Escolar Documentos
Profissional Documentos
Cultura Documentos
F321 Module 3 Practice 5
Enviado por
coughsyrup123Direitos autorais
Formatos disponíveis
Compartilhar este documento
Compartilhar ou incorporar documento
Você considera este documento útil?
Este conteúdo é inapropriado?
Denunciar este documentoDireitos autorais:
Formatos disponíveis
F321 Module 3 Practice 5
Enviado por
coughsyrup123Direitos autorais:
Formatos disponíveis
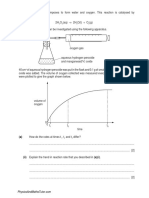
F321 module 3 Practice 5:
1.
An ore of strontium contains strontium carbonate, SrCO3.
To obtain metallic strontium,
the SrCO3 is converted into strontium oxide, SrO;
SrO is then reduced to produce strontium.
(i)
Suggest how strontium carbonate is converted into strontium oxide.
.........................................................................................................................
[1]
(ii)
Aluminium can be used to reduce strontium oxide.
Balance the equation below for this conversion.
............ SrO(s) + ............ Al(s) ............ Sr(s) + ............ Al2O3(s)
[1]
(iii)
A chemical company receives an order to supply 100 tonnes of strontium. The
company needs to work out how much ore to process
The ore typically contains 2% by mass of SrCO3.
Calculate the mass of ore that the company would need in order to produce 100
tonnes of strontium.
1 tonne = 106 g
[3]
(iv)
Suggest how the company could minimise the environmental impact of strontium
production from the ore.
.........................................................................................................................
.........................................................................................................................
[1]
[Total 6 marks]
The King's CE School
2.
In this question, one mark is available for the quality of written communication.
This question is about the Group 7 elements chlorine, bromine and iodine.
Explain why chlorine, bromine and iodine have different physical states at room
temperature and pressure.
[4]
An aqueous solution of bromine was added to an aqueous solution of chloride
ions and also to an aqueous solution of iodide ions.
State what you would see in each case.
Include an equation for any chemical reaction that takes place.
Explain the trend shown by these observations.
[4]
Quality of Written Communication [1]
[Total 9 marks]
3.
A household bleach contains sodium chlorate(I), NaClO, as its active ingredient.
The concentration of NaClO in the bleach can be found by using its reaction with
hydrogen peroxide, H2O2.
NaCIO(aq) + H2O2(aq) O2(g) + NaCI(aq) + H2O(I)
(a)
Chlorine has been reduced in this reaction.
Use oxidation numbers to prove this.
.........................................................................................................................
.........................................................................................................................
.........................................................................................................................
[2]
The King's CE School
(b)
A student added an excess of aqueous hydrogen peroxide to 5.0 cm3 of the
bleach. 84 cm3 of oxygen gas were released.
(i)
How many moles of O2 were released?
Assume that, under the laboratory conditions, 1.00 mol of gas molecules
occupies 24 dm3.
answer ............................. mol
[1]
(ii)
How many moles of NaCIO were in 5.0 cm3 of the bleach?
answer ............................. mol
[1]
(iii)
What was the concentration, in mol dm3, of NaCIO in the bleach?
answer ............................. mol dm3
[1]
(c)
The label on the bottle of household bleach states that the bleach contains a
minimum of 4.5 g per 100 cm3 of NaCIO.
Use your answer to (b)(iii) to decide whether or not the information on the label is
correct.
[3]
(d)
It is extremely important that household bleach is not used with acids. This is
because a reaction takes place that releases toxic chlorine gas.
Suggest an equation for the reaction of an excess of hydrochloric acid with
household bleach.
.........................................................................................................................
[2]
[Total 10 marks]
The King's CE School
4.
This question is about elements and compounds of Group 2 of the Periodic Table.
When calcium is added to water, a vigorous reaction takes place, releasing hydrogen
gas.
Ca(s) + 2H2O(I) Ca{OH)2(aq) + H2(g)
(i)
Suggest a value for the pH of the solution formed in this reaction.
.........................................................................................................................
