Escolar Documentos
Profissional Documentos
Cultura Documentos
Scheme of Work Science Stage 9.v1
Enviado por
gkawsar22Descrição original:
Direitos autorais
Formatos disponíveis
Compartilhar este documento
Compartilhar ou incorporar documento
Você considera este documento útil?
Este conteúdo é inapropriado?
Denunciar este documentoDireitos autorais:
Formatos disponíveis
Scheme of Work Science Stage 9.v1
Enviado por
gkawsar22Direitos autorais:
Formatos disponíveis
Scheme of Work Science stage 9
Introduction
This document is a scheme of work created by Cambridge as a suggested plan of delivery for Cambridge Secondary 1 Science stage 9. Learning objectives for the stage have been grouped into topic areas or !nits". These have then been arranged in a recommended teaching order but you are free to teach objectives in any order within a stage as your local re#uirements and resources dictate. The scheme for Science has assumed a term length of 1$ weeks% with three terms per stage and three units per term. &n overview of the se#uence% number and title of each unit for stage 9 can be seen in the table below. The scheme has been based on the minimum length of a school year to allow fle'ibility. (ou should be able to add in more teaching time as necessary% to suit the pace of your learners and to fit the work comfortably into your own term times. Scientific )n#uiry learning objectives are recurring% appearing in every unit. &ctivities and resources are suggested against the objectives to illustrate possible methods of delivery. There is no obligation to follow the published Cambridge Scheme of *ork in order to deliver Cambridge Secondary 1. +t has been created solely to provide an illustration of how delivery might be planned over the three stages. & step,by,step guide to creating your own scheme of work and implementing Cambridge Secondary 1 in your school can be found in the Cambridge Secondary 1Teacher -uide available on the Cambridge Secondary 1 website. .lank templates are also available on the Cambridge Secondary 1 website for you to use if you wish.
Overview
Term 1 1& !nit 9.1 /hotosynthesis and /lant -rowth 1. !nit 9.0 The /eriodic Table and /reparing Salts 1C !nit 9.4 )lectrostatics and )lectric Currents Term 2 0& 0. 0C !nit 9.1 Se'ual 2eproduction in 3lowering /lants !nit 9.6 2eactivity and 2ates of 2eaction !nit 9.8 9ovements% /ressure and :ensity Term 3 4& 4. 4C !nit 9.5 )cology !nit 9.7 Chemicals and Thermal )nergy !nit 9.9 The )nergy Crisis and ;uman +nfluences
<1 1($5
Science Stage 9
Scheme of Work Science stage 9
Unit 1A: 9.1 Photo !nthe i "nd P#"nt $rowth
+n this unit% pupils build on their previous knowledge of photosynthesis and water and the transport of water and minerals in flowering plants to develop their knowledge of The process of photosynthesis including the word e#uation. The importance of water and mineral salts to plant growth.
Scientific )n#uiry work focuses on=
:eciding whether to use evidence from first,hand e'perience or secondary sources. !sing appropriate sampling techni#ues where re#uired. Looking critically at sources of secondary data. Comparing results and methods used by others.
2ecommended <ocabulary for this unit=
/hotosynthesis chlorophyll chloroplasts control.
<1 1($5
Science Stage 9
%r"mework &ode 9.p1 9.p0
'e"rnin( O)*ective :efine and describe photosynthesis and use the word e#uation. The importance of water and mineral salts to plant growth.
Activitie 2eview the work on photosynthesis and the transport of water and mineral salts in plants in stage 7.
+e ource
&omment Link to Stage 7 !nit 1& and !nit 0&.
Time 8$ min
9.p1
:efine and describe photosynthesis and use the word e#uation. Select ideas and produce plans for testing based on previous knowledge% understanding and research. +nterpret results using scientific knowledge and understanding. :efine and describe photosynthesis and use the word e#uation.
:iscuss how to investigate the effect of light on growing plants. Set up some #uickly germinating seeds in advance% e.g. cress% and leave them in the dark to observe the effects. Some should be set up in the light as a comparison. Link the e#uation with transfer of energy along the food chain. Sun >producer > consumer. Construct the word e#uation for photosynthesis and e'plain it is an endothermic reaction because of the re#uirement for energy. Light ? Carbon dio'ide ?water > sugar ? o'ygen.
Cress seeds% petri dishes or shallow containers% filter paper or cotton wool.
