Escolar Documentos
Profissional Documentos
Cultura Documentos
Reactivo RB 5 P. Eryngii
Enviado por
ericjeriaTítulo original
Direitos autorais
Formatos disponíveis
Compartilhar este documento
Compartilhar ou incorporar documento
Você considera este documento útil?
Este conteúdo é inapropriado?
Denunciar este documentoDireitos autorais:
Formatos disponíveis
Reactivo RB 5 P. Eryngii
Enviado por
ericjeriaDireitos autorais:
Formatos disponíveis
Water Air Soil Pollut (2013) 224:1595 DOI 10.
1007/s11270-013-1595-0
Microbial Decolorization of an Azo Dye Reactive Black 5 Using White-Rot Fungus Pleurotus eryngii F032
Tony Hadibarata & Liyana Amalina Adnan & Abdull Rahim Mohd Yusoff & Adhi Yuniarto & Rubiyatno & Meor Mohd Fikri Ahmad Zubir & Ameer Badr Khudhair & Zee Chuang Teh & M. Abu Naser
Received: 17 March 2013 / Accepted: 9 May 2013 # Springer Science+Business Media Dordrecht 2013
Abstract The growth of white-rot fungus Pleurotus eryngii F032 in a suitable medium can degrade an azo dye Reactive Black 5 (RB5), because of its ability to produce ligninolytic enzymes such as lignin peroxidase (LiP), manganese peroxidase (MnP), and laccase that able to degrade and transform the complex structure of the dye into a less toxic compound. The effect of environmental factors such as initial concentration of Reactive Black 5, pH, temperature of growth medium,
T. Hadibarata (*) : L. A. Adnan : A. R. M. Yusoff : A. Yuniarto : Rubiyatno : M. M. F. A. Zubir : A. B. Khudhair : Z. C. Teh Institute of Environmental and Water Resources Management, Universiti Teknologi Malaysia, 81310 UTM Skudai, Johor Bahru, Malaysia e-mail: hadibarata@utm.my L. A. Adnan : A. R. M. Yusoff Department of Chemistry, Faculty of Science, Universiti Teknologi Malaysia, 81310 UTM Skudai, Johor Bahru, Malaysia A. Yuniarto Department of Environmental Engineering, Institut Teknologi Sepuluh Nopember (ITS), Surabaya, Indonesia M. A. Naser Department of Biosciences and Health Sciences, Universiti Teknologi Malaysia, 81310 UTM Skudai, Johor Bahru, Malaysia
surfactant (Tween 80), and agitation were also investigated. The productions of ligninolytic enzymes were enhanced by increasing the white-rot fungi growth in optimum conditions. The decolorization of Reactive Black 5 were analyzed by using UVvis spectrophotometer at the maximum absorbance of 596 nm. The whiterot fungus, P. eryngii F032 culture exhibited 93.56 % decolorization of 10 mg/L RB5 within 72 h of incubation in dark condition with agitation. The optimum pH and temperature for the decolorizing activity was recorded at pH 3 and 40 C, respectively. The addition of surfactant (Tween 80) increased the decolorization to 93.57 % and agitation of growth medium at 120 rpm enhanced the distribution of nutrients to the fungus thus optimized the enzymatic reaction that resulted maximum decolorization of RB5 which was 93.57 %. The molecular docking studies were performed using Chimera visualization software as to analyze the decolorization mechanism of RB5 at molecular level. Keywords Pleurotus eryngii F032 . Reactive Black 5 . Decolorization . Tween 80 . Environmental factors . Chimera visualization software
1 Introduction Most of the industries especially textile industry is the major contribution to the disposal of toxic dye into the water. The use of white-rot fungi and/or their extracellular enzymes are currently an effective solution for
1595, Page 2 of 9
Water Air Soil Pollut (2013) 224:1595
removal of synthetic dye containing wastewater (Ali 2010). There are two important mechanisms for treatment of dye by white-rot fungi which are by biosorption of dye to the fungal biomass and biodegradation of dye into another compound by extracellular enzyme (Banat et al. 1996). The dyes are hardly removed from wastewater by conventional biological, physical, or chemical treatment. It is really important to remove dyes in wastewater because dyes are very toxic and are characterized by high chemical oxygen demand, biological oxygen demand, and highly aromatic conjugated and carcinogenic that can endanger human life (Bardi et al. 2010). Reactive Black 5 (RB5) is one of the reactive azo dye group that being dispelled into the river. This synthetic RB5 dye has been extensively used in textile industries around the world that producing dying cotton, woolen, and nylon fabric. However, the use of RB5 has drawbacks for human life which the maximally exposure to this dye may cause allergic reactions of respiratory tract and even worse cancer (Usha et al. 2006). So, this dye should be treated and removed from wastewater in an economical and efficient way. Azo-based dye contained chromophore and different types of reactive groups such as chlorotriazine, trichloropyrimidine, and difluorochloropyrimidine. The chromophore of RB5 molecule absorbs the visible light at 596 nm (Bayramonglu and Arica 2007). This azo-type dye in effluent may give nutrients to the residue thus, increase its fertility in the river. This phenomenon is known as eutrophication where the river is characterized by oxygen deficiency, growth of algal bloom, and death of aquatic life (Bardi and Marzona 2010). Eutrophication will also limit the penetration of light to the river or lake, thus, reduce the efficiency of photosynthesis process. The amount of oxygen in lake will reduce and aquatic life will die. There are a lot of treatments for decolorization of dyes such as electrocoagulation technique, coagulationflocculation, and adsorption. Most of these technologies, however suffer from several shortcomings, including high amount sludge generation, requirements of high cost of chemical and techniques (Guaratini et al. 2001). Furthermore, the use of bacteria in the biological treatment of wastewater may result in generation of colorless, dead-end aromatic amine which are generally more hazardous than the parent compounds and thus may have poor usage and
