Escolar Documentos
Profissional Documentos
Cultura Documentos
Glaucoma Essay
Enviado por
sujataravi6Direitos autorais
Formatos disponíveis
Compartilhar este documento
Compartilhar ou incorporar documento
Você considera este documento útil?
Este conteúdo é inapropriado?
Denunciar este documentoDireitos autorais:
Formatos disponíveis
Glaucoma Essay
Enviado por
sujataravi6Direitos autorais:
Formatos disponíveis
The Role of Biomechanics in Glaucoma The human eye is a remarkable organ with a complex anatomy.
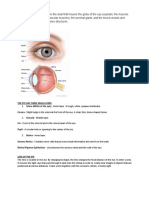
It is responsible for detecting, focussing and subsequently, converting photons into electrochemical signals that are then transmitted to the brain. The eye is roughly spherical and approximately one inch in diameter. Its outermost layer is made of two tough connective tissues; the cornea and the sclera. The middle layer consists of the iris, ciliary body and the choroid. The choroid contains blood vessels that nourish the retinal cells, and facilitate metabolite exchange. The iris is responsible for the changing size of the pupil to ensure that the right amount of light reaches the retina. Finally, the ciliary body, made up of the ciliary processes and the ciliary muscle is responsible for the production of aqueous humor and the accommodation of the lens to adapt to changing focal distances, respectively. The innermost layer of the eye, the retina, is the sensory layer containing neurons, and photoreceptors neurons known as rods and cones that convert the incident light into neuron firings that are transmitted to the brain via the optic nerve. The eyeball is divided into the anterior and posterior chambers, filled with aqueous humor and the vitreous body, filled with vitreous humor. In the absence of any bony processes, the eyeball maintains its rigidity by maintaining a small positive pressure with respect to the surroundings. This positive pressure is known as the Intraocular Pressure (IOP). The IOP is transmitted throughout the eye by the vitreous humor. Therefore, the eye remains spherical and rigid, and a stable distance between the lens and the retina is always maintained. The normal mean value of IOP for the human eye is 15.5 2.6 mm Hg (Ethier & Simmons, 2007).
Figure 1: Image of the anatomy of the human eye (Bonnick, 2013) A normal IOP is essential to functioning of the human eye. How is this IOP produced and why? Aqueous humor is responsible for the IOP. In the cornea and the lens, because of the need for optical
transparency, metabolite exchange with blood via perfusion is not possible. Therefore, clear and colourless aqueous humor also serves as a medium for nutrient exchange. Secreted by epithelial cells in the ciliary processes, the aqueous humor perfuses the lens and then moves into the anterior chamber of the eye. From here, it drains out of the eye through special channels in the angle formed by the iris and the cornea known as the angle of the anterior chamber. The rate of production of aqueous humor by the ciliary processes is very slow (2.4 0.6 l/min in the normal human eye). However, the resistance to outflow of aqueous humor during its drainage is what causes the considerable positive pressure. The drainage of aqueous humor in the eye occurs through two channels; the conventional route and the unconventional route. The unconventional route or the uveo-scleral route carries only 10% of the outflow, and does not account as the primary site for flow resistance. Majority of the aqueous humor drains through the conventional route; a series of specialised tissues: the trabecular meshwork, the canal of Schlemm, and the collector channels/aqueous veins. These are the main cause of the outflow resistance to aqueous humor (approximately 3-4mm Hg/l/min). Once aqueous humor leaves the aqueous veins, it drains back into the right side of the heart after joining the episcleral veins. At the normal values of outflow resistance, the IOP remains at a value of 15.5 mm Hg, which is normal. Ocular hypertension is a condition where the IOP is elevated beyond its normal value, in the absence of optic nerve damage or visual field loss. Ocular hypertension is the most important risk factor for glaucoma and thus, functions as a valuable screening tool for glaucoma. According to Casson et al (2012) glaucoma may be described as: .. a group of ocular disorders of multifactorial aetiology united by a clinically characteristic optic