Escolar Documentos
Profissional Documentos
Cultura Documentos
Chapter 15 Notes
Enviado por
Nicole BercegeayDescrição original:
Direitos autorais
Formatos disponíveis
Compartilhar este documento
Compartilhar ou incorporar documento
Você considera este documento útil?
Este conteúdo é inapropriado?
Denunciar este documentoDireitos autorais:
Formatos disponíveis
Chapter 15 Notes
Enviado por
Nicole BercegeayDireitos autorais:
Formatos disponíveis
Chem 2261 Notes
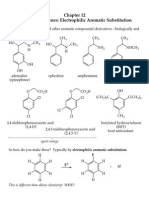
Chemistry of Benzene and Its Derivatives
(Bruice, Chapter 15)
Aromatic Compounds and the Concept of Aromaticity
Benzene and Other Compounds Possessing Cyclic Six Electron (Aromatic) Systems Aromatic compounds are those possessing a planar, continuous cyclic system which contains a total of 4n+2 electrons. Benzene and its derivatives are the best known examples. Benzene has six electrons (corresponding to 3 bonds) delocalized among a cyclic array six overlapping p-orbitals system of benzene. Other cyclic systems containing six electrons are cyclopentadienyl anion, tropyllium cation, cyclobutadienyl dianion. These structures, like benzene, are aromatic. Aromaticity confers a special stability on these systems.
benzene
cyclopentadienyl anion
tropyllium cation
N
cyclobutadienyl dianion
Pyridine, pyrrole, furan, and imidazole are examples of aromatic heterocyles:
N pyridine NH pyrrole O furan NH
imidazole
Other Cyclic 4n+2 Electron (Aromatic) Systems
2 electrons +
= =
+ NH 18 electrons N N HN
10 electrons
Antiaromatic Systems Planar, continuous cyclic systems which contain a total of 4n electrons are especially unstable and are said to be antiaromatic.
For 8 electrons delocalization is destablilizing For 4 electrons delocalization is destablilizing distorts to square delocalized antiaromatic UNSTABLE rectangular localized non-aromatic more stable
=
planar, delocalized antiaromatic UNSTABLE
distorts to nonplanar, localized non-aromatic more stable
Classify the following molecules as aromatic, antiaromatic, or non-aromatic:
N+
Chem 2261 Notes
Chemistry of Benzene and Its Derivatives
(Bruice, Chapter 15)
Electrophilic Aromatic Substitution
General
H E+ B H E E
E = D, Cl, Br, SO3H, NO2, R (alkyl), O=C-R (acyl), etc.
aromatic
resonance delocalized
aromatic
Resonance delocalization of cationic intermediate:
H E H E H E H E
resonance structures
hybrid structure
Specific Reactions
All electrophilic aromatic substitution reactions follow the same general mechanism. The only thing that varies is the specific electrophile which gets attached to the aromatic ring. Some specific electrophilic aromatic substitution reactions are described below. a) Sulfonation
H conc. H2SO4 SO3H Electrophile = OH S+ O O
+SO H 3
overall H replaced by SO3H
+SO H 3
H SO3H
SO3H
Sulfonation differs subtly from most other electrophilic aromatic substitution reactions in that it is reversible (note equilibrium arrows). b) Nitration
H HNO3 H2SO4 NO2
+
Electrophile = O N O NO2+
overall H replaced by NO2
NO2+
H NO2
NO2
How are the electrophiles generated in the sulfonation and nitration reactions above?
Chem 2261 Notes c) Halogenation
H X2 FeX3 (X = Br or Cl) H X X FeX3 B X
Chemistry of Benzene and Its Derivatives
(Bruice, Chapter 15)
Electrophile =
X X FeX3 equivalent to X+
overall H replaced by X (X = Br or Cl)
H X
d) Friedel-Crafts Alkylation
H R-Cl AlCl3 R Electrophile = R Cl AlCl3 R Cl AlCl3 H R Cl AlCl3 B H R R overall H replaced by R R = alkyl group
Carbocation rearrangement competes with substitution (see discussion of incipient primary carbocations on p.647 of your textbook):
H Cl AlCl3 Cl AlCl3 H Cl AlCl3 minor (35%) MAJOR (65%) major electrophile Cl AlCl3
e) Friedel-Crafts Acylation
H O R Cl AlCl3 O C R
+
R
+
Electrophile =
R C O acylium ion
O overall H replaced by R RCO = acyl group (R = alkyl group) O R Cl AlCl3
O H
While Friedel-Crafts acylation can be used to make aryl ketones, it cannot be used to make corresponding aldehydes due to the instability of formyl choride. 3
Chem 2261 Notes
H O H Cl AlCl3 O H
Chemistry of Benzene and Its Derivatives
(Bruice, Chapter 15)
O is not possible because H Cl is unstable
However, benzaldehyde (and other aryl aldehydes) can be prepared by means of the GattermanKoch formylation reaction whereby formyl chloride is generated in situ under conditions where it will react in an electrophilic aromatic substitution reaction upon its generation.