limited application in treatment of dye effluents (Guaratini et al. 2001). White-rot fungi are well-known for their outstanding ability to secrete non-specific extracellular oxidative enzymes such as laccase that involved in the degradation of lignin (Banat et al. 1996). The fungus Pleurotus eryngii F032 has been extensively investigated for its application in the degradation of naphthalene, a two-ring aromatic compound (Hadibarata et al. 2013). Laccase has the powerful mechanism to degrade phenolic and non-phenolic compounds, while the secretion of lignin peroxidase (LiP) by fungi will enhance the decolorization of dye (Hadibarata et al. 2012a, b; Hazeroual et al. 2006). Thus, the objective of this research was to investigate the optimum parameters for the growth of white-rot fungi thus maximized the decolorization of Reactive Black 5. This is the first report about the decolorization of Reactive Black 5 by P. eryngii F032.
2 Materials and Methods All the chemical used in this research were highly graded and the methodology were divided into four main stages which were, growth of WRF in agar medium, screening for best performance of WRF in agar medium, growth of WRF in liquid medium with different parameters (initial dye concentration, pH, temperature, volume of Tween 80, and agitation), and analytical method for decolorization of RB5. 2.1 Chemicals Malt extract agar was obtained from United States based on company Difco (Detroit, USA). Reactive Black 5, yeast extract, glucose, and all other chemicals with highest analytical grade were purchased from Milwaukee Wisconsin, USA company of SigmaAldrich (Milwaukee, USA). The characterization of RB5 is presented in Table 1. 2.2 Microorganism and Growth Conditions Various white-rot fungi were collected from recreational forest, Universiti Teknologi Malaysia (UTM) and stored in refrigerator to preserve their growth. The tissue of the fungi were cut and cultured in malt extract agar (MEA). MEA contained malt extract
Water Air Soil Pollut (2013) 224:1595 Table 1 Properties of Reactive Black 5
Page 3 of 9, 1595
Parameter C.I. Name Commercial Name Functional group CAS Number C.I. Number Mol. Mass (g/mol) Mol. Formula Chemical Formula
O
Value Remazol Black 5 Reactive Black 5 Azo 17095-24-8 13657666 990.87 C26H21N5Na4O19S6
-
O Na+ S O O S O N N O Na+-O S O O S O- Na+ NH2 OH N N O O S O O O
O Na+ S O
Lambda max Purity (%) Solubility Manufacturer pH of the stock solution
596 55 200 g/L in water at 80 C Sigma-Aldrich 5.5
(20 g/L), glucose (20 g/L), chloramphenicol (300 mg/L), and Reactive Black 5 (50 mg/L) (Hadibarata et al. 2011). The samples were incubated for 7 days to screen the best performance of white-rot fungi. To study the decolorization of RB5, inoculum (three agar plugs) were cut from the fungus mycelium that grow in agar medium and transferred into mineral medium (MM) containing yeast extract (20 g/L) and glucose (20 g/L). These reagents were added with distilled water until it reached the volume of 20 mL in 100 mL Erlenmeyer flask. Then, the appropriate amount of RB5 (50 mg/L) were dissolved in Tween 80 and chloramphenicol to solubilize the dye in distilled water and inhibit bacterial growth, respectively. These flasks were incubated for 72 h at 27 C under agitated condition to disperse the RB5 and ligninolytic enzymes of fungi in culture medium. The decolorization
of RB5 was monitored for 24, 48, and 72 h using UV vis spectrophotometer. In each experiment, 20 mL pregrown sample were prepared and autoclaved for positive control. All the experiments were performed in triplicate to obtain a validate result. 2.3 Effects of Different Parameters on Dye Decolorization The active growth of P. eryngii F032 in agar medium (three agar plugs) were cultured in MM at dark condition for 72 h. Various parameters affecting the decolorization of RB5 were investigated in this experiment including dye concentration (10, 20, and 30 mg/L), volume of Tween 80 (0.1, 0.5, and 1.0 mL), pH (3, 5, 7, and 10), temperature (15, 25, and 40 C), and agitation (80, 120, and 160 rpm). The decolorization of RB5 was monitored
1595, Page 4 of 9
Water Air Soil Pollut (2013) 224:1595
using PerkinElmer UVvis spectrophotometer at 0, 24, 48, and 72 h. 2.4 Analytical Method The liquid culture were filtrated and centrifuged at 4,000 rpm for 15 min. The supernatant were collected for further analysis. Decolorization was monitored by scanning the absorbance between 400 and 750 nm using PerkinElmer UVvis spectrophotometer and the maximum absorption of RB5 recorded at 596 nm. Reductions in the absorbance at 596 nm showed that decolorization have taken place. Dye concentrations were calculated from the calibration curve of absorbance and dye concentration (mg/L). The decolorization percentage of RB5 was calculated as follows: Decolorization% C 0 C t 100% C0
et al. 2009). Two-dimensional structures of RB5 and Tween 80 were collected from PubChem database and both these two-dimensional structures were then converted into three-dimensional structures using OpenBabel (Guha et al. 2006) software. The processed coordinates were used for docking using SwissDock (Grosdidier et al. 2011).
