Escolar Documentos
Profissional Documentos
Cultura Documentos
CHEM1201-Lecture - 4A - Organic Chemistry Lecture Notes
Enviado por
Youssef LatashTítulo original
Direitos autorais
Formatos disponíveis
Compartilhar este documento
Compartilhar ou incorporar documento
Você considera este documento útil?
Este conteúdo é inapropriado?
Denunciar este documentoDireitos autorais:
Formatos disponíveis
CHEM1201-Lecture - 4A - Organic Chemistry Lecture Notes
Enviado por
Youssef LatashDireitos autorais:
Formatos disponíveis
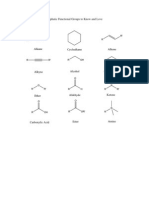
CHEM1201 Lecture Notes
Monday, 12 August 2013 DDT First synthesied in 1874. Its insecticidal properties werent discovered to the 80s, was originally used to control Malaria. In terms of restricting the number of malaria carrying mosquitos, is good, but it has a profound number of running consequence. These have toxic side eects. It was banned in 1972. The eects upon the rerpduction in birds by applying DDT to the environment, there were so few birds around giving rise to the silent spring. Its still used in some counties. Other Interesting and Important Compounds Containing halogens Fluorouracil is the analogue to uracil, which is used in cancer tratment. Halothan (anasthetic). Dicult to administer the right amounts, it tends to stick around in fatty tissue because its a realtively nonpolar compound. Even thhough the individual bonds themselves are very polar, the oerall molecules themselves are very lipophillic, or hydrophobic. So its hard to get the amount of anaesthetic right. So the reason for this is because this compond,w hen people inhale it, it nds its way into fatty tissue, and when they move around more, it gets released, etc. Its not a perfect structure. Chlorouorocarbons, known as freons, originally used as regrigerant. They are long lived compounds and have been demonstrated to lead to chemical reactions which deplete the ozone layer.
Amines
We have sp3 hydbridied tetrahedral geometry on the nitrogen atom. We esentially have a pyramidal shape. Structure and properties The presence of N means that we get hydrogen bonding between molecules of primary amines, and so dimethyl ehter and dimethylamine, despite having about the same molecular, have very dierent BPs. 1
Acid-Base Reactions Higher pKa of conjugate acid (ammonium salt) means more basic the base (amine). There is something curious about aniline, P hN H2 is quite a low pKa, 4.6, because theres a big dierence between this and triethylamine, ethyline, ammonia, which have pKa between 10.75 and 9.2. This is because there is conjugation with the aromatic ring. Generally, the longer the carbon chain, the less water soluble it becomes. You can turn it around by reacting this with a relatively mild acid, and because they are ionic, they become very soluble in water. Amines tend to be smelly compounds once their volatile. But when theyre produced into salts, theyre not AMines as Nucleophiles Amines have that lone pair to form new Nitrogen bonds. Theyll do it with a proton, but theyll also do it with electrophiles. You can go from primary to secondary amines, etc. You are performing a substitution reaction to form a new N-C bond, followed by an acid-base reaction to form that higher amine. It turns out that primary amines, when you react it with an alkyl halide, you create. Primary amines are less sterically hindered, whereas secondary amines are more sterically hindered. But we know that carbon subsitutients are electron donating. This makes secondary more reactive that primary, even though it may not necessarily be deduced from the steric considerations. Nicotine Produced naturally by many plants. Produced by the roots, but it ends up in the leaves. Its extremely widely used, and every cigarette releases 1mg. The lethal dose is 30mg. So the therapeutic index for this one is pretty bad. If you tried to market a medicine with a similar therapeutic index is bad. Ampetamine derivatives Methamphetamine is the secondary amine. Methamphetamine, crystal meth, are the hydrochloride salt of the substance, The free base version is a volatile liquid. These are oil, fatty substance, volatile substance. 2
Both enantiomers are biologically active. Generally on the street you get the racemic, and sometimes the single enantiomers, and the single enantiomier is what people hardered to the drug will want. Production and distribution of these substances is controlled by authorities. It is sometimes used in the clinic, but the bad side eects, means its restricted. Phenethylamine is found in chocolate. There was a theory in 80s that peoples craving for chcolate is because of this. But this is false, since it gets destroyed by enzymes into a carboxyic acid and doesnt ahve the biolgocial properties that MDMA and speed do. 25I-NBOme. Closely related to the other compounds. This is starting to replace LSD Crack Cocaine Coaine is the ammonium chloride salt, this is the stu that is extracted from the plant. But crack cocaine is a chrystalline solid. They have very dierent properties. MP: Cocaine = 195, Crack = 95. Thus people smoke crack. Its a similar reaction to if you take sh and add citric acid.