neuropathy with potentially progressive, clinically visible changes at the optic nerve head (ONH), comprising focal or generalized thinning of the neuroretinal rim with excavation and enlargement of the optic cup, representing neurodegeneration of retinal ganglion cell axons and deformation of the lamina cribrosa; corresponding diffuse and localized nerve-fibre-bundle pattern visual field loss may not be detectable in early stages; while visual acuity is initially spared, progression can lead to complete loss of vision.. Chronically elevated IOP is indicated in the development as well as the progression of glaucoma. A patients IOP may be considered elevated if it is greater than 97.5 percentile of the population (Casson et al, 2012). It may affect the pressure sensitive cells and tissues in the eye viz. the trabecular meshwork (TM), the optic nerve head (ONH) including the lamina cribrosa cells and optic nerve head astrocytes, the peripapillary sclera around the optic nerve head, retinal ganglion cells (RGC) and RGC axons in the retinal nerve fibre layer (Clark, 2012). This increase in IOP is caused by an increase in outflow resistance. In a glaucomatous eye, the resistance to outflow maybe as high as or greater than triple the normal value. There are also some forms of glaucoma in which this increase in IOP is not due to the increase in outflow resistance. These are categorized as angle-closure glaucoma, which occurs due to the iris pivoting forward and blocking the access to the drainage structures in the angle of the anterior chamber. This may be caused due to an anatomic predisposition. A few hypotheses have been put forth to explain the resistance to outflow in the conventional route for drainage: 1) Collapse of Canal of Schlemm - Channel collapse is governed by two factors. The underlying elastic TM tends to keep the Canal of Schlemm open. Whereas, the pressure drop across the TM and the inner wall of the canal tends to make it close. On solving the resulting equations drawn up to conserve mass, pressure drop and deformation it was found that for
typical values of input parameters, the resistance due to the canal of Schlemm would be negligible, except at very high pressures such as 50mm Hg where the canal collapses. Even with a collapsed Canal of Schlemm the outflow resistance is not as high as a glaucomatous eye. Therefore, channel collapse can be ruled out as a cause of Glaucoma (Ethier & Simmons, 2007).
Figure 2: Scanning Electron Micrograph showing an overview of conventional tissues for aqueous humor drainage (Ethier & Simmons, 2007) 2) Experimental evidence supports the conclusion that the source of normal and increased outflow resistance in glaucoma is attributable to the inner wall endothelium, its basement membrane, the juxtacanalicular connective tissue (JCT), or some combination of all three of these (Johnson, 2006). Like other epithelial basement membranes in the body, the basement membrane in the inner wall of the canal of Schlemm may be responsible for the generation of outflow resistance. However, due to the discontinuous nature of the membrane in the human eye, this resistance may not account for a large fraction. The porous nature of the JCT as evinced by transmission electron microscopy shows large open spaces, which may not cause significant outflow resistance. However, if these spaces were to contain glycosaminoglycans at physiological concentrations, then the flow resistance due to the JCT would be considerable. Upon further investigation using quick freeze/deep etch electron microscopy that may preserve GAGs in the sample, similar pores were still seen. Therefore, it remains unclear whether GAGs account for the outflow resistance, and if yes, till what extent. In the inner wall, the endothelial cells form a continuous layer attached to each other using tight junctions. The unique feature of these cells is the formation of vacuoles that are invaginations of the endothelium into the lumen of the canal of Schlemm, caused by the pressure drop across the endothelium. These invaginations may account for the outflow resistance. These vacuoles also contain membrane lined pores that connect the apical and basal side of the cell. A study has shown that the pore density in the glaucomatous eye is significantly reduced,
which may mean that these pores account for the increase in outflow resistance as experienced in glaucoma (Overby, Stamer & Johnson, 2008).
