Escolar Documentos
Profissional Documentos
Cultura Documentos
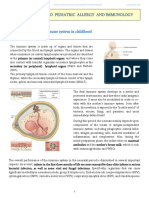
SI Nos Neonatos
Enviado por
Gabrielli AvelarDescrição original:
Direitos autorais
Formatos disponíveis
Compartilhar este documento
Compartilhar ou incorporar documento
Você considera este documento útil?
Este conteúdo é inapropriado?
Denunciar este documentoDireitos autorais:
Formatos disponíveis
SI Nos Neonatos
Enviado por
Gabrielli AvelarDireitos autorais:
Formatos disponíveis
J.
Arab Neonatal Forum 2005; 2: 5-11
Development of the Immune System in Neonates
Gaetano Chirico Division of Neonatology and Neonatal Intensive Care, Spedali Civili, Brescia, Italy Introduction Neonatal age is characterized by a delicate process of adaptation from intra- to extra-uterine life. The immune system is particularly subject to problems of adaptation; indeed, a mature immune competence could cause unfavorable effects due to maternal-fetal antigenic incompatibility, and is unnecessary to the fetus, which develops in a highly protective germ-free environment. The newborn infant, on the other hand, must be capable of defending himself against hostile micro-organisms in the surroundings. As a result of these contradictory requirements the immune system is incompletely developed at birth. The antigenic inexperience and the prevalence of suppression factors during fetal life are responsible for the physiological immaturity of the immune function in newborn infants. The high incidence of infectious disease (e.g. 1% of septicemia) in the perinatal period is a direct consequence of the precarious host-parasite balance. In preterm neonates the immunodeficiency is more severe and prolonged, and is associated with a higher incidence of infection and sepsis1, 2, and increased risk of morbidity, mortality and neurological sequelae3. In addition, due to immaturity of hematological system, anemia, thrombocytopenia or neutropenia are frequently observed during sepsis, particularly in very low birth weight infants. Detrimental consequences of immunodeficiency are mostly mitigated by some naturally occurring compensatory mechanisms. The transplacental passage of high avidity IgG antibodies4 (particularly of the IgG1 subclass5) from mother to fetus during intrauterine life is probably the most important one. After birth human milk maintains the mother-newborn immunological link by providing a host of protective components. Indeed, most of the cells and soluble factors that are deficient in the neonate are present in human milk (Table 1)6, 7.
Table 1. Immunological and Antiinfective Components in Human Milk _____________________________________________________________________________________________ Soluble Specific immunity factors: Immunoglobulins sIgA (11S), 7S IgA, IgG, IgM, IgE, IgD, Secretory component, Antiidiotypes, Histocompatibility antigens Cytokines, Chemokines and receptors: IL-1 IL-2, IL-4, IL-5, IL-6, IL-8, IL-10, IL-12, IL-13, IL-16, IL-18, IFN-, TNF-, G-CSF, M-CSF, GM-CSF, GRO- MCP-1, RANTES, TGF- 1 e 2, sCD14, Toll Like receptor, sFas, sFasL. Innate immunity factors: Complement, Chemotactic factors, Lactoferrin, Lysozyme, Properdin factors, Mannan binding lectin, Interferon, Alphafetoprotein, Antiadherence substances (oligosaccharides, mucins, lactadherin, glycans, k-casein), Antiviral factors, MiIk fat globule, Migration inhibition factor, sCD14, -defensin-1, Fatty acids, monoglycerids, pre- and postdigestion antimicrobial peptides Prebiotics, Bifidogenic factors, Oligosaccharides Hormones and growth factors: prolactin, cortisol, insulin, thyroxine, prostaglandins, erythropoietin, EGF, VEGF, NGF,TGF) Others: Carrier proteins, Enzymes, Nucleotides, LCPUFA, HAMLET (human -lactalbumin made lethal to tumor cells) Breast Milk Cells Total counts: Colostrum: 1-3 x 106/ml; mature milk: ~ 1 x 105/ml Cell types : o Macrophages: ~ 60% (Lisozime, Lactoferrine, IgA, Complement, Cytokines, Factor B, Phagocytosis, Bactericidal activity, APC) o Neutrophils: ~ 25% (Phagocytosis, Bactericidal activity) o Lymphocytes: ~ 10% (80% Activated T lymphocytes, Cytokines, IgA) _______________________________________________________________________________________________ Host defense mechanisms against pathogens are usually classified into two main components, the innate (natural, non specific) and the adaptive
____________________________________________
Correspondence to: Gaetano Chirico, Divisione di Neonatologia e Terapia Intensiva Neonatale, Spedali Civili, 25123 Brescia, Italy, Tel. No. +39 030 3995219; Fax No. +39 030 3700817 Email: gaechir@tin.it
(acquired, specific) immune systems. Skin and mucosal barriers, neutrophils, monocytes, macrophages, dendritic cells, antigen presenting cells derived cytokines, mast cells, T and B-1 lymphocytes, type I interferon, natural killer cells, complement, fibronectin, defensins, lysozyme, myeloperoxidase, natural antibodies and other natural antimicrobial agents provides protection 5
Development of the Immune System in Neonates DR and lineage committed antigens on CD34+ cells10. Human umbilical cord blood is considered a rich source of early hematopoietic progenitor cells. The percentage and the absolute number of CD34+ cells are significantly higher in very preterm foetuses less than 25 weeks of gestation compared to more mature preterm and term newborns, with a significant inverse correlation between number of CD34+ cells and advancing gestational age. The highest proliferative activity is observed at the lowest gestational ages, especially for the most undifferentiated hematopoietic stem cells, and progressively decreases with advancing gestational age. The extremely preterm foetus has a significantly more immature pool of circulating cord blood hematopoietic progenitor cells as compared to term infants, furthermore the process of development in foetal blood appear to be very active during the last two trimesters of pregnancy11. Lymphocytes and cytokines Neonatal T lymphocytes immunoproliferation to Concanavalin A, in both preterm and term infants, is significantly lower as compared to children and adults12. Most studies have reported significant phenotypic differences between neonatal and adult T cells, suggesting a reduction of cell mediated immune response, although more recent reports have documented that neonatal T cell, though nave, can raise competent immune responses under appropriate stimuli13. The percentage of CD4+ T lymphocytes is higher in newborns, particularly in preterm infants, than in children and adults. On the contrary, CD8+ cells are higher at older ages, with a resulting gradual decline with age of the CD4+/CD8+ ratio, suggesting a gradual cytotoxic response maturation with advancing age (Figure 1).