Carbonyl Group Chemistry
Aldehydes and Ketones These are compounds containing a carbonyl group, C = O. formaldehyde is the simplest molecule in this class. All four atomes lie on one plane. So in a ketone, you have 3 carbons and an oxygen in the same plane, but in an aldehyde, youd have two carbones, an oxygen and Hydrogen. All the bond angles are approximately 120 degrees. Functional groups containing a carbonyl absorb at around 1700cm Structure and Bonding We have a pi bond, sp2 hybridised. If you have an aldehyde,you end with -al Ketones end with -one Nomenclature Oxidation Levels of Functional Groups
Odiation of Aldehydes Aldehyles get oxidised to carboxylic acids because when theyre exposed to dchromate ion get it. We also saw that secondary alcohols get oxidised to ketones, but they dont get a reactions. This is because you cant break carbon-carbon bonds.
Você também pode gostar
- IEEE-Std-C57-149-IEEE Guide For The Application and Interpretation of Frequency Response Analysis For Oil-Immersed Transformers PDFDocumento72 páginasIEEE-Std-C57-149-IEEE Guide For The Application and Interpretation of Frequency Response Analysis For Oil-Immersed Transformers PDFJose Luis BarretoAinda não há avaliações
- Loudon Organic Chemistry Chapter 14Documento32 páginasLoudon Organic Chemistry Chapter 14JohnAinda não há avaliações
- Science Bowl Organic Chemistry NotesDocumento44 páginasScience Bowl Organic Chemistry Notestaosat11Ainda não há avaliações
- LaTeX Mathematical SymbolsDocumento4 páginasLaTeX Mathematical Symbolsjfreyre100% (9)
- PETREL 1 Structural Modeling PDFDocumento42 páginasPETREL 1 Structural Modeling PDFKuala Tambora100% (1)
- Organic Chemistry - Some Basic Principles and TechniquesDocumento16 páginasOrganic Chemistry - Some Basic Principles and TechniquesAbhayAinda não há avaliações
- Recognizing Endo and Exo - Master Organic ChemistryDocumento9 páginasRecognizing Endo and Exo - Master Organic ChemistryashishAinda não há avaliações
- Organic Chemistry Lectures on Alkanes, Cycloalkanes and AlkenesDocumento32 páginasOrganic Chemistry Lectures on Alkanes, Cycloalkanes and AlkenesAbdulHameedAinda não há avaliações
- Chemistry Assignment: Name: Asniza Binti Zul'AzmanDocumento5 páginasChemistry Assignment: Name: Asniza Binti Zul'AzmanAsAinda não há avaliações
- Organic Chemistry Compound Classes, Formulas, and ReactionsDocumento17 páginasOrganic Chemistry Compound Classes, Formulas, and ReactionsMichael lIuAinda não há avaliações
- Course Type Course Code Name of Course L T P Credit: Aromaticity: Introduction To Aromaticity, Anti-AromaticDocumento1 páginaCourse Type Course Code Name of Course L T P Credit: Aromaticity: Introduction To Aromaticity, Anti-AromaticOmkar KurlekarAinda não há avaliações
- Organic NomenclatureDocumento11 páginasOrganic NomenclatureAmalia SillerAinda não há avaliações
- Organic ChemistryDocumento40 páginasOrganic ChemistryGlenda ResultayAinda não há avaliações
- Organic Chemistry - Pertemuan KeduapptDocumento60 páginasOrganic Chemistry - Pertemuan Keduapptnadhilah shabrinaAinda não há avaliações
- Organic Chemistry: Alkene NotesDocumento11 páginasOrganic Chemistry: Alkene NotesDommie FranklinAinda não há avaliações
- Organic Chemistry QuestionsDocumento2 páginasOrganic Chemistry QuestionsKevin Dacre100% (1)
- Summary Notes Organic ChemistryDocumento1 páginaSummary Notes Organic ChemistryVernonAinda não há avaliações