8$ min
9)p1
9)c0 9.p1
Sugars are converted to starch.
4$min
9.p1
:efine and describe photosynthesis and use the word e#uation. 9ake observations and measurements
9)o4
@now the green parts of cells are called chloroplasts. Compare pond weed and onion skin cells under the microscope. Chloroplasts are identified.
9icroscopes% onion% pond weed% slides and coverslips.
8$min
<1 1($5
Science Stage 9
%r"mework &ode 9.p1
'e"rnin( O)*ective
Activitie
+e ource
&omment
Time
:efine and describe photosynthesis and use the word e#uation. :raw conclusions. :efine and describe photosynthesis and use the word e#uation. Suggest and use preliminary work to decide how to carry out and investigation. :ecide which measurements and observations are necessary and what e#uipment to use. 9ake sufficient observations and measurements to reduce error and make results more reliable. )'plain results using scientific knowledge and understanding.
9)c1 9.p1
@now that carbon dio'ide can enter and o'ygen escape through stoma. Leaf,peel techni#ues can be used to see stoma% possibly showing differences on upper and lower surfaces. 9easure rates of photosynthesis by using the work on testing the formation of o'ygen from pond weed AStage 7 !nit 1&B to develop further investigations on the rate of production of o'ygen e.g. by counting bubbles produced by pond weed% or the gas can be collected.
Suitable leaves include Tradescantia% +mpatiens and beans. Clear nail varnish% microscope slides% microscopes. )lodea% gas collection apparatus Atrough of water% filter funnel% test,tubeB. Link to Stage 7 !nit 1&. Link to Stage 9 !nit 0..
8$min
8$min
9)p6
9)p5
9)o1
9)c7
<1 1($5
Science Stage 9
%r"mework &ode 9.p0
'e"rnin( O)*ective !nderstand the importance of water and mineral salts to plant growth. :ecide whether to use evidence from first,hand e'perience or secondary sources. !nderstand the importance of water and mineral salts to plant growth. :ecide whether to use evidence from first,hand e'perience or secondary sources. !nderstand the importance of water and mineral salts to plant growth.
Activitie !se secondary sources to e'plain why water is important to a plant.
+e ource Secondary sources.
&omment
Time 4$min
9)p8
9.p0
+nvestigate the effect of different nutrients on plant growth. Students should appreciate the importance of the three essential elements% nitrogen% phosphorus and potassium. Summarise the re#uirements for plant growth in the form of a diagram of a plant showing the intake and output of items by arrows and including the transport routes of 'ylem and phloem.
9)p8
:uckweed is grown in shallow dishes containing solutions with certain minerals omitted to observe the effects. & control should be included. *ater should be prevented from leaving the containers by an oil film.
Secondary data should also used and a comparison with the primary data made.
7$min
9.p0
1$min
<1 1($5
Science Stage 9
Scheme of Work Science stage 9
Unit 1,: 9.2 The Periodic t")#e "nd Pre-"rin( S"#t
+n this unit% pupils build on their previous knowledge of the /eriodic Table% particle theory and chemical reactions to develop their knowledge of The structure of an atom. The methods and discoveries of 2utherford and other scientists. The structures of the first twenty elements of the /eriodic Table. Trends in groups and periods. /reparing some common salts by the reactions of metals or metal carbonates with acid. *riting word e#uations to describe reactions of metals or metal carbonates with acids.
Scientific )n#uiry work focuses on=
The importance of #uestions% evidence and e'planations% using historical e'amples. !sing e'planations to make predictions and then evaluate these against evidence. :iscussing the way that scientists work today and how they worked in the past% including reference to e'perimentation% evidence and creative thought. :eciding which apparatus to use and assess any haCards in the laboratory. !sing a range of materials and e#uipment and control risks.
2ecommended <ocabulary for this unit=
Ducleus proton neutron electron electronic shell AorbitB atomic AprotonB number group period evidence prediction evaluation reactants products carbonates sulfates nitrates chlorides neutraliCation filtration crystalliCation evaporation.
<1 1($5
Science Stage 9
%r"mework &ode 9Cp0
'e"rnin( O)*ective Compare the structures of the first twenty elements of the /eriodic Table.