[1]
(ii)
Complete the electronic configuration of calcium in
Ca(s)
1s22s22p6 .........................
Ca(OH)2(aq)
1s22s22p6 .........................
[2]
[Total 3 marks]
5.
When barium metal is added to water, the reaction taking place is much more vigorous
than with calcium.
Explain why barium is more reactive than calcium.
..................................................................................................................................
..................................................................................................................................
..................................................................................................................................
..................................................................................................................................
..................................................................................................................................
..................................................................................................................................
[Total 4 marks]
The King's CE School
Você também pode gostar
- Metal Catalysed Carbon-Carbon Bond-Forming ReactionsNo EverandMetal Catalysed Carbon-Carbon Bond-Forming ReactionsAinda não há avaliações
- Question Database For Amount of SubstancesDocumento23 páginasQuestion Database For Amount of SubstancesKamrul Alam MasumAinda não há avaliações
- Balanced Equations & Associated Calc's 05 QPDocumento8 páginasBalanced Equations & Associated Calc's 05 QPlmao lmaoAinda não há avaliações
- 2.4 2.5 2.6 Assessed HomeworkDocumento7 páginas2.4 2.5 2.6 Assessed HomeworkRabia Rafique100% (1)
- Cons. of Mass, Chemical Measurements - Eqns 2 QPDocumento18 páginasCons. of Mass, Chemical Measurements - Eqns 2 QPHusain KamaliAinda não há avaliações
- t2 Chem Revision Ex 22Documento19 páginast2 Chem Revision Ex 22Nicholas OwAinda não há avaliações
- Topic 09.3 Group IVDocumento3 páginasTopic 09.3 Group IVzafarchem_iqbalAinda não há avaliações
- Periodicity Past QuestionsDocumento17 páginasPeriodicity Past QuestionsMohammad KhanAinda não há avaliações
- Mytest 1Documento8 páginasMytest 1Aditi ChauhanAinda não há avaliações
- AQA C2 Past Paper Q&A Part 1Documento184 páginasAQA C2 Past Paper Q&A Part 1Junaid AsgharAinda não há avaliações
- Bonding - Structuce 3 QPDocumento14 páginasBonding - Structuce 3 QPtertianerima101Ainda não há avaliações
- As Level Chemistry: Topic 2 - Amount of Substance TestDocumento10 páginasAs Level Chemistry: Topic 2 - Amount of Substance Testkarima akterAinda não há avaliações
- BondingDocumento14 páginasBondingMuizzudin AzaliAinda não há avaliações
- 4.1 Reactivity of Metals 3 QPDocumento16 páginas4.1 Reactivity of Metals 3 QPDumpsterFireGamingAinda não há avaliações
- As Level Chemistry: Topic 6 - Redox, Group 2 and Group 7 TestDocumento9 páginasAs Level Chemistry: Topic 6 - Redox, Group 2 and Group 7 TestMahrukh FatimaAinda não há avaliações
- Redox Questions Igcse ChemDocumento7 páginasRedox Questions Igcse ChemCaylinAinda não há avaliações
- Redox 2 QPDocumento7 páginasRedox 2 QPPramitaAinda não há avaliações
- Ionic Bonding 2 QPDocumento7 páginasIonic Bonding 2 QPHuseyn AgazadeAinda não há avaliações
- P7 Oxidation Reduction and RedoxDocumento42 páginasP7 Oxidation Reduction and Redox/ “Nu” /Ainda não há avaliações