- Important Order and Facts of Organic ChemistryDocumento6 páginasImportant Order and Facts of Organic ChemistryDEEPAK KUMAR MALLICKAinda não há avaliações
- Introduction to Organic Chemistry GuideDocumento64 páginasIntroduction to Organic Chemistry GuideYuen Kim100% (1)
- Substitution ProcessDocumento5 páginasSubstitution ProcesselabagsAinda não há avaliações
- Chemistry 6310 Advanced Topics in Organic Chemistry: The Organic ChemistryDocumento5 páginasChemistry 6310 Advanced Topics in Organic Chemistry: The Organic Chemistryapi-20179616Ainda não há avaliações
- s5 Organic Chemistry 30-03-20Documento208 páginass5 Organic Chemistry 30-03-20ONAP PATRICK JOSEPHAinda não há avaliações
- EAMCET QR Chemistry SR Chem 17.organic Chemistry Carbonyl CompoundsDocumento11 páginasEAMCET QR Chemistry SR Chem 17.organic Chemistry Carbonyl CompoundsJagadeesh GoliAinda não há avaliações
- AnatomyDocumento7 páginasAnatomyM.AhsanAinda não há avaliações
- Worksheet: CaramelizationDocumento3 páginasWorksheet: CaramelizationLoreto T. Porcari JrAinda não há avaliações
- Metalated Hetero Cycles and Their Applications in Synthetic Organic ChemistryDocumento56 páginasMetalated Hetero Cycles and Their Applications in Synthetic Organic Chemistrygokay05Ainda não há avaliações
- Advances in Quinoline SynthesisDocumento96 páginasAdvances in Quinoline SynthesisMurali Venkat NagAinda não há avaliações
- Organic Chemistry - Lesson 2Documento17 páginasOrganic Chemistry - Lesson 2knlsinhaAinda não há avaliações
- ASSIGNMENT of Organic ChemistryDocumento8 páginasASSIGNMENT of Organic ChemistryWania AliAinda não há avaliações
- Division of Human AnatomyDocumento3 páginasDivision of Human Anatomyriffyjean0% (1)
- Anatomy Review Block 1Documento32 páginasAnatomy Review Block 1ngAinda não há avaliações
- General Organic Chemistry (GOC)Documento33 páginasGeneral Organic Chemistry (GOC)Jitendra Verma100% (1)
- Surface Anatomy: Clinical Correlations: - Gray's Pp. 200-208Documento14 páginasSurface Anatomy: Clinical Correlations: - Gray's Pp. 200-208speedy.catAinda não há avaliações
- IGCSE chemistry section 3 alkanes and alkenesDocumento2 páginasIGCSE chemistry section 3 alkanes and alkenesNayeemAhmedAinda não há avaliações
- Anatomy 2 QuestionsDocumento9 páginasAnatomy 2 QuestionshumanupgradeAinda não há avaliações
- Organic Chemistry 2Documento6 páginasOrganic Chemistry 2mydreamcometrueAinda não há avaliações
- AnatomyDocumento14 páginasAnatomyMaliha SiddiquiAinda não há avaliações
- Neck AnatomyDocumento47 páginasNeck AnatomyGiuseppe DattolaAinda não há avaliações
- Self Study Intraoral AnatomyDocumento79 páginasSelf Study Intraoral AnatomyNadia VidirachmillaAinda não há avaliações
- Organic ChemistryDocumento1 páginaOrganic ChemistryRaj KumarAinda não há avaliações
- Anic Chemistry PDFDocumento30 páginasAnic Chemistry PDFHakim Abbas Ali PhalasiyaAinda não há avaliações
- Organic Chemistry I-Edit PDFDocumento132 páginasOrganic Chemistry I-Edit PDFJeevitha SivamAinda não há avaliações
- AnatomyDocumento4 páginasAnatomyvinAinda não há avaliações
- Organic Chemistry Lab 3Documento4 páginasOrganic Chemistry Lab 3Presley OmorodionAinda não há avaliações
- 15 - DNA Affinity Screening of Plants, BJC. v.27, n.2, 2010Documento4 páginas15 - DNA Affinity Screening of Plants, BJC. v.27, n.2, 2010Bolivian Journal of ChemistryAinda não há avaliações
