Escolar Documentos
Profissional Documentos
Cultura Documentos
VV
Enviado por
Lisaam De YesteDescrição original:
Título original
Direitos autorais
Formatos disponíveis
Compartilhar este documento
Compartilhar ou incorporar documento
Você considera este documento útil?
Este conteúdo é inapropriado?
Denunciar este documentoDireitos autorais:
Formatos disponíveis
VV
Enviado por
Lisaam De YesteDireitos autorais:
Formatos disponíveis
Kinetics 1.
The kinetics of the hydrolysis of the halogenoalkane RCH2Cl with aqueous sodium hydroxide (where R is an alkyl group) was studied at 50 C. The following results were obtained: Experiment 1 2 3 (i) (ii) (iii) (iv) [RCH2Cl] 0.050 0.15 0.10 [OH] 0.10 0.10 0.20 Initial rate/mol dm3 s1 4.0 104 1.2 103 1.6 103
Deduce the order of reaction with respect to the halogenoalkane, RCH2Cl, and with respect to the hydroxide ion, OH, giving reasons for your answers. (4) Hence write the rate equation for the reaction. Calculate the value of the rate constant with its units for this reaction at 50 C. Using your answer to part (ii), write the mechanism for this reaction. (1) (2) (3)
(Total 10 marks)
2. In an experiment to determine the rate of the reaction between persulphate ions and iodide ions in aqueous solution
2 S2O 8 + 2I 2SO 2 4 + I2
the following data were obtained:
Concentration/mol dm 3
S2 O 2 8
I 0.100 0.100 0.200
Initial rate /mol dm3 s 1 0.36 0.72 1.44
0.100 0.200 0.200
(a)
(i) (ii) (iv)
Deduce the order of reaction with respect to each of the reagents and hence write the rate equation for the reaction. (3) With reference to this reaction state what is meant by the overall order of a reaction. (1) (2) Explain why the rate equation cannot be written directly from the stoichiometric equation for the reaction. (1) Suggest a suitable experimental technique that would enable you to determine the rate of the reaction given opposite. (1) Suggest a necessary condition that would help to ensure accurate results. (1) (1)
(Total 10 marks)
(iii) Calculate the rate constant including units.
(b)
(i) (ii)
(iii) Suggest one advantage or disadvantage of your chosen experimental method. (a) (i) Explain what is meant by the following terms. Rate of reaction & Overall order of a reaction (ii) (b)
(2)
Explain why the order of reaction cannot be deduced from the stoichiometric equation for a reaction. (1)
Substitution reactions of halogenoalkanes, can proceed via an SN1 or SN2 mechanism. When 1-bromobutane,
CH3CH2CH2CH2Br, 2-bromobutane, CH3CH2CHBrCH3, and 2-bromo-2-methylpropane, (CH3)3CBr, are reacted separately with aqueous sodium hydroxide solution each gives the corresponding alcohol. (i) (iii) Give the mechanism for the SN1 reaction between 2-bromobutane and hydroxide ions.(3) In an experiment designed to find the mechanism of the reaction between 2-bromo-2methylpropane and hydroxide ions the following data were obtained at constant temperature. Initial concentration of 2-bromo-2-methylpropane /mol dm3 0.10 0.20 0.30 Initial concentration of OH / mol dm3 0.10 0.10 0.20 Initial rate of reaction /mol dm3 s1 1.2 102 2.4 102 3.6 102
Use the data to deduce the rate equation for the reaction of 2-bromo-2- methylpropane with sodium hydroxide solution. (3) (c) Suggest, in outline, a method you could use to follow the progress of the reaction between a bromoalkane and aqueous sodium hydroxide. (3)
(Total 14 marks)
