Escolar Documentos
Profissional Documentos
Cultura Documentos
Conversion of Waste Tyres Into Carbon Black and Their Utilization As Adsorbent
Enviado por
Hartono PrayitnoDescrição original:
Título original
Direitos autorais
Formatos disponíveis
Compartilhar este documento
Compartilhar ou incorporar documento
Você considera este documento útil?
Este conteúdo é inapropriado?
Denunciar este documentoDireitos autorais:
Formatos disponíveis
Conversion of Waste Tyres Into Carbon Black and Their Utilization As Adsorbent
Enviado por
Hartono PrayitnoDireitos autorais:
Formatos disponíveis
Journal of the Chinese Chemical Society, 2006, 53, 1085-1089
1085
Conversion of Waste Tyres into Carbon Black and their Utilization as Adsorbent
Jasmin Shah,* M. Rasul Jan, Fazal Mabood and M. Shahid
Department of Chemistry, University of Peshawar, N.W.F.P., Pakistan
Pyrolysis has the potential of transforming used tyres into useful recyclable products. Pyrolytic carbon black is one of the most important products of tyre pyrolysis. Waste tyres were pyrolysed at 450 C in a batch reactor under atmospheric pressure. The recovered pyrolytic carbon black residues were studied to investigate their characteristics for use as a possible adsorbent. EDX elemental analysis and surface area determinations were used to investigate the distinctive features of pyrolytic carbon black. Due to various inorganic additives of the original tyre that contaminated the carbon black obtained, it was treated with acid for demineralization. The demineralized carbon black was activated at 900 C in a furnace. It was observed that acid treatment and activation increased the surface areas and decreased the concentration of contaminants. Furthermore, adsorption characteristics of methylene blue on acid-treated and activated carbon black (prepared via acid treatment) were compared with those of commercial activated carbon in liquid phase adsorption. It was found that adsorption capacity of methylene blue on acid-treated activated carbon black was greater. Keywords: Pyrolytic carbon black; Demineralization; Adsorption; Methylene blue.
INTRODUCTION Scrap tyres represent a major environmental problem. When tyres are no longer usable, they are usually dumped in landfill sites, or burned in cement kilns or in brick kilns.1 However, because of the elevated emissions of polyaromatic hydrocarbons (PAH) and of heavy metals (e.g. zinc), expensive gas cleaning systems have to be installed in order to perform this process in an environmentally responsible fashion.2 Pyrolysis may be an environmentally friendly process to transform used tyres into useful products. This process transforms used tyres into gas, oil and pyrolytic carbon black.3-5 Researchers have shown that the conversion of used tyres into oil is a feasible process.6-9 The derived oils may be used directly as fuel or added to petroleum refinery feedstock. They may also be an important source of refined chemicals.10 The gaseous products are also useful as fuel, and the carbon black may be used as reinforcing filler in rubber, especially in tyres,11 or as activated carbon because of its high carbon content.12-16 Activated carbon is widely used for water purification, air purification and also in bat-
teries and fuel cells. The main purpose of the present work was to prepare activated carbon from waste tyres, which are suitable for adsorbing relatively large molecules. The adsorption characteristics of the obtained carbon were investigated after acid treatment and activation for organic molecules like methylene blue.
EXPERIMENTAL Characteristics of the Sample Pyrolysed Tyres are composed of very different compounds (rubber, carbon black, fillers, stearic acid, zinc oxide, etc.).17 In order to use representative samples of the whole tyre, cross section pieces of 5-10 mm wide of a commercial passenger car tyre were used for the pyrolysis experiments. Thermogravimetric Analysis The thermogravimetric experiment was performed in a Perkin-Elmer TGA 7 type instrument. The sample was heated from 40 C to a final temperature of 1000 C at a rate
* Corresponding author. Fax: 92-91-9216652; E-mail: jasminshah2001@yahoo.com
1086
J. Chin. Chem. Soc., Vol. 53, No. 5, 2006
Shah et al.
