Escolar Documentos
Profissional Documentos
Cultura Documentos
Thermoset
Enviado por
galati123450 notas0% acharam este documento útil (0 voto)
92 visualizações12 páginasthermoset
Direitos autorais
© Attribution Non-Commercial (BY-NC)
Formatos disponíveis
PDF, TXT ou leia online no Scribd
Compartilhar este documento
Compartilhar ou incorporar documento
Você considera este documento útil?
Este conteúdo é inapropriado?
Denunciar este documentothermoset
Direitos autorais:
Attribution Non-Commercial (BY-NC)
Formatos disponíveis
Baixe no formato PDF, TXT ou leia online no Scribd
0 notas0% acharam este documento útil (0 voto)
92 visualizações12 páginasThermoset
Enviado por
galati12345thermoset
Direitos autorais:
Attribution Non-Commercial (BY-NC)
Formatos disponíveis
Baixe no formato PDF, TXT ou leia online no Scribd
Você está na página 1de 12
89
Thermoset Powder
Coatings
89.1. Introduction 89-1
Power Coatings Defined» The First Dwr Coatings ©
Thezmoset Beginnings» The Begining of Groth +The World
Discovers Powter Castings
89.2. Processing and Equipment 89.3,
Premature Extrusion + Grinding Sifting and Clasiying ©
Application Equipment
89.3. Chemisty. 89-6
Epoxy Systems» Pojester Spats» Acrylic Syatems
89.4 Formulation. 89-11
Resin Systems Pigments and Filer Aditves
Lawrence R. Waelde 895. End Uses 89-12
Boy Corporation Relerences. 89-12
89.1 Introduction
In recent years, avateness of environmental conservation and pollution prevention has risen steadily
Governmental regulation and true concern for the environment have motivated chemists to modify all,
types of coatings to reduce environmental impact. The concept of environmental “iriendliness” has
dramatically changed the way that coatings ate formulated,
Powder coatings are arguably the most environmentally “iriendly” coatings. They do not contain
solvents to be released as hazardous air pollutants (HAPS). Powder coatings release very low amounts of
volatile organic compounds (VOCs) during the baking cycle. They
‘And, they contain very few hazardous chemicals. (Note: The few hazardous chemicals that have found
their way into powder coatings are decreasing as they are replaced with safer materials.)
roduce virtually no waste material
89.1.1 Powder Coatings Defined
Powder coatings can be described as “ground up dry paint.” They may also be referred to as pulverized.
plastics. They have properties in common with both materials. The polymeric resins that ate used in
producing powder coatings are similar in nature to those used in both paints and plasties. All three
‘materials are composed of combinations of resin, pigment, fille, and various additive materials. They
say be thermoplastic or thermosetting.
‘The primary diflerence between the three types of compounds is the molecular weight range of the
polymers used as binders. Plastics use the highest molecular weight resins, paint the next highest, and.
powder coatings the lowest. Paint, of course also contains various solvents to dissolve or dilute the coating,
for easy application
89.1
89.2 Coatings Technology Handbook, Third Edition
89.1.2 The First Powder Coatings
Powder coatings are relatively new finishes. The first application of a “dry paint” occurred in the 1940s."
At that time, pulverized thermoplastic formulations were flame sprayed over metallic substrates. The
plastics melted in the flame, flowed onto the substrate, and cooled there to form a protective coating
‘This was a poor way to apply a protective barrier, as it was very wasteful, Material decomposed in the
flame and was lost. Furthermore, there was very little contzol of film uniformity or thickness that was
built up on the substrate. It was not uncommon for films to exceed 100 mils (~2500 microns).
In 1950, British patent was issued to Schori Metallzing Process, Lt, fora “hot-dip” coating process?
By this process, a heated metal part was dipped or rolled ina thermoplastic powder. Loss through decom-
position was minimized, but film build was sil extremely high. It was also difficult to maintain clean plant
conditions, as handling the parts throughout the coating process produced high dust conditions.
‘The next innovation in the application of thermoplastic powders occurred in 1953. A patent was
applied for by Erwin Gemmer of Knapsack-Griesheim AG (later acquired by Hoechst) for a “fluidized
bed” coating process” By this process, pulverized material is“Mloated” in a container by upward movement
of airstreams that are forced to diffuse through a bed of porous material. The floating powder takes on
properties of a fluid in that the particles “flow” in the aitstreams much like molecules of boiling water
bubbling in a saucepan, A German patent was issued for this process in 1955 and a US. patent in 1958
In luidized bed application, the metal substrate is heated above the melting point of the powder and
dipped into the fluidized material. Powder that touches the metal melts and adheres to the part. The past
js then transported to the following stage of production by conveyor. The process is much cleaner than
dipping or rolling, but film build is tll ery high,
89.
Upto this point, all powder coatings were thermoplastic in nature, Thermoset powder coatings were just
beginning to be explored. The first trials were made with existing resins or slightly modified polymers,
‘They had low glass transition points (T,) and tended to sinter (cake) upon standing for any length of time
In the early 1960s, two developments occurred almost simultaneously. The first development was the
invention of storage stable thermoset powder coatings.® They were epoxies based on diglyeidyl ethers of
Bisphenol A. The second invention was the electrostatic spray gun, which was capable of applying a
powder coating under controlled conditions."
Electrostatic application of powder coatings is performed by pumping a stream of coating particles in
an airstream past a igh voltage cascade generator. The powder acquires an electrostatic charge and flows
through the generated electrical field to a grounded metal substrate, The substrate is then transported
to an oven where the coating melts, lows out, and is cured,
3. Thermoset Beginnings
89.1.4 The Beginning of Growth
‘The electrostatic spray gun is probably the single most important invention in the rapid growth of
thermoset powder coatings. With this equipment, vitually all of a powder coating product can be used.
in application, as oversprayed material can be reclaimed and used. Film build can be reduced to les than,
5 mils (~125 microns). In fact, film thickness similar to that possible with liquid coatings can now be
achieved (<1.0 mil or ~25 microns)
It has been reported that the world production of thermoset powder coatings in 1962 was around
16,000 Ib (ca. 73 metric tons). All of these coatings were epoxies. By 1966, the volume had grown to
100,000 ib (ca. 45.4 metric tons).