Figure 3: Scanning Electron Micrograph of the inner wall of Schlemms Canal (Johnson, 2006) Apart from the elevated outflow resistance, glaucoma causes extensive and progressive neuropathy. ONH cupping caused by the death of the neurons in the optic nerve is a clinical feature of glaucoma (Downs, Roberts & Sigal, 2012). RGC axons converge to form the optic nerve that pierces the sclera to join the brain. Since this is a weak point of discontinuity in the otherwise continuous sclera, it is additionally susceptible to concentration of stresses. The lamina cribrosa is the porous connective tissue that spans the sclera canal and supports the RGC axons as they leave the eye as the optic nerve. Two theories attempt to explain the damage to the RGCs. The mechanical theory of glaucomatous optic neuropathy postulates that the increased mechanical stresses acting in lamina cribrosa cause axonal damage. This damage may be mediated through astrocytes. The astrocytes divide and form a glial scar and fail to provide nutrition and guidance the neurons, thus leading to neuronal death. The second theory, vasogenic theory, states that the glaucomatous insult results from inadequate vascular perfusion in the laminar cribrosa, resulting in insufficient oxygen delivery. The resulting ischemia triggers neuronal cell death (Ethier & Simmons, 2007). In conclusion, glaucoma is a progressive eye pathology caused by chronically elevated IOP that leads to irreversible vision loss and if left untreated, blindness. Since the effects may not be clear till they have progressed considerably, early screening is a must. Once diagnosed, the treatment for glaucoma involves reduction of the IOP via medication, or surgical treatment. In addition to IOP and ONH biomechanics, the biomechanics of the cornea and the corneal thickness may also play a role in the development of glaucoma, especially the progression of ocular hypertension into full fledged glaucoma (Brown & Congdon, 2006). Therefore, further research into the biomechanics of glaucoma will help identify risk, early diagnosis, treatment modalities as well as patient prognosis.
References Bonnick (2013) CSEC - The Eye - functions of the various parts. Biologs. Weblog. [Online]. Available from http://thebiologs.blogspot.co.uk/2013/09/csec-eye.html. Accessed 24th October 2013. Brown, K. E. & Congdon, N. G. (2006) Corneal structure and biomechanics: impact on the diagnosis and management of glaucoma. Current Opinion in Ophthalmology.17 (4), 338-343. Casson, R. J., Chidlow, G., Wood, J. P., Crowston, J. G. & Goldberg, I. (2012) Definition of glaucoma: clinical and experimental concepts. Clinical & Experimental Ophthalmology. 40 (4), 341349. Clark, A. F. (2012) The cell and molecular biology of glaucoma: biomechanical factors in glaucoma. Investigative Ophthalmology & Visual Science. 53 (5), 2473-2475. Crawford Downs, J., Roberts, M. D. & Sigal, I. A. (2011) Glaucomatous cupping of the lamina cribrosa: a review of the evidence for active progressive remodeling as a mechanism. Experimental Eye Research. 93 (2), 133-140. Ethier, C. R., Johnson, M. & Ruberti, J. (2004) Ocular biomechanics and biotransport. Annual Review of Biomedical Engineering. 6, 249-273. Ethier, C. R. & Simmons, C. A. (2007) Introductory biomechanics: from cells organisms. Cambridge texts in biomedical engineering. Cambridge, Cambridge University Press. to
Johnson, M. (2006) 'What controls aqueous humour outflow resistance?'. Experimental Eye Research. 82 (4), 545-557. Overby, D. R., Stamer, W. D. & Johnson, M. (2009) The changing paradigm of outflow resistance generation: towards synergistic models of the JCT and inner wall endothelium. Experimental Eye Research. 88 (4), 656-670.
Você também pode gostar
