Escolar Documentos
Profissional Documentos
Cultura Documentos
Scheme Britanny Nelson
Enviado por
Britanny NelsonDireitos autorais
Formatos disponíveis
Compartilhar este documento
Compartilhar ou incorporar documento
Você considera este documento útil?
Este conteúdo é inapropriado?
Denunciar este documentoDireitos autorais:
Formatos disponíveis
Scheme Britanny Nelson
Enviado por
Britanny NelsonDireitos autorais:
Formatos disponíveis
Scheme Sample Description : (solid , crystal, powder) Flame test for Na+ , K+ and H4 N+ Flame test: Put in flame
for 3-5 seconds Bright yellow and long-lasting = Na+ ________ , _______ Red/orange = Ca2+ Purple = K+ but possibly NH4+ _______ , ________ Further testing needs to be done No color NH4+is present ,Na+ and K+ are absent _____ , _______ CO3 2-/HCO3- :
Britanny Nelson
Dissolve a pinch of solid unknown in a few drops of 6M HNO3. If bubbles appear either CO3 2or HCO3- is present. Carbonates interfere with flame test of solid Na and Ca salts. NH4+ test: Method 1: First treat with 1 M NaOH, ( NH4+ formed to NH3) .Suspend a piece of damp red litmus paper above solution ( make sure it does not touch). Make need to warm up tube to make the reaction go faster, in a small test tube. If it turns blue , NH4+ is present . Method 2: Place portion of sample in a crucible and heat it up. If gray fumes and smoke are emitted, then NH4+ is present. K+ test: If NH4+ is present, then place small portion of the same sample in a crucible and heat strongly until fumes and smoke are no longer discharged. Next, dissolve any residue in 1-2 mL of DIW. In a small test tube or vial , or in a spot plate, add a drop of 6 M HAc and 2-3 drops of sodium cobaltinitrite reagent .If yellow precipitate forms then K+ is present If NH4+ is not present: Dissolve a small portion of the sample in 1-2 mL of DIW and place in spot plate. Add a drop of 6 M HAc and 2-3 drops sodium cobaltnitrite. If yellow precipitate forms K+ is present. Test pHs: HMO: 3 (R) 4 (OR) 5 (Y) ; HBtb: 6(Y) 7(G) 8(B) ; HIC 12( B) 13(G) 14(Y); HMV 0 (YG) 1(BG) 2(B) 3(BV) 3.5 (V) pH for HSO4- , NH4+, OH- ; use litmus paper pH : add 2 drops of indicator ; test pH of DIW 5.5-7 is okay. For pH dissolve pea size amount in 2 mL water. use indicator HBtb , HMO , HIC HSO4- = low acidity pH 1-2 , OH- is absent _____ NH4+ pH 4-5 ____
OH- very basic pH = 13____ Cl- test: Place around 20 drops of sample solution in a vial and add 1 M HNO3 until solution is acidic. ( Test this with blue litmus paper, it should turn red when acidic) Add 1 drop of 0.02 M AgNO3. If cloudy white solution/ precipitate forms then Cl- is present. SO42- /HSO4- test : Place 10-20 drops of sample solution in a vial and add 1M HNO3 until acidic. ( test with blue litmus paper- red means its acidic)Add 1 drop of 0.25 M Ba(NO3)2. If cloudy white precipitate forms, SO42- is present. To see if it has HSO4- test pH of solution if it is closer to a pH of 2 then it has HSO4- in it. NO3- test : Place crystals of Fe (OH2)6 (NH4)2 (SO4)2 in a spot plate. Cover crystals with a few drops of sample solution. Immediately add 18 M H2SO4-. If brown solution forms (not precipitate) NO3is present. Mg(H2O)2+ / Al(H2O)3+ If no precipitate forms after the addition of NH3, then Mg (H2O)6 2+ is not present. If precipitate forms after the addition of NH3 with the addition of excess 2M NaOH then Mg(H2O)6 2+ is present and Al(H2O)3+ is absent. CO3 2- / HCO3- test Treat the sample solution with 1 M HCl to observe generation of colorless odorless gas (CO2) To distinguish between CO3 2- and HCO3- test pH. If test is positive for CO2 and isnt soluble in H2O, it is CO3 2-. S/HS Add H2SO4 to sample; if it smells of sulfur released S/HS is present. Ca2+ separation : treat sample with excess 0.5M Na2CO3. After ppt has formed, centrifuge in test tube. Treat centrifuged sample with more Na2CO3 and repeat until Ca2+ is out.