During 1969, the frst polyester resins designed for thermoset electrostatically applied powder coatings
were developed.’ They took a long time to cure and had caking problems, Nevertheless, powder coating
production continued to increase.
By 1972, the world production of thermoset powder coatings had grown to 11.6 million pounds (ca.
5300 metric tons). That same year, the first carboxyl-terminated polyester resins were produced,” They
‘Thermoset Powder Coatings 89.3
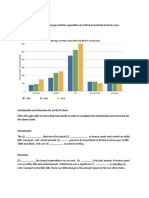
Pounds (000s)
1962. 1966 1969 1972 1975 1980 1984 1988 1992 1996 2000 2003
FIGURE
“Worldwide growth of powder coating production for the years 1962 through 2003,
were the first polyester resins that eliminated the problem of outgassing due to reaction products forming,
bubbles and pinholes in the coating film.
Polyester resins with higher reactivity and, therefore, faster cure, were first introduced in 1974.7 They
started to gain in popularity in the next couple of years, and by 1983, the first trimellitic anhydride-ree
(TMA) polyesters were produced?” These were the earliest attempts to eliminate hazardous chemicals.
from powder coating formulae.
89.1.5 The World Discovers Powder Coatings
Powder production continued to grow at a fantastic rate, World production had grown from 162.8 million
pounds (74,000 metric tons) in 1980 to 633 million pounds (ca. 290,000 metric tons) in 1990.8
About that time, it was discovered that TIC (triglycidylisocyanurate) could cause mutation in the
chromosomes of lab mice. TGIC had been used as a cross-linker with certain carboxyl-terminated
polyesters to produce extremely durable exterior coatings.
Immediately, chemists began to search for nonmutagenic alternatives. And, in 1990, the first non-
‘TGIC cross-linkers for polyester resins were introduced.” The first was a f-hydroxyalkyl polyamide. It
was followed in 1991 by tetramethoxymethyl glycoluril
Even with the concern over mutagenic materials, powder production has continued to grow rapidly
Worldwide production of thermoset powder coatings for 1996 exceeded 1.13 billion pounds (over 1/
2 million metric tons). The projected growth for the year 2002 is more than 1.8 billion pounds
(827,000 metric tons) worldwide and for 2003, it was over 1.9 billion pounds (> 880,000 metric
tons) (see Figure 89.1).
89.2 Processing and Equipment
‘The process of producing a powder coating is somewhat more complex than that for other types of
coatings. The equipment needs are more numerous, The production time is also somewhat longer. This
section will describe each of the necessary pieces of equipment in the order that they are used during
the process of making a powder coating,
89.2.1 Premixture
‘The first step in the manufacture of a powder coating is the premix stage. This is the most crucial phase
of production. The various sizes of resin flake, pigment powder, ete, must be transformed into a
homogenous blend of similar particle size before they enter the following stage — extrusion
89.4 Coatings Technology Handbook, Third Edition
FIGURE 9.2. Generalized schematic of premix equipment.
Because the extruder is a simple melt mixer, and not a very efficient disperser, the premix stage must
allow for the best possibility of good dispersion of all ingredients in the final product. Thus, premixture
js performed in equipment with suficient ability to break down the large resin flakes, thoroughly blend
any liquid components, and disperse the smaller quantity constituents, such as additives.
89.2.1.1 Premixers
Most premix equipment consists of a mixing bowl with a locking cover. The equipment is usually fitted
with a cooling jacket capable of being filled with running water of, in rare cases, cryogenic gasses, The
‘mixing blades within the bowl may have various configurations. However, there are generally two or
three blades placed at diferent heights and orientation to one another (Figure 89.2).
89.2.2 Extrusion
‘The second stage of powder coating manufacture is extrusion of the premix. The extruder is basically
composed of a horizontally placed stainless stel tube or “barrel” Within the barrel are one or two“screws”
that are slightly smaller than the barrel in diameter. The serews turn and move the premix forward
through heated “mixing zones” The mixing zones have special attachments, called mixing paddles or
blades. The paddles knead the melted premix and blend the various ingredients
‘Contrary to what may be imagined, the extruder is not a very good disperser. The space between the
screws and the barrel walls is relatively large and allows material to travel through without dispersing it
thoroughly. Also, the coating mixture passes through the barrel fairly rapidly and does not have time to
be dispersed efficiently
89.2.2.1 Extruders
‘There are three basic types of extruder. The first type, the single screw extruder, is most offen used in
the plastics industry. It does not move material forward as rapidly as other types, and thermosetting,
coatings could react in the bareel and gel prematurely. Thermoplastic materials used in injection molding
are commonly prepared in single screw extruders. However, there is one type of single screw extruder
used in the thermosetting powder coatings industry. It has special attachments along the sides of the
barrel that aid in the blending of the ingredients
‘Thermoset Powder Coatings 89.5
Forwarding ting Faveraing
(open tights) (wadcles) | |_(camel back)
Twin Seven Agitator for Powder Coatings
FIGURE $9.3 Typical configuration for twin screw corotating extruder
‘The second type of extruder isthe counter-rotating twin screw extruder. It is composed of two screws
placed side-by-side and turning in opposite directions (ie., one turns clockwise and the other counter-
clockwise). This type is also usually used in the plastics industry.
‘The third kind of extruder, which is used in powder coating production, is the corotating twin screw
extruder (see Figure 89.3). In this type of equipment, the two side-by-side screws turn in the same
direction, This greatly increases throughput and decreases residence time. Reduced residence time is
important in thermoset coatings, especially in the most reactive systems with short gel times.
