Escolar Documentos
Profissional Documentos
Cultura Documentos
04-Process Description and MEBC
Enviado por
Bhuneshwar ChelakDireitos autorais
Formatos disponíveis
Compartilhar este documento
Compartilhar ou incorporar documento
Você considera este documento útil?
Este conteúdo é inapropriado?
Denunciar este documentoDireitos autorais:
Formatos disponíveis
04-Process Description and MEBC
Enviado por
Bhuneshwar ChelakDireitos autorais:
Formatos disponíveis
5.
PROCESS DESCRIPTION
The detailed process for the batch process manufacture of 3, 5-dichloroaniline is described below. The entire plant can be split into two main sections:( I ) Deamination section. ( II ) Hydrogenation section and subsequent purification.
Section ( I ) 2, 6-dichloro-4-nitroaniline is major raw material. It is deaminated in first section to give 3, 5-dichloroaniline. Since, 2, 6-dichloro-4-nitroaniline is solid, sufficient amount of solvent is used for dissolving it. Here, we used iso-propanol as a solvent. Around 90% of solvent is recycled back. Detailed description: Initially, solid 2, 6-dichloro-4-nitroaniline is charged to R1002. Sufficient amount of solvent, iso-propanol is added to R1002 from day tank. Solid NaNO 2 is charged to R1001 and to this sufficient amount of water is added which dissolves NaNO2 and forms NaNO2 solution. Proper heat transfer arrangement is provided for removing heat of solution. This NaNO2 solution at 250C is charged toR1002. In addition to this required amount of 98% H 2SO4 and process water is added to R1002 from respective day tanks. Reaction takes place at 500 C, so it is necessary to heat all reactants to 500 C. After this constant temperature is achieved reaction takes place in around 3 hours. This reaction is exothermic so constant heat removal is carried out. Here we get one mole of 3, 5-dichloro-4-nitroaniline per mole of 2,6-dichloro-4-nitroaniline with 100% selectivity and 100% conversion of 2,6-dichloro-4-nitroaniline.
16
During this deamination reaction, nitrogen is evolved which is vented out of the reactor to the atmosphere. Product, 3, 5-dichloro-4-nitroaniline, melts at 65 0 C so it will be in solid state when it comes out of reactor. Thus 3, 5-dichloro-4nitroaniline is separated by filtration in the solid form by using agitated nutsche type of filter. Mother liquor contains mainly H2O and iso-propanol and inorganic salts. Isopropanol is recovered by distillation and recycled back. Since, 3, 5-dichloro-4nitroaniline is insoluble in water, it will be absent in mother liquor.
Section ( II ) Hydrogenation is effected in a CSTR which is Gas-Inducing-Dead-End type of reactor. Since, hydrogen can form explosive mixture; Dead-End type of reactor is preferred to ensure safety. Dead-End type of reactor is that in which there is total consumption of hydrogen in the reactor and no hydrogen gas is vented out. Detailed description: 3, 5-dichloro-4-nitroaniline which is separated by filtration is heated and converted into liquid form and fed to R1003 which is hydrogenation reactor. It is simple CSTR. Sufficient quantity of 50% H2SO4 is prepared in D1004. Required amount of 50% H2SO4 is charged to R1003 from day tank. Apart from this, R1003 is charged with Raney-Nickel catalyst and hydrogen gas sparged at five bar pressure in stoichiometric amount. Hydrogenation of 3, 5-Dichloronitrobenzene is carried out at 700C for around 2.5 hours. Thus all these reactants are to be heated to reaction temperature and same temperature is maintained in the reactor. Here, all reactants are preheated and added to R1003. Hydrogenation reaction is exothermic so liberated heat need to be removed by providing proper cooling arrangement. Here we will get 100% yield as meta oriented chlorine is not get removed under such mild conditions.
17
After reaction gets over, output of R1003 contains 3, 5-dichloroaniline, water formed in the reaction, suphuric acid charged and catalyst. This mixture is then filtered at more than 700C to remove catalyst. Mother liquor will contain 3, 5dichloroaniline, water formed in the reaction and sulphuric acid charged in the reactor. Mother liquor from F1002 is fed to phase separator (P1001) to separate aqueous and organic phases. Aqueous phase will contain most of water and sulphuric acid which is sent to effluent treatment plant. Solubility of 3, 5-dichloroaniline in water is very less (0.6 gm/lit) so only small amount of solid will go into water. Organic phase will mainly contain 3,5-dichloroaniline along with very less amount of sulphuric acid and water. Since we require 3, 5-dichloroaniline more than 99% pure we need to remove this associated sulphuric acid and water. 50% solution of NaOH is added for neutralizing sulphuric acid and water associated with it will dissolve salt formed and excess NaOH. After this water wash is required to remove any inorganic part associated with organic phase. Then aqueous and organic phases are separated by P1002 to obtain 3, 5-dichloroaniline in organic phase which will be more than 99% pure. Since there is not any other organic compound with 3, 5-dichloroaniline in organic phase we need not require distillation unit.
