdersDocumento34 páginasCRPT1 Insect OrdersCharles Russell TabelismaAinda não há avaliações
- Seedling Functional Types in A Lowland Rain Forest in MexicoDocumento13 páginasSeedling Functional Types in A Lowland Rain Forest in MexicoJim VillenaAinda não há avaliações
- Asociación Planta OrmigaDocumento4 páginasAsociación Planta OrmigaDaniel Aparicio HilaresAinda não há avaliações
- Enchytraeids PotwormsDocumento1 páginaEnchytraeids PotwormsLifko DeiviAinda não há avaliações
- Phytoliths As Indicators of Pedogenesis and Paleoenvironmental Changes in The Brazilian CerradoDocumento5 páginasPhytoliths As Indicators of Pedogenesis and Paleoenvironmental Changes in The Brazilian Cerradokaname10Ainda não há avaliações
- Pages From Beat Wermelinger - Forest Insects in Europe - Diversity, Functions and Importance (2021, CRC Press) - Libgen - Li PDFDocumento9 páginasPages From Beat Wermelinger - Forest Insects in Europe - Diversity, Functions and Importance (2021, CRC Press) - Libgen - Li PDFrahmat ridhoAinda não há avaliações
- Camponotus GigasDocumento3 páginasCamponotus Gigasdesi cuteAinda não há avaliações
- Briefs: Tree Species Resistant To TermitesDocumento2 páginasBriefs: Tree Species Resistant To TermitesVanny Gimotea BaluyutAinda não há avaliações
- Fleming Et Al. 2009 - The Evolution of Bat Pollination - A Phylogenetic PerspectiveDocumento27 páginasFleming Et Al. 2009 - The Evolution of Bat Pollination - A Phylogenetic PerspectiveMarcelo MorettoAinda não há avaliações
- Diversity and Genomes of Uncultured Microbial Symbionts in The Termite GutDocumento7 páginasDiversity and Genomes of Uncultured Microbial Symbionts in The Termite Gut20-067 Dinda Hairani Br BangunAinda não há avaliações
- Boone 2008Documento13 páginasBoone 2008joaopauloramosdemeloAinda não há avaliações
- Papel de Los Mamíferos en Los Procesos de Dispersión y Depredación de Semillas de Mauritia Flexuosa (Arecaceae) en La..Documento13 páginasPapel de Los Mamíferos en Los Procesos de Dispersión y Depredación de Semillas de Mauritia Flexuosa (Arecaceae) en La..Mariana Gutierrez MuneraAinda não há avaliações
- Zoology BonDocumento118 páginasZoology BonRupkathar Pagol Kra RajkumariAinda não há avaliações
- Review Chiroptological Studies in IndiaDocumento40 páginasReview Chiroptological Studies in Indiaradioactive.greenmonsterAinda não há avaliações
- Ant Id KeyDocumento30 páginasAnt Id Keyafira1809Ainda não há avaliações
- Guia Morfologia Heces BrasilDocumento24 páginasGuia Morfologia Heces BrasilTom BombadilAinda não há avaliações
- Avila & Medelin. 2004. Ecological, Taxonomic, and Physiological Correlates of Cave Use by Mexican Bats PDFDocumento14 páginasAvila & Medelin. 2004. Ecological, Taxonomic, and Physiological Correlates of Cave Use by Mexican Bats PDFHenry CondoriAinda não há avaliações
- 2006 Willmott Cladistics IthomiiniphylogenyDocumento73 páginas2006 Willmott Cladistics IthomiiniphylogenyRoberta MirandaAinda não há avaliações
- 121 1995 Biodiversity at Its UtmostDocumento14 páginas121 1995 Biodiversity at Its UtmostArghya PaulAinda não há avaliações
- Termites Survey in Secondary Dry Dipterocarp Forest atDocumento6 páginasTermites Survey in Secondary Dry Dipterocarp Forest atale46Ainda não há avaliações
- Biotechnology Investigatory ProjectDocumento6 páginasBiotechnology Investigatory ProjectEphraim Soriano50% (4)