89.2.3. Grinding
‘The grinding phase in the manufacture of a powder coating is important to its final performance. The
average particle size and distribution are important in the application properties and final surface
appearance of the coating.
‘Once a powder coating has been extruded, it passes through one or more stages to prepare it for
grinding, The molten material is dropped onto chilled metal rollers, where itis pressed flat and cooled,
‘The coating is then broken into flakes or granulated and fed to the grinder.
89.2.3.1 Grinders
‘There are three favored types of grinding equipment used in the powder coating industry. They ate the
impact or hammer mill, the air jet mill, and the air classifier mill.
‘The impact or hammer mill grinds the coating materials by throwing them or hitting them against a
stationary sereen or stator.” The particles are broken down until they are small enough to fit through,
the screen, The particles are then passed to various sieves or cyclonic separators for final particle size
distribution adjustments.
"The ait jet mill isan extremely efficient grinder. Powder coatings are fed into a high volume and high
velocity airstream, The particles circle the cylindrical grinding chamber striking other particles and
breaking them into very fine powders. The fine material rises out of the chamber with the escaping air,
through conduits, to separation devices that structure the particle size distribution,
‘The air classifier has elements of both of the previous types. There is an impact chamber that is
supplemented with airstreams that rapidly break down the coating materials. This provides excellent
89.6 Coatings Technology Handbook, Third Edition
control of particle size distribution, and the moving air tends to cool the powder, minimizing heat effects,
such as impact fusion.
89.2.4. Sifting and Classifying
‘The inal structuring of particle size disteibution is accomplished by passing the ground materials through,
sieves or cyclonic separators. Sieves “scalp” the largest or smallest particles, which narrows the particle
size distribution. By judicious use of sieves, the distribution and average particle size may be chosen for
best application and surface properties.
Cyclonic separators or “classifiers” function by forcing powders in arstreams to circle conical chambers.
‘The heaviest (largest) particles will fall to the bottom of the chamber and will be collected for use or
dumping. The smallest particles will flow out through the top with escaping air, will flow through
conduits, and will be saved or discarded as the need arises. By adjusting the volume and velocity of the
airstream, a particle size distribution can be varied.
89.2.5 Application Equipment
‘There are three primary methods of applying powder coatings. The first uses a fluidized bed. As described
calie, the fluidized bed transmits a dilfuse steam of air upward through the bottom of a container holding
the coating to be applied. The airstream is kept at low enough pressure so that the powder rises into the
air but is not expelled from the container. Asair escapes past the floating particles, the surface ofthe powder
‘mass appears to bubble or “percolate” The term “fluidized” refers to this luid-like motion. ‘The metal part
tobe coated is heated above the melting point ofthe coating and is “dipped” into the fluidized bed. Powder
touching the part melts and adheres to the metal. The part is then transported to a curing oven,
‘The second and third methods of application utilize electrostatic spray guns. There ate two basic types
of gun: corona-charged and “tribo” -charged. The corona-charged spray gun applies an electrostatic charge
to the powder as the material flows through an electrical field generated at the gun tip of the gun. The
twibo-charged gun has a polytetraflouroethylene (PTFE) barrel, and powder flowing through it acquires
an electrical charge from friction. The charged powders ate sprayed onto grounded metal parts, where
they adhere through electromagnetic attraction. The parts are then transported to the curing oven,
89.3 Chemistry
‘The chemistry of powder coatings is not very different from that of liquid coatings. The main resin types
used in thermosetting powder coatings ae all familiar to the liquid coatings chemist. The epoxy, polyester,
acrylic polymers are well known in liquid coatings. However, the molecular weight and curing agents
are somewhat different. From these three resin types, a number of different cross-linked systems are
possible, The most common will be discussed here. Note that each subsection describing a cross-linking.
pair includes a common industry “nickname” in parentheses
89.3.1 Epoxy Systems
‘The most common epoxy resins in use today are diglycidyl ethers of Bisphenol A made from reaction of
epichlorohydrin with Bisphenol A. Diglycidyl ethers of Novolac resins also have significant commercial
impact
A f° i A
Diglycerde Ether of Bisphenol A Resin
‘Thermoset Powder Coatings 89.7
"The epoxy groups terminating each molecule react with acidic or basic curing agents. The three most
common are the phenols, dicyandiamides (DICY), and carboxylic acids, including carboxy-terminated.
polyesters. Various acids, anhydrides, amines, and imidazoles ae also used as crosslinkers with epoxy resins
°,
JN
o—cn.-oh on,
0,
chronmoiro—{ pois one o-cH—ch be,
Diglciay Ether of Novolac Resin
89.3.1.1 Epoxy-Phenols (Phenolic)
‘The curing of epoxy resins with phenols results in the opening ofthe epoxide ring and the formation of
hydroxyl group, at either the primary oF secondary position. The hydroxyl group is available for reaction
inthe cross-linking ofthe resin. The aromatic ring attaches to the unreacted carbon of the epoxide.
A e
89.3.1.2 _ Epoxy-Dicyandiamide (DICY)
ICY cured epoxy coatings eact in a similar manner to that ofthe previous type, where nitrogen-bearing
groups replace the aromatic ring, All four functional groups will react with the epoxide, acting as a
primary oF secondary amine
on
ocd on
oH
Meg Ni —on-di-chtro—a
N-< a ;
W\, ¢ CHP CH-cHro—R
NH -
icy Epoxy Resin Crosslink intermediate
89.3.1.3 Epoxy-Polyester (Hybrid)
Epoxy esins reat with carboxy-functional polyesters in the same way a carboxylic acids. The hydroxyl portion
of the acid group reacts with the epoxide. The rest of the reaction follows as we have seen in the previous
examples, Because both reactants are considered primary resins the system is referred to as a “hybrid.”
