Escolar Documentos
Profissional Documentos
Cultura Documentos
1 7 10 Wound Healing
Enviado por
kep1313Direitos autorais
Formatos disponíveis
Compartilhar este documento
Compartilhar ou incorporar documento
Você considera este documento útil?
Este conteúdo é inapropriado?
Denunciar este documentoDireitos autorais:
Formatos disponíveis
1 7 10 Wound Healing
Enviado por
kep1313Direitos autorais:
Formatos disponíveis
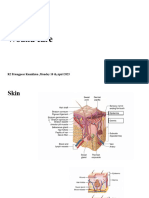
1-7-10 Wound Healing What is wound healing? Wound healing is the restoration of tissue continuity.
It is the restoration of epidermis (but not the dermis and can occur in tissues other than the skin. Most wounds heal with minimal physical care due to overlapping, redundant mechanisms. Lack of healing=death! There are many sequential stages to wound healing with significant overlap. The earlier stages are better understood than later ones: I. Hemostatic phase: occurs seconds to hours after injury when fibrin/platelet clot becomes a provisional matrix that cells remodel into new tissue. Platelets rlease TGFbeta and PDGF to initate inflammatory and synthetic phases. II Hemostasis and Inflammatory phase: hemostasis and platelet plug form 1-5 minutes, followed by increased blood flow and oxygen by 20-30 minutes. III. Inflammatory phase: Inflammation follows the blood, bringing PMNs, acute reactants, and eventually macrophages. Inflammation induces ROS, edema, and proteases that impair healing. It is initiated by denatured tissue, preformed chemokines, FSPs, and loss of barrier. Local PMN response induces edema and oxidant formation. There is an eventual transition to macrophages with cytokines and growth factor release. Continued inflammation causes disordered healing. IV. Proliferation and synthesis: Angiogenesis and vasculogenesis that is growth factor induced. Matrix synthesis by fibroblasts requiring oxygen, energy, protein synthesis, and growth factors. Re-epithelialization (epithelial replication and migration) requiring proteinase release of cells from BMoccurs more efficiently in a moist environment. Sub P induces IL-8, which causes pain that activates wound healing. More on epithelialization: Adjacent to wound, cytokines trigger MMP, which causes detachment of cells from BM and chemotaxis across the wound. Distal to the wound, growth factor induces cell replication Keratinocytes originate from adjacent skin and skin appendages This process is regenerative More on angiogenesis: Inflammatory cells migrate towards hypoxic matrix Release of VEGF and FGF-2 MMP-mediated detachment of cells allows for directional migration of endothelial cells Cells coalesce into sprouting tubes that mature into vessels and reconnect They are stabilized by pericytes and smooth muscle cells V. Bone marrow chimeric wound healing VI. Connective tissue formation: collagen subunit production in fibroblasts requiring oxygen and protein as substrates. Collagen is excreted as fibers into the wound and cross-linked. Ground substance is interstitial matrix composed of proteoglycans and chondoritin sulfates, and crosslinks with collagen. VII. Remodeling phase: Begins 2-6 weeks after injury with wound contraction by myofibroblasts. Vascularity and edema wane, and the wound loses pink/purple color and
becomes pale. Proteases facilitate remodeling in response to mechanical signals form collagen and proteoglycans. Collagen content equilibrates, and there is reorganization and crosslinking of that collagen to improve tensile strength. Sebaceous function returns. Note that remodeled tissue can only be 80% as strong as the original, and the process continues for years. What can go wrong in wound healing? The stages of wound healing must progress to completion for healing to be the best. Obstacles to proper wound healing include either systemic or local deficits: Systemic: malnutrition, diabetes, drugs like steroids, age, chronic medical conditions, immunodeficiency Local: wound infection, necrotic tissue, irradiated tissue, repeated trauma, neoplasm, foreign bodies A chronic wound is characterized by impaired