- Key For Identification of The Hymenopteran Parasitoids of The African Citrus Psylla Trioza Erytreae Del Guercio (Hemiptera: Triozidae) in CameroonDocumento7 páginasKey For Identification of The Hymenopteran Parasitoids of The African Citrus Psylla Trioza Erytreae Del Guercio (Hemiptera: Triozidae) in CameroonSushivanyaAinda não há avaliações
- Coleoptera WPS OfficeDocumento15 páginasColeoptera WPS OfficeAl Francis MendozaAinda não há avaliações
- Molecular Phylogenetics and Evolution: Sanna A. Leppänen, Ewald Altenhofer, Andrew D. Liston, Tommi NymanDocumento11 páginasMolecular Phylogenetics and Evolution: Sanna A. Leppänen, Ewald Altenhofer, Andrew D. Liston, Tommi NymanViniciusAinda não há avaliações
- Cactus ReviewDocumento20 páginasCactus ReviewLinx_AAinda não há avaliações
- 18157-Article Text-55730-1-10-20160202Documento12 páginas18157-Article Text-55730-1-10-20160202Lucìa SolerAinda não há avaliações
- Diversity and Endemicity of Chilimo Forest, Central EthiopiaDocumento4 páginasDiversity and Endemicity of Chilimo Forest, Central Ethiopiaasmeraamde21Ainda não há avaliações
- Morphology and Classification of MossesDocumento53 páginasMorphology and Classification of MossesIlham ArfiansyahAinda não há avaliações
- Best Animal BiomesDocumento6 páginasBest Animal Biomessalman672003Ainda não há avaliações
- Termite: Not To Be Confused With,, or - This Article Is About Social Insects. For Other Uses, SeeDocumento13 páginasTermite: Not To Be Confused With,, or - This Article Is About Social Insects. For Other Uses, SeeArun KumarAinda não há avaliações
- Identification of Invertebrate Taxonomic CharacterDocumento6 páginasIdentification of Invertebrate Taxonomic CharacterDaisy KavinskyAinda não há avaliações
- Gleaning Bats As Underestimated Predators of Herbivorous Insects: Diet of Micronycteris Microtis (Phyllostomidae) in PanamaDocumento10 páginasGleaning Bats As Underestimated Predators of Herbivorous Insects: Diet of Micronycteris Microtis (Phyllostomidae) in PanamaTiate TdatdnttAinda não há avaliações
- 07 Rodents Pdf-Module-7Documento13 páginas07 Rodents Pdf-Module-7jemuelpachecoAinda não há avaliações
- Twelv: ArthropodsDocumento20 páginasTwelv: ArthropodsMaharani Putri ChaniaAinda não há avaliações
- Molecular Phylogenetics and Evolution: Sciverse SciencedirectDocumento11 páginasMolecular Phylogenetics and Evolution: Sciverse SciencedirectVermilion~Ainda não há avaliações
- 7 - Biotic InteractionsDocumento22 páginas7 - Biotic InteractionsJorge Botia BecerraAinda não há avaliações
- Entomology: University of Zakho Faculty of Science Department of BiologyDocumento20 páginasEntomology: University of Zakho Faculty of Science Department of BiologySerdar AgidAinda não há avaliações
- 1.1 Taxonomy of ActinomycetesDocumento8 páginas1.1 Taxonomy of ActinomycetesLinguumAinda não há avaliações
- 01 PDFDocumento22 páginas01 PDFDeboraAinda não há avaliações
- Bijlage 12 Regulations For Enrolment Non Regular 2021-2022 - ENG - DefDocumento12 páginasBijlage 12 Regulations For Enrolment Non Regular 2021-2022 - ENG - DefmrusdihAinda não há avaliações
- Reclamation of Municipal Domestic Wastewater by Aquaponics of Tomato PlantsDocumento8 páginasReclamation of Municipal Domestic Wastewater by Aquaponics of Tomato PlantsmrusdihAinda não há avaliações
- Alo Ever A Quality StandardDocumento2 páginasAlo Ever A Quality StandardmrusdihAinda não há avaliações
- Interpretation of Infrared Spectra, A Practical ApproachDocumento24 páginasInterpretation of Infrared Spectra, A Practical ApproachLucas TimmerAinda não há avaliações