89.8 Coatings Technology Handbook, Third Edition
a
(giroH-cHro— Re
on
054.9 i
Ho S68 on P—CHsCH-cHO—AE
Dene-nt eg,
oa
Epony Resin CCrossnk intermediate
89.3.2 Polyester Systems
‘There are two types of polyester resins used in thermoset powder coatings. They are carboxy-and hydroxy-
functional. There are several diferent curing agents used with each. We will examine the four most
common systems,
893.
‘Triglycidyisocyanurate (TGIC) is a heterocyclic tri-epoxy curing agent. As such, it reacts with earboxy-
functional polyester resins similar to those used in hybrid coating systems. The major difference i in the
wif unctionality. One mole of TGIC will tact with three moles of polyester resin. The eross-link network
is aso more complex because of the extra reactive sites available
1 Polyester-Triglycidyl Isocyanurate (TGIC or Polyester)
°. °
cH ch—cH—N~ ced,
TeIc coon Polyester
Crosaink intermediate
‘Thermoset Powder Coatings 89.9
89.3.2.2 Polyester-Isocyanate (Polyurethane)
Polyester resins used to produce urethanes have hydroxyl functionality. They react at the carbon-nitrogen
bonds in an isocyanate. The two isocyanates most used in powder coatings are isophorone diisocyanate
(IPDI) and toluene diisocyanate (TDD. However, TDI melts at room temperature, and IPDI is a liquid,
‘This would cause any powder coating in which they were used to have poor package stability. The coating
would sinter (cake) into a solid very quickly. Furthermore, they would react with the polyester and gel
during the heat of extrusion, again making them unusable.
‘Consequently, isocyanates are reacted with triol materials to give them higher melting points and
better package stability when compounded in powder coatings. Then they are blocked, most commonly
with (epsilon) e-caprolactam, to prevent them from reacting with the polyester until they unblock
naturally at curing temperatures inthe oven
° ot Gio
bn +0, on
omy
ot N77
8
§ |
Ha6 ‘ori N=c=0 OH
capolcam _lophrone Biscoan Tene Deseyanate We
3
chy cH, 8
Me cH nt
8. g on
Sound oF n-chSons ;
a + HO—R1I—OH —»
i cit
gn AT oi,
g
%
ED
Cepromtan Bost 01 Ho-Foyenee
oa i
° °
Hom t—o—ton. wb ot. on
CCrosslink Intermediate ‘Caprolactam
89.10 Coatings Technology Handbook, Third Edition
893.23. Polyester-Non-TGIC ({-Hydroxyalkylamide, Tetramethoxymethyl Glycoluril)
As we discussed eatlier, 1
vas found to have mutagenic properties. While some controversy still
remains as to its hazardous nature, many coatings companies and regulatory bodies have taken a con-
servative stance and limited its use. There have been regulations enacted that requite warnings to be
placed on labels of coatings that contain TGIC. Coatings companies have also turned to curing agents
that do not have these types of hazards associated with them,
‘The two types that are used most often are the (j-hydroxyalkylamides and tetramethoxymethyl gly-
coluril. The hydroxy-amides are tetrafunctional, which makes them highly reactive at curing temper
tures. Theit major drawback is that they release water from the reaction that must escape the curing film,
‘This requites further formulation to insute defect-ire films. The glycolurl is also teteafunetional and
releases a VOC, methanol, as part of the reaction process
@ a
wo-bi-ce 9 9 _om-dh-on
Sy dane bow .
wo-cn-o% Nuicgion
i it
Pyne COOH Poet
g m » 8
asgarcell—o—dh cm, qustiro— const
OH é . ‘OH
of
a -0,,
N—GaRe- + Wy
° .
ats Oo
aac coat cncgio—g-o-feed
tom ot
Cea knee vir
89.3.3 Acrylic Systems
‘There ate two primary acrylic systems: those based on hydroxy-functional acrylic resins and those using
epoxy- or glycidyl-functional polymers. Carboxy-functional materials have been produced. However,
they have not made much progeess into the industry
89.3.3.1 Acrylic-Isocyanate (Acrylic-Urethane)
‘Acrylic-urethanes are formed in exactly the same way as their polyester counterparts. The acrylic resins
are linear instead of aromatic. However, they use hydroxy-functionality and blocked-isocyanates to form
the urethane bonds.
89.3.3.2 Acrylic-Diacid (Glycidyl-Acrylic)
Epoxy- (glycidyl) functional acrylic resins can be compared tothe hybrid systems discussed earlier. They
are generally reacted with dicarboxylic acids or anhydrides. The most common crosslinker is 1,12.
decanoie acid (1,12-dodecane dioie acid).
‘Thermoset Powder Coatings 89-11
89.4 Formulation
89,
Formulation of thermoset powder coatings is much the same as that for liquid coatings used for similar
purposes. A coating may be chosen for functional or decorative purposes. All of the coating types used
in powder coatings can offer decorative options. Nonetheless, itis function that dictates system choice
‘The resin chemistry must be chosen to suit service needs. Various pigments, filles, and additive materials
are then included to enhance decorative or functional requirements.
“There are two primary functions for any protective coating, They are chemical protection and exterior
durability. As with many coating properties, these two tend to be in opposition, The best systems for
chemical protection ate usually the poorest for exterior durability.
Epoxy powder coating systems deliver the best chemical and corrosion resistance. However, they have
the least effective exterior durability. The double bonds in the aromatic rings are easily broken by the
ultraviolet (UV) light from the sun. Glossy finishes will go flat with as litle as 6 months of exposure,
with film degradation following soon thereafter.
Urethane systems are also very good for chemical resistance, and they have fairly good exterior
durability, aswell. Hybrids offer good chemical resistance, however the polyester component makes them
less effective. They are also poor in relation to exterior exposure due to the epoxy portion of the eross-
link network.