of 20 C/min. Pyrolysis Procedure The carbon black samples were produced from waste tyre pyrolysis. For pyrolysis about 10 g of waste tyre rubber particles were put into a batch reactor of glass tube. The reactor was heated from room temperature to a final temperature of 280, 300, 350, 400, 450 and 500 C for 2 h. The initial gas atmosphere in the reactor consisted of air. Upon heating, however, decomposition of the tyre sample started. The gases released by these reactions gradually replaced the air in the reactor. The gaseous products passed through a trap, where the liquid hydrocarbon was collected. Liquid and solid (carbon black) pyrolysis yields were determined in each experiment by weighing the amount of each obtained and calculating the corresponding percentage. The gas yields were determined by difference. Sample Characterization a. Surface area measurements Surface areas were measured using iodine adsorption number (ASTM 1510). b. EDX elemental analysis The elemental composition of carbon black was determined using an Inca 200 (UK) Energy Dispersive X-Ray (EDX) Analyzer. c. Acid demineralization An acid demineralization process was carried out to decrease the inorganic impurities as well as to remove undesirable (ash) contents from the carbon black. Hydrochloric acid (HCl) and sulphuric acid (H2SO4) were selected for the acid demineralization. 1 N hydrochloric acid and sulphuric acid solutions were used, and demineralization was conducted at room temperature. The samples were demineralized for a period of 24 h, a length of time that was considered to be long enough not to limit the demineralization process. After 24 h the samples were filtered and thoroughly rinsed with distilled water to remove the residual acid. The samples were then dried in an oven at 110 C for 24 h. The carbon black sample was ground and sieved through a 45 mm sieve. In the activation step, the known weight of an oven dried acid treated carbon black sample was placed in a furnace at 900 C for 2 h, which was already optimized. Liquid Phase Adsorption The adsorption characteristics of carbon black acid
treated and activated were investigated. Methylene blue, which is a relatively large molecule, was employed as the adsorbate in the adsorption experiments. An aqueous solution with a concentration of 1000 mg/dm3 was prepared by mixing an appropriate amount of methylene blue with distilled water. Adsorption experiments were conducted by placing 0.5 g of the adsorbent and 50 cm3 of the aqueous solution in a 250 cm3 glass stopper flask. The flasks were agitated at room temperature until the equilibrium was attained. The solutions were filtered and finally their unadsorbed concentrations were measured using a UV-Visible spectrophotometer (Unico 2100, USA) at wavelengths of 610 nm. The equilibrium adsorption capacities (qe) at different methylene blue concentrations were determined according to the mass balance on the adsorbate: qe = (Ci - Ce) V/m where Ci is the initial concentration, Ce is the equilibrium concentration, V is the volume of the liquid phase, and m is the mass of the carbon black.
RESULTS AND DISCUSSION Thermogravimetric analysis of waste tyre rubber was performed to determine the optimum temperature for pyrolysis using a batch reactor. A thermogravimetric curve for waste tyre rubber is shown in Fig. 1. In the first part 3.4% weight loss occurred at 169.48 C to 219.51 C and then 62.9% weight loss occurred from 239.22 C to 463.60 C. The pyrolysis completed in the temperature range of 239.22 C to 463.60 C with a constant weight (33.7%) of carbon black. The results obtained in the tyre pyrolysis experiment using a batch reactor at different temperatures are presented in Fig. 2. With an increase of temperature from 300 C to 450 C the %wt of solid decreased to 34%, almost equivalent at 450 C and 500 C as well as to the yield from thermogravimetric analysis (33.7%). The liquid yield increased from 300 C to 450 C but at 500 C there was a decrease due to stronger thermal pyrolysis. Therefore a carbon black sample was obtained from waste tyre pyrolysis in a batch reactor at 450 C. Surface area The surface area (A) of the carbon black obtained by
Waste Tyres into Carbon Black
J. Chin. Chem. Soc., Vol. 53, No. 5, 2006
1087
Fig. 1. TGA curve of waste tyre rubber at a heating rate program of 20 C/min.
pyrolysis at 450 C was approximately 85 m2g-1 (Table 1). This value is close to the average value of surface areas of commercial carbon blacks used in passenger car tyres such
Fig. 2. Effect of temperature on the yields of gas, liquid and carbon black from pyrolysis of used automotive tyres.
as N330 (A = 82 m2g-1) and N339 (A = 90 m2g-1). Other authors also have reported the similar values.13,18,19 This suggests that the carbon black structure changed only slightly during pyrolysis. To enhance the commercial value of carbon black from pyrolysis and to increase its potential usage as an activated carbon, further treatment was necessary. Hydrochloric acid and sulphuric acid were used for the demineralization of the pyrolytic carbon black, and the surface area increased after acid treatment, drying, crushing and sieving through 45 mm. The surface areas were found to be 870 m2g-1 and 800 m2g-1, respectively. Carbon black treated with hydrochloric acid and sulphuric acid were further activated at 900 C, and a substantial increase in the measured surface area was observed in both acid treated carbon black samples (Table 1). These values compare favorably with those of commercially available activated carbons (specific surface areas ranging between 800 and 1100
1088
J. Chin. Chem. Soc., Vol. 53, No. 5, 2006
Shah et al.