without the need for prior sensitization to the foreign antigen and represents a rapid first line barrier against pathogens. Most cells of the innate immunity contribute to the antigen processing and presentation8. T and B lymphocytes, antibodies, natural killer cells, colony stimulating factors, T cell derived effector and immunoregulatory cytokines, and Interferon-, are the main components of the adaptive immunity. A previous exposure and sensitization is necessary for the acquired specific response against antigens. The two components are strictly interconnected; several factors of both innate and adaptive immune system are usually involved and may simultaneously participate in the response against infection. Hematopoietic progenitor cells The ontogeny of immune system starts early in the embryo, continues during fetal life and is completed only several years after birth. Hematopoietic cells appear in the embryo at the 3rd-4th week of gestation, migrate from the yolk sac to the liver and spleen during the 5th-12th week and ultimately reach the bone marrow through the fetal circulation at the 2nd-3rd trimester of gestation. T lymphocyte differentiation initiates in the thymus after the 7th week of gestation, but T-cells only colonize the fetal liver, spleen and bone marrow after the 13th week. Helper and suppressor activities of fetal thymocytes and splenocytes are acquired between the 12th and the 16th week. HLA antigens are first detected in the lymphocytes at the beginning of the second trimester9. Hematopoietic progenitor cells usually express the CD34 surface antigen and the so-called stem cells may be identified by the absence of CD38, HLA-
Figure 1. Percentage of CD4+ and CD8+ T lymphocytes and CD4+/CD8+ ratio in very preterm newborns of 20-29 weeks gestational age, preterm newborns of 30-37 weeks gestational age, term newborns, children and adults10.
J. Arab Neonatal Forum 2005; 2: 5-11 Percentages of CD4+/CD45RA+ are significantly higher and of CD4+/CD45RO+ T cells lower in newborns than in children and adult. The fetal-neonatal predominant nave phenotype is likely to be related to the absence of antigenic stimulation in the germ free intrauterine environment.12 Under standard activation conditions, naive neonatal T cells are functionally (although not intrinsically) deficient, both in vitro and in vivo14. Th1 derived IFN- and TNF- are lower in the neonate15. A reduction of CD40-ligand expression by neonatal T cells may contribute to the delayed differentiation of nave T cell into Th1 effector cells16. The percentage of IL-2 producing cells is higher, both for CD4+ and CD8+ T cells, in newborns, while the percentage of IL-4 producing cells is higher for CD8+ and lower for CD4+ T cells in cord blood than at older ages. On the other hand, IFN- synthesis is significantly impaired in both CD4+ (about ten folds) and CD8+ cells (about five folds).12 The decreased percentage production of IL-4 and IFN- in cord blood as compared to that in children and adults may be justified by a switch from CD45RA+ to CD45RO+ phenotype, since in adults both IL-4 and IFN- are mostly produced by CD45RO+ cells17,18. A reduced production of IL-12 by mononuclear and dendritic cells may contribute to the neonatal IFN- deficiency.19, 20, 21 The percentage of IL-10 secreting cells is reduced in the CD4+ population and increased in CD8+ population during fetal life.12 This finding may partly explain the apparent discrepancy in studies reporting low or high IL-10 production in neonates.22, 23 The high IL-10 levels produced by neonatal T cells may play an important role in the reduced incidence of GVHD that is associated with cord blood transplantation24. On the whole, cord blood T lymphocytes are less able to perform Th1 and Th2-like responses25. Due to the more important IFN- deficiency, it is suggested that the Th1-like response is more compromised in neonates26, 27 and that a progressive maturation towards the Th1-like response occurs with age28. It should be noted, however, that neonatal T cells are capable of raising type 1 and 2 immune responses upon appropriate stimulus.29, 30 Several differences of B cell surface phenotype, as increased CD10 and CD38 and reduced CD21, CD32, adhesion molecules and class II MHC expression have been observed in neonates.1 The immaturity of T and B lymphocytes and of antigen presenting cells (in particular reduced level of expression of HLA-DR, CD1a, CD40, CD80, ICAM-1 and IL-12) are responsible for the marked deficiency of antibody production in the neonate31. Indeed, the switch from IgM to other immunoglobulin isotypes is delayed, only low levels of IgM are produced during the first month of life in response to antigenic challenge, and no response to lipopolysaccharide
antigen may be observed. In addition, levels of IgG are low in preterm infants because transplacental passage from the mother mostly occurs during the last trimester of gestation. Therefore, preterm neonates may lack the protection ensured by maternal derived pathogenspecific IgG32. Neonatal immunization does not generally lead to rapid antibody responses; however, it may result in efficient immunologic priming which can act as a basis for future responses. It is therefore possible to induce early protection, for instance against hepatitis B infection33 or pertussis disease34, by immunization at birth, even in preterm infants35. Monocytes, macrophages and dendritic cells. Main activities of these components of immune system are phagocytosis and subsequent antigen processing and presentation to lymphocytes, or pathogen killing. This last activity depends on the secretions of cytokines, mainly IFN-, IL-12 and IL-18. The reduced synthesis of these cytokines in newborn infants may results in increased susceptibility to infection by intracellular bacteria.8 