- Neurofeedback AdhdDocumento8 páginasNeurofeedback AdhdAlbertoAinda não há avaliações
- Psychiatric Nursing Notes by Dr. FaustoDocumento377 páginasPsychiatric Nursing Notes by Dr. Faustopertru08100% (2)
- 1 BrainBeeUSM MBBC2019 Learning Resources PDFDocumento157 páginas1 BrainBeeUSM MBBC2019 Learning Resources PDFwan muhd100% (1)
- The Handbook of Clinical NeuropsychologyDocumento1.439 páginasThe Handbook of Clinical NeuropsychologyFabiana AriasAinda não há avaliações
- Neurology MCQDocumento6 páginasNeurology MCQMuhammad Qasum43% (7)
- Back PropagationDocumento106 páginasBack PropagationSergio SisternaAinda não há avaliações
- All 3rd Form Course Outlines 2020-2021 WBSDocumento182 páginasAll 3rd Form Course Outlines 2020-2021 WBSSANDS ENGINEERING DESIGNS & CONSTRUCTION CO. LTD.Ainda não há avaliações
- (Aging, Exercise, and Cognition Series, v. 3) Wojtek J Chodzko-Zajko - Arthur F Kramer - Leonard W Poon - Enhancing Cognitive Functioning and Brain Plasticity, Volume 3-Human Kinetics (2009) PDFDocumento250 páginas(Aging, Exercise, and Cognition Series, v. 3) Wojtek J Chodzko-Zajko - Arthur F Kramer - Leonard W Poon - Enhancing Cognitive Functioning and Brain Plasticity, Volume 3-Human Kinetics (2009) PDFIra SariAinda não há avaliações
- LESSON PLAN Sensation and PerceptionDocumento13 páginasLESSON PLAN Sensation and PerceptionBrylle DeeiahAinda não há avaliações
- Abbey Pain Assessment Scale (Follow On Assessment) : Date AND Time Date AND Time Date AND Time Date AND Time Date AND TimeDocumento2 páginasAbbey Pain Assessment Scale (Follow On Assessment) : Date AND Time Date AND Time Date AND Time Date AND Time Date AND TimeMuhammad RezgiaAinda não há avaliações
- Glaucoma PPT For Final YearDocumento57 páginasGlaucoma PPT For Final YearDarshan SinghAinda não há avaliações
- Anatomy and Physiology of Cornea: Dr. Joseph JohnDocumento46 páginasAnatomy and Physiology of Cornea: Dr. Joseph JohnBIBI MOHANAN100% (1)
- GlaucomaDocumento18 páginasGlaucomaOncología CdsAinda não há avaliações
- Primary Open-Angle Glaucoma: SeminarDocumento10 páginasPrimary Open-Angle Glaucoma: SeminarFebry LuthunananaAinda não há avaliações
- GlaucomaDocumento57 páginasGlaucomaAmmad ShahidAinda não há avaliações
- Makalah Glaucoma Group 1Documento27 páginasMakalah Glaucoma Group 1VeronicaAinda não há avaliações
- Glaucoma OverviewDocumento33 páginasGlaucoma Overviewc/risaaq yuusuf ColoowAinda não há avaliações
- Pathogenesis of Glaucoma: Determinants of Intraocular PressureDocumento17 páginasPathogenesis of Glaucoma: Determinants of Intraocular PressureAdi TriAinda não há avaliações
- Aqueous Humor Outflow:: Anterior Cavity: The Lens Divides The Anterior Cavity Into Further Two ChambersDocumento20 páginasAqueous Humor Outflow:: Anterior Cavity: The Lens Divides The Anterior Cavity Into Further Two ChambersNavjot BrarAinda não há avaliações
- Aqueous Humour: Anatomy & Physiology: by Tahir Muneeb - September 7, 2012 6 3502Documento9 páginasAqueous Humour: Anatomy & Physiology: by Tahir Muneeb - September 7, 2012 6 3502nnurulhudaaAinda não há avaliações
- Case Report Absolute GlaucomaDocumento22 páginasCase Report Absolute GlaucomaDewi Permatasari100% (2)
- Understanding Trabecular Meshwork Physiology: A Key To The Control of Intraocular Pressure?Documento5 páginasUnderstanding Trabecular Meshwork Physiology: A Key To The Control of Intraocular Pressure?RaissaAinda não há avaliações
- Discuss Physiology of Aqueous Formation and Its Drainage and Relate It To How Intraocular Pressure Is ControlledDocumento8 páginasDiscuss Physiology of Aqueous Formation and Its Drainage and Relate It To How Intraocular Pressure Is ControlledPrince ChipunguAinda não há avaliações
- INTRODUCTION NeewDocumento33 páginasINTRODUCTION NeewMuhammad MustafaAinda não há avaliações
