Escolar Documentos
Profissional Documentos
Cultura Documentos
Eng-Maximizing of Solar Absorption-Farhan Lafta Rashid
Enviado por
Impact JournalsTítulo original
Direitos autorais
Formatos disponíveis
Compartilhar este documento
Compartilhar ou incorporar documento
Você considera este documento útil?
Este conteúdo é inapropriado?
Denunciar este documentoDireitos autorais:
Formatos disponíveis
Eng-Maximizing of Solar Absorption-Farhan Lafta Rashid
Enviado por
Impact JournalsDireitos autorais:
Formatos disponíveis
IMPACT: International Journal of Research in Engineering & Technology (IMPACT: IJRET) ISSN(E): 2321-8843; ISSN(P): 2347-4599 Vol.
2, Issue 1, Jan 2014, 87-90 Impact Journals
MAXIMIZING OF SOLAR ABSORPTION BY (TiO2-WATER) NANOFLUID WITH GLASS MIXTURE
FARHAN LAFTA RASHID1, KHUDHAIR ABASS DAWOOD2 & AHMED HASHIM3
1,2
Ministry of Science and Technology, Iraq
3
Babylon University, Babylon, Iraq
ABSTRACT
Suspensions of nanoparticles (i.e., particles with diameters < 100 nm) in liquids, termed nanofluids, show remarkable thermal and optical property changes from the base liquid at low particle loadings. Recent studies also indicate that selected nanofluids may improve the efficiency of direct absorption solar thermal collectors. Nanofluids are prepared by dispersing and stably suspending nanometer sized solid particles in conventional heat transfer fluids. Past researches have shown that a very small amount of suspending nanoparticles have the potential to enhance the thermo physical, transport and radiative properties of the base fluid. At this research adding very small quantities of nano particle (TiO2) to pure water with different weights percent ranged 0.1, 0.2, 0.3 and 0.4wt.%, we found that the best weight percent is 0.2 that gave more heat absorbed. Then adding glass impurities ranged 10, 20 and 30wt.% to the nanofluid in order to enhancing the absorbed heat so energy storage. The best glass weights percent is 0.3.
KEYWORDS: Glass Impurities, Energy Storage, Enhancement Absorbed Heat, Solar Energy INTRODUCTION
Nanofluids, or suspensions of nanoparticles in liquids, have been studied for at least 15 years and have shown promise to enhance a wide range of liquid properties. Generally, nanofluids are formed by dispersing nanometer-sized particles (1-100 nm) or droplets into HTFs. Nanoparticles have unique properties, such as large surface area to volume ratio, dimension-dependent physical properties, and lower kinetic energy, which can be exploited by the nanofluids. At the same time, the large surface area make nanoparticles better and more stably dispersed in base fluids. Compared with micro-fluids or milli-fluids, nanofluids stay more stable, so nanofluids are promising for practical applications without causing problems mentioned above. Nanofluids well keep the fluidic properties of the base fluids, behave like pure liquids and incur little penalty in pressure drop due to the fact that the dispersed phase (nanoparticles) are extremely tiny, which can be very stably suspended in fluids with or even without the help of surfactants[1]. Nanotechnology provides new area of research to process and produce materials with average crystallite sizes below 100 nm called nanomaterial. The term Nano materials encompasses a wide range of materials including Nano crystalline materials, Nano composites, carbon nanotubes and quantum dots[2]. Very high thermal conductivity and extreme stability have always been desired for heat transfer fluids with particles. Fluids having this important combination of features did not exist till the advent of Nano fluids. Nano fluid technology could make the process more energy efficient and cost effective. These Nano fluids could be used in a wide range of industrial applications. Demand for ultra-high-performance cooling in electronics has been increasing, and
88
Farhan Lafta Rashid, Khudhair Abass Dawood & Ahmed Hashim