- ANRES GuidelineDocumento12 páginasANRES GuidelinemrusdihAinda não há avaliações
- Kramer Bress An 2015Documento20 páginasKramer Bress An 2015mrusdihAinda não há avaliações
- FAO Fisheries CircularDocumento114 páginasFAO Fisheries CircularmrusdihAinda não há avaliações
- Written & Compiled By: John Clare, B.A., PH.DDocumento20 páginasWritten & Compiled By: John Clare, B.A., PH.DmrusdihAinda não há avaliações
- Sicilian Dwarf ElephantDocumento24 páginasSicilian Dwarf ElephantMaria BarbozaAinda não há avaliações
- 01 The Living WorldDocumento16 páginas01 The Living Worldjocoge4337Ainda não há avaliações
- Efektifitas Trico Dan Fungisida Propineb Dalam Pengendalian Penyakit Karat Pada Bawang DaunDocumento14 páginasEfektifitas Trico Dan Fungisida Propineb Dalam Pengendalian Penyakit Karat Pada Bawang DaunMuhammad AzisAinda não há avaliações
- Tree Palms at Arecaceae Phylogeny PosterDocumento1 páginaTree Palms at Arecaceae Phylogeny Posterivan gonzalezAinda não há avaliações
- Kunci KSN-K Biologi Sma 2020Documento1 páginaKunci KSN-K Biologi Sma 2020Ilham NurhidayahAinda não há avaliações
- Biology Chapter 19 NotesDocumento6 páginasBiology Chapter 19 NotesJonah MartinAinda não há avaliações
- Written Work 3Documento7 páginasWritten Work 3Jellie May RomeroAinda não há avaliações
- Gitt - Did God Use EvolutionDocumento155 páginasGitt - Did God Use EvolutionAbel SaraivaAinda não há avaliações
- Xenogenesis JumpChainDocumento11 páginasXenogenesis JumpChainSilva TAinda não há avaliações
- Tutorial Letter 001/0/2021: Year Module: 2021Documento13 páginasTutorial Letter 001/0/2021: Year Module: 2021Zizzie NzimaAinda não há avaliações
- Food Science Dissertation IdeasDocumento6 páginasFood Science Dissertation IdeasWritingPaperServicesUK100% (1)
- IMHM321 Lecture Trans 3Documento9 páginasIMHM321 Lecture Trans 3Brix BrixAinda não há avaliações
- Vocab For First UnitDocumento6 páginasVocab For First UnitCharmaine LIEAinda não há avaliações
- Zhang 2022Documento10 páginasZhang 2022Statistics LearningAinda não há avaliações
- Rationale (Insecticide) g10Documento5 páginasRationale (Insecticide) g10Ma. Ruffa Mae MejiaAinda não há avaliações
- Cambridge O Level Biology (5090) : Movement Into and Out of CellsDocumento55 páginasCambridge O Level Biology (5090) : Movement Into and Out of CellsMoinuddin SummitAinda não há avaliações
- Introduction and Methodology Pr2 - Group8-BlDocumento9 páginasIntroduction and Methodology Pr2 - Group8-BlBrile Quirap100% (1)
- Air Bubbles The Agar: Pglo Lab Report 20 PointsDocumento3 páginasAir Bubbles The Agar: Pglo Lab Report 20 PointsSatoshi SugaAinda não há avaliações
- Electrophoresis Product GuideDocumento40 páginasElectrophoresis Product GuideDolphingAinda não há avaliações
- Intracrine Sex Steroid Synthesis and Signaling in HumanDocumento17 páginasIntracrine Sex Steroid Synthesis and Signaling in HumanMarcela DiasAinda não há avaliações
- Toxic Legacy IntroductionDocumento8 páginasToxic Legacy IntroductionChelsea Green Publishing100% (1)
- Gene BanksDocumento13 páginasGene BanksHARSHIT MAHESHWARIAinda não há avaliações
- Winogradsky Column: Marawi CampusDocumento13 páginasWinogradsky Column: Marawi CampusErnie Mark Patosa MaratasAinda não há avaliações
- Sintesis Protein Lengkap 311008Documento46 páginasSintesis Protein Lengkap 311008Indah Indryani UAinda não há avaliações