3. Manganate(VII) ions react with ethanedioate ions in acidic solution.
2MnO4 (aq) + 16H (aq) + 5C2O4 (aq) 2Mn (aq) + 8H2O(l) + 10CO2(g)
+ 2 2+
(a)
In a particular experiment 200 cm3 of aqueous potassium manganate(VII), KMnO4, of concentration 0.0500 mol dm3 were mixed with 50.0 cm3 of ethanedioic acid, HOOCCOOH, of concentration 0.500 mol dm3, and 80 cm3 of 1.0 mol dm3 sulphuric acid. (i) (ii) Show by calculation that the starting concentration of the manganate(VII) ions was 3.03 102 mol dm3. The concentration of the manganate(VII) ions was determined over a period of time. Time / s 0 400 800 1200 1600 2000 2400 Concentration of manganate(VII) ions/ mol dm
3
3.03 102 2.98 102 2.86 102 2.75 102 1.90 102 7.50 103 2.50 103
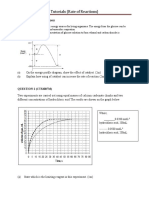
Plot a graph of the concentration of manganate(VII) ions against time and from it determine the initial rate of the reaction and the rate at 1600 s.(5) (b) A second experiment was set up involving the same chemicals in the same concentrations as in experiment 1 but this time some solid manganese(II) sulphate was dissolved in the ethanedioic acid before the potassium manganate (VII) solution was poured in. The plot of the concentration of manganate(VII) ions against time is given below:
(i) (ii)
Determine the order of the reaction with respect to manganate(VII) ions by considering the time taken for the concentration to fall by half, using the concentrations at 0, 800 and 1600 s. (3) Compare this graph with the one you plotted in (a) (ii) and give two pieces of evidence that manganese(II) sulphate is a catalyst for this reaction. (2)
3.5 10 2 3.0 10 2
concentration /mol dm 3
2.5 10 2 2.0 10 2 1.5 10 2 1.0 10 2 5.0 10 3 0 0 400 800 1200 1600 2000 2400 time / s
4. Hydrogen peroxide decomposes to form water and oxygen. (a) (b) Suggest a method for following the rate of this reaction. (2) The reaction is first order with respect to hydrogen peroxide. (i) (ii) Explain what is meant by the term first order. (1)
H2O2(aq) H2O(l) +
1 2
O2(g)
The overall order of the reaction is one. Give the rate equation for the reaction.(1)
(iii) How would you use a graph of hydrogen peroxide concentration against time to show that the reaction is first order? (2) (c) The data in the table show the effect of temperature on the rate of this reaction. T /K 293 302 314 323 (i) (ii) (iii) Rate / mol dm3 s1 1.6 106 4.2 106 14.4 106 33.8 106 1/T /K1 3.41 103 3.31 103 3.19 103 3.10 103 ln(rate) 13.3 12.4 11.1 10.3
On the axes below, sketch graphs for two temperatures, T1 and T2, where T2 is greater than T1, and use them to explain why increasing temperature has a dramatic effect on the rate of this reaction.(4) Plot a graph of ln(rate), on the vertical axis, against 1/temperature, on the horizontal axis, on the grid below.(3) Use your graph and the equation below to calculate the activation energy, EA, for this reaction.
ln(rate) = constant
EA R
(1/T)
where R = 8.31 J K1 mol1
You should include the sign and units with your answer which should be given to two significant figures.(3) 5. This question is about the kinetics of the reaction in which ammonium cyanate, NH4CNO, turns into urea, NH2CONH2, in aqueous solution. NH4CNO(aq) 2CONH2(aq) The table below shows the mass of urea, mt, which formed at different times in a solution of ammonium cyanate of known starting concentration. When the reaction stopped the mass of urea, mfinal, was 20.3 g. Time / min 0 25 50 75 100 150 200 300 (a) (b) (c) Mass of urea, mt /g 0 12.5 15.7 17.1 17.5 18.7 19.1 20.0 mfinal mt/g 20.3 7.8 4.6 3.2 2.8 1.6
Complete the final column of the table.(1) Plot a graph of mfinal mt (on the vertical axis) against time (on the horizontal axis)(2) The graph can be used to work out a rate equation for the reaction. What term in the rate equation for the reaction is proportional to mfinal mt? (1)
(d)
(i) (ii)
Show THREE successive half-life measurements on your graph, and give their values.(2) Use the half-lives to decide whether the reaction is zero order, first order or second order. Explain how you decided the order.(2)
(iii) Suggest a possible rate equation for the reaction. (1) (e) A student thought that water might take part in the rate-determining step of the reaction. (i) (ii) What is meant by the rate-determining step? (1) The solution of ammonium cyanate used in the experiment initially contained 0.35 moles of ammonium cyanate in approximately 1 dm3 (55.5 moles) of water. Is the order you calculated in (d)(ii) an order with respect to ammonium cyanate, or could it include the water as well? Explain your answer. (2)
(Total 12 marks)
6.
(a) (b)
In a rate of reaction experiment between two substances, A and B, the overall order of the reaction was found to be 2. Write three possible rate equations for such a second order reaction between A and B. (3) At a certain temperature the rate of reaction between nitrogen monoxide, NO, and hydrogen, H2, was investigated. The following data were obtained.