conventional enhanced surface techniques have reached their limit with regard to improving heat transfer. Since nanoparticles are relatively much smaller than the diameter of micro channel flow passages, smooth-flowing Nano fluids could provide the solution. Since Nano fluids can flow in micro channels without clogging, they would be suitable coolants. These could enhance cooling of MEMS under extreme heat flux conditions [3]. Specific heat capacity of solvents can also be enhanced by doping with nanoparticles. Nelson et al. [4] reported that the specific heat capacity of polyalphaolefin was enhanced by 50% on addition of ex-foliated graphite nanoparticles at 0.6%concentration by weight. Shin and Banerjee [5] synthesized molten salt-based silica nanofluid and observed 26% enhanced specific heat capacity for 1% weight concentration. Thermal energy storage _TES_ systems at high temperatures are required to improve the operational efficiencies and reliability of solar thermal energy conversion systems. The materials that are compatible for these applicationssuch as alkali-nitrates, alkalicarbonates, and alkali-chlorides _as well as their eutectics_have very low specific heat capacities, usually less than 2 J /g While, to compare, the specific heat of water is 4.2 J /g K at room temperature. It has been shown that nanoparticles with higher thermal conductivity than their surrounding liquid can increase the effective thermal conductivity of suspension. For example, a small amount (<1% volume fraction) of Cu nanoparticles or carbon nanotubes dispersed in water or oil was reported to increase the inherently poor thermal conductivity of the liquid by 74% and 150% [6, 7]. Specific heat capacity of solvents can also be enhanced by doping with nanoparticles. Nelson et al. [8] reported that the specific heat capacity of polyalphaolefin was enhanced by 50% on addition of ex-foliated graphite nanoparticles at 0.6% concentration by weight. Shin and Banerjee [9] synthesized molten salt-based silica nanofluid and observed 26% enhanced specific heat capacity for 1% weight concentration. In this work we will use glass impurities that is available everywhere with nanofluid (TiO2-water) to enhancement thermal energy storage.
RESULTS AND DISCUSSIONS
Results were obtained for the present work in the purpose of enhancement of solar energy storage by mixture of nanofluid (TiO2-water) with glass impurities ranged 10, 20 and 30wt.% were compared with the case of nanofluid alone (0.1, 0.2, 0.3 and 0.4 wt.%). Figure 1 represent variation of nanofluid temperature with losing time (without adding glass impurities), we can see that all nanofluid weight percent's gave less in absorbing and releasing solar heat than the case of pure water. In order to make an increasing in absorbing solar heat we add glass impurities with weights percent of 10 wt.% as in figure 2, 20 wt.% as in figure 3 and 30 wt.% as in figure 4. From figures we can see that the best case is of figure 4 which is 30 wt.% of glass impurities, this because of the enhancing of reflection to the solar radiation which lead to increase the solar heat absorbed by the mixture.
CONCLUSIONS
Our research gave us the following conclusions: Adding glass impurities can enhancement heat absorbing. Increasing the glass impurities weights percent increase amount of solar heat absorbed.
Maximizing of Solar Absorption by (TiO2-Water) Nanofluid with Glass Mixture
89
The best nanofluid weight percent is 0.2%. which gave the largest heat absorbed. Solar heat absorbed decreases with time.
REFERENCES
1. Farhan et al., (2013), Design Study of Nanofluid Solar Absorption Refrigeration system, International Journal of Innovative Research in Engineering & Science ISSN 2319-5665, (June 2013, issue 2 volume 6). 2. Hani et al., (2013), Thermal Energy Storage by Nanofluids, Journal of Energy Technologies and Policy, ISSN 2224-3232 (Paper) ISSN 2225-0573 (Online), Vol.3, No.5, 2013. 3. A. K. Singh, (2008), Thermal Conductivity of Nanofluids, Defense Science Journal, Vol. 58, No. 5, September 2008, pp. 600-607. 4. I.C. Nelson, D. Banerjee, R. Ponnappan, Flow loop experiments using polyalphaolefin, J. Thermophys. Heat Transfer 23 (2009) 752761. 5. D. Shin, D. Banerjee, Enhanced specific heat of silica nanofluid, ASME J. Heat Transfer 133 (2011) 024501024504, doi:10.1115/1.4002600. 6. 7. 8. S. Jana, A.S. Khojin, W.H. Zhong, Thermochim. Acta 462 (2007) 4555. S.U.S. Choi, Z.G. Zhang, W. Yu, et al., Appl. Phys. Lett. 79 (2001) 22522254. I.C. Nelson, D. Banerjee, R. Ponnappan, Flow loop experiments using polyalphaolefin, J. Thermophys. Heat Transfer 23 (2009) 752761. 9. D. Shin, D. Banerjee, Enhanced specific heat of silica nanofluid, ASME J. Heat Transfer 133 (2011) 024501024504, doi:10.1115/1.4002600.