migration of keratinocytes, altered fibroblast phenotypes resulting in decreased proliferation/migration/response to growth factors, excessive neovascularization, and presence of increased proportion to senescent cells. From a biochemical perspective, there is an increase in proteases, decrease in protease regulation, breakdown of the cell-trophic matrix, deficiency in growth factor, and excesses of inflammatory cytokines. A biofilm can also cause chronic wounds. Why do some wounds heal poorly? Inadequate hemostasis: patient bleeds to death Excessive hemostasis: FSP associated with increased inflammation, venous stasis wounds Ongoing release of oxidants damages cells and proteins Continued inflammation induces continued protease activity Inflammatory signals interfere with necessary signaling. This results from inflammatory cytokines in wounds such as TNF, IL-6, and IL-8. Excess inflammation can be caused by infection, occult infection, bacterial growths in tissues such as BIOFILMS, and necrotic tissue. Biofilms must either be debrided or sterilized by topical antimicrobials. Never treat biofilms with systemic antibiotics. MMP-9: matrix metalloproteinase type 9 has a role in both acute surgical wounds and chronic wounds. Excessive amount of MMP-o is associated with non-healing, while wound healing is associated with the disappearance of MMP-9. It is postulated that TNF-alpha mediates downregulation of TIMP-1 and activation of proMMP-9. The function of MMP-9 is to inhibit cell spreading and alteration in cellular cytoskeleton. Thus it impairs migration of collagen, fibronectin, and laminin. It also inhibits growth of keratinocytes in vitro. In summary, MMP-9 is induced and activated by inflammatory mediators. It delays wound healing via a mechanism of loss of migrating epithelial cells. Specific causes Early causes of failed healing: excessive inflammation, infection, necrotic tissue, bacterial
imbalance (BIOFILM), foreign body, lack of inflammation from steroids Late causes of failed healing: infection, increased inflammation due to bacterial imbalance (BIOFILM), malnutrition* (from increased needs, decreased eating, or increased catabolism) Granulation tissue is failed healing, comprising of overgrowth of vessels and matrix without epidermis. *To quantify nutrition, one should use albumin for measurement of chronic protein stores, and pre-albumin for measurement of acute protein stores. At LAC+USC Burn Center, all serous burns get nutritional support. Failed remodeling is characterized by excessive and ongoing matrix synthesis and contraction. This results in HTS/keloid and contractures, as well as epidermal fragility. Hyertrophic scars (HTS) are caused by excessive TGF-beta, so treat by reducing the cytokines that induce TGFb. Early wound closure limits scarring. Epidermal fragility is common after slow healing or large burns. One must treat subclinical infection and provide nutritional support. Doxycyline may benecessary What are the two possible outcomes of abnormal wound healing? Chronic wound Hypertrophic scar How else does excess inflammation affect wound healing? TNF-alpha suppresses TGF-beta promotion of myofibroblasts (suppresses TGF-beta transcription) thus inducing changes in stiffness of collagen-fibroblast matrix, inhibits Smad3 phosphorylation in normal human dermal fibroblasts, and decreases alpha-SMA mRNA stability. What to do clinically in cases of impaired wound healing? Address the inflammation: treat wounds to remove infection and necrotic tissue, change wound from chronic to acute via debridement, and physiologic interventions to heal wounds in the future. Proper wound care improves wound healing Debride necrotic tissue, maintain cell supportive environment (most wound), prevent infection (topical antimicrobials, NOT systemic ones), and limit pain and expense (fewer dressing changes) Increasing healing with tech Growth factors PDGF (US), bFGF (Japan), GM-CSF (China) Bioengineered tissues Negative pressure therapy Stem cells in the future Technology helps, but does not replace good surgical judgment.