[NO]/mol dm3 1.0 1.0 3.0 (i) (ii) (c)
[H2]/mol dm3 1.0 3.0 1.0
Rate/mol dm3 s1 0.02 0.06 0.18
Use the data above to deduce the rate equation for this reaction.(3) Use your answer to (b)(i) above to calculate the value of the rate constant, with units(2)
The investigation described in part (b), above, was repeated, but at a higher temperature, and the rate of the reactions increased. Explain, in terms of particles, why an increase in temperature increases the rate of a reaction. (3) State the effect of an increase in temperature on the value of the rate constant, k. (1) Explain the effect of a catalyst on the rate of a reaction (3) (Total 15 marks)
(d) (e)
Você também pode gostar
- How FastDocumento54 páginasHow FastKaushal Silva RanpatabendigeAinda não há avaliações
- Chapter 1 Reaction KineticsDocumento8 páginasChapter 1 Reaction KineticsDinesh RamaAinda não há avaliações
- NSS Chemistry Part 13 Industrial Chemistry - IDocumento36 páginasNSS Chemistry Part 13 Industrial Chemistry - Izwindows123456789Ainda não há avaliações
- CHM 212 Assignment DR AbdulwahabDocumento2 páginasCHM 212 Assignment DR Abdulwahabfortress generator servicesAinda não há avaliações
- Chemcal Kinetics (Tutorial Questions)Documento3 páginasChemcal Kinetics (Tutorial Questions)renAinda não há avaliações
- ALEVELREVISIONQUESTIONSDocumento7 páginasALEVELREVISIONQUESTIONSAnthony AndyAinda não há avaliações
- AssignmentDocumento6 páginasAssignmentHuang YingAinda não há avaliações
- A2 Chemistry ExamzoneDocumento4 páginasA2 Chemistry ExamzoneSan SiddzAinda não há avaliações
- Chapter 1: Rate of Reaction: Larning Task 1.2 Problem SolvingDocumento29 páginasChapter 1: Rate of Reaction: Larning Task 1.2 Problem Solvingamin_zamanAinda não há avaliações
- 3 (N) (Special Mock Exam 37)Documento6 páginas3 (N) (Special Mock Exam 37)Vinaigrette HeAinda não há avaliações
- Chemical KineticsDocumento5 páginasChemical KineticsTimothy HandokoAinda não há avaliações
- Tutorial 2 StudentDocumento6 páginasTutorial 2 StudentIrsyad KamilAinda não há avaliações
- t11 Reaction Kinetics 19-26Documento7 páginast11 Reaction Kinetics 19-26lorraine_cuaAinda não há avaliações
- Que Bank 12 ChemDocumento8 páginasQue Bank 12 Chemtechblogger098Ainda não há avaliações
- TR 02Documento2 páginasTR 02SuryaAinda não há avaliações
- 4.3 Reaction Rates and Reversible ReactionsDocumento18 páginas4.3 Reaction Rates and Reversible ReactionsVictor VC100% (5)
- Tutorial-Manual CH1002Documento18 páginasTutorial-Manual CH1002Gift Chulu100% (2)
- Electro Kinetics Coordination Set MDocumento3 páginasElectro Kinetics Coordination Set MShivam SahuAinda não há avaliações
- A Level Chemistry Paper 2 Exam 5Documento5 páginasA Level Chemistry Paper 2 Exam 5Anthony AndyAinda não há avaliações
- 6 EqDocumento4 páginas6 Eqchihingho19930903130Ainda não há avaliações
- Class 12 Monthly Test 2016-17 (JULY)Documento3 páginasClass 12 Monthly Test 2016-17 (JULY)rahulAinda não há avaliações
- SHCC - 2023 - Chem Paper2 - AnnaDocumento8 páginasSHCC - 2023 - Chem Paper2 - AnnaOof GucciAinda não há avaliações
- Tutorials (Rate of Reactions) : QUESTION 1 (2006 CT3 Jan)Documento7 páginasTutorials (Rate of Reactions) : QUESTION 1 (2006 CT3 Jan)Subesh ShanmugamAinda não há avaliações
- Chemistry 2Documento5 páginasChemistry 2Rak boyAinda não há avaliações
- Revision STPM Term 1Documento15 páginasRevision STPM Term 1Wong WengSiongAinda não há avaliações
- Chemistry 12 Term 1 (2023 24)Documento8 páginasChemistry 12 Term 1 (2023 24)lardemuydiAinda não há avaliações
- 1-8 Reaction Kinetics PDFDocumento8 páginas1-8 Reaction Kinetics PDFBerry101Ainda não há avaliações
- 3.chemical KineticsDocumento2 páginas3.chemical KineticsAnshumyAinda não há avaliações