Activitie 2evise the symbols for the first twenty elements. )ach group of students can make a poster of the structure of a chosen element. &rrange the first 0$ elements with atomic AprotonB numbers into a simple /eriodic Table. & game of cards can be played. )ach card has a symbol and as they are drawn from a pile they are laid out on a blank copy of the table. The winner completes his table first. Look at the information given for each element on the /eriodic Table and relate this to atomic structure. :iagrams show the arrangement of electrons in their shells around the nucleus. /upils should learn to build them up with increasing atomic number.
+e ource
&omment
Time 4$min
9Cp0
Compare the structures of the first twenty elements of the /eriodic Table.
.lank /eriodic Tables% sets of cards of first 0$ elements Aincluding atomic numbersB.
1$min
9Cp1
:escribe the structure of an atom and learn about the methods and discoveries of 2utherford.
6$min
9)p1
:iscuss and e'plain the importance of #uestions% evidence and e'planations% using historical and contemporary e'amples. :iscuss the way that scientists work today and how they worked in the past% including reference to e'perimentation% evidence and creative thought.
9)p4
Learn about the work of 2utherford and other scientists associated with the development of atomic structure and the /eriodic Table e.g. 9endeleev and .ohr. !se secondary sources to find out about the methods and discoveries of 2utherford. /repare a poster or a presentation about 2utherford.
1$min
<1 1($5
Science Stage 9
%r"mework &ode 9Cp4
'e"rnin( O)*ective :escribe trends in groups and periods.
Activitie Look at the vertical pairs of elements and seek similarities% e.g. inert gases% alkali metals% halogens. Students could make predictions about the ne't member of the group and compare the predictions with the actual properties of the element Compare reactivity between vertical pairs of elements where appropriate. i.e. the reaction of lithium E sodium with water% magnesium and calcium with dilute acid% physical properties of chlorine% bromine and iodine. 9ake further predictions about other elements within the groups studied. 2ecognise -roups and /eriods by colouring in according to the properties of the elements e.g..metals and non,metals or solids% li#uids and gases Aat room temperatureB.
+e ource /eriodic Tables.
&omment
Time 8$min
Safety goggles must be worn by students and teacher and screens used for sodium and lithium. :emonstration only.
9Cp4
:escribe trends in groups and periods.
2elate atomic structure to /eriods. !se diagrams to show the electron shells and relate these to position of elements in the /eriodic Table. :iscuss the elements in carbonate and Sulphate ions. Link )lements to compounds and comple' ions.
4$min
9Cc6
)'plain how to prepare some common salts by the reactions of metals and metal carbonates and be able to write word e#uations for these reactions. 'e"rnin( O)*ective
1$min
%r"mework &ode
Activitie
+e ource
&omment
Time
<1 1($5
Science Stage 9
9Cc6
)'plain how to prepare some common salts by the reactions of metals and metal carbonates and be able to write word e#uations for these reactions. :ecide which measurements and observations are necessary and what e#uipment to use. 9ake sufficient observations and measurements to reduce error and make results more reliable. )'plain results using scientific knowledge and understanding.
9)p5
:escribe the preparation of crystals of chloride or sulfate salts from carbonates and acids. )'cess carbonate is added to dilute acid until no more dissolves. The e'cess is filtered off. )vaporate until some solid appears and then leave to cool. 3ilter. Students to prepare an appropriate salt such as calcium chloride% magnesium nitrate% copper sulfate. Students assess the risks involved in the preparation. Students might discuss ways of producing different siCed crystals. Students to plan the preparation of Cinc nitrate /repare sodium chloride from sodium carbonate. *here there is no solid to indicate that a reaction is complete an indicator must be used. +n the reaction between sodium carbonate solution and dilute hydrochloric acid% an indicator can be used. The indicator can be removed with charcoal which is then filtered off. )vaporate until some solid appears and then leave to cool. 3ilter.
calcium carbonate% magnesium carbonate% copper carbonate% dil ;Cl soln% dil ;0SF1
*ord e#uations must be used.
8$min
9)o1
9)c7
9Cc6
)'plain how to prepare some common salts by the reactions of metals and metal carbonates and be able to write word e#uations for these reactions. :ecide which measurements and observations are necessary and what e#uipment to use. 9ake sufficient observations and measurements to reduce error and make results more reliable. )'plain results using scientific knowledge and understanding. 'e"rnin( O)*ective
Sodium carbonate soln% dil ;Cl% !+ soln% laboratory glassware and heating apparatus.