Table 1. Surface area and elemental composition of pyrolytic carbon black and commercial activated carbon (wt%) Sample Carbon black (not treated) Carbon black (HCl treated) Carbon black activated (HCl treated) Carbon black (H2SO4) Carbon black activated (H2SO4 treated) Activated carbon (commercial) Surface area (m2g-1) 085 870 940 800 910 990 C 83.1 93.0 93.9 87.0 90.0 96.8 O 6.0 5.1 4.3 5.9 4.4 2.9 Si 1.6 0.4 0.4 0.6 0.6 S 2.6 0.9 0.8 1.8 1.2 0.3 Zn 4.2 0.6 0.6 2.9 2.0 Ca 2.4 1.8 1.8 -
m2g-1). Acid demineralization The elemental compositions of carbon black determined on the pre and post acid demineralization and activated samples are presented in Table 1. In the acid demineralization process the feed sample contained 83.1% carbon, 2.6% sulphur, 4.2% zinc and 2.4% calcium. After the application of acid treatment of samples, the carbon concentration was significantly increased, indicating the removal of non-carbon phases with acid. The highest carbon concentration achieved with hydrochloric acid treatment was 93%. It can be seen that elements such as calcium appear to be completely removed from the sample and about 85.7% of zinc was removed from the carbon black with hydrochloric acid. While with sulphuric acid demineralization, carbon concentration increased up to 87% and the concentration of other elements decreased to a lesser extent. This may indicate that the majority of the elements in the carbon are present in acid insoluble form. The results of hydrochloric acid demineralization show that the process has potential for the production of a high carbon concentrate. In commercial carbon black, in addition to carbon, only oxygen and sulfur were found (hydrogen can not be detected by EDX). Liquid-phase adsorption isotherms The adsorption of methylene blue from aqueous solution on commercial activated carbon, acid treated and activated carbon black were studied and compared. The commercial grade activated carbon was employed to adsorb methylene blue for the purpose of comparison. The adsorp-
tion isotherms of methylene blue onto the acid treated carbon black and commercial activated carbon are shown in Fig. 3, while acid treated activated carbon black adsorption isotherms of methylene blue are shown in Fig. 4. The adsorption isotherms are sharp and the plateaus are relatively horizontal for both types of carbon. The adsorption capac-
Fig. 3. Comparison of adsorption isotherms of methylene blue on acid treated carbon black and commercial activated carbon (CAcarbon).
Fig. 4. Comparison of adsorption isotherms of methylene blue on acid treated activated carbon black.
Waste Tyres into Carbon Black
J. Chin. Chem. Soc., Vol. 53, No. 5, 2006
1089
ity of methylene blue on hydrochloric acid and sulphuric treated carbon black are similar. The commercial activated carbon was employed to adsorb methylene blue for the purpose of comparison. It has been shown that commercial activated carbon has a larger surface area than the acid treated carbon black. This suggests a larger methylene blue capacity for commercial activated carbon because of the monolayer adsorption on the carbon surface, and the adsorption capacity was found to be greater than the acid treated carbon black. Therefore, the acid treated carbon blacks were activated and again the adsorption capacity of methylene blue was studied. As can be seen from Fig. 4, the amounts of methyelene blue adsorbed increased considerably with acid treated carbon black after activation.
CONCLUSION An activated carbon was produced from waste tyre rubber through thermal pyrolysis and demineralized with acid prior to activation. Acid treatment is an efficient way to demineralize and increase the surface area of the carbon black after activation at 900 C. The liquid-phase adsorption results reveal that the acid treated activated carbon prepared from waste tyres is a suitable adsorbent, especially in systems which involve bulky organic molecules. The adsorption of methylene blue occurs as a monolayer.
Received January 6, 2006.
