Escolar Documentos
Profissional Documentos
Cultura Documentos
A2 Test 6 Notes - Analysis
Enviado por
Karanvir Singh BhallaTítulo original
Direitos autorais
Formatos disponíveis
Compartilhar este documento
Compartilhar ou incorporar documento
Você considera este documento útil?
Este conteúdo é inapropriado?
Denunciar este documentoDireitos autorais:
Formatos disponíveis
A2 Test 6 Notes - Analysis
Enviado por
Karanvir Singh BhallaDireitos autorais:
Formatos disponíveis
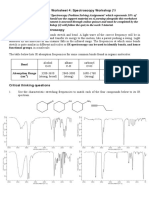
Topic 12 Spectroscopy Revision Notes 1)
Infrared spectroscopy
The bonds in organic molecules absorb certain frequencies of infrared radiation. The frequencies that are absorbed can be used to identify the presence of certain bonds. The absorptions you need to know are: a) b) c) d) C=O 1680 -OH in alcohols 3230 -OH in carboxylic acids 2500 C-O 1000 1750 3550 3300 1300 cm-1 cm-1 cm-1 (broad peak) cm-1
Example 1 propanone
Of the absorptions mentioned above, only the C=O peak will be present in propanones IR spectrum:
Example 2 ethanol
Of the absorptions mentioned above, the OH in alcohols and the C-O peaks will be present in ethanols IR spectrum:
! O-H
! C-O
Example 3 Ethanoic acid
Of the peaks mentioned above, the OH in acids, the C=O and the C-O peaks will be present in ethanols IR spectrum:
! O-H
C=O !
! C-O
2)
Mass spectrometry
In a mass spectrum for a compound, the peak furthest to the right (or highest m/z) is called the molecular ion peak and it gives the molar mass of the compound. Example ethanol
The molar mass of ethanol is 46. The molecular ion peak is indicated by the arrow.
"
3)
NMR spectroscopy a)
o o o
Introduction
NMR stands for nuclear magnetic resonance It can be used on the nuclei of atoms that contain an odd number of protons and neutrons e.g. 13C and 1H NMR involves the interaction of materials with low-energy radio waves
o o
NMR is the same technology as that use in magnetic resonance imaging (MRI) to obtain diagnostic information about internal structures in body scanners In NMR, the position of each peak is measured from the peak produced by a standard substance called tetramethylsilane, TMS, which is (CH3)4Si Solvents for running NMR spectra must not contain H atoms so deuterated solvents, such as CDCl3, are used
b)
Proton NMR
The peaks in a proton NMR spectrum give the following information about the hydrogen nuclei in an organic compound: The number of peaks shows the number of different types of proton The ratio of the areas under the peaks gives the number of protons of each type The distance along the axis (chemical shift) shows the environment of each type of proton (see data sheet) The splitting pattern shows how many neighbouring protons there are D2O can be used to identify OH groups Example 1 Ethanoic acid
Two different types of H so two peaks Size of peaks in ratio 3:1 -COCH3 at # 2.0-2.9 and COOH at # 11.0-11.7 No splitting as Hs not on adjacent atoms
Peak splitting 1) 2) 3) 4) A peak will be split if there are Hs on the next C or O. Splitting follows the n+1 rule 1H splits an adjacent peak into two (a doublet), 2Hs split an adjacent peak into three (a triplet) and 3Hs split an adjacent peak into four (a quartet) If splitting occurs from both sides a more complicated pattern is produced (a multiplet)
5)
In a doublet, the ratio of the areas under the peaks is 1:1. In a triplet, the ratio of the areas under the peaks is is 1:2:1. In a quartet, the ratio of the areas under the peaks is 1:3:3:1 Example 2 Ethanol