- C2 Useful Products IntermediateDocumento13 páginasC2 Useful Products IntermediatedownendscienceAinda não há avaliações
- Topical Questions For ElectrolysisDocumento6 páginasTopical Questions For Electrolysisokguserfucker idontgiveashitAinda não há avaliações
- Properties of Metals 4 QP PDFDocumento10 páginasProperties of Metals 4 QP PDFAli AshrafAinda não há avaliações
- My TestDocumento5 páginasMy TestMarin PesicAinda não há avaliações
- Periodic Table 2 QPDocumento12 páginasPeriodic Table 2 QPChibale Mchere BandaAinda não há avaliações
- Mixed Topic Revision 1 DiffusionDocumento23 páginasMixed Topic Revision 1 DiffusionYaakkwAinda não há avaliações
- Chapter 10 Exam Style QuestionsDocumento13 páginasChapter 10 Exam Style QuestionsAmanda AnushaAinda não há avaliações
- I2 Group 2 Alkaline Earth MetalsDocumento30 páginasI2 Group 2 Alkaline Earth Metalsasifh76543Ainda não há avaliações
- Electricity & Chemistry 1 QPDocumento11 páginasElectricity & Chemistry 1 QPValerine VictoriaAinda não há avaliações
- Unit 1 Chemistry Questions.Documento458 páginasUnit 1 Chemistry Questions.adalinefallingstar100% (1)
- Metal p4 Ws 4Documento10 páginasMetal p4 Ws 4NonnoonoAinda não há avaliações
- Water and HydrogenDocumento7 páginasWater and HydrogenOkumu KevinsAinda não há avaliações
- Igcse Electrochemistry Review PDFDocumento7 páginasIgcse Electrochemistry Review PDFbilly ogadaAinda não há avaliações
- Types of Reactions WsDocumento2 páginasTypes of Reactions WsTomáš Tommy NagyAinda não há avaliações
- I6 Reactions of Ions in Aqueous SolutionDocumento16 páginasI6 Reactions of Ions in Aqueous Solution/ “Nu” /Ainda não há avaliações
- Group 2 - The Alkaline Earth Metals 1 QPDocumento8 páginasGroup 2 - The Alkaline Earth Metals 1 QPWilliam TsuiAinda não há avaliações
- Topic 4 - Group 7Documento9 páginasTopic 4 - Group 7Abirame SivakaranAinda não há avaliações
- Periodic Table, Group 2 and The Halogens 2 QPDocumento14 páginasPeriodic Table, Group 2 and The Halogens 2 QPmalakAinda não há avaliações
- Class 10 Mid Exam 2019Documento12 páginasClass 10 Mid Exam 2019Khalid HassanAinda não há avaliações
- As Level Chemistry: Topic 6 - Redox, Group 2 and Group 7 Assessed HomeworkDocumento10 páginasAs Level Chemistry: Topic 6 - Redox, Group 2 and Group 7 Assessed HomeworkSumathi GanasenAinda não há avaliações
- Acids, Bases & Salts 5 QPDocumento7 páginasAcids, Bases & Salts 5 QPValerine VictoriaAinda não há avaliações
- Balanced Equations & Associated Calc's 10 QPDocumento8 páginasBalanced Equations & Associated Calc's 10 QPjade.davis0019Ainda não há avaliações
- 1.1.3 AcidsDocumento8 páginas1.1.3 AcidsKel1209Ainda não há avaliações
- 4CH0 2CR Que 20160615Documento20 páginas4CH0 2CR Que 20160615abhayAinda não há avaliações
- Chemical Energetics 2 QPDocumento11 páginasChemical Energetics 2 QPWilliam TsuiAinda não há avaliações
- Energy Changes, Rate, Reversible, NH3, H2SO4Documento5 páginasEnergy Changes, Rate, Reversible, NH3, H2SO4HudaAinda não há avaliações
- Cambridge IGCSE: Chemistry 0620Documento14 páginasCambridge IGCSE: Chemistry 0620PizzaAinda não há avaliações
- Electrochemistry A2Documento21 páginasElectrochemistry A2Umair AhmadAinda não há avaliações
- The Particulate Nature of Matter 3 QPDocumento10 páginasThe Particulate Nature of Matter 3 QPSajia EhsaniAinda não há avaliações