REFERENCES
1. Ariyadejwanich, P.; Tanthapanichakoon, W.; Nakagawa, K.;
Mukai, S. R.; Tamon, H. Carbon 2003, 41, 157-164. 2. Benallal, B.; Roy, C.; Pakdel, H.; Chabot, S.; Poirier, M. A. Fuel 1995, 74, 1589-1594. 3. Chaala, A.; Darmstadt, H.; Roy, C. Fuel Proc. Technol. 1996, 46, 1-15. 4. Cunliffe, A. M.; Williams, P. T. Environm. Technol. 1998, 19, 1177-1190. 5. Kuhner, G.; Voll, M. In Manufacture of Carbon Black ; Donnet, J. B., Bansal, R. C., Wang, M. J., Eds.; Carbon Black Science and Technology, 2nd Ed., Marcel Dekker: New York, USA, 1993; 1-65. 6. Lin, Y.; Teng, H. Micro Meso. Mater 2002, 54, 167-174. 7. Mastral, A. M.; Murillo, R.; Callen, M. S.; Garcia, T. Resour. Conserv. Recycl. 2000, 29, 263-272. 8. Merchant, A. A.; Petrich, M. A. AlChE J. 1993, 39, 13701376. 9. Pantea, D.; Darmstadt, H.; Kaliaguine, S.; Roy, C. J. Anal. Appl. Pyrol. 2003, 67, 55-76. 10. Rodriguez, I. M.; Laresgoiti, M. F.; Cabrero, M. A.; Torres, A.; Chomon, M. J.; Caballero, B. Fuel Proc. Technol. 2001, 72, 9-22. 11. Roy, C. Recovery of Commercially Valuable Products from Scrap Tyres; US patent No. 5229, 1993, 099. 12. Roy, C.; Labrecque, B.; Caumia, B. Resour. Conserv. Recycl. 1990, 4, 203-213. 13. Teng, H.; Serious, M. A.; Wajtowicz, M. A.; Bassilakis, R.; Solomon, P. R. Ind. Eng. Chem. Res. 1995, 34, 3102-3111. 14. Waddell, W. H.; Bhakuni, R. S.; Barbin, W. W.; Sandstrom, P. H. In Pneumatic Tyre Compounds ; Ohm, R. F., Ed.; The Vanderbilt Rubber Handbook, 13 th Ed., Vanderbilt Company: Norwalk, CT, USA, 1990, 596-621. 15. Williams, P. T.; Brindle, A. J. J. Anal. Appl. Pyrol. 2003, 67, 143-164. 16. Williams, P. T.; Brindle, A. J. Fuel 2003, 82, 1023-1031. 17. Zabanioton, A.; Lagoudakis, J.; Toumanidou, E.; Staropoulos, G. Energy Sources 2002, 24, 843-854. 18. Williams, P. T.; Besler, S.; Taylor, D. T. Fuel 1990 , 69 , 1474-1482. 19. Lin, S. H. AlChE J. 1993, 39, 1370-1376.
Você também pode gostar
- Book Index The Art of Heavy TransportDocumento6 páginasBook Index The Art of Heavy TransportHermon Pakpahan50% (2)
- DJI F450 Construction Guide WebDocumento21 páginasDJI F450 Construction Guide WebPutu IndrayanaAinda não há avaliações
- 5HP18Documento39 páginas5HP18Leonard67% (3)
- 5HP18Documento39 páginas5HP18Leonard67% (3)
- Samsung KS1A - AA41-00131H - CB21F52TS 5Q0765 TDA9381 LA7840 D2499 TDA8943-44Documento4 páginasSamsung KS1A - AA41-00131H - CB21F52TS 5Q0765 TDA9381 LA7840 D2499 TDA8943-44Hartono Prayitno86% (7)
- Addtional List Dissertation 040117Documento6 páginasAddtional List Dissertation 040117Sagar Kansara100% (2)
- Asteroids Prospective EnergyDocumento710 páginasAsteroids Prospective EnergySlavica Otovic100% (1)
- Office 2013 Product KeyDocumento2 páginasOffice 2013 Product KeyHartono Prayitno75% (4)
- FebvreDocumento449 páginasFebvreIan Pereira AlvesAinda não há avaliações
- Compiled LecsDocumento24 páginasCompiled LecsNur SetsuAinda não há avaliações
- Role of Recovered Carbon Black Ash Content CompositionDocumento12 páginasRole of Recovered Carbon Black Ash Content CompositionDimpi PatelAinda não há avaliações
- Gaffin, Biblical Theology and Westminster StandardsDocumento16 páginasGaffin, Biblical Theology and Westminster StandardstheoarticlesAinda não há avaliações
- Pilot-Scale Pyrolysis of Scrap Tires in A Continuous Rotary Kiln ReactorDocumento13 páginasPilot-Scale Pyrolysis of Scrap Tires in A Continuous Rotary Kiln ReactorAlexanderRodriguezGamboaAinda não há avaliações
- Rubber Tire Thermal Decomposition in A Used Oil Environment 2005 Journal of Analytical and Applied PyrolysisDocumento5 páginasRubber Tire Thermal Decomposition in A Used Oil Environment 2005 Journal of Analytical and Applied PyrolysismehdiAinda não há avaliações
- Tyre PyrolysisDocumento6 páginasTyre PyrolysisHande CambazAinda não há avaliações