Equations/possible compounds: Insoluble compounds: Ca(OH)2, CaSO4 (2H2O), CaCO3, MgCO3, Mg(OH)2
Você também pode gostar
- Menstruation and Bleeding Conditions in Women and AdolescentsDocumento123 páginasMenstruation and Bleeding Conditions in Women and AdolescentsBritanny NelsonAinda não há avaliações
- Approved Traumatic Brain Injury (TBI)Documento38 páginasApproved Traumatic Brain Injury (TBI)Britanny NelsonAinda não há avaliações
- Approved Hospice Discharge Disposition Meditech v2Documento8 páginasApproved Hospice Discharge Disposition Meditech v2Britanny NelsonAinda não há avaliações
- Management of Preconceptual Care, Normal PregnancyDocumento233 páginasManagement of Preconceptual Care, Normal PregnancyBritanny NelsonAinda não há avaliações
- Hca Retirement Readiness: Supporting Your Personal and Professional Goals While Promoting A Healthier, Happier LifeDocumento4 páginasHca Retirement Readiness: Supporting Your Personal and Professional Goals While Promoting A Healthier, Happier LifeBritanny NelsonAinda não há avaliações
- Tele Safety Bundle - StandardsDocumento2 páginasTele Safety Bundle - StandardsBritanny NelsonAinda não há avaliações
- Physical Assessment: HR: 100: Cervical Spine SubluxationDocumento1 páginaPhysical Assessment: HR: 100: Cervical Spine SubluxationBritanny NelsonAinda não há avaliações
- Critical Care Notes Clinical Pocket Guide - (Gastro-Urinary)Documento1 páginaCritical Care Notes Clinical Pocket Guide - (Gastro-Urinary)Britanny Nelson100% (1)
- Menopause Concept MapDocumento1 páginaMenopause Concept MapBritanny NelsonAinda não há avaliações
- Ati Med SurgDocumento1 páginaAti Med SurgBritanny NelsonAinda não há avaliações
- ATI SecretsDocumento9 páginasATI SecretsBritanny Nelson100% (11)
- Introduction To Critical CareDocumento21 páginasIntroduction To Critical CareBritanny NelsonAinda não há avaliações
- Critical Care Notes Clinical Pocket Guide - (Front Matter)Documento10 páginasCritical Care Notes Clinical Pocket Guide - (Front Matter)Britanny Nelson100% (1)
- Pharmacology NCLEX ReviewDocumento128 páginasPharmacology NCLEX ReviewBritanny NelsonAinda não há avaliações
- Critical Care Notes Clinical Pocket Guide - (Hematology Oncology)Documento17 páginasCritical Care Notes Clinical Pocket Guide - (Hematology Oncology)Britanny NelsonAinda não há avaliações
- Creating Study Guides Oregon StateDocumento5 páginasCreating Study Guides Oregon StateBritanny NelsonAinda não há avaliações
- R & C L W G: Esume Over Etter Riting UideDocumento15 páginasR & C L W G: Esume Over Etter Riting UideBritanny NelsonAinda não há avaliações
- Final 48 HouCram FullDocumento106 páginasFinal 48 HouCram FullRadu Urcan50% (2)
- Digestion and Absorption Study GuideDocumento6 páginasDigestion and Absorption Study GuideBritanny NelsonAinda não há avaliações
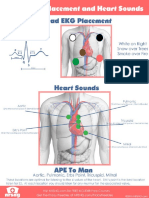
- 5 Lead EKG PlacementDocumento1 página5 Lead EKG PlacementBritanny NelsonAinda não há avaliações
- Order of Lab Draws PDFDocumento1 páginaOrder of Lab Draws PDFBritanny Nelson100% (1)
- The Yellow House: A Memoir (2019 National Book Award Winner)No EverandThe Yellow House: A Memoir (2019 National Book Award Winner)Nota: 4 de 5 estrelas4/5 (98)