- The Multifunctional ChoroidDocumento38 páginasThe Multifunctional ChoroidyinvilllAinda não há avaliações
- A. Primary GlaucomaDocumento5 páginasA. Primary GlaucomasekiannAinda não há avaliações
- Aqueous Humor Production 1Documento7 páginasAqueous Humor Production 1yesi eka molitaAinda não há avaliações
- Jia Et Al. - 2016 - Biomechanics of The Sclera and Effects On Intraocu PDFDocumento8 páginasJia Et Al. - 2016 - Biomechanics of The Sclera and Effects On Intraocu PDFFelicia SutarliAinda não há avaliações
- Johnson Ta MM 2010Documento10 páginasJohnson Ta MM 2010Lewi Esther SiahaanAinda não há avaliações
- Errors of RefractionDocumento27 páginasErrors of RefractionAminaAinda não há avaliações
- Jurnal Mata 1 PDFDocumento4 páginasJurnal Mata 1 PDFyayan latuconsinaAinda não há avaliações
- Pemicu 1 Pengindraan c2Documento113 páginasPemicu 1 Pengindraan c2CcAinda não há avaliações
- Pemicu 1 PENGINDRAAN C2Documento113 páginasPemicu 1 PENGINDRAAN C2CcAinda não há avaliações
- Sclera StructureDocumento15 páginasSclera Structureferdi151991Ainda não há avaliações
- Cet 5Documento4 páginasCet 5jumi26Ainda não há avaliações
- 2012.microinvasive Glaucoma Surgery-A Review of Schlemm's Canal-Based ProceduresDocumento10 páginas2012.microinvasive Glaucoma Surgery-A Review of Schlemm's Canal-Based ProceduresReza NaghibiAinda não há avaliações
- Pathophysiology of Acute AngleDocumento1 páginaPathophysiology of Acute AngleKusumaning AisahAinda não há avaliações
- Damage Control Surgery Ocular Traumatology (Injury)Documento7 páginasDamage Control Surgery Ocular Traumatology (Injury)Shirakawa AlmiraAinda não há avaliações
- Glaucoma Pathophysiology and DiagnosisDocumento10 páginasGlaucoma Pathophysiology and Diagnosisagus sanjayaAinda não há avaliações
- Glaucoma-1: Diagnosis: BMJ Clinical Research February 2004Documento4 páginasGlaucoma-1: Diagnosis: BMJ Clinical Research February 2004Hendry pramarthaAinda não há avaliações
- GlaukomaDocumento41 páginasGlaukomadanufarandiAinda não há avaliações
- AVICENNIA LBM 4 Mata SGD 20Documento28 páginasAVICENNIA LBM 4 Mata SGD 20Maylinda PutriAinda não há avaliações
- AVICENNIA LBM 4 Mata SGD 20Documento28 páginasAVICENNIA LBM 4 Mata SGD 20Maylinda Putri100% (1)
- Michael G. Glasspool FRCS, DO (Auth.) - Atlas of Ophthalmology-Springer Netherlands (1982) PDFDocumento117 páginasMichael G. Glasspool FRCS, DO (Auth.) - Atlas of Ophthalmology-Springer Netherlands (1982) PDFInna BujorAinda não há avaliações
- 2001.ageing of Schlemm's Canal in Nonglaucomatous SubjectsDocumento12 páginas2001.ageing of Schlemm's Canal in Nonglaucomatous SubjectsReza NaghibiAinda não há avaliações
- Chapter 3 - Introduction To GlaucomaDocumento16 páginasChapter 3 - Introduction To Glaucoma1330658Ainda não há avaliações
- Glaucoma Normo TensionDocumento38 páginasGlaucoma Normo TensionRiyang Pradewa AdmawanAinda não há avaliações
- Animal VertebrateDocumento10 páginasAnimal VertebrateAbdullahi AwwalAinda não há avaliações
- GlucomaDocumento14 páginasGlucomametalheavy9993Ainda não há avaliações
- Vitreous HemorrhageDocumento7 páginasVitreous HemorrhageAndreas OctavianoAinda não há avaliações
- Human EyeDocumento6 páginasHuman EyeclubsanatateAinda não há avaliações
- Ocular Biochemistry UpdatedDocumento42 páginasOcular Biochemistry UpdatedDivine OkolieAinda não há avaliações
- Orbital ExenterationDocumento9 páginasOrbital ExenterationJoji Dela pPeñaAinda não há avaliações
- SGD LBM 3 Modul MataDocumento12 páginasSGD LBM 3 Modul MataMillatiazmi Maulida ArdianiAinda não há avaliações
- JournalDocumento15 páginasJournalagungAinda não há avaliações
- NCP Rheum 0268Documento9 páginasNCP Rheum 0268drheriAinda não há avaliações
- Major Review: Ocular ColobomataDocumento20 páginasMajor Review: Ocular ColobomataPriyankaAinda não há avaliações