Three different types of H so three peaks Size of peaks in ratio 3:2:1 R-CH3 at # 0.7-1.6, -OCH2R at # 3.3-4.3 and R-OH at # 1.0-5.5 CH3 peak split into triplet by 2Hs on next C CH2 split into quartet by 3Hs on next C The OH peak is not split and does not split other peaks. Technically, this H is decoupled by fast proton exchange
Use of D2O with OH and NH groups Deuterium is an isotope of hydrogen that does not produce an NMR peak If an alcohol, carboxylic acid or amine is shaken with D2O, the H of the OH or NH group is replaced by D and the peak disappears from the spectrum
b)
Carbon-13 NMR
The peaks in a 13C NMR spectrum give the following information about the carbon nuclei in an organic compound: The number of peaks shows the number of different types of C The distance along the axis (chemical shift) shows the environment of each type of C (see data sheet)
Example 1 Ethanol
Two different types of C so two peaks C-O at # 50-70 and C-C at # 5-55
Source: http://www.chemguide.co.uk/analysis/nmr/interpretc13.html
Topic 13 Chromatography Revision Notes 1) Introduction
Chromatography is an analytical technique for separating mixtures It separates the components of the mixture between a mobile phase and a stationary phase The mobile phase can be a liquid or a gas In thin-layer chromatography, TLC, the stationary phase is a solid In gas chromatography, GC, the stationary phase is either a liquid or solid on a solid support A solid stationary phase separates by adsorption A liquid stationary phase separates by relative solubility
1)
Paper Chromatography
o o o o o o o o o Stationary phase is water adsorbed on paper Mobile phase is liquid solvent Separates by partition (relative solubility of solute between mobile and stationary phases) Rf values calculated from distance travelled by sample/distance travelled by solvent Rf values differ from solvent to solvent Components of mixture identified by comparing with known reference samples or using Rf values In two-way chromatography, the chromatogram is run in one solvent then rotated through 90 and run in a different solvent This is more effective than one-way chromatography because it is highly unlikely that 2 solvents have the same Rf values In the separation of amino acids, the spots are invisible and have to be shown up by adding ninhydrin or iodine (or by observing under u/v light)
2)
Thin-layer Chromatography
o o o o In TLC, the stationary phase is a thin layer of solid silica, SiO2, or alumina, Al2O3 coated onto a plate Stationary phase is silica/alumina Mobile phase is solvent A dry TLC plate separates by adsorption (solute molecules adsorbed onto stationary phase, rate of travel depends on solubility in mobile phase and strength of adsorption) A wet TLC plate separates by partition (water molecules adsorbed onto stationary phase act partially as stationary phase, solute molecules partitioned between stationary water phase and solvent) Rf values calculated from distance travelled by sample/distance travelled by solvent Rf values differ from solvent to solvent Components of mixture identified by comparing with known reference samples or using Rf values
o o o
3)
Gas Chromatography, GC
This is also called gas-liquid chromatography or GLC Mobile phase is a carrier gas e.g. nitrogen (which is inert) Sample mixture is injected into machine The retention time is the characteristic time it takes for a particular substance to pass through the system under set conditions The output is a graph of detector response (y-axis) against retention time (xaxis). This provides a spectrum of peaks for a sample representing the compounds present in a sample eluting from the column at different times Components of the mixture are identified by their retention times The use of retention times to identify substances is limited by the facts that similar compounds often have similar retention times and that unknown compounds have no reference retention times for comparison