- The Subtle Art of Not Giving a F*ck: A Counterintuitive Approach to Living a Good LifeNo EverandThe Subtle Art of Not Giving a F*ck: A Counterintuitive Approach to Living a Good LifeNota: 4 de 5 estrelas4/5 (5795)
- Elon Musk: Tesla, SpaceX, and the Quest for a Fantastic FutureNo EverandElon Musk: Tesla, SpaceX, and the Quest for a Fantastic FutureNota: 4.5 de 5 estrelas4.5/5 (474)
- A Heartbreaking Work Of Staggering Genius: A Memoir Based on a True StoryNo EverandA Heartbreaking Work Of Staggering Genius: A Memoir Based on a True StoryNota: 3.5 de 5 estrelas3.5/5 (231)
- Hidden Figures: The American Dream and the Untold Story of the Black Women Mathematicians Who Helped Win the Space RaceNo EverandHidden Figures: The American Dream and the Untold Story of the Black Women Mathematicians Who Helped Win the Space RaceNota: 4 de 5 estrelas4/5 (895)
- Never Split the Difference: Negotiating As If Your Life Depended On ItNo EverandNever Split the Difference: Negotiating As If Your Life Depended On ItNota: 4.5 de 5 estrelas4.5/5 (838)
- The Little Book of Hygge: Danish Secrets to Happy LivingNo EverandThe Little Book of Hygge: Danish Secrets to Happy LivingNota: 3.5 de 5 estrelas3.5/5 (400)
- The Hard Thing About Hard Things: Building a Business When There Are No Easy AnswersNo EverandThe Hard Thing About Hard Things: Building a Business When There Are No Easy AnswersNota: 4.5 de 5 estrelas4.5/5 (345)
- The Unwinding: An Inner History of the New AmericaNo EverandThe Unwinding: An Inner History of the New AmericaNota: 4 de 5 estrelas4/5 (45)
- Team of Rivals: The Political Genius of Abraham LincolnNo EverandTeam of Rivals: The Political Genius of Abraham LincolnNota: 4.5 de 5 estrelas4.5/5 (234)
- The World Is Flat 3.0: A Brief History of the Twenty-first CenturyNo EverandThe World Is Flat 3.0: A Brief History of the Twenty-first CenturyNota: 3.5 de 5 estrelas3.5/5 (2259)
- Devil in the Grove: Thurgood Marshall, the Groveland Boys, and the Dawn of a New AmericaNo EverandDevil in the Grove: Thurgood Marshall, the Groveland Boys, and the Dawn of a New AmericaNota: 4.5 de 5 estrelas4.5/5 (266)
- The Emperor of All Maladies: A Biography of CancerNo EverandThe Emperor of All Maladies: A Biography of CancerNota: 4.5 de 5 estrelas4.5/5 (271)
- The Gifts of Imperfection: Let Go of Who You Think You're Supposed to Be and Embrace Who You AreNo EverandThe Gifts of Imperfection: Let Go of Who You Think You're Supposed to Be and Embrace Who You AreNota: 4 de 5 estrelas4/5 (1090)
- The Sympathizer: A Novel (Pulitzer Prize for Fiction)No EverandThe Sympathizer: A Novel (Pulitzer Prize for Fiction)Nota: 4.5 de 5 estrelas4.5/5 (121)
- Eudragit L 100 and Eudragit S 100: Specification and Test MethodsDocumento7 páginasEudragit L 100 and Eudragit S 100: Specification and Test MethodscrackenworldAinda não há avaliações
- DF Prod & Sys SEADocumento103 páginasDF Prod & Sys SEApaimanAinda não há avaliações
- Generator 525mw MeilDocumento132 páginasGenerator 525mw MeilRaja VigneshAinda não há avaliações
- CBSE Class 10 Chemistry Worksheet - Chemical Reactions and EquationsDocumento4 páginasCBSE Class 10 Chemistry Worksheet - Chemical Reactions and EquationsMalancha high school HS100% (1)