H O CO/HCl high pressure AlCl3/CuCl H
The product of a Friedel-Crafts acylation can be reduced to a compound where the carbonyl group (C=O) is replaced by a methylene group (CH2). This can be accomplished through either Clemmenson reduction or Wolff-Kishner reduction.
O Clemmensen reduction Zn/Hg, HCl H R H EtOH, R
H2NNH2 HO, Wolff-Kishner reduction
Note that Clemmenson or Wolff-Kishner Reduction can be used in conjunction with FriedelCrafts Acylation to prepare alkyl benzene products which could not be made directly by FriedelCrafts Alkylation. For example:
O H O Cl AlCl3
As was shown above, attempted synthesis of butylbenzene by reaction of benzene with chorobutane/AlCl3 is thwarted by carbocation rearrangement leading instead to sec-butylbenzene as the major product. The acylation/reduction sequence provides a way around this problem. Alkylbenzenes can also be prepared from aryl halides by coupling reactions (see Chapter 11, p.454460). For example:
Br + R Cu R M R + R Cu + M+ Br-
How Some Substituents on a Benzene Ring Can Be Chemically Changed
a) Benzylic Halogenation with NBS A benzylic hydrogen can be replaced with a halogen by radical substitution reactions described in Chapter 12 (p.484-487).
H X2 or h X = Cl or Br X H NBS or h Br
Chem 2261 Notes
Chemistry of Benzene and Its Derivatives
(Bruice, Chapter 15)
b) Conversion of Alkyl Groups or Benzylic Alcohols to Carboxyl Groups with Cr+6 or Mn+7 Reagents Methyl, primary alkyl, and secondary alkyl groups are converted to carboxyl (CO2H) groups by Cr+6 and Mn+7 oxidizing agents.
Me or R R R Mn+7 or Cr+6 reagent CO2H
or
KMnO4 and H+, Na2Cr2O7 are commonly used oxidizing agents
The first step in these oxidation reactions is the removal of a benzylic hydrogen, so tertiary alkyl groups are unaffected by Cr+6 and Mn+7 oxidizing agents.
R R tertiary alkyl group R Mn+7 or Cr+6 reagent
NO REACTION
R R
The same reagents will oxidize benzylic alcohols to benzoic acids.
OH R OH OH Mn+7 or Cr+6 reagent CO2H
or
or
c) Oxidation of Benzylic Alcohols to Ketones or Aldehydes with MnO2
H H OH MnO2 1 benzyl alcohol aldehyde 2 benzyl alcohol H O H R OH MnO2 ketone R O
d) Reduction of Nitro Groups to Amino Groups A nitro substituent can be reduced to an amino substituent. Either a metal (tin, iron, or zinc) pul an acid (HCl) or catalytic hydrogenation can be used to carry out this reaction.