4)
GC-MS
Gas chromatography can be combined with mass spectrometry Components separated by GC are directed into a mass spectrometer A computer can analyse a components mass spectrum or compare it with a database for positive identification GC-MS is used in forensics, environmental analysis, airport security and space probes GC-MS is a far more powerful analytical technique than gas chromatography on its own
Você também pode gostar
- Selected Constants: Oxidation–Reduction Potentials of Inorganic Substances in Aqueous SolutionNo EverandSelected Constants: Oxidation–Reduction Potentials of Inorganic Substances in Aqueous SolutionAinda não há avaliações
- Topic 12 - Spectroscopy Revision Notes 1) Infrared SpectrosDocumento7 páginasTopic 12 - Spectroscopy Revision Notes 1) Infrared SpectrosChiwe Thando MatutaAinda não há avaliações
- Fourth International Conference on Non-Aqueous Solutions: Vienna 1974No EverandFourth International Conference on Non-Aqueous Solutions: Vienna 1974V. GutmannAinda não há avaliações
- Spectroscopy and ChromatographyDocumento7 páginasSpectroscopy and ChromatographyPa GesAinda não há avaliações
- Schaum's Easy Outline of Organic Chemistry, Second EditionNo EverandSchaum's Easy Outline of Organic Chemistry, Second EditionNota: 3.5 de 5 estrelas3.5/5 (2)
- Tutorial 5Documento9 páginasTutorial 5Ahmad WahideeAinda não há avaliações
- Analytical TechniquesDocumento7 páginasAnalytical TechniquesGideon CavidaAinda não há avaliações
- A Level OnlyDocumento27 páginasA Level OnlyTendaiAinda não há avaliações
- Nuclear Magnetic Resonance Spectroscopy: Origin of SpectraDocumento12 páginasNuclear Magnetic Resonance Spectroscopy: Origin of SpectrabhawanisrAinda não há avaliações
- NMR Hly09Documento44 páginasNMR Hly09Giang NguyenAinda não há avaliações
- Analytical TechniquesDocumento6 páginasAnalytical TechniquesahumanbeinginearthAinda não há avaliações
- CH 135 Exam II A KeyDocumento6 páginasCH 135 Exam II A Keynguyen ba trungAinda não há avaliações
- Introduction To Proton NMR SpectroscopyNJTDocumento13 páginasIntroduction To Proton NMR SpectroscopyNJTNicoleAinda não há avaliações
- As Topic 15 Notes - Analytical TechniquesDocumento4 páginasAs Topic 15 Notes - Analytical Techniquesrabs006Ainda não há avaliações
- NMR SpectraDocumento8 páginasNMR SpectraArshAinda não há avaliações
- Topic 20 NotesDocumento21 páginasTopic 20 NotesAmmaarah PatelAinda não há avaliações
- ChromatographyDocumento34 páginasChromatographyAakash SahaAinda não há avaliações
- Instrumental Analytical Techniques: An Overview of Chromatography and SpectrosDocumento52 páginasInstrumental Analytical Techniques: An Overview of Chromatography and SpectrosDella Fajar PAinda não há avaliações
- L4 NMR 2Documento18 páginasL4 NMR 2Cheng FuAinda não há avaliações
- Mass Spectra and IRDocumento7 páginasMass Spectra and IRSyed FahimAinda não há avaliações
- Analytical ChemistryDocumento40 páginasAnalytical ChemistryaqidahAinda não há avaliações
- CH 135 Exam II A KeyDocumento6 páginasCH 135 Exam II A KeyMark Cliffton BadlonAinda não há avaliações
- Chapter 3Documento43 páginasChapter 3George KingAinda não há avaliações
- Name: Hamidatun Nisa ID: 4133131023 Class: Chemistry Bilingual 2013Documento17 páginasName: Hamidatun Nisa ID: 4133131023 Class: Chemistry Bilingual 2013Wahyu HidayatAinda não há avaliações
- Chapter 13.2 - RothDocumento37 páginasChapter 13.2 - Rothkishan patelAinda não há avaliações