‘Acrylic and TGIC powder coatings provide the best exterior durability. Some systems can survive for up
to 20 years of exposure, They offer fair to good chemical resistance. Urethane systems seem to be the best
compromise between chemical and exposure properties. As mentioned, they are very good in both areas.
-1 Resin Systems
89.4.2 Pigments and Fillers
Most pigments and fillers used in liquid coatings ate suitable for use in powder coatings." There are only.
afew special requirements for use, They must be sufficiently heat stable so they withstand the heat of
extrusion and curing without degradation or color change. Normally, the heat of extrusion is 125°C or
less for a minute or two, The heat of cure is usually 160 to 200°C for 10 to 20 min
Second, they must be insoluble and nonreactive in the resin system, Blooming and color shift are the
‘most common results from pigments that are partially soluble or reactive with the binder. Some epoxy
system curing agents are especially susceptible to reaction with pigments
89.4.3 Additives!
89.4.3.1 Flow and Leveling
Flow and leveling agents are designed to minimize surface defects such as craters, pinholes, and orange
peel. The mechanism oftheir function alters the surface tension and rheology of the coating, The likelihood
of a smooth delect-free film is improved by reducing one (or both) of these properties ina coating,
Most flow and leveling agents are liquids. Many are blended with inert inorganic materials to offer
them in a conveniently solid form, The chemistries of these are usually polyacrylates or polysiloxanes. A
few new flow agents are available, however in solid organic form.
8943.2 Debubbling (Degassing)
‘The most common debubbling agent is benzoin (2-hydroxy-1,2-diphenyl ethanone). It is used to keep
the surface of a curing film open long enough to allow for entrained air and evolved gasses to escape
‘Trapped air and gas bubbles are cause for premature failure of coating films, because they make the
coating brittle. The one drawback tothe use of benzoin sits tendency to cause yellowing in lighter colors.
‘A number of new advances have entered the market in an attempt to match the efficiency of benzoin
Without the challenge of yellowing
89.12 Coatings Technology Handbook, Third Edition
89.4.3.3 UV Inhibitors
Various UV light inhibitors are available to aid coating resistance to degradation by the sun's rays. The
‘most common are hindered amines, phosphites, sulfates, and phenolics. Most will have some positive
effect on any coating’s UV resistance. However, each system will require testing to determine the best
combination of inhibitors. Some systems, like epoxies and hybrids, will not develop any substantial UV
resistance due to their aromatic nature.
89.43.46 Catalysts
Catalysts or accelerators are used to reduce the reaction time or curing temperature of the resin and
cxoss-linke. They allow for faster production time by shortening the gel or “se” time ofthe thermosetting
coating. Energy can be conserved, because full cure may be attained at lower oven temperatures. The
‘most common catalysts are thiazole (usd in polyestes), phosphines and ammonium halides (used in
epoxies) and thiocarbamates (used in urethanes)
89.5 End Uses
‘Thefollowing table details some of the applications currently using the chemistries discussed in this chapter.
Type “Typical Applications
Epoxy Shelving, transformer cases, primers, bathoom fixtures, veligerator racks, sweepers, sewing machines, power
tool, 100m air conditioners, office faentue, nstumeat cass, garden wos kitchen furitue ee
etinguishers, toys, efrgerato liners dryer drums, microwave ovens, mixersand blenders, fries spreader,
Screening ol filters, automobile springs, hospital equipment, bus seat fames, business machines, lass Doles
Hybrids Toolbox, farm equipment, clerical contol bores, ho water heaters, hot water radiators, primevsuracrs,
sain storage ins, transformer covers! ites and ait cleaners, ar conditioner housings, re extinguishers,
toys, screening wit, power tools, shelving ofice furniture
Urethane Fluorescent ight fixtures, eel and aluminum weds, patio furniture playground equipment, fence ftings,
home wheels and trim, garden tractors, range side panels and broier, ornamental fon ai conditioner
cabinets restaurant furnace supports, wansformer cases
TGIC —_Irvigaton pipe and fires, outdor furnace air conditioning units, sel and aluminum, wheels, wie fencing,
fence poles and fitings, frm equipment, aluminum extrusions, transformers
Acrylic Range ide panes refrigerator cabinets and doors, washing machine pats, dishwasher exterior, aluminum
extrusions, microwave ovens, garden tractors, aulomotive rim coating
References
1. Douglas 8. Richart, “Powder coatings — A review of the technology” Am. Paint & Coat. J, April
22,(1991),
2. P.G. Clements. Patent GB 643,691; 9/27/50, Schori Metallizing Process, Ltd.
3. E, Gemmer, Patent DE 933,019; 9/15/35, Knapsack-Griesheim AG.
4. E.Gemmer, Patent US. 2,844.489; 7/22/58, Knapsack-Griesheim AG.
5. Patent GB 915,575.
6. E.P Miler, “Electrostatic finishing methods,” paper presented at the Annual Meeting of the
"National Paint, Varnish, & Lacquer Association, Colorado Springs, CO, September 12, 1963.
7. DLA. Bates, The Science of Powder Coatings, Vol. 1. London: Scholium Intl, 1990.
8. Statistics from Powder Coatings Institute, Alexandria, VA,
9. Rohm and Haas Brochure 82F2, Primid XL-352 — A Novel Crossinker for Powder Coatings, 1990.
10. Pulverizing Machinery (product brochure), Mikropul Corp. Summit, NJ.
11. R. Campbell and R. Kumar, “Organi pigments for powder coatings” Am, Paint & Coat. J, Apeil
22.(1991),
12, Josel H. lek, Powder Coatings (monograph). Blue Bel, PA: Federation of $
‘Technology, 1991,
cities for Coatings
©2009 Tyr Ar Gap.