3 Result and Discussion All the results were obtained from triplicate experiment as to obtain high accuracy and precision of the results. 3.1 Decolorization of the Dye in Liquid Culture of P. eryngii F032 The UVvis spectrum in Fig. 1 shows the absorbance of dye after treated with P. eryngii F032. The maximum absorbance of RB5 was at 596 nm. It was characterized by the long conjugated -system that linking the two azo bond of RB5 (Kaushik and Malik 2009). The calibration curve that is shown in Fig. 2 indicates that Reactive Black 5 follows Beer Lambart law (Lucas and Peres 2007). 3.2 Effect of Initial Dye Concentration on the Decolorization of Reactive Black 5 The decolorization of RB5 was investigated by varying the concentration of dye (10, 20, and 30 mg/L). Based on the graph in Fig. 3a, the maximum dye
Where, C0 refer to the absorbance of control, Ct refer to the absorbance of sample, and t refer to the incubation time (h). 2.5 Molecular Docking Studies The molecular docking studies were performed to gain insight into the decolorization mechanism at molecular level. The crystal structure of versatile peroxidase from P. eryngii (PBD ID: 3FJW) was collected from protein data bank and dispensable chemical components from the raw peroxidase pdb file were deleted manually and missing hydrogen atoms were added using a python script from autodock4 suite (Morris
Fig. 1 The UV vis spectrum of Reactive Black 5 (RB5) after treated with P. eryngii F032 for 0, 24, 48, and 72 h. The pH value and the temperature of liquid medium kept at 3 and 40 C. Maximum absorbance at 596 nm
Water Air Soil Pollut (2013) 224:1595
Page 5 of 9, 1595
Fig. 2 Calibration curve of Reactive Black 5
decolorization was found to be in the concentration of 10 mg/L which was 93.56 % after treated with P. eryngii F032 for 72 h. The decolorization of RB5 indicated the degradation of azo bond and further treatment by fungi can lead to the cleavage of aromatic
a
Decolorization (%)
100
80 60 40 20 10 mg/L 20 mg/L 30 mg/L
rings (Hadibarata et al. 2012a, b). Further increase the dye concentration to 20 and 30 mg/L decreased the rate of decolorization to 74.26 and 67.63 %, respectively. This is due to the increase in the toxicity of dye that inhibited the growth and ligninolytic enzyme of fungi. Moreover, azo dye is a recalcitrant and complex molecule for degradation as it consists of fused aromatic rings (Kumari et al. 2007). Despite this, P. eryngii F022 still have the capability to decolorize high concentration of dye up to 67.63 %. There was inverse correlation between dye concentration and efficiency of decolorization of dye (Liao et al. 2012). Similar result was observed in the biodegradation of Remazol Brilliant Blue R (RBBR) by laccase of Polyporus sp. S133. The decolorization of dye was at 95 % when the concentration used below 300 mg/L and started to decline when the concentration increased to 500 mg/L. This result indicated that the rate of substrate oxidation by enzyme (laccase) increased with the substrate concentration until it reached the limitation of enzymatic activity or saturation of the available active site of enzyme (Lucas et al. 2008; Lu et al. 2009).