- Adsorptive Desulfurization of Dibenzothiophene From Fuels by Rubber Tyres-Derived Carbons: Kinetics and Isotherms EvaluationDocumento11 páginasAdsorptive Desulfurization of Dibenzothiophene From Fuels by Rubber Tyres-Derived Carbons: Kinetics and Isotherms EvaluationPrativa BeheraAinda não há avaliações
- Conversion of Waste Tires Into Activated Carbon Through Pyrolysis and Physical Activation With CO2Documento8 páginasConversion of Waste Tires Into Activated Carbon Through Pyrolysis and Physical Activation With CO2Isaac Meza TrujilloAinda não há avaliações
- Activation of Waste Tire Char Upon Cyclic Oxygen Chemisorption#DesorptionDocumento8 páginasActivation of Waste Tire Char Upon Cyclic Oxygen Chemisorption#DesorptionFrancisco HerasAinda não há avaliações
- Preparation of Activated Carbon by Thermal Decomposition of Waste Tires For Pollution ControlDocumento12 páginasPreparation of Activated Carbon by Thermal Decomposition of Waste Tires For Pollution ControlabdulqadirAinda não há avaliações
- Palm Oil Shell JURNALDocumento6 páginasPalm Oil Shell JURNALAmin SulaimanAinda não há avaliações
- Activated Carbon Pellets From Eucalyptus Char and Tar TG StudiesDocumento2 páginasActivated Carbon Pellets From Eucalyptus Char and Tar TG StudiesEduardo Gil LancherosAinda não há avaliações
- Tyre Pyrolysis by Using Nano Catalyst ToDocumento6 páginasTyre Pyrolysis by Using Nano Catalyst ToSaif Khan MubinAinda não há avaliações
- Microporous and Mesoporous MaterialsDocumento6 páginasMicroporous and Mesoporous MaterialskristianAinda não há avaliações
- Journal of The Taiwan Institute of Chemical Engineers: Tawfik A. Saleh, Gaddafi I. DanmalikiDocumento9 páginasJournal of The Taiwan Institute of Chemical Engineers: Tawfik A. Saleh, Gaddafi I. DanmalikiPrativa BeheraAinda não há avaliações
- Cunliffe - Composition of Oils Derived From The Batch Pyrolysis of Tyres - 1998Documento22 páginasCunliffe - Composition of Oils Derived From The Batch Pyrolysis of Tyres - 1998brayancortes077Ainda não há avaliações
- Effect of Activated Carbons Modification On Porosity, Surface Structure and Phenol AdsorptionDocumento8 páginasEffect of Activated Carbons Modification On Porosity, Surface Structure and Phenol AdsorptionCFAinda não há avaliações
- Evaluation of Response of Brown Coal To Selective Oild Agglomeration by Zeta Potential MeasurementDocumento6 páginasEvaluation of Response of Brown Coal To Selective Oild Agglomeration by Zeta Potential MeasurementdelycocukAinda não há avaliações
- Synthesis of Acrolein From Glycerol Using FePO4 CaDocumento9 páginasSynthesis of Acrolein From Glycerol Using FePO4 Calu canal do amorAinda não há avaliações
- Catalytic PyrolysisDocumento7 páginasCatalytic Pyrolysiseksilivut100% (1)
- Combustion Analysis of Coal-Water Slurry Fuel Prepared From Plant Coal and Recovered Coal FinesDocumento7 páginasCombustion Analysis of Coal-Water Slurry Fuel Prepared From Plant Coal and Recovered Coal Finesarsalan322Ainda não há avaliações
- 004 10199ny0509 18 25Documento8 páginas004 10199ny0509 18 25Erin WalkerAinda não há avaliações
- Applied Energy: Young Nam Chun, Seong Cheon Kim, Kunio YoshikawaDocumento8 páginasApplied Energy: Young Nam Chun, Seong Cheon Kim, Kunio YoshikawaCarlos AlvarezAinda não há avaliações
- Fractional DistillationDocumento0 páginaFractional DistillationTushar BhingradiyaAinda não há avaliações
- Hydrogen-Rich Gas Production With A Ni-Catalyst in A Dual Fluidized Bed Biomass GasifierDocumento15 páginasHydrogen-Rich Gas Production With A Ni-Catalyst in A Dual Fluidized Bed Biomass GasifieraberahAinda não há avaliações
- Fuel Processing Technology: Jun Wang, Tian-Lu Liu, Qun-Xing Huang, Zeng-Yi Ma, Yong Chi, Jian-Hua YanDocumento7 páginasFuel Processing Technology: Jun Wang, Tian-Lu Liu, Qun-Xing Huang, Zeng-Yi Ma, Yong Chi, Jian-Hua YanIulia StanciuAinda não há avaliações
- 1 Li Bat Rice Husk 2001 Print OkDocumento5 páginas1 Li Bat Rice Husk 2001 Print OkMuslimahdzakiyahAinda não há avaliações