Você também pode gostar
- Epoxy - WikipediaDocumento11 páginasEpoxy - Wikipediaramthecharm_46098467Ainda não há avaliações
- Chopper FanDocumento2 páginasChopper FanJulio CAinda não há avaliações
- 73fc3013fec43c9 - Ek Bond Energy and ImpactorDocumento4 páginas73fc3013fec43c9 - Ek Bond Energy and ImpactorKallol MahalanabisAinda não há avaliações
- Thermosets: A "Thermoset" Is A Cross-Linked Polymer Formed by An Irreversible Exothermic Chemical ReactionDocumento58 páginasThermosets: A "Thermoset" Is A Cross-Linked Polymer Formed by An Irreversible Exothermic Chemical ReactionyigitilgazAinda não há avaliações
- Chapter 5Documento25 páginasChapter 5LAURA MILENA VALLES CALDERONAinda não há avaliações
- Optical Properties of Paints and CoatingsDocumento4 páginasOptical Properties of Paints and CoatingsJustine CabuayAinda não há avaliações
- MetallizingDocumento26 páginasMetallizingShubham KumarAinda não há avaliações
- Experiment 6Documento3 páginasExperiment 6Subhasis BiswalAinda não há avaliações
- NYLON 6,6 (Nylon 6) : OverviewDocumento4 páginasNYLON 6,6 (Nylon 6) : OverviewKaruppiah VigneshAinda não há avaliações
- Brosch - Biokunststoffe Web v01 - 1 PDFDocumento68 páginasBrosch - Biokunststoffe Web v01 - 1 PDFMike AndersonAinda não há avaliações
- Overview Nano MaterialsDocumento34 páginasOverview Nano MaterialsAparna AkhileshAinda não há avaliações
- Ha SpongeDocumento45 páginasHa SpongeSantoso NugrohoAinda não há avaliações
- PolytetrafluoroethyleneDocumento27 páginasPolytetrafluoroethyleneTzuyu Chou100% (1)
- Polyethylene Demand & SupplyDocumento4 páginasPolyethylene Demand & Supplysadam_madas2050% (2)
- Effect of Phase Transformation On Optical Properties of Zntio3 Ceramic Powder Prepared by Sol-Gel MethodDocumento18 páginasEffect of Phase Transformation On Optical Properties of Zntio3 Ceramic Powder Prepared by Sol-Gel MethodIJAR JOURNALAinda não há avaliações
- Fabrication of Ceramic Matrix Composites by Liquid Phase InfiltrationDocumento5 páginasFabrication of Ceramic Matrix Composites by Liquid Phase InfiltrationyoukahoAinda não há avaliações
- 6830 en 7 Myths About PVC DebunkedDocumento5 páginas6830 en 7 Myths About PVC DebunkedNishi JhaAinda não há avaliações
- Chenghong Li Siloxane Magnetic FluidDocumento150 páginasChenghong Li Siloxane Magnetic FluidniebelungenAinda não há avaliações
- Xylene From MethylationDocumento8 páginasXylene From Methylationalicia1990Ainda não há avaliações
- Flexible Intermediate Bulk Containers Specifications PDFDocumento13 páginasFlexible Intermediate Bulk Containers Specifications PDFZahir Khira100% (1)
- PolytetrafluoroethyleneDocumento19 páginasPolytetrafluoroethyleneMulyanto MulyonoAinda não há avaliações
- PolyetheretherkeytoneDocumento4 páginasPolyetheretherkeytoneGriffin BeemillerAinda não há avaliações
- Silica Gel Technology For Tailor-Made Matting AgentsDocumento4 páginasSilica Gel Technology For Tailor-Made Matting AgentsNez ArdenioAinda não há avaliações
- Characterization of METHOCEL Cellulose Ethers by Aqueous SEC With Multiple DetectorsDocumento11 páginasCharacterization of METHOCEL Cellulose Ethers by Aqueous SEC With Multiple DetectorsCastoriadisAinda não há avaliações
- 1112impact of Petroleum Jelly On The Ageing of Telephone Wire FinalDocumento6 páginas1112impact of Petroleum Jelly On The Ageing of Telephone Wire FinalNavneet SinghAinda não há avaliações
- Metal Bearing Waste Streams: Minimizing, Recycling and TreatmentNo EverandMetal Bearing Waste Streams: Minimizing, Recycling and TreatmentAinda não há avaliações
- Atomic Force Microscope (AFM) : Block Copolymer Polymer BlendDocumento71 páginasAtomic Force Microscope (AFM) : Block Copolymer Polymer Blendsvo svoAinda não há avaliações
- An Example: These Guidelines Applied To The Safe Automation of A Batch Polymerization ReactorDocumento15 páginasAn Example: These Guidelines Applied To The Safe Automation of A Batch Polymerization ReactorIamsAinda não há avaliações
- Overall Aspects of Non-Traditional Glasses: Synthesis, Properties and ApplicationsNo EverandOverall Aspects of Non-Traditional Glasses: Synthesis, Properties and ApplicationsAinda não há avaliações
- Cross-Linking Organic Coating With BlockedDocumento6 páginasCross-Linking Organic Coating With Blockedalfi alfathanaAinda não há avaliações
- Emulsion Polymerization 2Documento13 páginasEmulsion Polymerization 2ismahAinda não há avaliações
- STruture and Properties of PolymersDocumento35 páginasSTruture and Properties of PolymersWasif Razzaq100% (1)
- Characteristics, Applications and Processing of Polymers Chapter 15Documento53 páginasCharacteristics, Applications and Processing of Polymers Chapter 15tjandelkier100% (1)
- Blocked and Deblocked Isocyanate With Sodium BisulfiteDocumento19 páginasBlocked and Deblocked Isocyanate With Sodium BisulfiteAdlyLubis100% (1)