Polymorphonuclear Granulocytes Since neonates have an already compromised neutrophil storage pool, during neonatal sepsis the marrow stores of neutrophils are rapidly exhausted. In peripheral blood high neutrophil counts can be found initially during infection but later neutropenia develops frequently. A severe leucopenia may often be observed during early sepsis, particularly by Group B Streptococcus. Together with the reduced leukocyte numbers during severe infections or stress, various abnormalities have been demonstrated in the function of neonatal polymorphonuclear granulocytes, like decreased deformability, adhesion, chemotaxis, phagocytosis, receptor expression, depressed oxidative metabolism and bacterial killing.1, 36 The quantitative (compromised neutrophil storage pool and rapid exhaustion of the marrow stores of neutrophils during sepsis) and qualitative deficiency of the phagocyte system is considered one of the most important contributory cause of the neonatal increased susceptibility to infection. The combined neonatal deficiency of immunoglobulin, complement and neutrophil activity results in increased susceptibility to systemic infections from encapsulated pathogens, such as Group B Streptococcus, Staphylococci and Klebsiella sp, which require opsonization for efficient phagocytosis and killing37. Hematopoietic growth factors Granulocyte Colony Stimulating Factor (G-CSF) and Granulocyte-Macrophage Colony Stimulating Factor (GM-CSF) are important cytokines for proliferation, differentiation, survival and functional activation of phagocytes. G-CSF and GM-CSF have been shown to stimulate myeloid progenitor proliferation, to increase bone marrow neutrophil storage pool, to induce 7
Development of the Immune System in Neonates neutrophilia in the peripheral blood, to prime mature cell function including chemotaxis, oxidative metabolism, phagocytosis and antibody dependent cellular cytotoxicity, to modulate C3bi, FcRIII and CR3 receptor expression and to enhance the bactericidal activity of polymorphonuclear neutrophils.38, 39, 40 A reduced GM-CSF and G-CSF gene expression, due to mRNA post-transcriptional instability, and protein production from mononuclear cells41 and GM-CSF production by T cells42 was observed in neonates. We found a significant reduction of G-CSF serum concentration in preterm infants, the lower the gestational age or birth weight, the greater the deficiency, and a reduced response to infection43. The neutropenia observed during sepsis may be partly due to inability of infants to produce G-CSF.44, 45 The use of G-CSF and GM-CSF is considered a first line treatment for the severe congenital neutropenia (Kostmanns syndrome)46. Other possible indications for the use of G-CSF in newborns are neutropenia in infants of mothers with preeclampsia47, 48 and neonatal isoimmune (alloimmune) neutropenia49. The deficiency of G-CSF and GM-CSF production, particularly in preterm infants, and a resistance, or delayed response, of myeloid precursors to the higher concentration of endogenous G-CSF in infected term newborn infants have justified therapeutic trials with pharmacological doses of G-CSF and GM-CSF. These cytokines have been evaluated in neutropenic infected term infants, to accelerate the onset of neutrophilia, or in preterm infected infants, even in the absence of severe neutropenia, to improve neutrophil function, or in neutropenic term and preterm infants for prophylaxis of infection50. Preliminary clinical trials on the use of G-CSF and GM-CSF for prevention or treatment of sepsis have given promising results, suggesting that 510 g/kg/day of G-CSF or GM-CSF for 3-10 days may improve neutrophil counts or prevent infection in neutropenic infants. Hematopoietic growth factors were well tolerated, and no significant short or long term side effects were observed51. These reports were evaluated in two meta-analysis52, 53, the most recent including seven studies with 257 infected neonates, and three infection prophylaxis trials involving 359 high risk newborns. The conclusions drawn from these meta-analyses were that no significant effect was observed of CSFs treatment of sepsis in non neutropenic infants, while a significant improvement of mortality at 14 days was found when the infants were neutropenic, as compared to untreated controls [RR 0.34 (95% CI 0.12, 0.92); RD -0.18 (95% CI -0.33, -0.03); NNT 6 (95% CI 3-33)], and that GMCSF use did not appear significantly effective in infection prophylaxis [RR 0.59 (95% CI 0.24,1.44); RD -0.03 (95% CI -0.08,0.02)]. Although further studies on larger population of infants are necessary to confirm the efficacy and to rule out the possibility of significant side effects, such as nonlymphoblastic leukemia observed in infants with Kostmann syndrome treated for long periods, G-CSF and GM-CSF may represent a valuable therapeutic tool for treating neonatal neutropenia and improve the depressed neutrophil function of the neonate. Evaluation of association of several cytokines or combination with other factors, as intravenous immunoglobulin, may be the next step in neonatal research. Hematopoietic growth factors (in particular G-CSF and erythropoietin solutions for enteral administration) are currently being evaluated to improve intestinal maturation during total parenteral nutrition or after intestinal surgery for necrotizing enterocolitis or malformation54. Complement Synthesis of classic and alternative complement pathway factors is reduced in neonates, the lower the gestational age, the greater the deficiency. In addition, kinetics of both classical and alternative pathway activity of complement are delayed, particularly in preterm newborns. C3b and C5a complement factors deficiency is responsible for the severe impairment of neonatal serum opsonic and chemotactic activities55.