100 80 60
40 20
a
0.1 mL
0.5 mL
0
Decolorization (%)
0 24 48 Incubation Time (h) 72
b
Decolorization (%)
100 80 60 40 20 0
0 24 48 Incubation Time (h) 72
pH 3
pH 5 pH 7 pH 10
1.0 mL
0 0 24 48 72 Incubation Time (h) 100 80 60
40 20
b
80 rpm 120 rpm 160 rpm
Decolorization (%)
100 80 60 40 20 0
15
25 40
Decolorization (%)
24 48 Incubation Time (h)
72
0 0 24 48 72 Incubation Time (h)
Fig. 3 Effect of initial dye concentration (a), pH (b), and temperature (C) (c) of the liquid medium on the decolorization of Reactive Black 5 by P. eryngii F032
Fig. 4 Effect of non-ionic surfactant, Tween 80 (a) and agitation (b) on the decolorization of RB5
1595, Page 6 of 9
Water Air Soil Pollut (2013) 224:1595
dye groups to it. The absorption of acidic dye was optimum at acidic condition because there was an increased in the protonation of weak base group (biomass) that bound and degraded the anionic group of acidic dye (Palmieri et al. 2005).
3.4 Effect of Temperature on the Decolorization of Reactive Black 5
Fig. 5 Docking of RB5 on P. eryngii F032 peroxidase
3.3 Effect of pH on the Decolorization of Reactive Black 5 The pH regulation of the growth medium is really crucial because it can affect the ligninolytic enzyme activity and biodegradation process by P. eryngii F032. An experiment was carried out by changing the pH of the liquid medium between 3 and 10 at 27 C for 72 h. As shown in Fig. 3b, the optimum pH for the decolorization of RB5 was at pH 3 because it showed the highest decolorization (93.56 %). The increase in pH to pH 10 decreased the decolorization to 25.9 %. Our results were in agreement with previous study that investigated the removal rate of phenolic compound by Phanerochaete chrysosporium. The optimum pH was between pH 3 and 5 because the pH range is suitable for the growth and enzymatic production by white-rot fungi (OMahony et al. 2002). On the other hand, the biodegradation of synthetic dye by white-rot fungus Lentinus polychrous was optimum at pH 3 (Osma et al. 2010). The pH of the liquid medium will change the charge of the enzyme thus influences the adsorption of charge
The effect of temperature on the decolorization of Reactive Black 5 by P. eryngii F032 was studied between 15 and 40 C with an optimum pH 3.0 for 72 h. As shown in Fig. 3c, the decolorization of RB5 increases from 46.37 to 96.77 % when the temperature of liquid medium increases from 15 to 40 C. The decolorization of dye increases with temperature because the solubility of enzyme (laccase) was increased at high temperature thus, enhanced the enzymatic activity of fungus in degrading substrate (dye) (Saratale et al. 2009). Each strain of fungus showed different microbial activity at different temperature. Newly isolated white-rot fungus Cladosporium cladosporioides decolorized 1:2 metal complex dye Acid Blue 193 completely at 40 C (Vijaykumar et al. 2006). Plus, the biosorption and degradation of Direct Blue-1 was optimum at 35 C because the available active site of ligninolytic enzymes and the kinetic energy of dye increased (Senthilkumar et al. 2011; Yesilada et al. 1998). The enzymatic activity of fungus was optimum at higher temperature because warmer condition was favorable for the growth and mitosis of the microbial cell (fungi). Furthermore, the decomposition of dead animal by decomposers (fungi or bacteria) was faster during summer than winter (Yi-Chin et al. 2003).
Table 2 Hydrogen bonds formed between RB5 and enzyme
No. 1 2 3 4 5 6 7
BR5 group (atom) Sulfide (O) Sulfate (O) Sulfate (O) Sulfate (O) Sulfate (O) Sulfide (O) Naphthalene (N)
Residue name (atom) ASN256 (H) ARG257 (H) ARG257 (H) ARG257 (H) LYS272 (H) LYS272 (H) Naphthalene (O)
Bond distance (A) 2.508 2.785 1.976 2.011 1.969 2.164 2.755
Water Air Soil Pollut (2013) 224:1595
Page 7 of 9, 1595
3.5 Effect of Non-ionic Surfactant, Tween 80 on the Decolorization of Reactive Black 5 The effect of non-ionic surfactant, Tween 80 was investigated in this experiment by varying the volume added in the liquid medium (0.1, 0.5, and 1.0 mL). As shown in Fig. 4a, highest decolorization of RB5 was obtained when 0.5 mL of Tween 80 was added in liquid medium (93.57 %) and upon addition of surfactant to 1.0 mL depleted the decolorization of RB5 to 48.37 %. These results agreed with previous research, that indicated the addition of Tween 80 in decolorization of RBBR by laccase of Polyporus sp. S133 increase the decolorization to 82.5 %. The addition of Tween 80 increases the solubility of dye in water, thus enhancing the enzymatic activity of fungus in degrading dye (Lu et al. 2009).