- Practice Problem Set Mixed Chromatography QuestionsDocumento14 páginasPractice Problem Set Mixed Chromatography QuestionsMelinda AndersonAinda não há avaliações
- NMR Spectropscopy HkaurDocumento42 páginasNMR Spectropscopy Hkaurakshatgoyal643Ainda não há avaliações
- 3 - Carbon-13-NMRDocumento21 páginas3 - Carbon-13-NMRahmed mohamedAinda não há avaliações
- NMR Solving StrategyDocumento2 páginasNMR Solving Strategysorrow Lemon100% (1)
- MSC Analytical ChromatographyDocumento10 páginasMSC Analytical ChromatographySachin ashokAinda não há avaliações
- CHM 312 Fall 2008: ChromatographyDocumento20 páginasCHM 312 Fall 2008: ChromatographylordniklausAinda não há avaliações
- Capítulo 4 - Técnicas ExperimentalesDocumento53 páginasCapítulo 4 - Técnicas Experimentalesleizar_death64Ainda não há avaliações
- Mass Spectroscopy & NMR Spectroscopy: Big PictureDocumento13 páginasMass Spectroscopy & NMR Spectroscopy: Big PictureluyawinAinda não há avaliações
- 6spectroscopy and ChromatographyDocumento15 páginas6spectroscopy and ChromatographyThinaya JayarathneAinda não há avaliações
- C-13 NMR and DEPTDocumento41 páginasC-13 NMR and DEPTV G Viju Kumar100% (1)
- Assessment Schedule: Chemistry 91388 Identifying The Reaction ProductsDocumento3 páginasAssessment Schedule: Chemistry 91388 Identifying The Reaction Productsjthamilton4Ainda não há avaliações
- NMR SpectrumDocumento5 páginasNMR SpectrumNabaa KhaldoonAinda não há avaliações
- Analytical ChemistryDocumento14 páginasAnalytical ChemistryFAB 5Ainda não há avaliações
- Lecture 8 - NMR Spectroscopy-Ii. Chem 216 Fall 2013 PDFDocumento8 páginasLecture 8 - NMR Spectroscopy-Ii. Chem 216 Fall 2013 PDFPhillip WachowiakAinda não há avaliações
- HPLC Training New LatestDocumento60 páginasHPLC Training New LatestMuhammadAmdadulHoqueAinda não há avaliações
- S Olvent Extraction of Rhodium Chloride From Aqueous Solutions and Its Separation From Palladium and PlatinumDocumento6 páginasS Olvent Extraction of Rhodium Chloride From Aqueous Solutions and Its Separation From Palladium and Platinummladen lakicAinda não há avaliações
- ws4 PDFDocumento5 páginasws4 PDFmohammedabubakrAinda não há avaliações
- Edexcel Chemistry A-Level: Topic 19: Modern Analytical Techniques IIDocumento8 páginasEdexcel Chemistry A-Level: Topic 19: Modern Analytical Techniques IILulwa KhaskiehAinda não há avaliações
- ChromatographyDocumento4 páginasChromatographyDIPAK DASAinda não há avaliações
- Chromatography MethodDocumento19 páginasChromatography MethodHafiz AzizAinda não há avaliações
- Chromatographic Techniques of AnaylsisDocumento39 páginasChromatographic Techniques of Anaylsispratik satiAinda não há avaliações
- 02 SpectroscopystratDocumento3 páginas02 SpectroscopystratDhiraj PatilAinda não há avaliações
- Curs HPLCDocumento132 páginasCurs HPLCSimon RobertaAinda não há avaliações
- NMR SpectrosDocumento57 páginasNMR SpectrosAnuki PereraAinda não há avaliações
- Essential Parts of A ChromatographDocumento13 páginasEssential Parts of A ChromatographTayyab IftikharAinda não há avaliações
- Final Examination Question Solve For ChromatogrphyDocumento44 páginasFinal Examination Question Solve For ChromatogrphyMunna IslamAinda não há avaliações
- Guía de Estudio EspectrosDocumento5 páginasGuía de Estudio EspectrosCésar CidAinda não há avaliações
- EC-Mid PreparationDocumento9 páginasEC-Mid PreparationanandswarupAinda não há avaliações
- Carbon - 13 NMR: Nuclear Magnetic Resonance SpectrosDocumento35 páginasCarbon - 13 NMR: Nuclear Magnetic Resonance SpectrosSri Rezeki SamosirAinda não há avaliações
- Pemisahan Problem Set TutorDocumento3 páginasPemisahan Problem Set TutorPutriNurHamidahAinda não há avaliações