Você também pode gostar
- Wound Healing Normal and Abnormal Mechanisms and Closure TechniquesDocumento40 páginasWound Healing Normal and Abnormal Mechanisms and Closure TechniquesAhmad Fakhrozi Helmi100% (1)
- Wound HealingDocumento45 páginasWound HealingmaleehaAinda não há avaliações
- Wound Healing & Care Guide: Stages, Factors & TreatmentDocumento55 páginasWound Healing & Care Guide: Stages, Factors & TreatmentClaudio Luis VenturiniAinda não há avaliações
- Kuliah UKI - Wound HealingDocumento32 páginasKuliah UKI - Wound HealingFina Rachma Destafany100% (1)
- Chronic WoundsDocumento7 páginasChronic WoundstaniaAinda não há avaliações
- GROUP6Documento22 páginasGROUP6AYUSHI PATELAinda não há avaliações
- Wound Healing, Tissue Repair, and FibrosisDocumento28 páginasWound Healing, Tissue Repair, and FibrosisRibka Theodora100% (1)
- Wound Healing Phases and ProcessesDocumento24 páginasWound Healing Phases and ProcessesSrishti SrivastavaAinda não há avaliações
- Wound Healing, Tissue Repair, and FibrosisDocumento28 páginasWound Healing, Tissue Repair, and FibrosisRibka TheodoraAinda não há avaliações
- (SURGERY SGD) Wound HealingDocumento8 páginas(SURGERY SGD) Wound HealingPaulene RiveraAinda não há avaliações
- Chap-1a Wound HealingDocumento60 páginasChap-1a Wound HealingAlex HaileyesusAinda não há avaliações
- Chap1 Wound HealingDocumento35 páginasChap1 Wound HealingAlex HaileyesusAinda não há avaliações
- A Practical Guide To Wound Healing: Learning ObjectivesDocumento15 páginasA Practical Guide To Wound Healing: Learning ObjectivesVanessaGGSAinda não há avaliações
- Woundcare Monday10423Documento63 páginasWoundcare Monday10423Santanico De CVT deozaAinda não há avaliações
- WOUND HEALING: A COMPLEX PROCESSDocumento35 páginasWOUND HEALING: A COMPLEX PROCESSGayathri MaigandanAinda não há avaliações
- Ijms 18 01068 v2 PDFDocumento17 páginasIjms 18 01068 v2 PDFfahira septianiAinda não há avaliações
- The Role of Phytochemicals in The Inflammatory Phase of Wound HealingDocumento17 páginasThe Role of Phytochemicals in The Inflammatory Phase of Wound Healingfahira septianiAinda não há avaliações
- Wound Healing and Its Impairment in The Diabetic Foot: ReviewDocumento9 páginasWound Healing and Its Impairment in The Diabetic Foot: ReviewJoey TsaiAinda não há avaliações
- Wound Healing and Dental Therapies Repair and RegenerationDocumento44 páginasWound Healing and Dental Therapies Repair and RegenerationAthenaeum Scientific PublishersAinda não há avaliações
- Wound Healing: Ziv Peled, M.DDocumento8 páginasWound Healing: Ziv Peled, M.Dapi-26007957Ainda não há avaliações
- Basic Science of Wound HealingDocumento4 páginasBasic Science of Wound HealingMarnia SulfianaAinda não há avaliações
- Tissue Regeneration and Repair ProcessDocumento23 páginasTissue Regeneration and Repair Processmilton7777Ainda não há avaliações
- 3a. Wound HealingDocumento37 páginas3a. Wound HealingOlivia Avriyanti HanafiahAinda não há avaliações
- Wound Healing and Its PatologyDocumento18 páginasWound Healing and Its PatologyAnggie MutmainnahAinda não há avaliações
- Trisha Sando Lecture on Wound Healing Stages and FactorsDocumento51 páginasTrisha Sando Lecture on Wound Healing Stages and FactorsMonasterio Tan KennethAinda não há avaliações
- Repair and HealingDocumento22 páginasRepair and Healingمحمد آل راشدAinda não há avaliações
- Tissue Injury and Healing: Brent Kincaid, DDS, John P. Schmitz, DDS, PHDDocumento10 páginasTissue Injury and Healing: Brent Kincaid, DDS, John P. Schmitz, DDS, PHDAmith HadhimaneAinda não há avaliações