Fig. 7 Docking of Tween 80 on P. eryngii F032 peroxidase
3.6 Effect of Agitation on the Decolorization of Reactive Black 5 The Erlenmeyer flask containing growth liquid media were shook using shaker at different revolutions per minute (80,120, and 160 rpm). Samples were grown in
dark places at 27 C for 72 h. Based on graph in Fig. 4b, the decolorization increased to 93.57 % with the agitation of 120 rpm and started to decrease to 35.03 % when we increased the agitation to 160 rpm. Shaking condition enhanced nutrient distribution (carbon sources and nitrogen sources) and oxygen transfer in the liquid medium thus, promoted cell growth and biodegradation process by white-rot fungi (Banat et al. 1996). Similar report indicated that the decolorization of Astrazon Red FBL by Funalia Trogii was optimum at 100150 rpm (Zouari-Mechichi et al. 2006). 3.7 Docking of Reactive Black 5 and Tween 80 The SwissDock predicted 31 potential binding sites of RB5 and also generated about eight conformations for
Fig. 6 Structure of RB5 at the docking site and electron transfer path of P. eryngii F032 peroxidase
Fig. 8 Structure of Tween 80 at docking site and electron transfer path of P. eryngii F032 peroxidase
1595, Page 8 of 9
Water Air Soil Pollut (2013) 224:1595
each of the binding site. All the conformations were visually inspected using Chimera visualization software (Pettersen et al. 2004). The RB5 binding site with the lowest free energy of binding (Fig. 5) was found to be consistent with the experimental evidence (Perez-Boadal et al. 2005). In this binding mode RB5 is stabilized by seven hydrogen bonds (Table 2). Temperature and agitation might have a role in breaking these hydrogen bonds in releasing product into the solution. The RB5 is docked about 4 away from Trp 164 (Fig. 6) which accept electron from RB5 and pass the electron on to Leu 165 and finally to heme group (Perez-Boadal et al. 2005). Perez-Boadal et al. (2005) also showed that Trp 164 is the vital component for the oxidation of RB5. The numbers of predicted binding modes for Tween 80 were 37 and a total of 256 conformations were also generated by SwissDock. Previously mentioned approach and criterion were used for visual inspection and conformation selection, respectively. The docking results (Figs. 7 and 8) suggested that Tween 80 is not chemically involved in enhancing decolorization. However, the results suggested that there is some loss of conformational entropy for both the Tween 80 and enzyme. In order to compensate this entropy loss, conformation of the enzyme may have changed to facilitate RB5 docking on its surface.
since it can only be used once. Thus, a new technology of fungal immobilization should be proposed as to overcome this problem.
Acknowledgments A part of this research was financially supported by a Research University Grant of Universiti Teknologi Malaysia (Vote QJ1.3000.2522.02H65), which is gratefully acknowledged.
References
Ali, H. (2010). Biodegradation of synthetic dyesa review. Water, Air, and Soil Pollution, 213, 251273. Banat, I. M., Nigam, P., & Singh, D. (1996). Microbial decolourization of textile dye containing effluentsa review. Bioresource Technology, 58, 217227. Bardi, L., & Marzona, M. (2010). Factors affecting the complete mineralization of azo dyes. Biodegradation of azo dyes. Handbook Environment Chemistry, 9, 195210. Bayramonglu, G., & Arica, M. Y. (2007). Biosorption of benzidine based textile dyes Direct Blue 1 and Direct Red 128 using native and heat treated biomass of Trametes versicolor. Journal of Hazardous Materials, 143, 135143. Grosdidier, A., Zoete, V., & Michielin, O. (2011). SwissDock, a protein-small molecule docking web service based on EADock DSS. Nucleic Acid Research, 39, W270W277. Guaratini, C. C. I., Fogg, A. G., & Zanoni, M. V. B. (2001). Assessment of the application of cathodic stripping voltammetry to the analysis of diazo reactive dyes and their hydrolysis products. Dyes and Pigments, 50, 211221. Guha, R., Howard, M. T., Hutchison, G. R., Murray-Rust, P., Rzepa, H., Steinbec, C., et al. (2006). The blue obeliskinteroperability in chemical informatics. Journal of Chemical Information and Modeling, 46, 991998. Hadibarata, T., Tachibana, S., & Askari, M (2011). Identification of metabolites from phenanthrene oxidation by phenoloxidase and dioxygenase of Polyporus sp. S133. Journal of Microbiology and Biotechnology, 21, 299304. Hadibarata, T., Yusoff, A. R. M., Aris, A., Salmiati, Hidayat, T., & Kristanti, R. A. (2012a). Decolorization of azo, triphenylmethane and anthraquinone dyes by laccase of a newly isolated Armillaria sp. F022. Water, Air, and Soil Pollution, 223, 10451054. Hadibarata, T., Yusoff, A. R. M., & Kristanti, R. A. (2012b). Decolorization and metabolism of anthraquinone-type by laccase of white-rot fungi Polyporus sp. S133. Water, Air, and Soil Pollution, 223, 933941. Hadibarata, T., Teh, Z. C., Rubiyatno, Zubir, M. M. F. A., Khudhair, A. B., Yusoff, A. R. M., et al. (2013). Identification of naphthalene metabolism by white rot fungus Pleurotus eryngii. Bioprocess and Biosystems Engineering. doi:10.1007/s00449-013-0884-8. Hazeroual, Y., Kim, B. S., Kim, C. S., Blaghen, M., & Lee, K. M. (2006). Biosorption of bromophenol blue from aqueous solution by Rhizopus stolonifer biomass. Water, Air, and Soil Pollution, 177, 135146.