- Edexcel & Cambridge Syllabus: Unit 4: Analytical Chemistry Alauddin Sir A & O Level Chemistry TeacherDocumento8 páginasEdexcel & Cambridge Syllabus: Unit 4: Analytical Chemistry Alauddin Sir A & O Level Chemistry TeacherMaliha Ishrat JarinAinda não há avaliações
- 3017-M.ahmad-BS Chem 4th-E Analytical ChemistryDocumento13 páginas3017-M.ahmad-BS Chem 4th-E Analytical ChemistryAhmad AhmadAinda não há avaliações
- 13C NMRDocumento40 páginas13C NMRKrishna BurakaAinda não há avaliações
- Analytical techniques-TLC, HPLC, GLCDocumento27 páginasAnalytical techniques-TLC, HPLC, GLCKudzayi TusaumweAinda não há avaliações
- Lesson PlanDocumento2 páginasLesson Plannicole rigonAinda não há avaliações
- Broken BondsDocumento20 páginasBroken Bondsapi-316744816Ainda não há avaliações
- Introduction-: Microprocessor 68000Documento13 páginasIntroduction-: Microprocessor 68000margyaAinda não há avaliações
- Chapter 8 Data Collection InstrumentsDocumento19 páginasChapter 8 Data Collection InstrumentssharmabastolaAinda não há avaliações
- Quick Help For EDI SEZ IntegrationDocumento2 páginasQuick Help For EDI SEZ IntegrationsrinivasAinda não há avaliações
- Lec 33 - Householder MethodDocumento11 páginasLec 33 - Householder MethodMudit SinhaAinda não há avaliações
- Wilcoxon Matched Pairs Signed Rank TestDocumento3 páginasWilcoxon Matched Pairs Signed Rank TestDawn Ilish Nicole DiezAinda não há avaliações
- What Is TranslationDocumento3 páginasWhat Is TranslationSanskriti MehtaAinda não há avaliações
- Essay Rough Draft 19Documento9 páginasEssay Rough Draft 19api-549246767Ainda não há avaliações
- Global Geo Reviewer MidtermDocumento29 páginasGlobal Geo Reviewer Midtermbusinesslangto5Ainda não há avaliações
- 5c3f1a8b262ec7a Ek PDFDocumento5 páginas5c3f1a8b262ec7a Ek PDFIsmet HizyoluAinda não há avaliações
- All You Need To Know About Egg YolkDocumento7 páginasAll You Need To Know About Egg YolkGolden Era BookwormAinda não há avaliações
- Modulo EminicDocumento13 páginasModulo EminicAndreaAinda não há avaliações
- The Mantel Colonized Nation Somalia 10 PDFDocumento5 páginasThe Mantel Colonized Nation Somalia 10 PDFAhmad AbrahamAinda não há avaliações
- MGMT Audit Report WritingDocumento28 páginasMGMT Audit Report WritingAndrei IulianAinda não há avaliações
- Networker Performance Tuning PDFDocumento49 páginasNetworker Performance Tuning PDFHarry SharmaAinda não há avaliações
- Application of Graph Theory in Operations ResearchDocumento3 páginasApplication of Graph Theory in Operations ResearchInternational Journal of Innovative Science and Research Technology100% (2)
- Online Extra: "Economists Suffer From Physics Envy"Documento2 páginasOnline Extra: "Economists Suffer From Physics Envy"Bisto MasiloAinda não há avaliações
- Recommendations For Students With High Functioning AutismDocumento7 páginasRecommendations For Students With High Functioning AutismLucia SaizAinda não há avaliações
- Waterstop TechnologyDocumento69 páginasWaterstop TechnologygertjaniAinda não há avaliações
- B122 - Tma03Documento7 páginasB122 - Tma03Martin SantambrogioAinda não há avaliações
- Existential ThreatsDocumento6 páginasExistential Threatslolab_4Ainda não há avaliações
- Business Analytics Emphasis Course GuideDocumento3 páginasBusiness Analytics Emphasis Course Guidea30000496Ainda não há avaliações
- GT-N7100-Full Schematic PDFDocumento67 páginasGT-N7100-Full Schematic PDFprncha86% (7)
- Pt3 English Module 2018Documento63 páginasPt3 English Module 2018Annie Abdul Rahman50% (4)
- Review1 ScheduleDocumento3 páginasReview1 Schedulejayasuryam.ae18Ainda não há avaliações
- Assembler Pass 2Documento5 páginasAssembler Pass 2AnuAinda não há avaliações
- DNA ReplicationDocumento19 páginasDNA ReplicationLouis HilarioAinda não há avaliações