Natural Killer (NK) activity with effector:target ratios 100:1, 30:1in and 10:1 in very preterm Figure Figure 22 .. Natural Killer (NK) cell cell activity with effector: target ratios of 100:1,of 30:1 and 10:1 very preterm newborns of newborns of 20-29 weeks gestational age, preterm newborns of 30-37 weeks gestational age, term newborns, 20-29 weeks gestational age, preterm newborns of 30-37 weeks gestational age, term newborns, children and adults10 children and adults.12
%
60 50 40 30 20 10 0 NK 100:1 NK 30:1
Very preterm Preterm Term Children Adults
NK 10:1
J. Arab Neonatal Forum 2005; 2: 5-11 Natural killer cell Natural killer (NK) cell activity, that is implicated in GVHD pathogenesis and represents an important innate immune defense mechanism against tumors and viral infections, is significantly reduced in preterm and term infants56 as compared to children and adults, although in adults a tendency to a reduction may be observed (figure 2). In addition, NK activity is significantly correlated with gestational age.12 A reduced CD56high and increased CD56low expression is characteristic of neonatal immature NK cells.16 The reduction of NK activity could be related to the IL-12 and IL-15 deficiency57. The NK insufficiency can contribute to increased incidence, severity and tendency to chronicity of perinatal viral infections. In conclusion, the reduced cytotoxic response during fetal life, the poor T lymphocytes response to mitogens, the immaturity of T and B lymphocytes, the inadequate cytokine synthesis, the marked deficiency of antibody production and the reduced neutrophil, complement and natural killer cell activity are important contributory factors to the complex deficiency of immunological function in the neonate and may represent the biological basis for the increased susceptibility to various infections and the reduced clearance of intracellular pathogens. At the same time, these characteristics of the neonatal immunity may contribute to the reduced incidence and severity of GVHD in patients transplanted with umbilical cord blood (CB) compared to bone marrow58, 59. Reference 1. Lewis DB, Tu W. The physiologic immunodeficiency of immaturity. In: Stiehm ER, Ochs HD, Winkelstein JA (eds). Immunologic disorders in infants and children. Philadelphia: Elsevier Saunders Company, fifth Edition, 2004:687-760. Lewis DB, Wilson CB. Developmental immunology and role of host defenses in fetal and neonatal susceptibility to infection. In: Remington JS, Klein JO (eds). Infectious Diseases of the Fetus and Newborn Infant. Philadelphia: WB Saunders Company, Fifth Edition, 2001:25-138. Fanaroff AA, Korones SB, Wright LL, Verter J, Poland RL, Bauer CR, et al. Incidence, presenting features, risk factors and significance of late onset septicemia in very low birth weight infants. The National Institute of Child Health and Human Development Neonatal Research Network. Pediatr Infect Dis J 1998;17:593-598. Avanzini MA, Pignatti P, Chirico G, Gasparoni A, Jalil F, Hanson LA. Placental transfer favours high avidity IgG antibodies. Acta Paediatr 1998;87:180-185. Gasparoni A, Avanzini A, Ravagni Probizer F, Chirico G, Rondini G, Severi F. IgG subclasses compared in maternal and cord serum and breast milk. Arch Dis Child 1992; 67:41-43. 14. 15. 16. 6. 7. 8. Newburg DS. Innate immunity and human milk. J Nutr 2005;135:1308-1312. Field CJ. The immunological components of human milk and their effect on immune development in infants. J Nutr 2005;135:1-4. La Pine TR, Hill HR. Host defense mechanisms against bacteria. In: Polin RA, Fox WW, Abman SH (eds). Fetal and Neonatal Physiology. Philadelphia: Saunders; 3rd ed. 2004: 1475-1486. Royo C, Touraine JL, de Bouteiller O. Ontogeny of T lymphocyte differentiation in the human fetus: acquisition of phenotype and functions. Thymus 1987;10:57-73. DArena G, Musto P, Cascavilla N, Di Giorgio G, Zendoli F, Carotenuto M. Human umbilical cord blood: immunophenotipic heterogeneity of CD34+ hematopoietic progenitor cells. Haematologica 1996; 81:404-409. Gasparoni A, Ciardelli L, Avanzini MA, Bonfichi M, Di Mario M, Piazzi G, et al. Immunophenotypic changes of foetal cord blood hematopoietic progenitor cells during gestation. Pediatr Res 2000;47:825-829. Gasparoni A, Ciardelli L, Avanzini A, Castellazzi AM, Carini R, Rondini G, Chirico G. Age-related changes in intracellular TH1/TH2 cytokine production, immunoproliferative T lymphocyte response and natural killer cell activity in newborns, children and adults. Biol Neonate 2003;84:297-303. Chipeta J, Komada Y, Zhang XL, Azuma E, Yamamoto H, Sakurai M. Neonatal (cord blood) T cells can competently raise type 1 and 2 immune responses upon polyclonal activation. Cell