- Chemistry 2 Pre NectaDocumento4 páginasChemistry 2 Pre NectabhaijanAinda não há avaliações
- Vakev Chemistry-Examination-Of-The-Third-Term-2021-For-S6Documento15 páginasVakev Chemistry-Examination-Of-The-Third-Term-2021-For-S6vigiraneza0Ainda não há avaliações
- H2 Chemistry Prelims 2011 (Planning)Documento12 páginasH2 Chemistry Prelims 2011 (Planning)iuhihzAinda não há avaliações
- Eee L-1, T-2 (2016-2017) PDFDocumento26 páginasEee L-1, T-2 (2016-2017) PDFআশিক পালোয়ানAinda não há avaliações
- Kinetics & Photochemistry Tutorial ProblemsDocumento4 páginasKinetics & Photochemistry Tutorial ProblemsAmbuj Yadav 4-Year B.Tech. Chemical EngineeringAinda não há avaliações
- Chemistry Worksheet 2Documento3 páginasChemistry Worksheet 2LemontAinda não há avaliações
- 09 (2) PhysChem Exam-AnswersDocumento10 páginas09 (2) PhysChem Exam-Answerstiffanyyy00Ainda não há avaliações
- Chapter 4Documento3 páginasChapter 4khalidAinda não há avaliações
- Examen 29 de Mayo - Termoquimica AcidoDocumento8 páginasExamen 29 de Mayo - Termoquimica AcidoMaria Elena SalgadoAinda não há avaliações
- Mark Scheme: University of Malta Matriculation Certificate Examination Intermediate Level MAY 2010Documento17 páginasMark Scheme: University of Malta Matriculation Certificate Examination Intermediate Level MAY 2010Bernice JohnsonAinda não há avaliações
- Chemical KineticsDocumento9 páginasChemical KineticsTrung VõAinda não há avaliações
- PT-1 Chemistry (SET-B) 2023-24Documento4 páginasPT-1 Chemistry (SET-B) 2023-24karthikeyan cocAinda não há avaliações
- Mock Paper I SuggestedAnswerDocumento10 páginasMock Paper I SuggestedAnswerIndrik WijayaAinda não há avaliações
- Rate of ReactionDocumento44 páginasRate of Reactionpokyik cheungAinda não há avaliações
- Chemistry 2 - Exam N AnswersDocumento16 páginasChemistry 2 - Exam N AnswersMakame AliAinda não há avaliações
- 08a. Chemical Kinetics SheetDocumento33 páginas08a. Chemical Kinetics SheetVIKRANTH KUMAR JAKKOJUAinda não há avaliações
- pt-1 Xii ChemDocumento2 páginaspt-1 Xii ChemPrempal KumarAinda não há avaliações
- Tutorial 1 SolutionsDocumento20 páginasTutorial 1 Solutionsanushka shagunAinda não há avaliações
- Chem Electro Kinetics Coordination Set PDocumento2 páginasChem Electro Kinetics Coordination Set PShivam SahuAinda não há avaliações
- 99prepare SolDocumento53 páginas99prepare SolPopa ElenaAinda não há avaliações
- MSS 1718MockPaper2Documento8 páginasMSS 1718MockPaper2Kelvin ChowAinda não há avaliações
- 6 Chemical Kinetics Practice Questions.Documento2 páginas6 Chemical Kinetics Practice Questions.Greg The MedicAinda não há avaliações
- A Level Chemistry Paper 2 Exam 28Documento3 páginasA Level Chemistry Paper 2 Exam 28Anthony AndyAinda não há avaliações
- Chemistry 1 - ExamDocumento6 páginasChemistry 1 - Examnassorussi9Ainda não há avaliações
- Set 2 SonDocumento4 páginasSet 2 SonJerson Mendoza CAinda não há avaliações
- Tutorial Sheet 4 - CHEMICAL KINETICSDocumento4 páginasTutorial Sheet 4 - CHEMICAL KINETICSSaviour SichizyaAinda não há avaliações
- AL Chemistry 1995-1998 Paper 1Documento18 páginasAL Chemistry 1995-1998 Paper 1api-3734333Ainda não há avaliações
- 01) Xii Theory Paper 24-01-24Documento3 páginas01) Xii Theory Paper 24-01-24bbfnpsy2cdAinda não há avaliações
- Chemical Kinetics Board Questions 2010Documento5 páginasChemical Kinetics Board Questions 2010amone nAinda não há avaliações
- 31 Prepare ThaiDocumento52 páginas31 Prepare ThaiHuyềnTrânCôngChúaAinda não há avaliações
- 12th Revision Test Chap. 1,2&3Documento4 páginas12th Revision Test Chap. 1,2&3Bloody DemonAinda não há avaliações