- giảm dầu bằng vlxt.- Hong-2011-Reduction of tar using cheap catalysDocumento4 páginasgiảm dầu bằng vlxt.- Hong-2011-Reduction of tar using cheap catalysThành LợiAinda não há avaliações
- 4373 - DR Published Research AbsractsDocumento10 páginas4373 - DR Published Research AbsractsSalah OsmanAinda não há avaliações
- 10 ReviewComparisonofagriculturalby-productsactivatedcarbon PDFDocumento11 páginas10 ReviewComparisonofagriculturalby-productsactivatedcarbon PDFRohan ChauguleAinda não há avaliações
- Dilek Angin: HighlightsDocumento8 páginasDilek Angin: HighlightsMathilda Jowito PasaribuAinda não há avaliações
- 1 s2.0 S0165237003001852 Main PDFDocumento18 páginas1 s2.0 S0165237003001852 Main PDFU.G.Ainda não há avaliações
- ASB PyrolisisDocumento4 páginasASB PyrolisisJaffimJaffimAinda não há avaliações
- Bash Kova 2009Documento7 páginasBash Kova 2009CFAinda não há avaliações
- Wei Li, Shan Tan, Yun Shi, Sujing Li: SciencedirectDocumento8 páginasWei Li, Shan Tan, Yun Shi, Sujing Li: SciencedirectAnindita IndrianaAinda não há avaliações
- Wang 2005Documento10 páginasWang 2005hellna284Ainda não há avaliações
- Adsorption of Anionic and Cationic Dyes On Activated Carbons With Different Surface ChemistriesDocumento10 páginasAdsorption of Anionic and Cationic Dyes On Activated Carbons With Different Surface ChemistriessureshbabuchallariAinda não há avaliações
- 16-10 Hrich - IDDocumento7 páginas16-10 Hrich - IDSylab InstrumentsAinda não há avaliações
- ACTIVATEDCARBONDocumento28 páginasACTIVATEDCARBONAnonymous xn6NbytuBgAinda não há avaliações
- Optimization of Conditions For The Preparation of Activated Carbon From Mango Nuts Using HCLDocumento12 páginasOptimization of Conditions For The Preparation of Activated Carbon From Mango Nuts Using HCLAJER JOURNALAinda não há avaliações
- Hydrogen From Waste TyresDocumento3 páginasHydrogen From Waste Tyresbrayancortes077Ainda não há avaliações
- Optimization of Conditions For The Preparation of Activated Carbon From Mango Nuts Using ZNCLDocumento7 páginasOptimization of Conditions For The Preparation of Activated Carbon From Mango Nuts Using ZNCLIJERDAinda não há avaliações
- ST 15267Documento10 páginasST 15267Anonymous slaSbtN0Ainda não há avaliações
- Research Article: Improvement of Waste Tire Pyrolysis Oil and Performance Test With Diesel in CI EngineDocumento9 páginasResearch Article: Improvement of Waste Tire Pyrolysis Oil and Performance Test With Diesel in CI Enginemontie3Ainda não há avaliações
- Autothermal Reforming of Simulated Gasoline and Diesel FuelsDocumento8 páginasAutothermal Reforming of Simulated Gasoline and Diesel FuelsMOHAMMAD HASHIM KHANAinda não há avaliações
- 75 Environment (Pollution, Health Protection, Safety) : Fuel and Energy Abstracts March 1996Documento1 página75 Environment (Pollution, Health Protection, Safety) : Fuel and Energy Abstracts March 1996سید حسین عارفیAinda não há avaliações
- Adsorption of Hexavalent CR From Aq Soln Using Chemically Activated C Prepared From Waste of BambooDocumento23 páginasAdsorption of Hexavalent CR From Aq Soln Using Chemically Activated C Prepared From Waste of BambooChern YuanAinda não há avaliações
- Characterization of Mesoporous Activated Carbons Prepared by Pyrolysisnext Term of Sewage Sludge With PyrolusiteDocumento5 páginasCharacterization of Mesoporous Activated Carbons Prepared by Pyrolysisnext Term of Sewage Sludge With PyrolusiteyemresimsekAinda não há avaliações
- 4 Catalytic Performances ofDocumento5 páginas4 Catalytic Performances ofAchyut Kumar PandaAinda não há avaliações
- Supramono 2019Documento9 páginasSupramono 2019Arifah Sukasri Jurusan Teknik KimiaAinda não há avaliações
- Azargohar 2005Documento7 páginasAzargohar 2005gueabdelkaderAinda não há avaliações
- Advanced Treatment ofDocumento9 páginasAdvanced Treatment ofangginoviariAinda não há avaliações