18
6.1 MATERIAL BALANCE
Product: 500 TPA of 3, 5-dichloroaniline Basis: Plant operates for 328 days and hence 1525 Kg of product need to be produced per day.
Material Balances across the equipments: Mixer(R1001)
NaNO2 which is nitrosating agent is in the solid form and hence dissolved in water in this equipment. NaNO2 and water are introduced at 250 C. IN Quantity Component NaNO2 H2O Total (kg / day) 1176.4 1646.9 2823.3 Component (kg / day) NaNO2 2823.3 Solution Total 2823.3 OUT Quantity
Reactor (R1002)
2,6-Dichloro-4-nitrobenzene is added to reactor through fresh material and dissolved in iso-propanol which acts as solvent. NaNO 2 solution prepared in mixer is also added to this reactor. In addition to that 98 % H2SO4 and H2O are also added in sufficient amount. In Quantity Component (kg / day) 2,6-Dichloro-41948.1 nitrobenzene Iso-propanol Water 98% H2SO4 1694 2823.3 H2SO4-1797.5 19 3,5-Dichloronitrobenzene Iso-propanol Water Na2SO4 1806.9 1694 4507.9 1336.4 Component (kg / day) Out Quantity
H2O-37.64 NaNO2-1176.4 NaNO2 Solution H2O-1646.9 Total 11123.9 H2SO5 N2 Total 1072.8 263.5 11123.8 HNO2 442.3
Filter (F1001)
The outlet stream from Reactor (1002) enters the filter and gets separated into solid and liquid phases. Since solubility of 3,5-Dichloronitrobenzene in water is zero, we take the balance for both the phases with no 3,5-Dichloronitrobenzene in the mother liquor. We assume that 3,5-Dichloronitrobenzene is sparingly soluble in organic solvent that is iso-propanol and we consider this quantity as negligible. Thus all 3,5-Dichloronitrobenzene will be in the form of cake. We will add water, 50% by weight of cake for washing. Thus amount of water need to be added is 900 kg.
Cake Quantity Component (kg / day) 3,51806.9 Dichloronitrobenzene
Mother liquor Quantity Component (kg / day) Iso-propanol Water Na2SO4 HNO2 H2SO5 Total 1694 5407.9 1336.4 442.3 1072.8 9953.4
Total
1806.9
Distillation Column (D1001) :
Mother liquor obtained after filtration contains iso-propanol which is need to be separated. Since, iso-propanol is soluble in water we need to employ distillation to separate it. Thus after separation of iso-propanol, remaining aqueous phase is sent to
20
ETP for further treatment. This iso-propanol which is separated is recycled back to main storage tank. IN Quantity Component Iso-propanol Water Na2SO4 HNO2 H2SO5 Total (kg / day) 1694 4507.9 1336.4 442.3 1072.8 9053.4 (kg / day) 1694 5.13 0 0 0 1699.13 1336.4 442.3 1072.8 7354.3 (kg / day) 0 4502.8 Distillate OUT Bottom
Reactor (R1003)
Solid product obtained after filtration is turned into liquid by heating before it is fed to this reactor. In addition to 3,5-Dichloronitrobenzene small amount 50% H2SO4 and required amount of Raney Nickel catalyst is charged into the reactor. Here, hydrogenation of 3,5-Dichloronitrobenzene is carried out which gives 3,5Dichloroaniline and water. For this process hydrogen is sparged from bottom at 5 bar pressure. Hydrogen is charged in no excess. IN Quantity Component (kg / day) 3,51806.9 Dichloronitrobenzene H2SO4-1.497 50% H2SO4 Catalyst H2 Total H2O-1.497 44.737 56.47 1911.1 H2SO4 Water Catalyst H2 Total 1.497 340.3 44.737 0 1911.1 3,5-Dichloroaniline 1524.6 Component (kg / day) OUT Quantity
Filter (F1002):
21
Output stream from reactor (R1003) is filtered at reaction temperature for removing catalyst from solution. Cake Quantity Component Catalyst Total (kg / day) 44.737 44.737 Component 3,5-Dichloroaniline H2SO4 Water Total (kg / day) 1524.6 1.497 340.3 1866.4 Mother liquor Quantity
Phase separator (PS1001):
Input to this unit is nothing but the mother liquor obtained after filtration. Thus this outlet stream from filter enters into the separator and gets separated in the aqueous and organic phases. Since solubility of 3,5-Dichloroaniline in water is 0.6 gm/lit some amount of 3,5-Dichloroaniline will go in aqueous phase. But majority part of 3, 5-Dichloroaniline will be in organic phase. After separation some amount of H2SO4 along with some water will go into organic phase.