4 Conclusion Microbial organisms were affected by changing of environment and/or parameter of the growth medium. So, the condition for the growth of fungi should be strictly monitored to enhance the secretion of ligninolytic enzymes that will decolorize dye completely. White-rot fungus P. eryngii F032 decolorize RB5 efficiently at the concentration of 10 mg/L with agitation (120 rpm). In addition, the decolorization was optimal at a temperature of 40 C and at pH 3. The addition of non-ionic surfactant (Tween 80) enhanced the decolorization to 93.57 %. The decolorization mechanism of RB5 can be visualized at molecular level by using Chimera visualization software. These results will be useful for further analysis on the biodegradation and identification of metabolite products of Reactive Black 5 by white-rot fungi. However, the problem that might occur from this research is the reproducibility limitation of the fungus
Water Air Soil Pollut (2013) 224:1595 Kaushik, P., & Malik, A. (2009). Fungal dye decolourization: recent advances and future potential. Environment International, 35, 127141. Kumari, K., & Abraham, T. E. (2007). Biosorption of anionic textile dyes by nonviable biomass of fungi and yeast. Bioresource Technology, 98, 17041710. Liao, C. S., Hung, C. H., & Chao, S. L. (2012). Decolorization of azo dye Reactive Black B by Bacillus cereus strain HJ-1. Chemosphere, 90, 21092114. Lu, Y., Yan, L., Wang, Y., Zhou, S., Fu, J., & Zhang, J. (2009). Biodegradation of phenolic compounds from coking wastewater by immobilized white-rot fungus Phanerochaete chrysosporium. Journal of Hazardous Materials, 165, 10911097. Lucas, M. S., & Peres, J. A. (2007). Degradation of Reactive Black 5 by Fenton/UV-C and ferrioxalate/ H2O2/ solar light processes. Dyes and Pigments, 74, 622629. Lucas, M., Meryens, V., & Vanhull, S. (2008). Synthetic dyes decolourisation by white-rot fungi; development of original micro titre plate method and screening. Enzyme and Microbial Technology, 42, 97106. Morris, G. M., Huey, R., Lindstorm, W., Sanner, M. F., Belew, R. K., Goodsell, D. S., et al. (2009). Autodock4 and AutoDockTools4: automated docking with selective receptor flexibility. Journal of Computational Chemistry, 16, 27852791. OMahony, T., Guibal, E., & Tobin, J. M. (2002). Reactive dye biosorption by Rhizopus arrhizus biomass. Enzyme and Microbial Technology, 31, 456463. Osma, J. F., Toca-Herrera, L., & Rodriguez-Couto, S. (2010). Transformation pathway of Remazol Brilliant Blue R by immobilised laccase. Bioresource Technology, 101, 85098514. Palmieri, G., Cennamo, G., & Sannia, G. (2005). Remazol Brilliant Blue R decolourization by the fungus Pleurotus Ostreatus and its oxidative enzymatic system. Enzyme and Microbial Technology, 36, 1724. Perez-Boadal, M., Ruiz-Dueas, F. J., Pogni, R., Basosi, R., Choinowski, T., Martinez, M. J., et al. (2005). Versatile peroxidase oxidation of high redox potential aromatic compounds: site-directed mutagenesis, spectroscopic and
Page 9 of 9, 1595 crystallographic investigation of three long-range electron pathways. Journal of Molecular Biology, 354, 385402. Pettersen, E. F., Goddard, T. D., Huang, C. C., Couch, G. S., Greenblatt, D. M., Meng, E. C., et al. (2004). UCSF Chimeraa visualization system for exploratory research and analysis. Journal of Computational Chemistry, 25, 10651612. Saratale, R. G., Saratale, G. D., Kalyani, D. C., Chang, J. S., & Govindwar, S. P. (2009). Enhanced decolorization and biodegradation of textile azo dye Scarlet R by using developed microbial consortium-GR. Bioresource Technology, 100, 24932500. Senthilkumar, S., Perumalsamy, M., & Prabhu, H. J. (2011). Decolourisation potential of white-rot fungus Phanerochaete Chrysporium on synthetic dye bath effluent containing Amino Black 10B. Journal of Saudi Chemical Society. doi:10.1016/j.jscs.2011.10.010. Usha, M. S., Sasirekha, B., Bela, R. B., Devi, S., Kamalini, C., Manasa, G. A., et al. (2006). Optimization of Reactive Black 5 dye and Reactive Red 120 dye degradation. Journal of Chemical and Pharmaceutical Research, 3(6), 450 457. Vijaykumar, M. H., Veeranagouda, Y., Neelakanteshwar, K., & Karegoudar, T. B. (2006). Decolorization of 1:2 metal complex dye Acid blue 193 by a newly isolated fungus, Cladosporium cladosporioides. World Journal of Microbiology and Biotechnology, 22, 157162. Yesilada, O., & Ozcan, B. (1998). Decolourization of Orange II dye with the crude culture filtrate of white-rot fungus, Coriolus versicolor. Turkish Journal of Biology, 22, 463476. Yi-Chin, T., Lin, Y. J., Obbard, J. P., & Yen-Peng, T. (2003). Decolourisation of azo-dyes by white-rot fungi (WRF) isolated in Singapore. Enzyme and Microbial Technology, 33, 569575. Zouari-Mechichi, H., Mechichi, T., Dhouib, A., Sayadi, S., Martinez, A. T., & Martinez, M. J. (2006). Laccase purification and characterization from Trametes trogii isolated Tunisia: decolorization of textile dyes by the purified enzyme. Enzyme and Microbial Technology, 39, 141148.