- Synthesis of Waste Cooking Oil-Based Biodiesel Via Effectual RecyclableDocumento8 páginasSynthesis of Waste Cooking Oil-Based Biodiesel Via Effectual RecyclableDiko FernandoAinda não há avaliações
- Activated Carbon From Cherry StonesDocumento6 páginasActivated Carbon From Cherry StonesQussay AhmedAinda não há avaliações
- Environmental TechnologyDocumento12 páginasEnvironmental TechnologySara NaseriAinda não há avaliações
- Adsorption of Crystal Violet Dye From Aqueous Solution Onto Zeolites From Coal Fly and Bottom AshesDocumento13 páginasAdsorption of Crystal Violet Dye From Aqueous Solution Onto Zeolites From Coal Fly and Bottom AshessamsudinhafidAinda não há avaliações
- Production of Active Carbons From Waste Tyres - A Review: Edward L.K. Mui, Danny C.K. Ko, Gordon MckayDocumento17 páginasProduction of Active Carbons From Waste Tyres - A Review: Edward L.K. Mui, Danny C.K. Ko, Gordon MckayJeas Grejoy AndrewsAinda não há avaliações
- Tetracycline Adsorption Onto Activated Carbons Produced by KOH Activation of Tyre Pyrolysis CharDocumento9 páginasTetracycline Adsorption Onto Activated Carbons Produced by KOH Activation of Tyre Pyrolysis CharRoshanAinda não há avaliações
- Pulp and Paper - de InkingDocumento1 páginaPulp and Paper - de InkingYonas MuluAinda não há avaliações
- Preparation and Characterization of Activated CarbonDocumento5 páginasPreparation and Characterization of Activated Carbonmd_kureshiAinda não há avaliações
- Valorisation of Waste Tyre by Pyrolysis in A Moving Bed ReactorDocumento5 páginasValorisation of Waste Tyre by Pyrolysis in A Moving Bed Reactorkartik521Ainda não há avaliações
- 214 384 1 SMDocumento10 páginas214 384 1 SMHartono PrayitnoAinda não há avaliações
- TG Industrial WeldingDocumento1 páginaTG Industrial WeldingHartono PrayitnoAinda não há avaliações
- Industrial Electronics - Eng Walid Matlab Tutorial PDFDocumento3 páginasIndustrial Electronics - Eng Walid Matlab Tutorial PDFHartono PrayitnoAinda não há avaliações
- In Situ: BurningDocumento23 páginasIn Situ: BurningHartono PrayitnoAinda não há avaliações
- Absorptive and Swelling Properties of Clay-Water SystemDocumento8 páginasAbsorptive and Swelling Properties of Clay-Water SystemHartono PrayitnoAinda não há avaliações
- 1 Erlin BWMRHDocumento10 páginas1 Erlin BWMRHGibran AfifAinda não há avaliações
- Investigations of The Scaling Criteria For A Mild Combustion BurnerDocumento9 páginasInvestigations of The Scaling Criteria For A Mild Combustion BurnerHartono PrayitnoAinda não há avaliações
- Hydrogen Production From Biomass Wastes by Hydrothermal GasificationDocumento15 páginasHydrogen Production From Biomass Wastes by Hydrothermal GasificationHartono PrayitnoAinda não há avaliações
- Cooking Options in Refugee SituationsDocumento49 páginasCooking Options in Refugee SituationshailemebrahtuAinda não há avaliações
- The Future For Biomass Pyrolysis and Gasification: Status, Opportunities and Policies For EuropeDocumento30 páginasThe Future For Biomass Pyrolysis and Gasification: Status, Opportunities and Policies For EuropeHartono PrayitnoAinda não há avaliações
- 4-1 DR - Yan RongDocumento27 páginas4-1 DR - Yan RongHartono PrayitnoAinda não há avaliações
- 1.2.1 - Different Types of Gasifiers and Their Integration With Gas TurbinesDocumento11 páginas1.2.1 - Different Types of Gasifiers and Their Integration With Gas TurbinesRene GonzalezAinda não há avaliações
- Suitability of Tyre Pyrolysis Oil (Tpo) As An Alternative Fuel For Internal Combustion EngineDocumento5 páginasSuitability of Tyre Pyrolysis Oil (Tpo) As An Alternative Fuel For Internal Combustion EngineHartono PrayitnoAinda não há avaliações
- Biofuel From Efb Pyrolysis - OkDocumento7 páginasBiofuel From Efb Pyrolysis - OkNursyamin ZafirahAinda não há avaliações