AQUEOUS Quantity Component 3,5-Dichloroaniline H2SO4 Water Total (kg / day) 0.17 1.47 333.5 335.14
ORGANIC Quantity Component 3,5-Dichloroaniline H2SO4 Water Total (kg / day) 1524.4 0.03 6.81 1531.24
Neutralizer (N1001):
Organic phase obtained from phase separation is charged into this unit for removing unwanted H2SO4. This is need to be done since we require more than 99% pure product. Here, we add 50% NaOH solution to neutralize H 2SO4 accompanying organic phase. 22
IN Quantity Component 3,5-Dichloroaniline H2SO4 Water 50% NaOH solution Total H2O-0.025 1531.3 Total (kg / day) 1524.4 0.03 6.81 NaOH-0.025 Component
OUT Quantity 3,5-Dichloroaniline Na2SO4 Water (kg / day) 1524.4 0.043 6.85
1531.3
Washing Unit (W1001):
After neutralization unit washing of product with water is done in order to remove salt formed during neutralization and also excess NaOH. Thus, 2 kg of water is added to output of neutralizer.
Phase separator (PS1002)
Input to this unit is output of neutralizer and extra 2 kg of washing water added. Organic phase and aqueous phases are separated after this to get more than 99% pure 3,5-Dichloroaniline. AQUEOUS Quantity Component 3,5-Dichloroaniline Na2SO4 Water Total Thus purity of final product is = (1524.4/1524.58) =99.98% (kg / day) 0.005 0.043 8.67 8.72 Component 3,5-Dichloroaniline Water Total (kg / day) 1524.4 0.18 1524.58 ORGANIC Quantity
100
23
Amount of product formed per day =9.4099 162 1000 =1524.4 Kg/ day
6.2 ENERGY BALANCE
Product: 500 TPA of 3,5-dichloroaniline Basis: Plant operates for 328 days and hence 1525 Kg of product need to be produced per day. Energy balance across the equipments:
In all the calculations the reference temperature is taken as 250 C. The formula used for calculating the enthalpy of a stream is,
Q = m C P T
The condenser load is calculated by using the formula,
24
QC = m
Where is latent heat of vaporization of the component which is to be condensed. The Reboiler load is calculated by taking overall energy balance across the distillation column as,
Q R = Q D + Q B + QC Q F
Where the subscripts R, D, B, C and F refers to Reboiler, distillate, bottoms, condenser and feed respectively.
Mixer (R1001)
NaNO2 which is nitrosating agent is in the solid form and hence dissolved in water in this equipment. NaNO2 and water are introduced at 250 C. IN Temperature Component NaNO2 H2O Total C 25 25
0
Enthalpy Component KJ 0 0 0 NaNO2
OUT Temperature
0
Enthalpy KJ 0 0
25 solution Total
Heat of solution =-256800 KJ
25
Thus heat need to be removed by cooling water so as to keep outlet stream temperature constant at 250C = 256800 KJ
Reactor (R1002)
All the reactants are charged at 25 0C. Reaction takes place at 500 C. Internal coils are used for reaction mixture to reaction temperature.