NO2 1. Sn, HCl 2. NaOH NH2
H2 Pd/C
Você também pode gostar
- DAT Practice TestDocumento75 páginasDAT Practice TestJustinTsui88% (8)
- SEJ446 Final Report 2014Documento43 páginasSEJ446 Final Report 2014Busiku SilengaAinda não há avaliações
- Naoh Vs Oxalic Acid - TitrationDocumento3 páginasNaoh Vs Oxalic Acid - TitrationffffffgAinda não há avaliações
- Benzene and Aromatic Compounds-fazli-In ClassDocumento57 páginasBenzene and Aromatic Compounds-fazli-In Classjokowi123Ainda não há avaliações
- Aromatic ChemistryDocumento16 páginasAromatic ChemistrysimbaleoAinda não há avaliações
- AromaticDocumento38 páginasAromaticDerrick Maatla MoadiAinda não há avaliações
- Aromatic CompoundsDocumento25 páginasAromatic CompoundsElizabeth Vivar100% (1)
- Romatic Ydrocarbons: CH CH CH C HDocumento7 páginasRomatic Ydrocarbons: CH CH CH C HMukesh BishtAinda não há avaliações
- Chemistry of Benzene: Electrophilic Aromatic Substitution: Based On Mcmurry'S Organic Chemistry, 6 Edition, Chapter 16Documento89 páginasChemistry of Benzene: Electrophilic Aromatic Substitution: Based On Mcmurry'S Organic Chemistry, 6 Edition, Chapter 16Nur Anis HidayahAinda não há avaliações
- 3 ArenesDocumento7 páginas3 ArenesAnonymous 8VJhV1eI2yAinda não há avaliações
- Hydrocarbon Compounds: AromaticDocumento49 páginasHydrocarbon Compounds: AromaticUMMU MARDHIAH ABDUL HALIMAinda não há avaliações
- BR BR BR: O-Bromotuloene P-Bromotuloene M-BromotuloeneDocumento46 páginasBR BR BR: O-Bromotuloene P-Bromotuloene M-Bromotuloenejan100% (1)
- 4.8 Further Organic Chemistry PDFDocumento11 páginas4.8 Further Organic Chemistry PDFMohamed ZaidhanAinda não há avaliações
- Ochem Notes StereoDocumento4 páginasOchem Notes StereoDmitry HeisenbergAinda não há avaliações
- Reaction of Alkenes and Alkynes For StudentsDocumento53 páginasReaction of Alkenes and Alkynes For StudentsGlen MangaliAinda não há avaliações
- Electophilic Aromatic Substitution: K.Sowjanya Associate Professor Department of PH - Chemistry Nirmala College of PharmacyDocumento44 páginasElectophilic Aromatic Substitution: K.Sowjanya Associate Professor Department of PH - Chemistry Nirmala College of Pharmacysowjanya kAinda não há avaliações
- Aromatics HandoutDocumento8 páginasAromatics HandoutJan ChretienAinda não há avaliações
- Chemistry Form 6 Sem 3 03Documento39 páginasChemistry Form 6 Sem 3 03Ng Swee Loong StevenAinda não há avaliações
- Electrophilic Aromatic Substitution + The Chemistry of BenzeneDocumento28 páginasElectrophilic Aromatic Substitution + The Chemistry of BenzeneTrescia Mae EstilloreAinda não há avaliações
- 15 CH242 Benzene & AromaticityDocumento68 páginas15 CH242 Benzene & Aromaticityali mu'adAinda não há avaliações
- Nucleophilic Aromatic SubstitutionDocumento5 páginasNucleophilic Aromatic Substitutionnjwaghmare7392100% (1)
- Reaction of Aromatic Chapter18Documento70 páginasReaction of Aromatic Chapter18Glen Mangali100% (1)
- Benzene 2Documento9 páginasBenzene 2claimstudent3515Ainda não há avaliações
- Corrected Fundamentals of Organic ChemistryDocumento71 páginasCorrected Fundamentals of Organic ChemistryDAM2120Ainda não há avaliações
- Chemistry Form 6 Sem 3 Chapter 3Documento39 páginasChemistry Form 6 Sem 3 Chapter 3Yuzamrah Awang NohAinda não há avaliações
- Derivatives of Aromatic HydrocarbonDocumento43 páginasDerivatives of Aromatic HydrocarbonShariar ShawoŋAinda não há avaliações
- ArenesDocumento5 páginasArenes林琪Ainda não há avaliações
- ArenesDocumento4 páginasArenesNkemzi Elias NzetengenleAinda não há avaliações
- Chapter 11Documento30 páginasChapter 11kanilkadianAinda não há avaliações
- Aromatic Compound Theory - EDocumento23 páginasAromatic Compound Theory - Ethinkiit100% (1)
- Aromatic Notes 2 PDFDocumento6 páginasAromatic Notes 2 PDFChris_Barber09100% (1)
- Benzene StructureDocumento30 páginasBenzene StructureAnuki PereraAinda não há avaliações
- Chapter 4Documento9 páginasChapter 4luckshimiAinda não há avaliações