APPENDICES
Figure 1: Variation of Nanofluid (TiO2-Water) Temperature with Losing Time without Glass Impurities
90
Farhan Lafta Rashid, Khudhair Abass Dawood & Ahmed Hashim
Figure 2: Variation of Nanofluid (TiO2-Water) Temperature with Losing Time for Adding 10 wt.% of Glass Impurities
Figure 3: Variation of Nanofluid (TiO2-Water) Temperature with Losing Time for Adding 20 wt.% of Glass Impurities
Figure 4: Variation of Nanofluid (TiO2-Water) Temperature with Losing Time for Adding 30 wt.% of Glass Impurities
Você também pode gostar
- A Heartbreaking Work Of Staggering Genius: A Memoir Based on a True StoryNo EverandA Heartbreaking Work Of Staggering Genius: A Memoir Based on a True StoryNota: 3.5 de 5 estrelas3.5/5 (231)
- The Sympathizer: A Novel (Pulitzer Prize for Fiction)No EverandThe Sympathizer: A Novel (Pulitzer Prize for Fiction)Nota: 4.5 de 5 estrelas4.5/5 (119)
- Never Split the Difference: Negotiating As If Your Life Depended On ItNo EverandNever Split the Difference: Negotiating As If Your Life Depended On ItNota: 4.5 de 5 estrelas4.5/5 (838)
- Devil in the Grove: Thurgood Marshall, the Groveland Boys, and the Dawn of a New AmericaNo EverandDevil in the Grove: Thurgood Marshall, the Groveland Boys, and the Dawn of a New AmericaNota: 4.5 de 5 estrelas4.5/5 (265)
- The Little Book of Hygge: Danish Secrets to Happy LivingNo EverandThe Little Book of Hygge: Danish Secrets to Happy LivingNota: 3.5 de 5 estrelas3.5/5 (399)
- The World Is Flat 3.0: A Brief History of the Twenty-first CenturyNo EverandThe World Is Flat 3.0: A Brief History of the Twenty-first CenturyNota: 3.5 de 5 estrelas3.5/5 (2219)
- The Subtle Art of Not Giving a F*ck: A Counterintuitive Approach to Living a Good LifeNo EverandThe Subtle Art of Not Giving a F*ck: A Counterintuitive Approach to Living a Good LifeNota: 4 de 5 estrelas4/5 (5794)
- Team of Rivals: The Political Genius of Abraham LincolnNo EverandTeam of Rivals: The Political Genius of Abraham LincolnNota: 4.5 de 5 estrelas4.5/5 (234)
- The Emperor of All Maladies: A Biography of CancerNo EverandThe Emperor of All Maladies: A Biography of CancerNota: 4.5 de 5 estrelas4.5/5 (271)
- The Gifts of Imperfection: Let Go of Who You Think You're Supposed to Be and Embrace Who You AreNo EverandThe Gifts of Imperfection: Let Go of Who You Think You're Supposed to Be and Embrace Who You AreNota: 4 de 5 estrelas4/5 (1090)
- The Hard Thing About Hard Things: Building a Business When There Are No Easy AnswersNo EverandThe Hard Thing About Hard Things: Building a Business When There Are No Easy AnswersNota: 4.5 de 5 estrelas4.5/5 (344)
- Hidden Figures: The American Dream and the Untold Story of the Black Women Mathematicians Who Helped Win the Space RaceNo EverandHidden Figures: The American Dream and the Untold Story of the Black Women Mathematicians Who Helped Win the Space RaceNota: 4 de 5 estrelas4/5 (890)
- Elon Musk: Tesla, SpaceX, and the Quest for a Fantastic FutureNo EverandElon Musk: Tesla, SpaceX, and the Quest for a Fantastic FutureNota: 4.5 de 5 estrelas4.5/5 (474)
- The Unwinding: An Inner History of the New AmericaNo EverandThe Unwinding: An Inner History of the New AmericaNota: 4 de 5 estrelas4/5 (45)