- Chronic Inflammation Healing and Repair GuideDocumento51 páginasChronic Inflammation Healing and Repair Guideahmed mokhtarAinda não há avaliações
- PBL 4 Wound Healing and Tissue AtrophyDocumento3 páginasPBL 4 Wound Healing and Tissue AtrophyadaumedAinda não há avaliações
- Stem CellDocumento14 páginasStem CellsattwikaAinda não há avaliações
- الاصلاحDocumento9 páginasالاصلاحNajwa AbdualgaderAinda não há avaliações
- Manejo de HeridasDocumento17 páginasManejo de HeridassaortizpAinda não há avaliações
- Healing & Repair Guide for DentistryDocumento50 páginasHealing & Repair Guide for DentistryVrushali BhoirAinda não há avaliações
- Skin Wound HealingDocumento9 páginasSkin Wound HealingOEDIEN-kmbAinda não há avaliações
- 3 Chronic InflammationDocumento25 páginas3 Chronic InflammationAnkushh RAinda não há avaliações
- Luka (Injury) : Materi Kuliah Bedah, FK UNAYADocumento58 páginasLuka (Injury) : Materi Kuliah Bedah, FK UNAYADina DinaAinda não há avaliações
- Wound Healing )Documento14 páginasWound Healing )miftahuldursinaAinda não há avaliações
- Tissue Repair and Wound Healing ProcessDocumento70 páginasTissue Repair and Wound Healing ProcessRegi SeptianAinda não há avaliações
- Luka (Injury) Healing StagesDocumento58 páginasLuka (Injury) Healing Stagesmasyuni cantika sariAinda não há avaliações
- Leoni 2015Documento10 páginasLeoni 2015Yunita SaharawatiAinda não há avaliações
- Combine Class Chronic Inflammation & Wound Healing (Power Point)Documento35 páginasCombine Class Chronic Inflammation & Wound Healing (Power Point)Suleiman KikulweAinda não há avaliações
- Mucosal wound repair role of immune-epithelial interactionsDocumento10 páginasMucosal wound repair role of immune-epithelial interactionsBrilliantAinda não há avaliações
- Wound Healing and RepairDocumento13 páginasWound Healing and RepairShiva KumarAinda não há avaliações
- Growth Factors and Cytokines in Wound HealingDocumento17 páginasGrowth Factors and Cytokines in Wound HealingCitra AryantiAinda não há avaliações
- Relation Between Hypothermia and SSIDocumento7 páginasRelation Between Hypothermia and SSIRekkiAinda não há avaliações
- Chapter 7: Wound HealingDocumento12 páginasChapter 7: Wound HealingpoddataAinda não há avaliações
- 0003IADDocumento12 páginas0003IADRika AzyenelaAinda não há avaliações
- Wound Healing and RepairDocumento54 páginasWound Healing and RepairnyangaraAinda não há avaliações
- Wound Healing1Documento8 páginasWound Healing1muralidhar_mettaAinda não há avaliações
- Inflammation and HealingDocumento23 páginasInflammation and Healingatiqullah tarmiziAinda não há avaliações
- Review Article: Nanotechnology-Based Therapies For Skin Wound RegenerationDocumento12 páginasReview Article: Nanotechnology-Based Therapies For Skin Wound RegenerationDina Arwina DalimuntheAinda não há avaliações
- Principles of Wound HealingDocumento28 páginasPrinciples of Wound HealingRafael BagusAinda não há avaliações
- First Foundations in Pathology Part 3: Growth and RepairDocumento46 páginasFirst Foundations in Pathology Part 3: Growth and RepairmadhuAinda não há avaliações
- Stechmiller JK. Understanding The Role of Nutrition and Wound Healing. Nutr Clin PractDocumento8 páginasStechmiller JK. Understanding The Role of Nutrition and Wound Healing. Nutr Clin PractOctavianus KevinAinda não há avaliações
- Wounds and Wounds HealingDocumento9 páginasWounds and Wounds HealingJuma AwarAinda não há avaliações
- Basic Mechanism Involved in Process of Inflammation and RepairDocumento32 páginasBasic Mechanism Involved in Process of Inflammation and RepairDeepakAinda não há avaliações