- Organic Chemistry: An Indian JournalDocumento5 páginasOrganic Chemistry: An Indian Journalsnigdha shromaAinda não há avaliações
- CCH Anatomy SyllabusDocumento6 páginasCCH Anatomy SyllabusNikhil SinghAinda não há avaliações
- Organic Chemistry Uses of Organic Compounds: SR - No Organic Compound UsesDocumento1 páginaOrganic Chemistry Uses of Organic Compounds: SR - No Organic Compound UsesAnmol AgarwalAinda não há avaliações
- Organic Chemistry: Prepared By: Goce, Ivan Rei LDocumento35 páginasOrganic Chemistry: Prepared By: Goce, Ivan Rei LReiVanAinda não há avaliações
- Enoolate Chemistry ExcerciesDocumento15 páginasEnoolate Chemistry ExcerciesClara CarreraAinda não há avaliações
- Organic ChemistryDocumento21 páginasOrganic ChemistryCHRISTINE JOY PASTURANAinda não há avaliações
- Anatomy QuesDocumento7 páginasAnatomy QuesShabab AliAinda não há avaliações
- College of Arts and Sciences: CHM 215 Organic Chemistry IDocumento3 páginasCollege of Arts and Sciences: CHM 215 Organic Chemistry INajmul Puda PappadamAinda não há avaliações
- Notes Organic Chemistry and AlkanesDocumento17 páginasNotes Organic Chemistry and Alkanessrk78Ainda não há avaliações
- Anatomy AxillaDocumento2 páginasAnatomy AxillaFroi Jovanni PerezAinda não há avaliações
- Organic Chemistry '13Documento33 páginasOrganic Chemistry '13Princess KimAinda não há avaliações
- Organic ChemistryDocumento5 páginasOrganic Chemistryapi-233187566Ainda não há avaliações
- Human Anatomy - ReadingDocumento3 páginasHuman Anatomy - ReadingzuzanamAinda não há avaliações
- Sample Formal Report in Organic ChemistryDocumento10 páginasSample Formal Report in Organic ChemistryAudrey CobankiatAinda não há avaliações
- Application of PullulanDocumento2 páginasApplication of PullulanKumar Organic Products LimitedAinda não há avaliações
- Peter Barbera's BSC2085 Unit 3 reportDocumento3 páginasPeter Barbera's BSC2085 Unit 3 reportjohnnysackAinda não há avaliações
- Organic LectureDocumento3 páginasOrganic LectureSymonette OcturaAinda não há avaliações
- Useful Organic MoleculesDocumento70 páginasUseful Organic MoleculesMonika GuliaAinda não há avaliações
- Structural Elucidation in ChemistryDocumento49 páginasStructural Elucidation in ChemistryYoussef LatashAinda não há avaliações
- Reading 05 - Violent Videogames (Jeffrey Goldstein)Documento17 páginasReading 05 - Violent Videogames (Jeffrey Goldstein)Youssef LatashAinda não há avaliações
- ANU Chemistry I HPO Lect 2012Documento44 páginasANU Chemistry I HPO Lect 2012Youssef LatashAinda não há avaliações
- LaTeX SymbolsDocumento22 páginasLaTeX SymbolstamgiangAinda não há avaliações
- Yeats' Poetry and the Significance of DesireDocumento3 páginasYeats' Poetry and the Significance of DesireYoussef Latash100% (1)
- Spanish Language Around the WorldDocumento18 páginasSpanish Language Around the WorldYoussef LatashAinda não há avaliações
- Shake It OutDocumento9 páginasShake It OutYoussef LatashAinda não há avaliações
- EditDocumento2 páginasEditEvey HernándezAinda não há avaliações
- TDS PH MacTexMXL May2014Documento1 páginaTDS PH MacTexMXL May2014Catherine MagnayeAinda não há avaliações
- cp3 TrussdesignDocumento106 páginascp3 Trussdesignznyaphotmail.comAinda não há avaliações
- Sludge Dewatering Tube Utilization - Palm Oil HunterDocumento7 páginasSludge Dewatering Tube Utilization - Palm Oil Hunteruma shankar balakrishnanAinda não há avaliações