- 10 Haloalkanes and Haloarenes PPT-1Documento107 páginas10 Haloalkanes and Haloarenes PPT-1Dhruv JainAinda não há avaliações
- Benzene: Draw Out Suitable Structures Which Fit The Molecular Formula C HDocumento8 páginasBenzene: Draw Out Suitable Structures Which Fit The Molecular Formula C HmuhajireenAinda não há avaliações
- Lesson 13 Reactions of Benzene and Its Derivatives-Hannah-PcDocumento22 páginasLesson 13 Reactions of Benzene and Its Derivatives-Hannah-Pcdela2Ainda não há avaliações
- Hydrocarbons - AlkenesDocumento11 páginasHydrocarbons - Alkenesanish.chandrasekar.bloreAinda não há avaliações
- Organic Chemistry WordDocumento19 páginasOrganic Chemistry Wordisnaini safitriAinda não há avaliações
- Aldehida Dan KetonDocumento88 páginasAldehida Dan KetonSITI FARAS RAHMAWATI 2021Ainda não há avaliações
- Trinitrotoluene (TNT), A Pale Yellow, Solid Organic Nitrogen Compound Used Chiefly As An Explosive, Prepared by Stepwise Nitration of TolueneDocumento76 páginasTrinitrotoluene (TNT), A Pale Yellow, Solid Organic Nitrogen Compound Used Chiefly As An Explosive, Prepared by Stepwise Nitration of TolueneVidhan PatniAinda não há avaliações
- AK Alc PhenolDocumento3 páginasAK Alc PhenolFelix Joshua.B 10 BAinda não há avaliações
- Chapter 12Documento23 páginasChapter 12Hải NguyễnAinda não há avaliações
- Chapter 4 Aromatic CompoundsDocumento61 páginasChapter 4 Aromatic CompoundsapayAinda não há avaliações
- 4.1.1 ArenesDocumento8 páginas4.1.1 ArenesFin BrickmanAinda não há avaliações
- Reasoning Questions in Organic ChemistryDocumento6 páginasReasoning Questions in Organic ChemistryPavankumar SAinda não há avaliações
- Reactions of BenzeneDocumento7 páginasReactions of BenzeneCleveland BrownAinda não há avaliações
- F334 - What's in A Medicine?Documento11 páginasF334 - What's in A Medicine?Becky Tenney100% (1)
- AlcoholDocumento78 páginasAlcoholVarnit Mittal100% (1)
- Aromatic CompoundsDocumento27 páginasAromatic CompoundsScience AcademyAinda não há avaliações
- Aromatic CompoundsDocumento36 páginasAromatic CompoundsMaria Ruela Agodera SumogAinda não há avaliações
- Chemistry Presentation.Documento5 páginasChemistry Presentation.Bushra PervaizAinda não há avaliações
- Organic ReviewerDocumento4 páginasOrganic ReviewerRanie MagpocAinda não há avaliações
- Electrophillic Aromatci SubstitutionDocumento114 páginasElectrophillic Aromatci SubstitutionAbe KobAinda não há avaliações
- Chapter 4Documento51 páginasChapter 4Wai Kwong ChiuAinda não há avaliações
- AromaticsDocumento70 páginasAromaticsEceDiril100% (1)
- Assignment 4 Reactions of Aromatic Compounds AnswersDocumento11 páginasAssignment 4 Reactions of Aromatic Compounds AnswersJonathan Yeung100% (1)
- Physical Organic Chemistry — 3: Plenary Lectures Presented at the Third IUPAC Conference on Physical Organic Chemistry, Montpellier, France, 6 - 10 September, 1976No EverandPhysical Organic Chemistry — 3: Plenary Lectures Presented at the Third IUPAC Conference on Physical Organic Chemistry, Montpellier, France, 6 - 10 September, 1976A. FruchierAinda não há avaliações
- Physical Organic Chemistry—Ii: Specially Invited Lectures Presented at the Second IUPAC Conference on Physical Organic Chemistry Held at Noordwijkerhout, Netherlands, 29 April–2 May 1974No EverandPhysical Organic Chemistry—Ii: Specially Invited Lectures Presented at the Second IUPAC Conference on Physical Organic Chemistry Held at Noordwijkerhout, Netherlands, 29 April–2 May 1974Th. J. De BoerAinda não há avaliações
- Practice Makes Perfect in Chemistry: Acids, Bases, and SaltsNo EverandPractice Makes Perfect in Chemistry: Acids, Bases, and SaltsAinda não há avaliações
- Practice Makes Perfect in Chemistry: Acids, Bases, and Salts with AnswersNo EverandPractice Makes Perfect in Chemistry: Acids, Bases, and Salts with AnswersAinda não há avaliações