- Polishing and Etching Coal Samples For Microscopical Analysis by Reflected LightDocumento4 páginasPolishing and Etching Coal Samples For Microscopical Analysis by Reflected LightGnanavel GAinda não há avaliações
- Full Isolation of Mono-Atomic Elements From Dead Sea SaltDocumento2 páginasFull Isolation of Mono-Atomic Elements From Dead Sea SaltWigwamman100% (2)
- Soda AshDocumento59 páginasSoda AshDurvasula Aditya0% (1)
- 11 - Hot Pack Cold PackDocumento6 páginas11 - Hot Pack Cold PackMoon KimAinda não há avaliações
- Chem Stoichio QnsDocumento9 páginasChem Stoichio Qnsemily_liu_5Ainda não há avaliações
- Climax-Catalog LUBRICANTES Y SELLANTES PDFDocumento30 páginasClimax-Catalog LUBRICANTES Y SELLANTES PDFNini Jhoana Ardila CamachoAinda não há avaliações
- Vinegar PDFDocumento16 páginasVinegar PDFmuhammadjati_yhAinda não há avaliações
- Water TreatmentDocumento37 páginasWater TreatmentAMAL MATHEWAinda não há avaliações
- Lab Report Experiment 2 Determination of Ka Value of A Weak AcidDocumento17 páginasLab Report Experiment 2 Determination of Ka Value of A Weak AcidarisyahariffAinda não há avaliações
- Solubility of Organic Compounds: Kirstin Blaire A. Magdadaro Nathalie ReloxDocumento5 páginasSolubility of Organic Compounds: Kirstin Blaire A. Magdadaro Nathalie ReloxKirstin Blaire MagdadaroAinda não há avaliações
- QP-117 Organic Impurities in Fine Aggregate - Develop FormDocumento4 páginasQP-117 Organic Impurities in Fine Aggregate - Develop FormResearcherAinda não há avaliações
- AspirinDocumento3 páginasAspirinMAYUR CHATURAinda não há avaliações
- NB DhavalDocumento140 páginasNB DhavalDEVESH SINGH100% (2)
- Week010 LaboratoryExercise003 AcidsandBasesSolubilityEquilibriaDocumento9 páginasWeek010 LaboratoryExercise003 AcidsandBasesSolubilityEquilibriaMae Borja MisadorAinda não há avaliações
- Arabian Journal For Science and Engineering, 2022, 47, 5587-5599Documento13 páginasArabian Journal For Science and Engineering, 2022, 47, 5587-5599DanCosminAinda não há avaliações
- Survey Results Bulk HandlingDocumento102 páginasSurvey Results Bulk HandlingAnda LankuAinda não há avaliações
- Corporate ProfileDocumento40 páginasCorporate ProfileVinay ChoudharyAinda não há avaliações
- Mercurous Nitrate Test For Copper AlloysDocumento4 páginasMercurous Nitrate Test For Copper Alloysandrea assanelliAinda não há avaliações
- Humastar 200Documento286 páginasHumastar 200Dinesh SreedharanAinda não há avaliações
- Cambridge Ordinary Level: Cambridge Assessment International EducationDocumento16 páginasCambridge Ordinary Level: Cambridge Assessment International EducationPrince YugAinda não há avaliações
- Viskositas NaOH AkhirnyaaDocumento2 páginasViskositas NaOH AkhirnyaaLisa Ayu Wulandari0% (1)
- Experiment 3: Calorimetry: Chemistry For Engineers LaboratoryDocumento16 páginasExperiment 3: Calorimetry: Chemistry For Engineers Laboratoryjamila milanoAinda não há avaliações
- PAG Activity For Biological MoleculesDocumento19 páginasPAG Activity For Biological Moleculesahmedpadela84Ainda não há avaliações
- Chapter 7Documento39 páginasChapter 7jonathen jaganAinda não há avaliações
- Copper Cycle Report SP16Documento12 páginasCopper Cycle Report SP16BirobaAinda não há avaliações
- Experiment Number 2 Coffee Cup CalorimetryDocumento7 páginasExperiment Number 2 Coffee Cup Calorimetryapi-529605052Ainda não há avaliações