Você também pode gostar
- The Subtle Art of Not Giving a F*ck: A Counterintuitive Approach to Living a Good LifeNo EverandThe Subtle Art of Not Giving a F*ck: A Counterintuitive Approach to Living a Good LifeNota: 4 de 5 estrelas4/5 (5794)
- The Little Book of Hygge: Danish Secrets to Happy LivingNo EverandThe Little Book of Hygge: Danish Secrets to Happy LivingNota: 3.5 de 5 estrelas3.5/5 (400)
- Hidden Figures: The American Dream and the Untold Story of the Black Women Mathematicians Who Helped Win the Space RaceNo EverandHidden Figures: The American Dream and the Untold Story of the Black Women Mathematicians Who Helped Win the Space RaceNota: 4 de 5 estrelas4/5 (895)
- The Yellow House: A Memoir (2019 National Book Award Winner)No EverandThe Yellow House: A Memoir (2019 National Book Award Winner)Nota: 4 de 5 estrelas4/5 (98)
- The Emperor of All Maladies: A Biography of CancerNo EverandThe Emperor of All Maladies: A Biography of CancerNota: 4.5 de 5 estrelas4.5/5 (271)
- A Heartbreaking Work Of Staggering Genius: A Memoir Based on a True StoryNo EverandA Heartbreaking Work Of Staggering Genius: A Memoir Based on a True StoryNota: 3.5 de 5 estrelas3.5/5 (231)
- Never Split the Difference: Negotiating As If Your Life Depended On ItNo EverandNever Split the Difference: Negotiating As If Your Life Depended On ItNota: 4.5 de 5 estrelas4.5/5 (838)
- Elon Musk: Tesla, SpaceX, and the Quest for a Fantastic FutureNo EverandElon Musk: Tesla, SpaceX, and the Quest for a Fantastic FutureNota: 4.5 de 5 estrelas4.5/5 (474)
- Devil in the Grove: Thurgood Marshall, the Groveland Boys, and the Dawn of a New AmericaNo EverandDevil in the Grove: Thurgood Marshall, the Groveland Boys, and the Dawn of a New AmericaNota: 4.5 de 5 estrelas4.5/5 (266)
- The Unwinding: An Inner History of the New AmericaNo EverandThe Unwinding: An Inner History of the New AmericaNota: 4 de 5 estrelas4/5 (45)
- Team of Rivals: The Political Genius of Abraham LincolnNo EverandTeam of Rivals: The Political Genius of Abraham LincolnNota: 4.5 de 5 estrelas4.5/5 (234)
- The World Is Flat 3.0: A Brief History of the Twenty-first CenturyNo EverandThe World Is Flat 3.0: A Brief History of the Twenty-first CenturyNota: 3.5 de 5 estrelas3.5/5 (2259)
- The Gifts of Imperfection: Let Go of Who You Think You're Supposed to Be and Embrace Who You AreNo EverandThe Gifts of Imperfection: Let Go of Who You Think You're Supposed to Be and Embrace Who You AreNota: 4 de 5 estrelas4/5 (1090)
- The Hard Thing About Hard Things: Building a Business When There Are No Easy AnswersNo EverandThe Hard Thing About Hard Things: Building a Business When There Are No Easy AnswersNota: 4.5 de 5 estrelas4.5/5 (344)
- The Sympathizer: A Novel (Pulitzer Prize for Fiction)No EverandThe Sympathizer: A Novel (Pulitzer Prize for Fiction)Nota: 4.5 de 5 estrelas4.5/5 (121)
- The Effect of Temperature On The Hatching Success of Brine ShrimpDocumento2 páginasThe Effect of Temperature On The Hatching Success of Brine ShrimptahamidAinda não há avaliações
- The Influence of Giberelin On Stem GrowthDocumento11 páginasThe Influence of Giberelin On Stem GrowthDevina AlifahAinda não há avaliações
- General Biology - Chapter IDocumento10 páginasGeneral Biology - Chapter IG.k. Vinnan Rao100% (2)
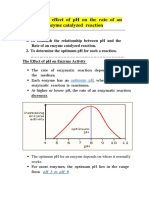
- Effect of PH On Enzyme ActivityDocumento12 páginasEffect of PH On Enzyme ActivityAb AbAinda não há avaliações