- The Yellow House: A Memoir (2019 National Book Award Winner)No EverandThe Yellow House: A Memoir (2019 National Book Award Winner)Nota: 4 de 5 estrelas4/5 (98)
- VTU Tribology Lesson PlanDocumento3 páginasVTU Tribology Lesson Plankumar100% (1)
- Design of Hose Reel System: - Nozzle: P 200 Kpa 0.4 L/SDocumento36 páginasDesign of Hose Reel System: - Nozzle: P 200 Kpa 0.4 L/Sمحمد القدومي100% (1)
- Optimization Design Study of Low-Reynolds-number High-Lift Airfoils For The High-Efficiency Propeller of Low-Dynamic Vehicles in StratosphereDocumento16 páginasOptimization Design Study of Low-Reynolds-number High-Lift Airfoils For The High-Efficiency Propeller of Low-Dynamic Vehicles in StratospherepuhumightAinda não há avaliações
- Heat Transfer: Download Free Books atDocumento155 páginasHeat Transfer: Download Free Books atDurgaPrasadKrishnaAinda não há avaliações
- Sizing Three Phase Horizontal SeparatorDocumento1 páginaSizing Three Phase Horizontal SeparatorekabudiartiAinda não há avaliações
- 19-11-2022-1668837986-6-Impact - Ijrhal-2. Ijrhal-Topic Vocational Interests of Secondary School StudentsDocumento4 páginas19-11-2022-1668837986-6-Impact - Ijrhal-2. Ijrhal-Topic Vocational Interests of Secondary School StudentsImpact JournalsAinda não há avaliações
- 19-11-2022-1668838190-6-Impact - Ijrhal-3. Ijrhal-A Study of Emotional Maturity of Primary School StudentsDocumento6 páginas19-11-2022-1668838190-6-Impact - Ijrhal-3. Ijrhal-A Study of Emotional Maturity of Primary School StudentsImpact JournalsAinda não há avaliações
- 14-11-2022-1668421406-6-Impact - Ijrhal-01. Ijrhal. A Study On Socio-Economic Status of Paliyar Tribes of Valagiri Village AtkodaikanalDocumento13 páginas14-11-2022-1668421406-6-Impact - Ijrhal-01. Ijrhal. A Study On Socio-Economic Status of Paliyar Tribes of Valagiri Village AtkodaikanalImpact JournalsAinda não há avaliações
- 12-11-2022-1668238142-6-Impact - Ijrbm-01.Ijrbm Smartphone Features That Affect Buying Preferences of StudentsDocumento7 páginas12-11-2022-1668238142-6-Impact - Ijrbm-01.Ijrbm Smartphone Features That Affect Buying Preferences of StudentsImpact JournalsAinda não há avaliações
- 09-12-2022-1670567289-6-Impact - Ijrhal-06Documento17 páginas09-12-2022-1670567289-6-Impact - Ijrhal-06Impact JournalsAinda não há avaliações
- 25-10-2022-1666681548-6-IMPACT - IJRHAL-4. IJRHAL-Challenges of Teaching Moral and Ethical Values in An Age of Instant GratificationDocumento4 páginas25-10-2022-1666681548-6-IMPACT - IJRHAL-4. IJRHAL-Challenges of Teaching Moral and Ethical Values in An Age of Instant GratificationImpact JournalsAinda não há avaliações
- 15-10-2022-1665806338-6-IMPACT - IJRBM-1. IJRBM Theoretical and Methodological Problems of Extra-Budgetary Accounting in Educational InstitutionsDocumento10 páginas15-10-2022-1665806338-6-IMPACT - IJRBM-1. IJRBM Theoretical and Methodological Problems of Extra-Budgetary Accounting in Educational InstitutionsImpact JournalsAinda não há avaliações
- 28-10-2022-1666956219-6-Impact - Ijrbm-3. Ijrbm - Examining Attitude ...... - 1Documento10 páginas28-10-2022-1666956219-6-Impact - Ijrbm-3. Ijrbm - Examining Attitude ...... - 1Impact JournalsAinda não há avaliações