- Armstrong Flooring Catalog 2012-13Documento166 páginasArmstrong Flooring Catalog 2012-13Steven PentonAinda não há avaliações
- Colour Subs F CMDocumento70 páginasColour Subs F CMLucia OchovaAinda não há avaliações
- Tie Layers Development For Triple Bubble® TechnologyDocumento81 páginasTie Layers Development For Triple Bubble® TechnologyElizabeth OlivaresAinda não há avaliações
- NylonDocumento65 páginasNylonAkash YadavAinda não há avaliações
- Polyethylene Properties - VinidexDocumento8 páginasPolyethylene Properties - VinidexalexAinda não há avaliações
- Quantification of The Maleic Anhydride Grafted Onto Polypropylene by Chemical and Viscosimetric Titrations, and FTIR Spectros PDFDocumento11 páginasQuantification of The Maleic Anhydride Grafted Onto Polypropylene by Chemical and Viscosimetric Titrations, and FTIR Spectros PDFThinh DangAinda não há avaliações
- Natural Foam Blowing AgentsDocumento178 páginasNatural Foam Blowing AgentssohailAinda não há avaliações
- Inorganic Chemistry 4 - SCH 304Documento10 páginasInorganic Chemistry 4 - SCH 304Arsalan ChoudharyAinda não há avaliações
- Facts About PET - 25 March 2013Documento7 páginasFacts About PET - 25 March 2013Cátia CoelhoAinda não há avaliações
- Handbook Adhesive TechnologyDocumento29 páginasHandbook Adhesive TechnologyVansala GanesanAinda não há avaliações
- New Stabilizer Solutions For Polyolefin Film GradesDocumento52 páginasNew Stabilizer Solutions For Polyolefin Film GradesZirve Polimer100% (1)
- Review EVOHDocumento39 páginasReview EVOHMaximiliano SalazarAinda não há avaliações
- Breathability & Moisture Vapour Permeability: Technical DigestDocumento4 páginasBreathability & Moisture Vapour Permeability: Technical DigestcormolioAinda não há avaliações
- How to Name an Inorganic Substance: A Guide to the Use of Nomenclature of Inorganic Chemistry: Definitive Rules 1970No EverandHow to Name an Inorganic Substance: A Guide to the Use of Nomenclature of Inorganic Chemistry: Definitive Rules 1970Nota: 5 de 5 estrelas5/5 (1)
- Fluoropolymers Coatings - Extreme PerformanceDocumento5 páginasFluoropolymers Coatings - Extreme PerformanceAnselmo RibeiroAinda não há avaliações
- Volumetric DilatometryDocumento14 páginasVolumetric DilatometryNasim MalekiAinda não há avaliações
- Easing Your Way To Reliable Peelable Seals HahmDocumento8 páginasEasing Your Way To Reliable Peelable Seals HahmAbhineet ShrivastavaAinda não há avaliações
- Dimethyl TerephthalateDocumento9 páginasDimethyl Terephthalatehung_metalAinda não há avaliações
- Processing The DentureDocumento8 páginasProcessing The DentureMody ShaheenAinda não há avaliações
- PC and ABSDocumento8 páginasPC and ABSDaisyAinda não há avaliações
- Polymer Synthesis AND Characterization: Basic Laboratory Course For Polymer Science M. Sc. ProgramDocumento51 páginasPolymer Synthesis AND Characterization: Basic Laboratory Course For Polymer Science M. Sc. ProgramFA AyAinda não há avaliações
- Polyester WeatherabilityDocumento10 páginasPolyester Weatherabilitynikopigni2Ainda não há avaliações
- 5 Threads and FastenersDocumento126 páginas5 Threads and Fastenersgalati12345Ainda não há avaliações
- Immitance MeasurementDocumento20 páginasImmitance Measurementgalati12345Ainda não há avaliações
- TelemetryDocumento18 páginasTelemetrygalati12345Ainda não há avaliações
- Inductance MeasurementDocumento14 páginasInductance Measurementgalati12345Ainda não há avaliações
- Medical ImagingDocumento25 páginasMedical Imaginggalati12345Ainda não há avaliações
- Magnetic Field MeasurementDocumento34 páginasMagnetic Field Measurementgalati12345Ainda não há avaliações
- Gearsand GearingDocumento57 páginasGearsand Gearingcamohunter71Ainda não há avaliações
- Light DisplayDocumento11 páginasLight Displaygalati12345Ainda não há avaliações
- Optimal ControlDocumento15 páginasOptimal Controlgalati12345Ainda não há avaliações
- Area MeasurementDocumento13 páginasArea Measurementgalati12345Ainda não há avaliações
- Reading DeviceDocumento43 páginasReading Devicegalati12345Ainda não há avaliações
- Level MeasurementDocumento21 páginasLevel Measurementgalati12345Ainda não há avaliações
- Pid ControlDocumento9 páginasPid Controlgalati12345Ainda não há avaliações
- RoboticsDocumento17 páginasRoboticsgalati12345Ainda não há avaliações
- Carateristic of InstrumentationDocumento9 páginasCarateristic of Instrumentationgalati12345Ainda não há avaliações
- DistanceDocumento18 páginasDistancegalati12345Ainda não há avaliações
- Messurement StandardDocumento13 páginasMessurement Standardgalati12345Ainda não há avaliações
- Messurement AccuracyDocumento14 páginasMessurement Accuracygalati12345Ainda não há avaliações
- InhibitorsDocumento30 páginasInhibitorsgalati12345Ainda não há avaliações
- Thickness MeasurementDocumento10 páginasThickness Measurementgalati12345Ainda não há avaliações
- Static Dinsmic CaracteristicDocumento23 páginasStatic Dinsmic Caracteristicgalati12345Ainda não há avaliações