- 505 BorsodiDocumento6 páginas505 BorsodiHartono PrayitnoAinda não há avaliações
- 23 F Borsodi Full Paper IpcDocumento9 páginas23 F Borsodi Full Paper IpcHartono PrayitnoAinda não há avaliações
- 2 Nrel Stefan CzernikDocumento17 páginas2 Nrel Stefan CzernikHartono PrayitnoAinda não há avaliações
- 5B Jones ReviewKorean 2010Documento44 páginas5B Jones ReviewKorean 2010Hartono PrayitnoAinda não há avaliações
- Pyrolysis of Waste Plastic To Liquid HydroocaqrbonsDocumento5 páginasPyrolysis of Waste Plastic To Liquid HydroocaqrbonsGustavosalazarmAinda não há avaliações
- Investigations of The Scaling Criteria For A Mild Combustion BurnerDocumento9 páginasInvestigations of The Scaling Criteria For A Mild Combustion BurnerHartono PrayitnoAinda não há avaliações
- Cooking Options in Refugee SituationsDocumento49 páginasCooking Options in Refugee SituationshailemebrahtuAinda não há avaliações
- 03 Chapter 2Documento13 páginas03 Chapter 2Hartono PrayitnoAinda não há avaliações
- 04 Edwards 1Documento17 páginas04 Edwards 1Hartono PrayitnoAinda não há avaliações
- Absorptive and Swelling Properties of Clay-Water SystemDocumento8 páginasAbsorptive and Swelling Properties of Clay-Water SystemHartono PrayitnoAinda não há avaliações
- 15 Benefits of CyclingDocumento8 páginas15 Benefits of CyclingJoycs PintoAinda não há avaliações
- Conquest CXAX Air-to-Water Heat PumpDocumento6 páginasConquest CXAX Air-to-Water Heat PumpAlexandre LopesAinda não há avaliações
- Azure Machine Learning StudioDocumento17 páginasAzure Machine Learning StudioNurain IsmailAinda não há avaliações
- 331-10 331 Operators Manual enDocumento12 páginas331-10 331 Operators Manual enYahir VidalAinda não há avaliações
- Contoh CV / Daftar Riwayat HidupDocumento2 páginasContoh CV / Daftar Riwayat HiduprusmansyahAinda não há avaliações
- Isulat Lamang Ang Titik NG Tamang Sagot Sa Inyong Papel. (Ilagay Ang Pangalan, Section atDocumento1 páginaIsulat Lamang Ang Titik NG Tamang Sagot Sa Inyong Papel. (Ilagay Ang Pangalan, Section atMysterious StudentAinda não há avaliações
- Cold Regions Science and TechnologyDocumento8 páginasCold Regions Science and TechnologyAbraham SilesAinda não há avaliações
- AS and A Level: ChemistryDocumento11 páginasAS and A Level: ChemistryStingy BieAinda não há avaliações
- Asme b16-25Documento22 páginasAsme b16-25JamesAinda não há avaliações
- Diagnosis of TrypanosomiasisDocumento82 páginasDiagnosis of TrypanosomiasisDrVijayata Choudhary100% (1)
- ContempoDocumento4 páginasContempoPrincess Jonette YumulAinda não há avaliações
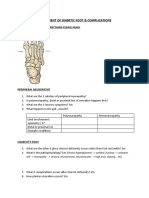
- Assessment of Diabetic FootDocumento7 páginasAssessment of Diabetic FootChathiya Banu KrishenanAinda não há avaliações
- Para Lec CombinedDocumento83 páginasPara Lec CombinedClent Earl Jason O. BascoAinda não há avaliações
- Manuscript FsDocumento76 páginasManuscript FsRalph HumpaAinda não há avaliações
- Clocks (New) PDFDocumento5 páginasClocks (New) PDFAbhay DabhadeAinda não há avaliações
- Chillers VoltasDocumento4 páginasChillers Voltasanil shuklaAinda não há avaliações
- FactSet London OfficeDocumento1 páginaFactSet London OfficeDaniyar KaliyevAinda não há avaliações
- Hevi-Bar II and Safe-Lec 2Documento68 páginasHevi-Bar II and Safe-Lec 2elkabongscribdAinda não há avaliações
- G10Mapeh Exam First QuaterDocumento8 páginasG10Mapeh Exam First QuaterJonas LamcisAinda não há avaliações
- Etoricoxib - Martindale 39thDocumento2 páginasEtoricoxib - Martindale 39thCachimbo PrintAinda não há avaliações
- YoungMan EN131 GUIDEDocumento16 páginasYoungMan EN131 GUIDErcpawar100% (1)
- Comparative Study On Serial and Parallel Manipulators - ReviewDocumento23 páginasComparative Study On Serial and Parallel Manipulators - ReviewShaik Himam SahebAinda não há avaliações
- Discrete Wavelet TransformDocumento10 páginasDiscrete Wavelet TransformVigneshInfotechAinda não há avaliações