- 11-10-2022-1665489521-6-IMPACT - IJRHAL-Analysing The Matrix in Social Fiction and Its Relevance in Literature.Documento6 páginas11-10-2022-1665489521-6-IMPACT - IJRHAL-Analysing The Matrix in Social Fiction and Its Relevance in Literature.Impact JournalsAinda não há avaliações
- 09-12-2022-1670567569-6-Impact - Ijrhal-05Documento16 páginas09-12-2022-1670567569-6-Impact - Ijrhal-05Impact JournalsAinda não há avaliações
- Effect of Sahaja Yoga Meditation in Reducing Anxiety Level of Class Vi Students Towards English As A Second LanguageDocumento8 páginasEffect of Sahaja Yoga Meditation in Reducing Anxiety Level of Class Vi Students Towards English As A Second LanguageImpact JournalsAinda não há avaliações
- 12-10-2022-1665566934-6-IMPACT - IJRHAL-2. Ideal Building Design y Creating Micro ClimateDocumento10 páginas12-10-2022-1665566934-6-IMPACT - IJRHAL-2. Ideal Building Design y Creating Micro ClimateImpact JournalsAinda não há avaliações
- Influence of Erotic Nollywood Films Among Undergraduates of Lead City University, Ibadan, NigeriaDocumento12 páginasInfluence of Erotic Nollywood Films Among Undergraduates of Lead City University, Ibadan, NigeriaImpact JournalsAinda não há avaliações
- 22-09-2022-1663824012-6-Impact - Ijranss-5. Ijranss - Periodontics Diseases Types, Symptoms, and Its CausesDocumento10 páginas22-09-2022-1663824012-6-Impact - Ijranss-5. Ijranss - Periodontics Diseases Types, Symptoms, and Its CausesImpact JournalsAinda não há avaliações
- 21-09-2022-1663757720-6-Impact - Ijranss-3. Ijranss - The Role and Objectives of Criminal LawDocumento8 páginas21-09-2022-1663757720-6-Impact - Ijranss-3. Ijranss - The Role and Objectives of Criminal LawImpact JournalsAinda não há avaliações
- 21-09-2022-1663757902-6-Impact - Ijranss-4. Ijranss - Surgery, Its Definition and TypesDocumento10 páginas21-09-2022-1663757902-6-Impact - Ijranss-4. Ijranss - Surgery, Its Definition and TypesImpact JournalsAinda não há avaliações
- 01-10-2022-1664607817-6-Impact - Ijrbm-2. Ijrbm - Innovative Technology in Indian Banking Sector-A Prospective AnalysisDocumento4 páginas01-10-2022-1664607817-6-Impact - Ijrbm-2. Ijrbm - Innovative Technology in Indian Banking Sector-A Prospective AnalysisImpact JournalsAinda não há avaliações
- 27-10-2022-1666851544-6-IMPACT - IJRANSS-2. IJRANSS - A Review On Role of Ethics in Modern-Day Research Assignment - 1Documento6 páginas27-10-2022-1666851544-6-IMPACT - IJRANSS-2. IJRANSS - A Review On Role of Ethics in Modern-Day Research Assignment - 1Impact JournalsAinda não há avaliações
- 02-09-2022-1662112193-6-Impact - Ijrhal-1. Ijrhal - Shifting Gender Roles A Comparative Study of The Victorian Versus The Contemporary TimesDocumento12 páginas02-09-2022-1662112193-6-Impact - Ijrhal-1. Ijrhal - Shifting Gender Roles A Comparative Study of The Victorian Versus The Contemporary TimesImpact JournalsAinda não há avaliações
- 05-09-2022-1662375638-6-Impact - Ijranss-1. Ijranss - Analysis of Impact of Covid-19 On Religious Tourism Destinations of Odisha, IndiaDocumento12 páginas05-09-2022-1662375638-6-Impact - Ijranss-1. Ijranss - Analysis of Impact of Covid-19 On Religious Tourism Destinations of Odisha, IndiaImpact JournalsAinda não há avaliações