Immunol 2000;205:110-119. Adkins B. T-cell function in newborn mice and humans. Immunol Today 1999;20:330-335. Rondini G, Chirico G. Hematopoietic growth factor levels in term and preterm infants. Curr Opin Hematol 1999;6:192-197. Lewis DB. Host defense mechanisms against viruses. In: Polin RA, Fox WW, Abman SH (eds). Fetal and Neonatal Physiology. Philadelphia: WB Saunders; 3rd ed. 2004: 1490-1511. Millet V, Lacroze V, Bodiou AC, Dubus JC, D'Ercole C, Unal D. Ontognie du systme immunitaire. Arch Pdiatr 1999;6:14-19. Chipeta J, Komada Y, Zhang XL, Deguchi T, Sugiyama K, Azuma E, Sakurai M. CD4+ and CD8+ cell cytokine profiles in neonates, older children, and adults: increasing T helper type 1 and T cytotoxic type 1 cell populations with age. Cell Immunol 1998;183:149-156. Lee SM, Suen Y, Chang L, Bruner V, Qian J, Indes J, et al. Decreased interleukin-12 (IL-12) from activated cord versus adult peripheral blood mononuclear cells and upregulation of interferongamma, natural killer, and lymphokine-activated killer activity by IL-12 in cord blood mononuclear cells. Blood 1996;88:945-954.
9.
10.
11.
12.
13.
2.
17. 18.
3.
4.
19.
5.
Development of the Immune System in Neonates 20. Marodi L. Down-regulation of Th1 responses in human neonates. Clin Exp Immunol. 2002;128:1-2 21. Langrish CL, Buddle JC, Thrasher AJ, Goldblatt D. Neonatal dendritic cells are intrinsically biased against Th-1 immune responses. Clin Exp Immunol. 2002;128:118-23. 22. Chheda S, Palkowetz KH, Garofalo R, Rassin DK, Goldman AS. Decreased interleukin-10 production by neonatal monocytes and T cells: relationship to decreased production and expression of tumor necrosis factor-alpha and its receptors. Pediatr Res 1996;40:475-483. 23. Splawski JB, Yamamoto K & Lipsky PE. Deficient interleukin-10 production by neonatal T cells does not explain their ineffectiveness at promoting neonatal B cell differentiation. Eur J Immunol 1998;28:42484256. 24. Rainsford E, Reen DJ. Interleukin 10, produced in abundance by human newborn T cells, may be the regulator of increased tolerance associated with cord blood stem cell transplantation. Br J Haematol 2002;116:702-709. 25. D'Arena G, Musto P, Cascavilla N, Minervini MM, Di Giorgio G, Maglione A, Carotenuto M. Inability of activated cord blood T lymphocytes to perform Th1-like and Th2-like responses: implications for transplantation. J Hematother Stem Cell Res 1999;8:381-385. 26. Vigano A, Esposito S, Arienti D, Zagliani A, Massironi E, Principi N, Clerici M. Differential development of type 1 and type 2 cytokines and beta-chemokines in the ontogeny of healthy newborns. Biol Neonate 1999;75:1-8. 27. Adkins B, Bu Y, Guevara P. The generation of Th memory in neonates versus adults: prolonged primary Th2 effector function and impaired development of Th1 memory effector function in murine neonates. J Immunol 2001;166:918-925. 28. Krampera M, Vinante F, Tavecchia L, Morosato L, Chilosi M, Romagnani S, et al. Progressive polarization towards a T helper/cytotoxic type-1 cytokine pattern during age-dependent maturation of the immune response inversely correlates with CD30 cell expression and serum concentration. Clin Exp Immunol 1999;117:291-297. 29. Schultz C, Reiss I, Bucsky P, Gopel W, Gembruch U, Ziesenitz S, Gortner L. Maturational changes of lymphocyte surface antigens in human blood: comparison between fetuses, neonates and adults. Biol Neonate 2000;78:77-82. 30. Perez-Cruz I, Fallen P, Madrigal JA, Cohen SB. Naive T cells from cord blood have the capacity to make Types 1 and 2 cytokines. Immunol Lett 2000;75:85-88. 31. Splawski JB, Jelinek DF, Lipsky PE. Delineation of the functional capacity of human neonatal lymphocytes. J Clin Invest 1991;87:545-553. 32. Chirico G, Rondini G, Plebani A, Chiara A, Massa M, Ugazio AG. Intravenous gammaglobulin therapy for prophylaxis of infection in high-risk neonates. J Pediatr 1987;110:437-442. 33. Chirico G, Belloni C, Gasparoni A, Cerbo RM, Rondini G, Klersy C, et al. Hepatitis B immunization in infants of hepatitis B surface antigen-negative mothers. Pediatrics 1993;92:717719 34. Belloni C, De Silvestri A, Tinelli C, Avanzini MA, Marconi M, Strano F, et al. Immunogenicity of a three-component acellular pertussis vaccine administered at birth. Pediatrics 2003;111:10421045. 35. Belloni C, Chirico G, Pistorio A, Orsolini P, Tinelli C, Rondini G. Immunogenicity of hepatitis B vaccine in term and preterm infants. Acta Paediatr 1998;87:336-338. 36. Chirico G, Marconi M, De Amici M, Gasparoni A, Mingrat G, Chiara A, et al. Deficiency of neutrophil bactericidal activity in term and preterm infants. A longitudinal study. Biol Neonate 1985;47:125-129. 