- Introduction To Building SectorDocumento13 páginasIntroduction To Building Sectorgalati12345Ainda não há avaliações
- Operational ModeDocumento8 páginasOperational Modegalati12345Ainda não há avaliações
- Welding ProcedureDocumento19 páginasWelding Proceduregalati12345Ainda não há avaliações
- ElectromagnetismDocumento18 páginasElectromagnetismgalati12345Ainda não há avaliações
- GasesDocumento31 páginasGasesgalati12345Ainda não há avaliações
- Maxwell TheoryDocumento93 páginasMaxwell Theorygalati12345Ainda não há avaliações
- Coordinate SystemDocumento8 páginasCoordinate Systemgalati12345Ainda não há avaliações
- GlasesDocumento73 páginasGlasesgalati12345Ainda não há avaliações
- Polymeric MaterialDocumento17 páginasPolymeric Materialgalati12345Ainda não há avaliações
- Prevent Cross Contamination and Mix UpDocumento2 páginasPrevent Cross Contamination and Mix UpPrince Moni100% (1)
- 1402AHS Prac Manual - 2023 - FINALDocumento200 páginas1402AHS Prac Manual - 2023 - FINALRuan BritsAinda não há avaliações
- LeasingDocumento18 páginasLeasingsunithakravi0% (1)
- 1896 - Pearson - Mathematical Contributions To The Theory of Evolution. III. Regression, Heredity, and PanmixiaDocumento67 páginas1896 - Pearson - Mathematical Contributions To The Theory of Evolution. III. Regression, Heredity, and PanmixiaNilotpal N SvetlanaAinda não há avaliações
- 2 Day ALS Programme 24 Candidates IO ARS (March 2016) PDFDocumento2 páginas2 Day ALS Programme 24 Candidates IO ARS (March 2016) PDFCojocariu Emanuel50% (2)
- Aigen Zhao, PHD, Pe, Gse Environmental, LLC, Usa Mark Harris, Gse Environmental, LLC, UsaDocumento41 páginasAigen Zhao, PHD, Pe, Gse Environmental, LLC, Usa Mark Harris, Gse Environmental, LLC, UsaCarlos Ttito TorresAinda não há avaliações
- Mitra SejatiDocumento2 páginasMitra Sejatiestu kurniaAinda não há avaliações
- Screw Take-Up Device Ur1 Ur7: Conveyor ComponentsDocumento1 páginaScrew Take-Up Device Ur1 Ur7: Conveyor ComponentsDxFxAinda não há avaliações
- Effect of Educational Environment On Personality and Adjustment of Female Students Studying in Colleges of UttarakhandDocumento5 páginasEffect of Educational Environment On Personality and Adjustment of Female Students Studying in Colleges of UttarakhandESSENCE - International Journal for Environmental Rehabilitation and ConservaionAinda não há avaliações
- PROD - Section 1 PDFDocumento1 páginaPROD - Section 1 PDFsupportLSMAinda não há avaliações
- Introduction To Mine SurveyingDocumento7 páginasIntroduction To Mine SurveyingJoshua Miguel MejiasAinda não há avaliações
- Benign Prostate Hyperplasia 2Documento125 páginasBenign Prostate Hyperplasia 2Danieal NeymarAinda não há avaliações
- Hoja Tecnica IS 320.1 Zona 1Documento2 páginasHoja Tecnica IS 320.1 Zona 1susanivar adrianoAinda não há avaliações
- Ceramic Fiber 23Documento28 páginasCeramic Fiber 23Kowsik RajendranAinda não há avaliações
- Annual Report - TakedaDocumento50 páginasAnnual Report - TakedaAbdullah221790Ainda não há avaliações
- Introduction To Nervous SystemDocumento4 páginasIntroduction To Nervous SystemErnie G. Bautista II, RN, MD100% (1)
- Wind Energy Wind Is Generated As The Fluid and Gaseous Parts of The Atmosphere Move Across The Surface of The EarthDocumento3 páginasWind Energy Wind Is Generated As The Fluid and Gaseous Parts of The Atmosphere Move Across The Surface of The EarthEphraim TermuloAinda não há avaliações
- CFM56 3Documento148 páginasCFM56 3manmohan100% (1)
- Sample Emg/Ncv Report - Normal StudyDocumento5 páginasSample Emg/Ncv Report - Normal StudyPhysiotherapist AliAinda não há avaliações
- JeromeDocumento2 páginasJeromeNads DecapiaAinda não há avaliações
- Am Jf211 - Jul 04Documento4 páginasAm Jf211 - Jul 04ilham_metallurgy6744Ainda não há avaliações
- Homeopathic Colour RemediesDocumento23 páginasHomeopathic Colour Remediesisadore100% (42)
- Carti Libraria Victor Papilian Ian 2015Documento8 páginasCarti Libraria Victor Papilian Ian 2015Petru AcozmeiAinda não há avaliações
- AppendicitisDocumento14 páginasAppendicitispreethijojo2003558288% (8)
- Chapter 8 Sensation and PerceptionDocumento66 páginasChapter 8 Sensation and Perceptionapi-726122866Ainda não há avaliações
- Curriculum Guide: Exploratory Course On Household ServicesDocumento5 páginasCurriculum Guide: Exploratory Course On Household ServicesJovanni Mancao PodadorAinda não há avaliações
- Ielts TASK 1 ExerciseDocumento3 páginasIelts TASK 1 Exerciseanitha nathenAinda não há avaliações
- Co JetDocumento4 páginasCo JetJaime PaulAinda não há avaliações
- Drying Operation: Meika Syahbana RusliDocumento69 páginasDrying Operation: Meika Syahbana RusliFrida GinaAinda não há avaliações
- Mycesmm2 Quiz: Please Circle Your Answer! Time Allocated To Answer Is 30 MinutesDocumento2 páginasMycesmm2 Quiz: Please Circle Your Answer! Time Allocated To Answer Is 30 MinutesSi Qian LuiAinda não há avaliações