- Born-Haber Cycles 1 QP PDFDocumento9 páginasBorn-Haber Cycles 1 QP PDFTalha KhanAinda não há avaliações
- 9701 s17 QP 42 RemovedDocumento18 páginas9701 s17 QP 42 RemovedSherise EeAinda não há avaliações
- Electricity & Chemistry 2 QPDocumento10 páginasElectricity & Chemistry 2 QPValerine VictoriaAinda não há avaliações
- Chemistry 1a N C Principles of Chemistry State of Matter Atoms Atomic StructureDocumento25 páginasChemistry 1a N C Principles of Chemistry State of Matter Atoms Atomic StructuresechosAinda não há avaliações
- Chlorine and Its Compounds Chemistry Form 3 Topical Questions and AnswersDocumento18 páginasChlorine and Its Compounds Chemistry Form 3 Topical Questions and Answersideal writersAinda não há avaliações
- Reversible ReactionDocumento17 páginasReversible ReactionNavyaa SinghAinda não há avaliações
- As-Level Paper 1 pp9Documento15 páginasAs-Level Paper 1 pp9ConorAinda não há avaliações
- 1 Hydrogen Peroxide Decomposes To Form Water and Oxygen. This Reaction Is Catalysed byDocumento9 páginas1 Hydrogen Peroxide Decomposes To Form Water and Oxygen. This Reaction Is Catalysed byKelvin DenhereAinda não há avaliações
- End of Week Chemistry TestDocumento4 páginasEnd of Week Chemistry TestOlolade OlaleyeAinda não há avaliações
- ChemicalanalysisDocumento90 páginasChemicalanalysismuhammadshadid4Ainda não há avaliações
- Properties of Metals 9 QPDocumento12 páginasProperties of Metals 9 QPAmogh KothariAinda não há avaliações
- CHEMJUNE2002C4Documento9 páginasCHEMJUNE2002C4api-3726022Ainda não há avaliações
- F321 Module 3 Practice 5 AnswersDocumento4 páginasF321 Module 3 Practice 5 Answerscoughsyrup123Ainda não há avaliações
- Further Mechanics Exam Pack MSDocumento45 páginasFurther Mechanics Exam Pack MScoughsyrup123Ainda não há avaliações
- F321 Module 3 Practice 3Documento10 páginasF321 Module 3 Practice 3coughsyrup123Ainda não há avaliações
- F321 Module 3 Practice 1Documento10 páginasF321 Module 3 Practice 1coughsyrup123Ainda não há avaliações
- F321 Module 3 Practice 2Documento7 páginasF321 Module 3 Practice 2coughsyrup123Ainda não há avaliações
- F321 Module 3 Practice 3 AnswersDocumento4 páginasF321 Module 3 Practice 3 Answerscoughsyrup123Ainda não há avaliações
- F321 Module 2 Practice 6Documento2 páginasF321 Module 2 Practice 6coughsyrup123Ainda não há avaliações
- F321 Module 1 Practice 4Documento7 páginasF321 Module 1 Practice 4coughsyrup123Ainda não há avaliações
- F321 Module 2 Practice 2 AnswersDocumento5 páginasF321 Module 2 Practice 2 Answerscoughsyrup123Ainda não há avaliações
- F321 Module 1 Practice 5Documento1 páginaF321 Module 1 Practice 5coughsyrup123Ainda não há avaliações
- F321 Module 1 Practice 1 AnswersDocumento3 páginasF321 Module 1 Practice 1 Answerscoughsyrup123Ainda não há avaliações
- F321 01 Jun12 OCR Chemistry Question PaperDocumento0 páginaF321 01 Jun12 OCR Chemistry Question PaperDaLilSPAinda não há avaliações
- Edexcel S1 NotesDocumento52 páginasEdexcel S1 Notesmystreet123100% (1)
- 2018 M SeriesDocumento15 páginas2018 M SeriesNhật Tân TrầnAinda não há avaliações
- Classified Chem U5 Questions With Mark SchemeDocumento197 páginasClassified Chem U5 Questions With Mark SchemeAzeem iftikhar0% (1)
- Artigo 1Documento7 páginasArtigo 1Rafael AmaranteAinda não há avaliações
- Cleaning of Paper Materials-K.K.GuptaDocumento2 páginasCleaning of Paper Materials-K.K.GuptavskanchiAinda não há avaliações