- Dihybrid ProblemsDocumento6 páginasDihybrid ProblemsVicky GuzmánAinda não há avaliações
- Protein Synthesis Notes Sheet 20-21Documento4 páginasProtein Synthesis Notes Sheet 20-21Cassandra KankamAinda não há avaliações
- Blue BrainDocumento20 páginasBlue BrainAnkitaPatel009Ainda não há avaliações
- Immune Response in The Human BodyDocumento30 páginasImmune Response in The Human BodyjohnosborneAinda não há avaliações
- Menigeal LymphoidDocumento10 páginasMenigeal LymphoidRenju KuriakoseAinda não há avaliações
- MotivationDocumento2 páginasMotivationPrachi Srivastava100% (1)
- PGA102 Presentation 1 (Eng. Ver.)Documento18 páginasPGA102 Presentation 1 (Eng. Ver.)Lee Wai KeatAinda não há avaliações
- Paired Passages - PresentationDocumento17 páginasPaired Passages - Presentationsuchi ravaliaAinda não há avaliações
- Eye - MCQs CorrectDocumento207 páginasEye - MCQs CorrectMaaz KhanAinda não há avaliações
- Activity No. 7.1 BloodDocumento2 páginasActivity No. 7.1 BloodDree SermanAinda não há avaliações
- Beckers World of The Cell 9th Edition Hardin Test Bank DownloadDocumento23 páginasBeckers World of The Cell 9th Edition Hardin Test Bank Downloaddaddockstudderyxeq100% (27)
- Guyabano Plant ResearchDocumento2 páginasGuyabano Plant ResearchAnnie OmlangAinda não há avaliações
- (English (Auto-Generated) ) Ep. 94 - Andres Ryan of BioAquatix - Com On Breeding Clown Loaches (DownSub - Com)Documento78 páginas(English (Auto-Generated) ) Ep. 94 - Andres Ryan of BioAquatix - Com On Breeding Clown Loaches (DownSub - Com)acromontiAinda não há avaliações
- 1 Human Anatomy Physiology With PathologyDocumento41 páginas1 Human Anatomy Physiology With PathologyEd dela PenaAinda não há avaliações
- Pathophysiology of Conjoined TwinsDocumento1 páginaPathophysiology of Conjoined TwinsPrincess Loreto AlegreAinda não há avaliações
- Summative Assessment QuizDocumento3 páginasSummative Assessment Quizapi-379149339Ainda não há avaliações
- Minimal Intervention Dentistry in General Dentistry PDFDocumento10 páginasMinimal Intervention Dentistry in General Dentistry PDFmostafahassan777Ainda não há avaliações
- Reference RangesDocumento8 páginasReference RangesKru PrimeAinda não há avaliações
- Dolly The SheepDocumento7 páginasDolly The SheepRhan AlcantaraAinda não há avaliações
- Car - Structural Bases For Shaping of DolinesDocumento18 páginasCar - Structural Bases For Shaping of DolinesCaegeoAinda não há avaliações
- 6 The Main PeriodsDocumento5 páginas6 The Main PeriodsUtami MAinda não há avaliações
- Arndt Schmidt-Denter Auer Weitere 2003Documento15 páginasArndt Schmidt-Denter Auer Weitere 2003Louis Fetilo FabunanAinda não há avaliações
- Immobilization of Catalase Via Adsorption Into NatDocumento6 páginasImmobilization of Catalase Via Adsorption Into NatErwin Guillermo Valdizón WinterAinda não há avaliações
- Lecture 3 Cell Cycle - QuestionsDocumento6 páginasLecture 3 Cell Cycle - Questions中华雅思王Ainda não há avaliações
- Dalandan (Citrus Aurantium Linn.) Peelings and Calamansi (Citrofortunella Microcarpa) Extract As An Alternative Fire Ant KillerDocumento6 páginasDalandan (Citrus Aurantium Linn.) Peelings and Calamansi (Citrofortunella Microcarpa) Extract As An Alternative Fire Ant KillerMaryknoll BernabeAinda não há avaliações
- 088 11MS9419 Print PDFDocumento11 páginas088 11MS9419 Print PDFNehaAinda não há avaliações