- Oxidation - Reduction Reactions and Titrations: Che 401: Analytical ChemistryDocumento16 páginasOxidation - Reduction Reactions and Titrations: Che 401: Analytical ChemistryScrappy WellAinda não há avaliações
- Saturated and Unsaturated HydrocarbonsDocumento4 páginasSaturated and Unsaturated HydrocarbonsgefegAinda não há avaliações
- 1 - Exp4 - Dynaz Aqilah Binti Mohd SamsulDocumento4 páginas1 - Exp4 - Dynaz Aqilah Binti Mohd SamsulDynazze 04Ainda não há avaliações
- Institute of Mathematics, Physics and Chemistry Department of ChemistryDocumento8 páginasInstitute of Mathematics, Physics and Chemistry Department of ChemistrykasuleAinda não há avaliações
- Niger ATCC9142Documento9 páginasNiger ATCC9142LiliOrangeLópezAinda não há avaliações
- Expt 7 Classification Tests For HydrocarbonsDocumento10 páginasExpt 7 Classification Tests For Hydrocarbonssean goAinda não há avaliações
- Alkaloids 3Documento5 páginasAlkaloids 3Nandya Nandiia100% (1)
- Fisa Purolite MZ 10 - EngDocumento5 páginasFisa Purolite MZ 10 - EngTudor TaranuAinda não há avaliações
- Experiment 2 Objective:: Kmno Serves As Self Indicator in Acidic SolutionDocumento4 páginasExperiment 2 Objective:: Kmno Serves As Self Indicator in Acidic Solutionfaxepe9472Ainda não há avaliações
- Water: Its Properties and Purification: Ust Chemical Engineering DepartmentDocumento13 páginasWater: Its Properties and Purification: Ust Chemical Engineering DepartmentKhristel PenoliarAinda não há avaliações
- Environmental Engg LabDocumento72 páginasEnvironmental Engg LabRajjan SinghAinda não há avaliações
- Cambridge International Advanced Subsidiary and Advanced LevelDocumento8 páginasCambridge International Advanced Subsidiary and Advanced LevelFaisal SyahrulAinda não há avaliações
- Is 12681 1989Documento15 páginasIs 12681 1989dipen royAinda não há avaliações
- SNI 0494-2008 Pulp - Cara Uji Bilangan KappaDocumento4 páginasSNI 0494-2008 Pulp - Cara Uji Bilangan KappaMar'atul FauziyahAinda não há avaliações
- Activity 6Documento26 páginasActivity 6Arra Maeva Gacusana0% (1)
- List of Chemicals For Cement AnalysisDocumento2 páginasList of Chemicals For Cement AnalysisChandra MouliAinda não há avaliações
- 27.2 Alcohols Ial Cie Chemistry QPDocumento16 páginas27.2 Alcohols Ial Cie Chemistry QPabdelrahmanAinda não há avaliações
- 04 Hydrogen PeroxideDocumento10 páginas04 Hydrogen Peroxidemacastillof100% (1)
- Biochemistry Lab Report: VNG - International UniversityDocumento19 páginasBiochemistry Lab Report: VNG - International UniversityHà Anh Minh LêAinda não há avaliações
- Inorganic Chemistry Lab ManualDocumento25 páginasInorganic Chemistry Lab ManualAbhik SarkarAinda não há avaliações
- Exprmt 1Documento25 páginasExprmt 1CionbasAinda não há avaliações
- Reboquio - experiment4.OxidationReductionReactions M7 C1Documento9 páginasReboquio - experiment4.OxidationReductionReactions M7 C1Denampo Ivan MikhaelAinda não há avaliações
- Chemistry Research AssignmentDocumento3 páginasChemistry Research AssignmentDavid AddisonAinda não há avaliações
- (DORDADO) Experiment No. 7 AlcoholsDocumento13 páginas(DORDADO) Experiment No. 7 AlcoholsBitterground Corp.Ainda não há avaliações
- Rapid Spectrophotometric Determination of CaffeineDocumento5 páginasRapid Spectrophotometric Determination of CaffeineKAinda não há avaliações
- Chemical Resistance Guide FPMDocumento26 páginasChemical Resistance Guide FPMjeanmarcauerAinda não há avaliações
- Redox TitrationDocumento6 páginasRedox TitrationMohammad RussellAinda não há avaliações
- Mole Concept1Documento40 páginasMole Concept1biswaranjan padhyAinda não há avaliações
- Caustic PermagnateDocumento3 páginasCaustic Permagnateg_sanchetiAinda não há avaliações
- CAPE - Chemistry LabsDocumento19 páginasCAPE - Chemistry Labskelliann george100% (3)