- Work ProblemsDocumento19 páginasWork ProblemsOfelia DavidAinda não há avaliações
- Law of EvidenceDocumento14 páginasLaw of EvidenceIsha ChavanAinda não há avaliações
- Sodium Bicarbonate: Nature's Unique First Aid RemedyNo EverandSodium Bicarbonate: Nature's Unique First Aid RemedyNota: 5 de 5 estrelas5/5 (21)
- Periodic Tales: A Cultural History of the Elements, from Arsenic to ZincNo EverandPeriodic Tales: A Cultural History of the Elements, from Arsenic to ZincNota: 3.5 de 5 estrelas3.5/5 (137)
- Process Plant Equipment: Operation, Control, and ReliabilityNo EverandProcess Plant Equipment: Operation, Control, and ReliabilityNota: 5 de 5 estrelas5/5 (1)
- Taste: Surprising Stories and Science About Why Food Tastes GoodNo EverandTaste: Surprising Stories and Science About Why Food Tastes GoodNota: 3 de 5 estrelas3/5 (20)
- The Nature of Drugs Vol. 1: History, Pharmacology, and Social ImpactNo EverandThe Nature of Drugs Vol. 1: History, Pharmacology, and Social ImpactNota: 5 de 5 estrelas5/5 (5)
- Monkeys, Myths, and Molecules: Separating Fact from Fiction, and the Science of Everyday LifeNo EverandMonkeys, Myths, and Molecules: Separating Fact from Fiction, and the Science of Everyday LifeNota: 4 de 5 estrelas4/5 (1)
- Chemistry for Breakfast: The Amazing Science of Everyday LifeNo EverandChemistry for Breakfast: The Amazing Science of Everyday LifeNota: 4.5 de 5 estrelas4.5/5 (14)
- AP® Chemistry Crash Course, For the 2020 Exam, Book + Online: Get a Higher Score in Less TimeNo EverandAP® Chemistry Crash Course, For the 2020 Exam, Book + Online: Get a Higher Score in Less TimeNota: 5 de 5 estrelas5/5 (1)
- Secrets of the Millionaire Mind: Mastering the Inner Game of WealthNo EverandSecrets of the Millionaire Mind: Mastering the Inner Game of WealthNota: 4.5 de 5 estrelas4.5/5 (197)
- It's Elemental: The Hidden Chemistry in EverythingNo EverandIt's Elemental: The Hidden Chemistry in EverythingNota: 4 de 5 estrelas4/5 (10)
- Chemistry for Breakfast: The Amazing Science of Everyday LifeNo EverandChemistry for Breakfast: The Amazing Science of Everyday LifeNota: 4.5 de 5 estrelas4.5/5 (90)
- Is That a Fact?: Frauds, Quacks, and the Real Science of Everyday LifeNo EverandIs That a Fact?: Frauds, Quacks, and the Real Science of Everyday LifeNota: 5 de 5 estrelas5/5 (4)
- An Applied Guide to Water and Effluent Treatment Plant DesignNo EverandAn Applied Guide to Water and Effluent Treatment Plant DesignNota: 5 de 5 estrelas5/5 (4)
- Guidelines for Chemical Process Quantitative Risk AnalysisNo EverandGuidelines for Chemical Process Quantitative Risk AnalysisNota: 5 de 5 estrelas5/5 (1)
- Well Control for Completions and InterventionsNo EverandWell Control for Completions and InterventionsNota: 4 de 5 estrelas4/5 (10)
- AP Chemistry Flashcards, Fourth Edition: Up-to-Date Review and PracticeNo EverandAP Chemistry Flashcards, Fourth Edition: Up-to-Date Review and PracticeAinda não há avaliações
- Proof of Heaven: A Neurosurgeon's Journey into the AfterlifeNo EverandProof of Heaven: A Neurosurgeon's Journey into the AfterlifeNota: 3.5 de 5 estrelas3.5/5 (165)
- Formulating, Packaging, and Marketing of Natural Cosmetic ProductsNo EverandFormulating, Packaging, and Marketing of Natural Cosmetic ProductsAinda não há avaliações
- Guidelines for Defining Process Safety Competency RequirementsNo EverandGuidelines for Defining Process Safety Competency RequirementsNota: 3 de 5 estrelas3/5 (1)
- Handbook of Formulating Dermal Applications: A Definitive Practical GuideNo EverandHandbook of Formulating Dermal Applications: A Definitive Practical GuideAinda não há avaliações