- Abd 91 05 0614Documento7 páginasAbd 91 05 0614Selvy Anriani GasperszAinda não há avaliações
- Noninfectious Corneal Ulcer PathogenesisDocumento23 páginasNoninfectious Corneal Ulcer PathogenesisAurelia SuryaniAinda não há avaliações
- Tissue Engineering and Wound Healing: A Short Case StudyNo EverandTissue Engineering and Wound Healing: A Short Case StudyNota: 5 de 5 estrelas5/5 (2)
- Case Studies 10Documento5 páginasCase Studies 10kep1313Ainda não há avaliações
- Case Studies 8Documento7 páginasCase Studies 8kep1313Ainda não há avaliações
- Case Studies 7Documento6 páginasCase Studies 7kep1313Ainda não há avaliações
- Case Studies 6Documento8 páginasCase Studies 6kep1313Ainda não há avaliações
- Case 1: Cystic FibrosisDocumento5 páginasCase 1: Cystic Fibrosiskep1313Ainda não há avaliações
- Case Studies 3Documento4 páginasCase Studies 3kep1313Ainda não há avaliações
- SeizuresDocumento3 páginasSeizureskep1313Ainda não há avaliações
- Case Studies 9Documento7 páginasCase Studies 9kep1313Ainda não há avaliações
- Case Studies 5Documento4 páginasCase Studies 5kep1313Ainda não há avaliações
- Case Studies 4Documento4 páginasCase Studies 4kep1313Ainda não há avaliações
- Virology 1 3Documento5 páginasVirology 1 3kep1313Ainda não há avaliações
- Biochem MedicineDocumento27 páginasBiochem Medicinekep1313Ainda não há avaliações
- Case Studies 2Documento4 páginasCase Studies 2kep1313Ainda não há avaliações
- Neurology Notes For Clerkship ReviewDocumento22 páginasNeurology Notes For Clerkship Reviewkep1313Ainda não há avaliações
- Case Studies 1Documento11 páginasCase Studies 1kep1313Ainda não há avaliações
- Week 5 Learning ObjectivesDocumento34 páginasWeek 5 Learning Objectiveskep1313Ainda não há avaliações
- Week 4 Learning ObjectivesDocumento24 páginasWeek 4 Learning Objectiveskep1313Ainda não há avaliações
- Week 2 Learning ObjectivesDocumento21 páginasWeek 2 Learning Objectiveskep1313Ainda não há avaliações
- Week 3 Learning ObjectivesDocumento12 páginasWeek 3 Learning Objectiveskep1313100% (1)
- Physiology - BSDocumento14 páginasPhysiology - BSkep1313Ainda não há avaliações
- Preventative Medicine PM - BSDocumento22 páginasPreventative Medicine PM - BSkep1313Ainda não há avaliações
- Genetics - MMDocumento25 páginasGenetics - MMkep1313Ainda não há avaliações
- MicroanatomyDocumento59 páginasMicroanatomykep1313Ainda não há avaliações
- Psychiatry - BSDocumento15 páginasPsychiatry - BSkep1313Ainda não há avaliações
- Biogenic AminesDocumento5 páginasBiogenic Amineskep1313Ainda não há avaliações
- Biochem MedicineDocumento27 páginasBiochem Medicinekep1313Ainda não há avaliações
- Case 1: Cystic FibrosisDocumento5 páginasCase 1: Cystic Fibrosiskep1313Ainda não há avaliações
- Physiology - BSDocumento14 páginasPhysiology - BSkep1313Ainda não há avaliações
- Anemia DifferentialDocumento1 páginaAnemia Differentialkep1313Ainda não há avaliações
- 2-5-10 HCT For Immunodeficiency and Autoimmune DisordersDocumento2 páginas2-5-10 HCT For Immunodeficiency and Autoimmune Disorderskep1313Ainda não há avaliações
- Lecture Nsche Engr Mafe SIWESDocumento38 páginasLecture Nsche Engr Mafe SIWESoluomo1Ainda não há avaliações
- Bushing Conductor TypesDocumento2 páginasBushing Conductor TypesSandeep BAinda não há avaliações
- Stages On The Empirical Program of RelativismDocumento9 páginasStages On The Empirical Program of RelativismJorge Castillo-SepúlvedaAinda não há avaliações
- Topographic Map of New WaverlyDocumento1 páginaTopographic Map of New WaverlyHistoricalMapsAinda não há avaliações