IN Temperature Component
0
OUT Enthalpy Component KJ 3,50 Temperature Enthalpy
0
KJ
NaNO2 25 solution H2O 25 0
Dichloronitrobenzene
50
66340
H2O
50
471500
98% H2SO4 2,6Dichloro-4nitroaniline Iso-
25
Na2SO4
50
32300
25
H2SO5
50
46350
25 propanol
Iso-propanol
50
64940
HNO2
50
411300
N2
50
6843
26
Total
Total
1099600
Total heat evolved during reaction=4115300 KJ Amount of heat need to be provided for heating reaction mixture to 500C = 591280 KJ Thus amount of heat need to be removed in order to keep reaction mixture temperature constant = 3608000 KJ
Distillation column (D1001):
Here we want to remove iso-propanol and water from the incoming stream. As isopropanol boils at around 790 C which is less temperature compared to temperature at which water boils. Thus we can get complete separation of iso-propanol and Water from rest of the mixture. The temperature of the incoming stream is 25 o C. thus the load on the reboiler is that of heating the mixture upto bubble point and then supplying the latent heat of vaporization. The load on the condenser is to condense the upcoming vapors using cooling water. The reboiler load is found to be 3831400 KJ/day. This heat is supplied by using saturated steam. The condenser load is found to be 3633200 KJ/day. This heat is removed by using cooling water coming in at 25 o C.
Melting operation:
During this operation 3,5- Dichloronitrobenzene is melted. Thus we need to consider heat of fusion of this compound.
27
Thus heat required to melt 3,5- Dichloronitrobenzene =279690 KJ
Reactor (R1003):
In this stage 3,5- Dichloronitrobenzene in molten form is added to reactor along with sulphuric acid and catalyst. Reaction is carried out at 70 0 C. Since reaction is exothermic large amount of heat need to be removed.
IN
Temperatur Enthalpy Component 3,5dichloronitrobenzene 50% H2SO4 solution H2 Total Heat of reaction =5023100 KJ Thus amount of heat need to be removed = 4889280 KJ/day = 56590 J/s 70 70 70 13267 417.53 43993 57680 e KJ
0
OUT
Temperatur Enthalpy Component 3,5Dichloroaniline H2SO4 Solution H2O H2 Total 70 70 70 70 119960 417.53 63788 7332 191498 e KJ
0
Neutraliser:
In this stage unwanted sulphuric acid associated with 3,5- dichloroaniline is removed. Thus 50% NaOH solution is used for this. Heat of neutralization =-57.1 KJ/mole =-57.1 6.114 0.0001
28
=-34.911 J Since, heat evolved in this operation is very small we do not require jacket. Also we will not try to remove that small quantity of heat.
29
Você também pode gostar
- Identifying Eugenol in Clove Oil Using Steam Distillation and IR SpectroscopyDocumento7 páginasIdentifying Eugenol in Clove Oil Using Steam Distillation and IR SpectroscopyMikeAinda não há avaliações
- Synthesis of P2PDocumento15 páginasSynthesis of P2PRiki Mandol83% (23)
- Ketamina SintesisDocumento11 páginasKetamina SintesisKepa Martinez GarciaAinda não há avaliações
- Advanced Pharmaceutical analysisNo EverandAdvanced Pharmaceutical analysisNota: 4.5 de 5 estrelas4.5/5 (2)
- 2C-B Synthesis Without LAH PDFDocumento4 páginas2C-B Synthesis Without LAH PDFatomosco100% (3)
- CyclohexeneDocumento11 páginasCyclohexeneanon-407590100% (10)
- ChECal SolutionsDocumento39 páginasChECal SolutionsMARGARET FLORESAinda não há avaliações
- Hobart Filler Metals CatalogDocumento244 páginasHobart Filler Metals CatalogBhrugu DhokaiAinda não há avaliações
- Ion Exchange Resins and Adsorbents in Chemical Processing: Second EditionNo EverandIon Exchange Resins and Adsorbents in Chemical Processing: Second EditionNota: 5 de 5 estrelas5/5 (1)
- Optimized 2C-B Synthesis via Nitrostyrene ReductionDocumento1 páginaOptimized 2C-B Synthesis via Nitrostyrene ReductionFermin GamboaAinda não há avaliações