- LAF TheoryDocumento22 páginasLAF TheoryNeeraj MehtaAinda não há avaliações
- Unit 4Documento76 páginasUnit 4raghuram67Ainda não há avaliações
- Lesson 1.3: General Properties of Indefinite IntegralsDocumento6 páginasLesson 1.3: General Properties of Indefinite IntegralsMarkAinda não há avaliações
- Module 1 - 2D FlowDocumento18 páginasModule 1 - 2D FlowRizal Irnandi HidayatAinda não há avaliações
- (06b) C2 - 025 Project Specification RVSDDocumento90 páginas(06b) C2 - 025 Project Specification RVSDmohammeddashtiAinda não há avaliações
- Getting Started With ANSYSDocumento19 páginasGetting Started With ANSYSThulasi RamAinda não há avaliações
- Divine Particles Pressnote by Sanatan SansthaDocumento4 páginasDivine Particles Pressnote by Sanatan SansthaHaindava KeralamAinda não há avaliações
- Online Courses From Top UniversitiesDocumento3 páginasOnline Courses From Top Universitiesephrem0% (2)
- Application of The Giroud - Han Design Method For Geosynthetic Reinforced Unpaved Roads With Tencate Mirafi GeosyntheticsDocumento7 páginasApplication of The Giroud - Han Design Method For Geosynthetic Reinforced Unpaved Roads With Tencate Mirafi GeosyntheticsFaten Abou ShakraAinda não há avaliações
- Simple and Inexpensive Microforge: by G. HilsonDocumento5 páginasSimple and Inexpensive Microforge: by G. Hilsonfoober123Ainda não há avaliações
- IRC Ammendments 2018Documento31 páginasIRC Ammendments 2018ਸੁਖਬੀਰ ਸਿੰਘ ਮਾਂਗਟ100% (1)
- MS27069GDocumento7 páginasMS27069Gawesome_600Ainda não há avaliações
- Astm d6218-00Documento19 páginasAstm d6218-00Francisco DelgadoAinda não há avaliações
- Calibration & Testing: ISO 17025 (NABL Accredited)Documento7 páginasCalibration & Testing: ISO 17025 (NABL Accredited)Spectro Analytical Labs LimitedAinda não há avaliações
- High Speed CastingDocumento7 páginasHigh Speed Castingferdlh9Ainda não há avaliações
- .Fraunhofer - Diffraction.at - The.slit.Documento6 páginas.Fraunhofer - Diffraction.at - The.slit.NCHE BORISAinda não há avaliações
- Assignment 4 - Written Component: Math 100 - Fall 2021 Due: Tuesday, October 5 at 11:59 PMDocumento2 páginasAssignment 4 - Written Component: Math 100 - Fall 2021 Due: Tuesday, October 5 at 11:59 PMameernot77Ainda não há avaliações
- Three-Phase Power Flow Calculations Using Direct ZBUS Method For Large-Scale Unbalanced Distribution NetworksDocumento8 páginasThree-Phase Power Flow Calculations Using Direct ZBUS Method For Large-Scale Unbalanced Distribution NetworksApikShafieeAinda não há avaliações
- Semester 1 Examinations 2018/2019: Programme (S)Documento6 páginasSemester 1 Examinations 2018/2019: Programme (S)fuckoffmanAinda não há avaliações
- ENGG 412 Materials Science and Engineering Composite MaterialsDocumento49 páginasENGG 412 Materials Science and Engineering Composite MaterialsVenus Abigail Gutierrez100% (1)
- Rabin CryptosystemDocumento41 páginasRabin CryptosystemArkadev GhoshAinda não há avaliações
- Pump TroubleshootingDocumento514 páginasPump Troubleshootingmohamed hamedAinda não há avaliações
- Civil Engineering: Reinforced Cement Concrete & Pre-Stressed ConcreteDocumento33 páginasCivil Engineering: Reinforced Cement Concrete & Pre-Stressed ConcreteDebendra Dev KhanalAinda não há avaliações
- CFD Application Tutorials 2Documento35 páginasCFD Application Tutorials 2Jubril AkinwandeAinda não há avaliações