- As PSGDocumento8 páginasAs PSGYusuff YuzuakkAinda não há avaliações
- Heart Disease SpeechDocumento3 páginasHeart Disease Speechshamim326150% (2)
- The Cambridge Guide To Pedagogy and Practice in Second Language TeachingDocumento4 páginasThe Cambridge Guide To Pedagogy and Practice in Second Language TeachingJessica GomesAinda não há avaliações
- Week Three Lesson Plan Bread and Jam For FrancesDocumento2 páginasWeek Three Lesson Plan Bread and Jam For Francesapi-29831576Ainda não há avaliações
- ACE Personal Trainer Manual Chapter 13Documento59 páginasACE Personal Trainer Manual Chapter 13Đạt NguyễnAinda não há avaliações
- 10 (2) Return To Play After Shoulder Instability in National Football League Athletes (Andi Ainun Zulkiah Surur) IIDocumento6 páginas10 (2) Return To Play After Shoulder Instability in National Football League Athletes (Andi Ainun Zulkiah Surur) IIainunAinda não há avaliações
- FET ExperimentDocumento4 páginasFET ExperimentHayan FadhilAinda não há avaliações
- System ThinkingDocumento18 páginasSystem Thinkingpptam50% (2)
- How TikTok Reads Your Mind - The New York TimesDocumento8 páginasHow TikTok Reads Your Mind - The New York Timesjoe smithAinda não há avaliações
- The German Tradition of Psychology in Literature and Thought 1700-1840 PDFDocumento316 páginasThe German Tradition of Psychology in Literature and Thought 1700-1840 PDFerhan savasAinda não há avaliações
- Astrolada - Astrology Elements in CompatibilityDocumento6 páginasAstrolada - Astrology Elements in CompatibilitySilviaAinda não há avaliações
- Lewatit VP OC 1600 LDocumento3 páginasLewatit VP OC 1600 Lphucuong2410Ainda não há avaliações
- Falcon Nir Online AnalyzerDocumento4 páginasFalcon Nir Online AnalyzerCesc MezaAinda não há avaliações
- Detect3D Fire and Gas Mapping Report SAMPLEDocumento29 páginasDetect3D Fire and Gas Mapping Report SAMPLEAnurag BholeAinda não há avaliações
- School Name Address Contact No. List of Formal Schools: WebsiteDocumento8 páginasSchool Name Address Contact No. List of Formal Schools: WebsiteShravya NEMELAKANTIAinda não há avaliações
- Graphical Machining Process SimulatorDocumento37 páginasGraphical Machining Process SimulatorFrodoAinda não há avaliações
- Reflection Paper About Educational Administration (Ivy M. Peralta)Documento1 páginaReflection Paper About Educational Administration (Ivy M. Peralta)Ivy peraltaAinda não há avaliações
- Management of Chronic ITP: TPO-R Agonist Vs ImmunosuppressantDocumento29 páginasManagement of Chronic ITP: TPO-R Agonist Vs ImmunosuppressantNiken AmritaAinda não há avaliações
- The Retired Adventurer - Six Cultures of PlayDocumento14 páginasThe Retired Adventurer - Six Cultures of Playfernando_jesus_58Ainda não há avaliações
- Journal Pre-Proof: Crop ProtectionDocumento34 páginasJournal Pre-Proof: Crop ProtectionKenan YılmazAinda não há avaliações
- Testing ReadingDocumento11 páginasTesting ReadingJoan Herbosa100% (1)
- Heat Transfer DoeDocumento32 páginasHeat Transfer DoeArt RmbdAinda não há avaliações
- Underground Cable FaultDocumento8 páginasUnderground Cable FaultMohammad IrfanAinda não há avaliações
- Replit Ubuntu 20 EnablerDocumento4 páginasReplit Ubuntu 20 EnablerDurval Junior75% (4)
- Peterbilt Essentials Module3 Sleepers PDFDocumento6 páginasPeterbilt Essentials Module3 Sleepers PDFLuis Reinaldo Ramirez ContrerasAinda não há avaliações
- Project Report: "Attendance Management System"Documento9 páginasProject Report: "Attendance Management System"SatendraSinghAinda não há avaliações