- Concentrating On ConcentrationsDocumento4 páginasConcentrating On ConcentrationsDrNareshSarafAinda não há avaliações
- Microsoft PowerPoint - Module 3-1 Sinner Circle Perf EffectsDocumento40 páginasMicrosoft PowerPoint - Module 3-1 Sinner Circle Perf EffectsLê LợiAinda não há avaliações
- Guidelines For Routine Environmental Cleaning of The Operating RoomDocumento13 páginasGuidelines For Routine Environmental Cleaning of The Operating RoomYnaffit Alteza UntalAinda não há avaliações
- Clean and Maintain Kitchen PremisesDocumento51 páginasClean and Maintain Kitchen PremisesMAE ANN MAGDADAROAinda não há avaliações
- Reuse of Water in Cotton PretreatmentDocumento43 páginasReuse of Water in Cotton PretreatmentMandal Souvik100% (1)
- STERILIZATION OF WATER USING BLEACHING POWDER Kartik (12Documento15 páginasSTERILIZATION OF WATER USING BLEACHING POWDER Kartik (12Atul AgarwalAinda não há avaliações
- Disposal Information For KB Internationals Polymer Systems 22032023GPDocumento3 páginasDisposal Information For KB Internationals Polymer Systems 22032023GPeconpile ep525Ainda não há avaliações
- Sodium Percarbonate-Oxygen Bleach TDSDocumento2 páginasSodium Percarbonate-Oxygen Bleach TDSajeetranadaksh11Ainda não há avaliações
- Cleaning in The LabDocumento8 páginasCleaning in The LabHon Kirimi Mwobobia IIAinda não há avaliações
- 18P31E0021Documento72 páginas18P31E0021GshshsAinda não há avaliações
- Project For InternshipDocumento63 páginasProject For Internshiptewele100% (1)
- BM480V2Documento215 páginasBM480V2Bình NguyênAinda não há avaliações
- March 2014 - Part 1 of 2Documento831 páginasMarch 2014 - Part 1 of 2SonjaWaltersAinda não há avaliações
- 8.1-8.6 Redox ReactionsDocumento65 páginas8.1-8.6 Redox ReactionsberonelleAinda não há avaliações
- Chemical Process Industries Report Paper and Pulp IndustriesDocumento46 páginasChemical Process Industries Report Paper and Pulp IndustriesRonalynAinda não há avaliações
- Autopsy Findings in Poisoning CasesDocumento53 páginasAutopsy Findings in Poisoning Casesabc apt0% (1)
- TLE LectureDocumento2 páginasTLE LectureKhunyneAinda não há avaliações
- Kids Zone Daycare - Covid 19 - Parent PoliciesDocumento12 páginasKids Zone Daycare - Covid 19 - Parent Policiesapi-252716157Ainda não há avaliações
- Chapter One Project 5 PagesDocumento5 páginasChapter One Project 5 PagesHIMANI PALAKSHAAinda não há avaliações
- MVC MSDS C 002PL 05 Sodium HypochloriteDocumento4 páginasMVC MSDS C 002PL 05 Sodium HypochloriteIan HiguitAinda não há avaliações
- Chemistry Project Project-2Documento21 páginasChemistry Project Project-2Jijin SajeevAinda não há avaliações
- CPT-Notes For B.Tech ChemicalDocumento47 páginasCPT-Notes For B.Tech ChemicalUnputdownable BishwarupAinda não há avaliações
- Used Producing Flour: Maturing and Bleaching AgentsDocumento6 páginasUsed Producing Flour: Maturing and Bleaching AgentsAprilia OanimaAinda não há avaliações
- 2l Fpps128 Exer5 Grp3Documento8 páginas2l Fpps128 Exer5 Grp3Anne CuadernoAinda não há avaliações
- Control of Microorganisms Unit 7Documento67 páginasControl of Microorganisms Unit 7Naseca100% (1)
- Effects of Different Desensitizing Agents On Bleaching TreatmentsDocumento7 páginasEffects of Different Desensitizing Agents On Bleaching TreatmentsFer TorresAinda não há avaliações