- Piric AcidDocumento2 páginasPiric Acidy_satyapAinda não há avaliações
- Environment Impact AssessmentDocumento11 páginasEnvironment Impact AssessmentBimal AntonyAinda não há avaliações
- The Chemistry of Fertilisers and Manure - Including Information on the Chemical Constituents and Types of Fertilisers and ManuresNo EverandThe Chemistry of Fertilisers and Manure - Including Information on the Chemical Constituents and Types of Fertilisers and ManuresNota: 5 de 5 estrelas5/5 (1)
- Ruchi Soya Project ReportDocumento45 páginasRuchi Soya Project ReportAnkita Toshniwal75% (4)
- Major Engineering ProblemsDocumento5 páginasMajor Engineering ProblemsaathiraAinda não há avaliações
- KOH synthetic routesDocumento6 páginasKOH synthetic routesCin D NgAinda não há avaliações
- Formal Report Chem 31.1Documento6 páginasFormal Report Chem 31.1Elah PalaganasAinda não há avaliações
- Cooling Gel Safety ReportDocumento15 páginasCooling Gel Safety ReportJacek Stanislawski100% (1)
- Production of Ethylene OxidefinalDocumento40 páginasProduction of Ethylene Oxidefinaljoshua amarnath100% (1)
- Project On NitrobenzeneDocumento65 páginasProject On NitrobenzeneAmit Khosla80% (10)
- HASTELLOY® C-22® Alloy PDFDocumento23 páginasHASTELLOY® C-22® Alloy PDFalextentwentyAinda não há avaliações
- (Faringita Streptococica:copii: 250 MG de 2-3 Ori Pe Zi Adolescenţi Şi Adulţi: 250 MG de 4 Ori Pe Zi Sau 500 MG de 2 Ori Pe Zi Timp de 10 ZileDocumento5 páginas(Faringita Streptococica:copii: 250 MG de 2-3 Ori Pe Zi Adolescenţi Şi Adulţi: 250 MG de 4 Ori Pe Zi Sau 500 MG de 2 Ori Pe Zi Timp de 10 ZileAlina C100% (1)
- Flow Diagram of Isopropyl AlcoholDocumento2 páginasFlow Diagram of Isopropyl Alcoholshamsullah100% (1)
- SYNTHESISDocumento7 páginasSYNTHESISJhonis Bentes MeirellesAinda não há avaliações
- Guide By:-Prof. Bhavi M. PandyaDocumento18 páginasGuide By:-Prof. Bhavi M. PandyaAbhi ButaniAinda não há avaliações
- Project 1 - Isopropanol and Acetone From Propylene PDFDocumento8 páginasProject 1 - Isopropanol and Acetone From Propylene PDFAnonymous RJkpep7D0rAinda não há avaliações
- Nitro Benzene Sai PDFDocumento65 páginasNitro Benzene Sai PDFDeepak Pandey100% (1)
- NaBH4 Reduction of CyclohaxanoneDocumento5 páginasNaBH4 Reduction of Cyclohaxanonenurul1110Ainda não há avaliações
- Adi Pic AcidDocumento8 páginasAdi Pic AcidTwas AnassinAinda não há avaliações
- Chemistry 125 Laboratory 11Documento5 páginasChemistry 125 Laboratory 11SmaeUBAinda não há avaliações
- 1 2 3 Properties 4 Different Product From Ipa 5 Different Processes 6 Ipa by Indirect Hydration 7 Process Flow Diagram 8 Uses 9 Safety 10 Toxicology 11 ReferencesDocumento12 páginas1 2 3 Properties 4 Different Product From Ipa 5 Different Processes 6 Ipa by Indirect Hydration 7 Process Flow Diagram 8 Uses 9 Safety 10 Toxicology 11 ReferencesArpit PatelAinda não há avaliações
- Exp 3-Reduction of Cyclohexanone With Sodium BorohydrideDocumento11 páginasExp 3-Reduction of Cyclohexanone With Sodium Borohydrideakuserai100% (3)
- Mild Adipic Acid SynthesisDocumento5 páginasMild Adipic Acid Synthesiskhaledegy10Ainda não há avaliações
- AADocumento30 páginasAAAhmed MajidAinda não há avaliações
- Sodium Borohydride Reduction of A KetoneDocumento5 páginasSodium Borohydride Reduction of A KetoneJulie Edington100% (1)
- 64788Documento35 páginas64788ghatak2100% (1)
- IPADocumento12 páginasIPAEr Bali Pandhare50% (2)
- Detailed Production Process of IPADocumento19 páginasDetailed Production Process of IPAJignesh Bhavsar75% (4)
- Chapter No 2Documento21 páginasChapter No 2Ali AhsanAinda não há avaliações