37. Ugazio AG, Chirico G, Plebani A, Duse M, Notarangelo LD, Burgio GR. Immunity and infection in term and preterm newborns. In: Xanthou M, Bracci R (eds). Neonatal Haematology and Immunology. Amsterdam:Elsevier Science Publishers,1990:219226. 38. Goldman S, Ellis R, Dhar V, Cairo MS. Rationale and potential use of cytokines in the prevention and treatment of neonatal sepsis. Clinics in Perinatology 1998;25:699-709. 39. Chirico G, Ciardelli L, Marconi M, Cecchi P, Gasparoni A, Rondini G. Effect of Granulocyte Colony Stimulating Factor on neutrophil bactericidal activity in preterm infants. Biol Neonate 1993, 64: 188. 40. Drossou-Agakidou V, Kanakoudi-Tsakalidou F, Sarafidis K, Tzimouli V, Taparkou A, Kremenopoulos G, Germenis A. In vivo effect of rhGM-CSF And rhG-CSF on monocyte HLA-DR expression of septic neonates. Cytokine. 2002;18:260-5. 41. min Lee S, Knoppel E, van de Ven C, Cairo MS. Transcriptional rates of granulocyte-macrophage colony-stimulating factor, granulocyte colonystimulating factor, interleukin-3, and macrophage colony-stimulating factor genes in activated cord versus adult mononuclear cells: alteration in cytokine expression may be secondary to posttranscriptional instability. Pediatr Res 1993;34:560-564. 42. English BK, Hammond WP, Lewis DB, Brown CB, Wilson CB. Decreased granulocytemacrophage colony-stimulating factor production by human neonatal blood mononuclear cells and T cells. Pediatr Res 1992;31:211-216. 43. Chirico G, Ciardelli L, Cecchi P, De Amici M, Gasparoni A, Rondini G. Serum concentration of granulocyte colony stimulating factor (G-CSF) in term and preterm infants. Eur J Pediatr 1997;156:269-271.
10
J. Arab Neonatal Forum 2005; 2: 5-11 44. Cairo MS. Therapeutic implications of dysregulated colony-stimulating factor expression in neonates. Blood 1993;82:2269-2272. 45. Kennon C, Overturf G, Bessman S, Sierra E, Smith KJ, Brann B. Granulocyte colony stimulating factor as a marker for bacterial infection in neonates. J Pediatr 1996;128:765-769. 46. Christensen RD, Calhoun DA. Congenital neutropenia. Clin Perinatol 2004;31:29-38. 47. Kocherlakota P, La Gamma EF. Preliminary report: rhG-CSF may reduce the incidence of neonatal sepsis in prolonged preeclampsiaassociated neutropenia. Pediatrics 1998;102:11071111. 48. Makhlouf RA, Doron MW, Bose CL, Price WA, Stiles AD. Administration of granulocyte colonystimulating factor to neutropenic low birth weight infants of mothers with preeclampsia. J Pediatr 1995;126:454-456. 49. Chirico G, Ciardelli L, Gasparoni A, Vigan C, Rondini G. Clinical and experimental aspects on the use of granulocyte colony stimulating factor (G-CSF) in the neonate. In: Bellanti JA, Bracci R, Prindull G and Xanthou M (eds). Neonatal Hematology and Immunology III. Amsterdam:Elsevier Science,1997:33-38. 50. Banarjea MC, Speer CP. The current role of colony-stimulating factors in prevention and treatment of neonatal sepsis. Semin Neonatol 2002;7:335-349. 51. Rosenthal J, Healey T, Ellis R, Gillan E, Cairo MS. A two-year follow-up of neonates with presumed sepsis treated with human granulocyte colony-stimulating factor during the first week of life. J Pediatr 1996;128:135-137. 52. Bernstein HM, Pollock BH, Calhoun DA, Christensen RD. Administration of recombinant granulocyte colony-stimulating factor to neonates with septicemia: A meta-analysis. J Pediatr 2001;138:917-920. Carr R, Modi N, Dore C. G-CSF and GM-CSF for treating or preventing neonatal infections. Cochrane Database Syst Rev 2003;(3):CD003066. Calhoun DA, Christensen RD. Hematopoietic growth factors in neonatal medicine: the use of enterally administered hematopoietic growth factors in the neonatal intensive care unit. Clin Perinatol 2004;31:169-182. Notarangelo LD, Chirico G, Chiara A, Colombo A, Rondini G, Plebani A, et al. Activity of classical and alternative pathways of complement in preterm and small for gestational age infants. Pediatr Res 1984;18:281-285. Chirico G, Maccario R, Montagna D, Chiara A, Gasparoni A, Rondini G. Natural Killer cell activity in preterm infants: effect of intravenous immune globulin administration. J Pediatr 1990;117:465-466. Qian JX, Lee YS, Knoppel E, Ven van de C, Cairo MS. Decreased interleukin-15 from activated cord versus adult peripheral blood mononuclear cells and the effect of interleukin-15 in upregulating antitumor immune activity and cytokine production in cord blood. Blood 1997;90:31063117. Newburger PE, Quesenberry PJ. Umbilical cord blood as a new and promising source of unrelateddonor hematopoietic stem cells for transplantation. Curr Opin Pediatr 1996;8:29-32. Cohen SB, Madrigal JA. Immunological and functional differences between cord and peripheral blood. Bone Marrow Transplant 1998;21 Suppl 3:S9-12.