- 07-09-2022-1662545503-6-Impact - Ijranss-2. Ijranss - Role of Bio-Fertilizers in Vegetable Crop Production A ReviewDocumento6 páginas07-09-2022-1662545503-6-Impact - Ijranss-2. Ijranss - Role of Bio-Fertilizers in Vegetable Crop Production A ReviewImpact JournalsAinda não há avaliações
- Effect of Organizational Commitment On Research and Development Performance of Academics: An Empirical Analysis in Higher Educational InstitutionsDocumento12 páginasEffect of Organizational Commitment On Research and Development Performance of Academics: An Empirical Analysis in Higher Educational InstitutionsImpact JournalsAinda não há avaliações
- Comparison of Soil Properties Collected From Nalgonda, Ranga Reddy, Hyderabad and Checking Its Suitability For ConstructionDocumento8 páginasComparison of Soil Properties Collected From Nalgonda, Ranga Reddy, Hyderabad and Checking Its Suitability For ConstructionImpact JournalsAinda não há avaliações
- 24-09-2022-1664015374-6-Impact - Ijranss-6. Ijranss - Patterns of Change Among The Jatsas Gleaned From Arabic and Persian SourcesDocumento6 páginas24-09-2022-1664015374-6-Impact - Ijranss-6. Ijranss - Patterns of Change Among The Jatsas Gleaned From Arabic and Persian SourcesImpact JournalsAinda não há avaliações
- 19-09-2022-1663587287-6-Impact - Ijrhal-3. Ijrhal - Instability of Mother-Son Relationship in Charles Dickens' Novel David CopperfieldDocumento12 páginas19-09-2022-1663587287-6-Impact - Ijrhal-3. Ijrhal - Instability of Mother-Son Relationship in Charles Dickens' Novel David CopperfieldImpact JournalsAinda não há avaliações
- 28-09-2022-1664362344-6-Impact - Ijrhal-4. Ijrhal - Understanding The Concept of Smart City and Its Socio-Economic BarriersDocumento8 páginas28-09-2022-1664362344-6-Impact - Ijrhal-4. Ijrhal - Understanding The Concept of Smart City and Its Socio-Economic BarriersImpact JournalsAinda não há avaliações
- 20-09-2022-1663649149-6-Impact - Ijrhal-2. Ijrhal - A Review of Storytelling's Role and Effect On Language Acquisition in English Language ClassroomsDocumento10 páginas20-09-2022-1663649149-6-Impact - Ijrhal-2. Ijrhal - A Review of Storytelling's Role and Effect On Language Acquisition in English Language ClassroomsImpact JournalsAinda não há avaliações
- 29-08-2022-1661768824-6-Impact - Ijrhal-9. Ijrhal - Here The Sky Is Blue Echoes of European Art Movements in Vernacular Poet Jibanananda DasDocumento8 páginas29-08-2022-1661768824-6-Impact - Ijrhal-9. Ijrhal - Here The Sky Is Blue Echoes of European Art Movements in Vernacular Poet Jibanananda DasImpact JournalsAinda não há avaliações
- Study and Investigation On The Red and Intangible Cultural Sites in Guangdong - Chasing The Red Mark and Looking For The Intangible Cultural HeritageDocumento14 páginasStudy and Investigation On The Red and Intangible Cultural Sites in Guangdong - Chasing The Red Mark and Looking For The Intangible Cultural HeritageImpact JournalsAinda não há avaliações
- 25-08-2022-1661426015-6-Impact - Ijrhal-8. Ijrhal - Study of Physico-Chemical Properties of Terna Dam Water Reservoir Dist. Osmanabad M.S. - IndiaDocumento6 páginas25-08-2022-1661426015-6-Impact - Ijrhal-8. Ijrhal - Study of Physico-Chemical Properties of Terna Dam Water Reservoir Dist. Osmanabad M.S. - IndiaImpact JournalsAinda não há avaliações