- Mat Balance DiagramDocumento21 páginasMat Balance DiagramKause MurugayaAinda não há avaliações
- Sodium Boronhydride Reduction of CyclohexanoneDocumento6 páginasSodium Boronhydride Reduction of CyclohexanoneWan Nur Amira91% (11)
- NPK fertilizer production via nitrophosphate routeDocumento9 páginasNPK fertilizer production via nitrophosphate routeHoàng TuấnAinda não há avaliações
- CHM 556 Organic Chemistry 2 Experiment 2: Reduction of CyclohexanoneDocumento5 páginasCHM 556 Organic Chemistry 2 Experiment 2: Reduction of CyclohexanoneAmirul Azhar100% (1)
- NaOCl Oxidation CamphorDocumento4 páginasNaOCl Oxidation CamphorLouiegi AlvarezAinda não há avaliações
- Absorption and DehydrationDocumento2 páginasAbsorption and Dehydrationemartey62Ainda não há avaliações
- CyclohexeneDocumento12 páginasCyclohexenePatricia CruzAinda não há avaliações
- Orgo IILab Midterm NotesDocumento3 páginasOrgo IILab Midterm NotesGeetha VipulanandanAinda não há avaliações
- NaBH4 Reduction of Cyclohexanone to Cyclohexanol (87Documento8 páginasNaBH4 Reduction of Cyclohexanone to Cyclohexanol (87hahadindongAinda não há avaliações
- Chapter 9 - Manufacture of C3 CompoundDocumento15 páginasChapter 9 - Manufacture of C3 CompoundMicrosoft GamingAinda não há avaliações
- H2O2 Manufacturing ProcessDocumento6 páginasH2O2 Manufacturing ProcessJames YatesAinda não há avaliações
- Hydrolysis of Methyl Salicylate ExpDocumento7 páginasHydrolysis of Methyl Salicylate ExpPradeep100% (1)
- Experimen 5 Organic ChemistryDocumento8 páginasExperimen 5 Organic ChemistryAbd RaHmanAinda não há avaliações
- Lecture 18: Isopropanol and Acetone From Propylene: Module 3: PetrochemicalsDocumento2 páginasLecture 18: Isopropanol and Acetone From Propylene: Module 3: Petrochemicalsshamsullah hamdardAinda não há avaliações
- Manufacturing Sodium Dodecyl Benzene Sulfonate (SDBSDocumento9 páginasManufacturing Sodium Dodecyl Benzene Sulfonate (SDBSamit_iffcoAinda não há avaliações
- Mat Balance Multi UnitDocumento20 páginasMat Balance Multi UnithAinda não há avaliações
- Experiment 9 - : Alkene Synthesis From Alcohol Preparation of Cyclohexene From CyclohexanolDocumento6 páginasExperiment 9 - : Alkene Synthesis From Alcohol Preparation of Cyclohexene From CyclohexanolSoo Hui Yan0% (2)
- Phenol Synthesis via Cumene Hydroperoxide Cleavage (Hock MethodDocumento10 páginasPhenol Synthesis via Cumene Hydroperoxide Cleavage (Hock MethodRizkyanto NugrohoAinda não há avaliações
- Project Presentation On Butadiene SulfoneDocumento69 páginasProject Presentation On Butadiene SulfoneDinesh DinnuAinda não há avaliações
- Patent Translate Method for 2,6-DiisopropylphenolDocumento3 páginasPatent Translate Method for 2,6-DiisopropylphenolBen de LangeAinda não há avaliações
- Process Discribtion:: So C H C H Alcl C H - C HDocumento3 páginasProcess Discribtion:: So C H C H Alcl C H - C HSharaf NourAinda não há avaliações
- Unit 3Documento29 páginasUnit 3ASHISH K.K.Ainda não há avaliações
- Project Report Draft On "Isopropyl Alcohol" BY: Jariwala Divyangkumar Rajeshkumar Exam No-701022 Roll No:-821Documento22 páginasProject Report Draft On "Isopropyl Alcohol" BY: Jariwala Divyangkumar Rajeshkumar Exam No-701022 Roll No:-821Arpit PatelAinda não há avaliações
- Transition Metal Catalyzed Furans Synthesis: Transition Metal Catalyzed Heterocycle Synthesis SeriesNo EverandTransition Metal Catalyzed Furans Synthesis: Transition Metal Catalyzed Heterocycle Synthesis SeriesAinda não há avaliações
- Hydroformylation: Fundamentals, Processes, and Applications in Organic SynthesisNo EverandHydroformylation: Fundamentals, Processes, and Applications in Organic SynthesisAinda não há avaliações