- University of Baghdad Alkindy College of Medicine Fifth Stage / GroupDocumento14 páginasUniversity of Baghdad Alkindy College of Medicine Fifth Stage / GroupNoor Al Zahraa AliAinda não há avaliações
- Ocular Biochemistry LECTURE NOTE-1Documento44 páginasOcular Biochemistry LECTURE NOTE-1Divine OkolieAinda não há avaliações
- Ophtho GuideDocumento24 páginasOphtho GuideNico7234100% (1)
- GlaucomaDocumento55 páginasGlaucomaMishiko DumbadzeAinda não há avaliações
- Li LBM 4 Mata 2Documento30 páginasLi LBM 4 Mata 2Salma SavitaAinda não há avaliações
- Ophthalmology: Anatomy of The LensDocumento35 páginasOphthalmology: Anatomy of The Lensمحمد عبدالوهاب ابراهيم الطباطبائيAinda não há avaliações
- Chronic Visual LossDocumento7 páginasChronic Visual LossJim Jose AntonyAinda não há avaliações
- Vitreous HemorrhageDocumento7 páginasVitreous HemorrhageindahAinda não há avaliações
- Ref 02Documento6 páginasRef 02Mohamad FachryAinda não há avaliações
- Chronic GlaucomaDocumento10 páginasChronic GlaucomaGepengCungkringAinda não há avaliações
- Anatomy and Physiology of EyeDocumento19 páginasAnatomy and Physiology of Eyeashwini priyaAinda não há avaliações
- Suprachoroidal Space InterventionsNo EverandSuprachoroidal Space InterventionsShohista SaidkasimovaAinda não há avaliações
- Head - Spinal - Injury SC1Documento48 páginasHead - Spinal - Injury SC1Jujhar BoparaiAinda não há avaliações
- Paper D (KMU) 2nd Year - 0Documento2 páginasPaper D (KMU) 2nd Year - 0G.R. JamesAinda não há avaliações
- Control & Cooridnation Previous Year Questiosn Class 10 ScienceDocumento3 páginasControl & Cooridnation Previous Year Questiosn Class 10 Sciencepdthedon007Ainda não há avaliações
- Chapter 13 - The Peripheral Nervous System and Reflex ActivityDocumento53 páginasChapter 13 - The Peripheral Nervous System and Reflex Activityramadan100% (1)
- Depression Diagnostic Criteria and Severity RatingDocumento1 páginaDepression Diagnostic Criteria and Severity Ratingapi-310299713Ainda não há avaliações
- Chapter IIDocumento4 páginasChapter IIIce cold83% (6)
- 6 Psychoanalytic Theory: Clues From The Brain: Commentary by Joseph LeDoux (New York)Documento7 páginas6 Psychoanalytic Theory: Clues From The Brain: Commentary by Joseph LeDoux (New York)Maximiliano PortilloAinda não há avaliações
- Nerve Supply and Muscle Fiber StimulationDocumento20 páginasNerve Supply and Muscle Fiber StimulationEdelrose LapitanAinda não há avaliações
- Neuromuscular Junction, or The Motor End PlateDocumento7 páginasNeuromuscular Junction, or The Motor End PlateabhilashkrishnantkAinda não há avaliações
- Lokomat DescriptionDocumento11 páginasLokomat Descriptionnadinne7Ainda não há avaliações
- Artículo en Ingles. TdahDocumento3 páginasArtículo en Ingles. TdahRaquel Marquez MoyaAinda não há avaliações
- Exercise 7 Embryology 1Documento10 páginasExercise 7 Embryology 1Jerjer KingAinda não há avaliações
- (G1) What Triggers ADHDDocumento24 páginas(G1) What Triggers ADHDhvtvan0702Ainda não há avaliações
- Atlas of Neuromuscular DiseasesDocumento364 páginasAtlas of Neuromuscular Diseasesandy sotoAinda não há avaliações
- Localization of Function WorksheetDocumento2 páginasLocalization of Function WorksheetCOVERSBYELIAinda não há avaliações
- Ulnar Nerve ReconstructionDocumento52 páginasUlnar Nerve ReconstructionDiyar Abdulwahid Salih100% (4)
- KayleeDocumento3 páginasKayleeKaylee RamsaroopAinda não há avaliações
- Humor For Good Health: Presented By: Michael Brodie, MBA, NHADocumento32 páginasHumor For Good Health: Presented By: Michael Brodie, MBA, NHAYuva Lakshmi Ramesh BabuAinda não há avaliações
- IntracranialsurgeryDocumento17 páginasIntracranialsurgeryJona Kristin EnclunaAinda não há avaliações
- Neural Network PDFDocumento3 páginasNeural Network PDFSUREKHA SAinda não há avaliações