53.
54.
55.
56.
57.
58.
59.
11
Você também pode gostar
- The Maturing Immune System: Neonatal Immunity: Table 1Documento2 páginasThe Maturing Immune System: Neonatal Immunity: Table 1Vania ParamithaAinda não há avaliações
- Immune System of ChildrenDocumento6 páginasImmune System of Childrenbholasimran93Ainda não há avaliações
- Rol de La Inmunidad Innata en La Infección Neonatal. 2013 PDFDocumento8 páginasRol de La Inmunidad Innata en La Infección Neonatal. 2013 PDFVictor AyalaAinda não há avaliações
- Immunity and Allergy. AsthmaDocumento21 páginasImmunity and Allergy. AsthmaSalman SathooAinda não há avaliações
- INTESTINO FELIZ, ANIMAL SALUDABLE EsDocumento22 páginasINTESTINO FELIZ, ANIMAL SALUDABLE EsurielAinda não há avaliações
- Reproductive Immunology: Basic ConceptsNo EverandReproductive Immunology: Basic ConceptsGil MorAinda não há avaliações
- Vaccination in Early LifeDocumento8 páginasVaccination in Early LifeLeonardo PalenciaAinda não há avaliações
- Neonatal SepsisDocumento22 páginasNeonatal Sepsisjake1014100% (1)
- Immune System - Development and Acquisition of Immunological Competence - 2020Documento8 páginasImmune System - Development and Acquisition of Immunological Competence - 2020Cláudia SilvaAinda não há avaliações
- Immunology in Pregnancy & Coagulation Failure in PregnancyDocumento30 páginasImmunology in Pregnancy & Coagulation Failure in PregnancySrikutty DevuAinda não há avaliações
- Neonatal SepsisDocumento17 páginasNeonatal SepsisDhilla Feroh Kesuma TAinda não há avaliações
- Sepsis Neonatorum UpdatedDocumento87 páginasSepsis Neonatorum Updated'Eduard Laab AlcantaraAinda não há avaliações
- Tutor Guide Astro BoyDocumento10 páginasTutor Guide Astro BoyMaria ArianAinda não há avaliações
- Original Research Paper Paediatric MedicineDocumento4 páginasOriginal Research Paper Paediatric MedicineSujoy DasAinda não há avaliações
- Mol3005 H08Documento13 páginasMol3005 H08DenizDerenAinda não há avaliações
- Infecciones NeonatalesDocumento23 páginasInfecciones NeonatalesAlejandra MedinaAinda não há avaliações
- Antiinfective Properties of Human MilkDocumento6 páginasAntiinfective Properties of Human Milkkiko arrojasAinda não há avaliações
- Chapter 2 - Immunological Changes in Pregnancy and - 2021 - Covid 19 InfectionsDocumento15 páginasChapter 2 - Immunological Changes in Pregnancy and - 2021 - Covid 19 InfectionsWalter MendozaAinda não há avaliações
- Fast Facts: Managing immune-related Adverse Events in Oncology: Early recognition, prompt intervention, effective managementNo EverandFast Facts: Managing immune-related Adverse Events in Oncology: Early recognition, prompt intervention, effective managementAinda não há avaliações
- Immune Responses in NeonatesDocumento26 páginasImmune Responses in NeonatesCony GSAinda não há avaliações
- Desarrollo Temprano Del Sistema Inmune PDFDocumento21 páginasDesarrollo Temprano Del Sistema Inmune PDFVictor AyalaAinda não há avaliações
- 2 Pa Tho Physiology of HIVDocumento8 páginas2 Pa Tho Physiology of HIVLeepin TanAinda não há avaliações
- 2 Pathophysiology of HIVDocumento8 páginas2 Pathophysiology of HIVRaga ManduaruAinda não há avaliações
- Non-inflammatory immunology: An introduction to the immune system and its pathologiesNo EverandNon-inflammatory immunology: An introduction to the immune system and its pathologiesAinda não há avaliações
- EbolaDocumento2 páginasEbolaJosipa ĆevidAinda não há avaliações
- AIDS and ImmunodeficiencyDocumento12 páginasAIDS and ImmunodeficiencyRashed ShatnawiAinda não há avaliações
- Innate Immunity: Fig 1 Table IDocumento9 páginasInnate Immunity: Fig 1 Table IforeveraldyAinda não há avaliações
- Imunology PregnancyDocumento9 páginasImunology PregnancyimeldafitriAinda não há avaliações
- Bookshelf: Chapter 8specific Acquired ImmunityDocumento12 páginasBookshelf: Chapter 8specific Acquired ImmunityBlake KamminAinda não há avaliações
- Peran Imunitas Seluler Pada Ibu Hamil: Arjilio T. Z. Runtukahu, Sylvia R. Marunduh, Hedison PoliiDocumento7 páginasPeran Imunitas Seluler Pada Ibu Hamil: Arjilio T. Z. Runtukahu, Sylvia R. Marunduh, Hedison PoliiPutri MaesarrahAinda não há avaliações
- 04 Intravenous Immune Globulin Clinical Applications in The NewbornDocumento11 páginas04 Intravenous Immune Globulin Clinical Applications in The NewbornMorales Eli Pediatra100% (1)