- STD ModelDocumento13 páginasSTD ModelBhuneshwar ChelakAinda não há avaliações
- Engineers Pocket Manual PDFDocumento51 páginasEngineers Pocket Manual PDFBhuneshwar ChelakAinda não há avaliações
- Technical Manual for Steel TubesDocumento122 páginasTechnical Manual for Steel TubesAlejandroBolanos100% (1)
- A 12, Bimleshwar Socity, Near TCS Company, Shubanpura Ellora Park, VadodaraDocumento1 páginaA 12, Bimleshwar Socity, Near TCS Company, Shubanpura Ellora Park, VadodaraBhuneshwar ChelakAinda não há avaliações
- Packed Bed TheoryDocumento6 páginasPacked Bed TheoryBhuneshwar ChelakAinda não há avaliações
- Friction FactorDocumento1 páginaFriction FactorBhuneshwar ChelakAinda não há avaliações
- Fluidised BedDocumento200 páginasFluidised Bedaravind991Ainda não há avaliações
- Reynolds Vs FrictionDocumento2 páginasReynolds Vs FrictionBhuneshwar ChelakAinda não há avaliações
- ΔP L D φ ε 3 Dpφ ε 3: Pressure Drop, Kpa/mDocumento2 páginasΔP L D φ ε 3 Dpφ ε 3: Pressure Drop, Kpa/mBhuneshwar ChelakAinda não há avaliações
- Fluidised BedDocumento200 páginasFluidised Bedaravind991Ainda não há avaliações
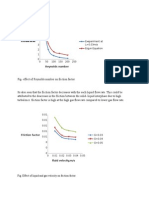
- Friction Factor: Fig. Effect of Liquid and Gas Velocity On Friction FactorDocumento2 páginasFriction Factor: Fig. Effect of Liquid and Gas Velocity On Friction FactorBhuneshwar ChelakAinda não há avaliações
- Pressure Drop CorrelationDocumento2 páginasPressure Drop CorrelationBhuneshwar ChelakAinda não há avaliações
- Drag CoefficientDocumento2 páginasDrag CoefficientBhuneshwar ChelakAinda não há avaliações
- LC Surface Roughness MeasurementsDocumento2 páginasLC Surface Roughness MeasurementsBhuneshwar ChelakAinda não há avaliações
- CoefficientDocumento3 páginasCoefficientBhuneshwar ChelakAinda não há avaliações
- OxygenDocumento2 páginasOxygenBhuneshwar Chelak0% (1)
- Axial Turnine CompressorDocumento35 páginasAxial Turnine CompressorBhuneshwar ChelakAinda não há avaliações
- More Than 100 Keyboard Shortcuts Must ReadDocumento3 páginasMore Than 100 Keyboard Shortcuts Must ReadChenna Keshav100% (1)
- Hastelloy C 276Documento16 páginasHastelloy C 276Bhuneshwar ChelakAinda não há avaliações
- Acabados de Acero Inoxidable 4Documento4 páginasAcabados de Acero Inoxidable 4FredyVenturaAinda não há avaliações
- Urface Finish ChartsSDocumento4 páginasUrface Finish ChartsSBhuneshwar ChelakAinda não há avaliações
- Reciprocating and Rotary Compressor TypesDocumento3 páginasReciprocating and Rotary Compressor TypesBhuneshwar ChelakAinda não há avaliações
- HR PumpDocumento1 páginaHR PumpBhuneshwar ChelakAinda não há avaliações
- Process Reactor Phase Temperature Pressure Catalyst Metal Conversion Per Pass (%) Yield By-ProductsDocumento2 páginasProcess Reactor Phase Temperature Pressure Catalyst Metal Conversion Per Pass (%) Yield By-ProductsBhuneshwar ChelakAinda não há avaliações
- Chapter Seven, Guidance For AbsorbDocumento6 páginasChapter Seven, Guidance For AbsorbBhuneshwar ChelakAinda não há avaliações
- Ace T AldehydeDocumento85 páginasAce T AldehydeBhuneshwar ChelakAinda não há avaliações
- Transfer Pump 2-1 and 2-2Documento1 páginaTransfer Pump 2-1 and 2-2Bhuneshwar ChelakAinda não há avaliações
- Transfer Pump 1-1 and 1-2Documento1 páginaTransfer Pump 1-1 and 1-2Bhuneshwar ChelakAinda não há avaliações
- WR Pump 2-1 & 2-2Documento1 páginaWR Pump 2-1 & 2-2Bhuneshwar ChelakAinda não há avaliações
- Pretreatment in Reverse Osmosis Seawater DesalinatDocumento9 páginasPretreatment in Reverse Osmosis Seawater DesalinataquaAinda não há avaliações
- Deeper Neet DCT - ChemistryDocumento8 páginasDeeper Neet DCT - Chemistryhbhaiya643Ainda não há avaliações