- Chapter 3 - Microscopic Displacement Eficiency - Part IIDocumento40 páginasChapter 3 - Microscopic Displacement Eficiency - Part IIMohamed ModerAinda não há avaliações
- Combustión: Análisis Termodinámico, Químico y Transferencia de MasaDocumento365 páginasCombustión: Análisis Termodinámico, Químico y Transferencia de MasaPaulo Cæsar SlowAinda não há avaliações
- UPVC Technical Data SheetDocumento1 páginaUPVC Technical Data SheetRND KencanaAinda não há avaliações
- FemDocumento10 páginasFemSeenu CnuAinda não há avaliações
- Solution To Problem 204 Stress-Strain Diagram - Strength of Materials ReviewDocumento3 páginasSolution To Problem 204 Stress-Strain Diagram - Strength of Materials ReviewimrancenakkAinda não há avaliações
- Fluid Mechanics (XE (B) ) PDFDocumento5 páginasFluid Mechanics (XE (B) ) PDFLucas SchroederAinda não há avaliações
- Principles of Momentum Transfer and ApplicationsDocumento9 páginasPrinciples of Momentum Transfer and ApplicationsjaiminAinda não há avaliações
- Manual RIDO - 4Documento37 páginasManual RIDO - 4messiahsAinda não há avaliações
- Mean Metal TempDocumento1 páginaMean Metal TempsankaranAinda não há avaliações
- RAC Servicing NC 1Documento10 páginasRAC Servicing NC 1PatrickAinda não há avaliações
- Water Supply CalculationsDocumento8 páginasWater Supply CalculationsAshiq NishmaAinda não há avaliações
- Modelling of Laser Welding of Aluminium Using COMSOL MultiphysicsDocumento73 páginasModelling of Laser Welding of Aluminium Using COMSOL MultiphysicsAli NasserAinda não há avaliações
- FinalDocumento18 páginasFinaltusharAinda não há avaliações
- Azanechiller Series 2010 (3) Final VersionDocumento12 páginasAzanechiller Series 2010 (3) Final VersionRajkumar GulatiAinda não há avaliações
- SCIENCE 4 Q3 W4 Activity Sheets 2Documento3 páginasSCIENCE 4 Q3 W4 Activity Sheets 2Rerai CaielleAinda não há avaliações
- Clarkson Strength of Materials Formula SheetDocumento1 páginaClarkson Strength of Materials Formula SheetMadi SilalahiAinda não há avaliações
- Mechanics of Solids PDFDocumento8 páginasMechanics of Solids PDFprashanthreddy26Ainda não há avaliações
- 10 1016@j Conbuildmat 2019 05 035Documento14 páginas10 1016@j Conbuildmat 2019 05 035Veronika PrymAinda não há avaliações
- Theoretical 3 Tornado SolutionDocumento7 páginasTheoretical 3 Tornado Solutionjas dwanAinda não há avaliações
- FVM - CFDDocumento44 páginasFVM - CFDgrkguptaAinda não há avaliações
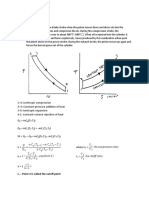
- Diesel Cycle: R - Point 3 Is Called The Cutoff PointDocumento7 páginasDiesel Cycle: R - Point 3 Is Called The Cutoff PointJethro Briza GaneloAinda não há avaliações
- Experiment 3 - 1st and 2nd Law of ThermodynamicsDocumento4 páginasExperiment 3 - 1st and 2nd Law of ThermodynamicsWee Chuan YeanAinda não há avaliações
- Mununga Day Secondary School: Candidate'S Name: ClassDocumento3 páginasMununga Day Secondary School: Candidate'S Name: ClassSimon SimuntalaAinda não há avaliações
- FRENKEL, Notes On Statistical Thermodynamics (UVA, 2001)Documento64 páginasFRENKEL, Notes On Statistical Thermodynamics (UVA, 2001)Marcio CameloAinda não há avaliações
- Spe 171590 MSDocumento14 páginasSpe 171590 MSDaniele OliveiraAinda não há avaliações