- Anna Rozaliyani: Department of Parasitology-Faculty of Medicine, Universitas IndonesiaDocumento27 páginasAnna Rozaliyani: Department of Parasitology-Faculty of Medicine, Universitas Indonesiainka.elseAinda não há avaliações
- Fetal-Specific CD8+ Cytotoxic T Cell Responses Develop During Normal Human Pregnancy and Exhibit Broad Functional CapacityDocumento10 páginasFetal-Specific CD8+ Cytotoxic T Cell Responses Develop During Normal Human Pregnancy and Exhibit Broad Functional Capacity石一Ainda não há avaliações
- Nutrients: Diet and Immune FunctionDocumento9 páginasNutrients: Diet and Immune FunctionjenniAinda não há avaliações
- Preclinical Immunology and Microbiology Review 2023: For USMLE Step 1 and COMLEX-USA Level 1No EverandPreclinical Immunology and Microbiology Review 2023: For USMLE Step 1 and COMLEX-USA Level 1Nota: 5 de 5 estrelas5/5 (1)
- Micronutrients Influencing The Immune Response in Leprosy: RevisiónDocumento11 páginasMicronutrients Influencing The Immune Response in Leprosy: RevisiónSherlocknovAinda não há avaliações
- Allergy: Back To IndexDocumento8 páginasAllergy: Back To IndexjnsenguptaAinda não há avaliações
- The Germinal Center Is An Important Site ForDocumento11 páginasThe Germinal Center Is An Important Site ForGopika SureshAinda não há avaliações
- Gut: Key Element On Immune System Regulation: Review - Human and Animal HealthDocumento14 páginasGut: Key Element On Immune System Regulation: Review - Human and Animal HealthyutefupAinda não há avaliações
- J Immunol 2010 Mueller 2182 90Documento10 páginasJ Immunol 2010 Mueller 2182 90winsarkarAinda não há avaliações
- Peran Imunitas Seluler Pada Ibu HamilDocumento8 páginasPeran Imunitas Seluler Pada Ibu Hamilgia amandaAinda não há avaliações
- Publishers Take NoteaxicvDocumento1 páginaPublishers Take Noteaxicvwoolonion5Ainda não há avaliações
- Neonatal Sepsis 2018Documento40 páginasNeonatal Sepsis 2018Abraham AnaelyAinda não há avaliações
- Cytokines and Prostaglandins in Immune Homeostasis and Tissue Destruction in Periodontal DiseaseDocumento32 páginasCytokines and Prostaglandins in Immune Homeostasis and Tissue Destruction in Periodontal DiseaseDiana GomezAinda não há avaliações
- Neonatal Sepsis in The Very Low Birth Weight Preterm Infants: Part 1: Review of Patho-PhysiologyDocumento10 páginasNeonatal Sepsis in The Very Low Birth Weight Preterm Infants: Part 1: Review of Patho-PhysiologyjukunkAinda não há avaliações
- Active Immunization Long DOCPDFDocumento31 páginasActive Immunization Long DOCPDFEnder IzaguirreAinda não há avaliações
- Inmunidad y SepsisDocumento6 páginasInmunidad y SepsisAmmarie SanchezAinda não há avaliações
- Cellular Immune Responses in The Pathophysiology of 2021Documento24 páginasCellular Immune Responses in The Pathophysiology of 2021Zakkiyatus ZainiyahAinda não há avaliações
- Role of Immunoglobulins in Neonatal SepsisDocumento6 páginasRole of Immunoglobulins in Neonatal SepsisMuhar RandiAinda não há avaliações
- Christ The King College College of Nursing and IHAP Calbayog CityDocumento4 páginasChrist The King College College of Nursing and IHAP Calbayog CityMannuelle GacudAinda não há avaliações
- Diagnostics 12 00453Documento15 páginasDiagnostics 12 00453Fabiola Vania FeliciaAinda não há avaliações
- Jurnal BPHDocumento15 páginasJurnal BPHdewasayogaAinda não há avaliações
- Immune System - 10pDocumento10 páginasImmune System - 10pLinh LeAinda não há avaliações
- Fast Facts: Managing Immune-Related Adverse Events in OncologyNo EverandFast Facts: Managing Immune-Related Adverse Events in OncologyAinda não há avaliações
- A Comprehensive Guide To Managing AutismDocumento89 páginasA Comprehensive Guide To Managing Autismapi-3696876100% (4)
- Infancy To Old Age Immune SysytemDocumento9 páginasInfancy To Old Age Immune Sysytemlinnet17Ainda não há avaliações
- Type 1 Diabetes Mellitus POSTERDocumento1 páginaType 1 Diabetes Mellitus POSTERLeandro FigueiraAinda não há avaliações
- Immunology of Pregnancy1Documento50 páginasImmunology of Pregnancy1Ekiran BabajideAinda não há avaliações
- Basic ImmunologyDocumento12 páginasBasic Immunologyjony_phurailatpamAinda não há avaliações