- Investigating Oxygen and Carbon Dioxide Levels in Inhaled and Exhaled AirDocumento2 páginasInvestigating Oxygen and Carbon Dioxide Levels in Inhaled and Exhaled AirAdy Phanterz100% (1)
- 8a-Advanced Waste Water TreatmentDocumento43 páginas8a-Advanced Waste Water TreatmentSathish GlAinda não há avaliações
- p1 Coverage Animal Nutrition - Chapter 1Documento13 páginasp1 Coverage Animal Nutrition - Chapter 1jayr ludoviceAinda não há avaliações
- Technical Data Sheet for Condenser On Load Tube Cleaning SystemDocumento46 páginasTechnical Data Sheet for Condenser On Load Tube Cleaning SystemSupriyaRongAinda não há avaliações
- Preview Unit Operations Handbook John J. McKetta PDFDocumento77 páginasPreview Unit Operations Handbook John J. McKetta PDFJkarlos TlAinda não há avaliações
- History of Hemostasis in Neurosurgery Paulo Et Al 2018Documento14 páginasHistory of Hemostasis in Neurosurgery Paulo Et Al 2018AlexAinda não há avaliações
- To Study The Quantity of Casein Present in Different Samples of MilkDocumento12 páginasTo Study The Quantity of Casein Present in Different Samples of MilkVartika MehrotraAinda não há avaliações
- Introduction To Cell BiologyDocumento43 páginasIntroduction To Cell BiologyEllemrac GageloniaAinda não há avaliações
- Materials Finer Than 75 - : Standard Test Method For M (No. 200) Sieve in Mineral Aggregates by WashingDocumento3 páginasMaterials Finer Than 75 - : Standard Test Method For M (No. 200) Sieve in Mineral Aggregates by WashingLuis Alejandro Sánchez LópezAinda não há avaliações
- Validation of Sterilization MethodsDocumento13 páginasValidation of Sterilization MethodsAshish NeupaneAinda não há avaliações
- Effect of Herbal Tablet Prepared From Moringa Oleifera Leaves ExtractDocumento7 páginasEffect of Herbal Tablet Prepared From Moringa Oleifera Leaves Extractsmail bendrissouAinda não há avaliações
- Tos Notes 3rd YrDocumento4 páginasTos Notes 3rd YrDEEPAK SINGHAinda não há avaliações
- Cemtec AL PU CoatingDocumento2 páginasCemtec AL PU CoatingAhmad ElghazolyAinda não há avaliações
- Dehradun Public School ASSIGNMENT (2019 - 2020) Subject: Science (086) Class - IxDocumento4 páginasDehradun Public School ASSIGNMENT (2019 - 2020) Subject: Science (086) Class - Ixbadnight4uAinda não há avaliações
- Fruit and Vegetable Waste: Bioactive Compounds, Their Extraction, and Possible UtilizationDocumento20 páginasFruit and Vegetable Waste: Bioactive Compounds, Their Extraction, and Possible UtilizationKrishi AgricultureAinda não há avaliações
- Essential Fatty Acids: Benefits, Sources & FunctionsDocumento7 páginasEssential Fatty Acids: Benefits, Sources & FunctionsRiya SugandhiAinda não há avaliações
- Explorer XRF: X-Ray Fluorescence SpectrometerDocumento11 páginasExplorer XRF: X-Ray Fluorescence Spectrometerhossam hamdyAinda não há avaliações
- Tough, UN Rated 55-Gallon Steel Drums Are Made For Hazardous Waste, So You Can Rely On Them To Deliver Safety and ComplianceDocumento2 páginasTough, UN Rated 55-Gallon Steel Drums Are Made For Hazardous Waste, So You Can Rely On Them To Deliver Safety and ComplianceBrijeshAinda não há avaliações
- Diffuser AugmentedDocumento8 páginasDiffuser Augmentedanthony100% (1)
- BSC2011 Animals Exam 2 ReviewDocumento72 páginasBSC2011 Animals Exam 2 ReviewDan TranAinda não há avaliações
- Astm g32 10Documento19 páginasAstm g32 10gidlavinayAinda não há avaliações
- Msds Super Gloss Oil PaintDocumento3 páginasMsds Super Gloss Oil PaintMD AbdullahAinda não há avaliações
- Drug Formulary 2219844Documento1.224 páginasDrug Formulary 2219844gszzq8cj4mAinda não há avaliações