- Organometallic Chemistry: Plenary Lectures Presented at the Fourth International Conference on Organometallic ChemistryNo EverandOrganometallic Chemistry: Plenary Lectures Presented at the Fourth International Conference on Organometallic ChemistryF. G. A. StoneAinda não há avaliações
- Lecture2 PDFDocumento18 páginasLecture2 PDFYavuz KaplanAinda não há avaliações
- Alpha Beta Gamma Nuclear Decay Activity SheetDocumento10 páginasAlpha Beta Gamma Nuclear Decay Activity SheetArlo RivasAinda não há avaliações
- Biochemistry Laboratory Activity No. 8: University of Perpetual Help System DALTA College of Medical TechnologyDocumento6 páginasBiochemistry Laboratory Activity No. 8: University of Perpetual Help System DALTA College of Medical TechnologyKristine Joy Abellar ResuentoAinda não há avaliações
- Experiment 3 Group 1Documento4 páginasExperiment 3 Group 1jamielAinda não há avaliações
- TiO2 Synthesis Using GlycineDocumento5 páginasTiO2 Synthesis Using GlycineRanjit KumarAinda não há avaliações
- Pears Pears Pears Pears Pears: Nitrogen (N)Documento4 páginasPears Pears Pears Pears Pears: Nitrogen (N)Zineil BlackwoodAinda não há avaliações
- Plastic Fuel Alka ZadgaonkarDocumento60 páginasPlastic Fuel Alka Zadgaonkarprashanth2812Ainda não há avaliações
- Lactic Acid Production From Glycerol Using CaO As Solid Base CatalystDocumento8 páginasLactic Acid Production From Glycerol Using CaO As Solid Base CatalystlarguedasAinda não há avaliações
- As 1221 Hose-ReelsDocumento2 páginasAs 1221 Hose-ReelsDo BuiAinda não há avaliações
- SF6 Circuit BreakerDocumento2 páginasSF6 Circuit BreakerkashifAinda não há avaliações
- ACV-12 Adjustable Choke Valves: For Wide Applications in Oil, Gas, and Water ServiceDocumento2 páginasACV-12 Adjustable Choke Valves: For Wide Applications in Oil, Gas, and Water ServiceDeyokeAinda não há avaliações
- Requirements of Dental Cements For Lining ApplicationsDocumento28 páginasRequirements of Dental Cements For Lining ApplicationsShahzadi SamanaAinda não há avaliações
- Study of Effect of Potassium Bisulphite As A Food PreservativeDocumento14 páginasStudy of Effect of Potassium Bisulphite As A Food PreservativeAntarctic SaverAinda não há avaliações
- AnthocyaninDocumento5 páginasAnthocyaninNguyen HoaAinda não há avaliações
- Local Anesthetics 2006Documento22 páginasLocal Anesthetics 2006Shashikant DrShashikant BagadeAinda não há avaliações
- Ger 3751a Understanding Diagnosing Repairing Leaks h20 Gen Stator WindingsDocumento28 páginasGer 3751a Understanding Diagnosing Repairing Leaks h20 Gen Stator Windingsnareshvkkd100% (1)
- Topic10 SeismicDesignofSteelStructuresHandoutsDocumento20 páginasTopic10 SeismicDesignofSteelStructuresHandoutsrk6482Ainda não há avaliações
- Bulk Electronic Structure of SrTiO3: Experiment and TheoryDocumento10 páginasBulk Electronic Structure of SrTiO3: Experiment and Theoryhoehoe1234Ainda não há avaliações
- Chemical Digestion and Absorption - A Closer Look - Anatomy and PhysiologyDocumento20 páginasChemical Digestion and Absorption - A Closer Look - Anatomy and PhysiologyMa Mayla Imelda LapaAinda não há avaliações
- VSI Week8 Lecture8 InstAnal 4th Stage Theory 2022Documento30 páginasVSI Week8 Lecture8 InstAnal 4th Stage Theory 2022Sozdar ArgoshiAinda não há avaliações
- ST2 PDFDocumento5 páginasST2 PDFKaraline MarcesAinda não há avaliações
- Ak 174 Photoetch Burnishing PDFDocumento1 páginaAk 174 Photoetch Burnishing PDFLea LeaAinda não há avaliações
- Measurement of Fluorescence Quantum YieldsDocumento4 páginasMeasurement of Fluorescence Quantum YieldsChaudhary Mandeep Singh Dalal100% (1)
- Case Study On Centrifugal PumpsDocumento2 páginasCase Study On Centrifugal PumpsRavindra Pawar0% (1)
- Assignment 1Documento2 páginasAssignment 1Sakshi Gupta0% (1)
- H-E Parts Data Sheet PT-80 Chromium CarbideDocumento1 páginaH-E Parts Data Sheet PT-80 Chromium CarbideJorge VillalobosAinda não há avaliações
- Mole Concept @kvpy - AspirantsDocumento7 páginasMole Concept @kvpy - AspirantssagarAinda não há avaliações