Safety goggles must be worn. *ord e#uations should be used.
8$min
9)p5
9)o1
9)c7 %r"mework &ode
Activitie
+e ource
&omment
Time
<1 1($5
Science Stage 9
9Cc6
)'plain how to prepare some common salts by the reactions of metals and metal carbonates and be able to write word e#uations for these reactions. :ecide which measurements and observations are necessary and what e#uipment to use. 9ake sufficient observations and measurements to reduce error and make results more reliable. )'plain results using scientific knowledge and understanding.
/repare crystals of chloride or sulfate crystals from metals and acids. )'cess metal is added to dilute acid until no more dissolves. The e'cess is filtered off. )vaporate until some solid appears and then leave to cool. 3ilter.
Ginc% magnesium ribbon% dil ;Cl% dil ;0SF1. Lab glassware and heating apparatus.
Safety goggles must be worn. *ord e#uations should be used.
9)p5
9)o1
9)c7
<1 1($5
Science Stage 9
1$
Scheme of Work Science stage 9
Unit 1&: 9.3 .#ectro t"tic "nd .#ectric &urrent
+n this unit% pupils build on their previous knowledge of different types of energy and energy transfers to develop their knowledge of )lectrostatics and the concept of charge% including digital sensors. Simple series and parallel circuits. ;ow common types of component% including cells AbatteriesB% affect current. ;ow current divides in parallel circuits. 9easuring current and voltage.
Scientific )n#uiry work focuses on=
Choosing apparatus and deciding which measurements and observations are necessary. &ssessing any haCards and controlling risk. Fbtaining reliable results. 9aking conclusions using scientific knowledge and understanding.
2ecommended <ocabulary for this unit=
)lectrostatic charge positive negative insulator attraction repulsion ammeters voltmeters parallel circuits series circuits circuit diagrams.
<1 1($5
Science Stage 9
11
%r"mework &ode 9/m1
'e"rnin( O)*ective :escribe electrostatics and the concept of charge% including digital sensors. 9ake observations and measurements. +nterpret results using scientific knowledge and understanding.
Activitie &fter charging by rubbing% plastic rulers pick up small pieces of paper% strips of cling film spring apart% balloons stick to walls% plastic rods deflect a steady stream of water etc. )'plain that only negative charges move in these circumstances and that by moving away from a neutral site they leave a net positive charge. They can also induce opposite charges on neutral material. The effect is only noticeable on insulators because conductors allow negative charge to pass to the hand and then to earth. +nvestigate the laws of attraction and repulsion. )stablish that there seem to be only two types of charge Aonly two effects are seenB. Suspending one charged item and approaching with another shows that similar charges repel and unlike charges attract. The electrostatic generator. This machine for generating electrostatic charge usually provides a memorable lesson. )ven a simple one can build up several thousand volts and cause lightning flashes% hair to rise% neon lights to light up% windmills to turn etc.
+e ource /lastic rulers% balloons% plastic rods% pieces of cloth e.g. dusterE t,shirt.
&omment Time spent ensuring the concept of charge is understood% will greatly benefit the understanding of electric circuits.
Time 4$min
9)o4 9)c0
9/m1
:escribe electrostatics and the concept of charge% including digital sensors. 9ake observations and measurements. +nterpret results using scientific knowledge and understanding. :escribe electrostatics and the concept of charge% including digital sensors. 9ake observations and measurements. +nterpret results using scientific knowledge and understanding
0$min
9)o4 9)c0 9/m1
)lectrostatic generator% +nsulating material Ato stand onB.
1$min
9)o4 9)c0
<1 1($5
Science Stage 9
10
%r"mework &ode 9/m1
'e"rnin( O)*ective :escribe electrostatics and the concept of charge% including digital sensors.
Activitie /upils investigate some problems and some advantages about electrostatics using secondary sources. /resentation of findings to whole class. 9ake a simple series electric circuit with switch% bulb and battery. :raw the circuit diagram. :iscuss the advantages of circuit diagrams. 9ake a simple parallel circuit by including a second bulb. :raw the circuit diagram. -ive a variety of circuit diagrams or circuits and decode if they are parallel or series circuits.
+e ource Secondary sources.
&omment Spray painting% risk of e'plosions with fuels% and combustible powders
Time 6$min
9/m0
+nterpret and draw simple parallel circuits.
Low voltage power supplies Ae.g. batteriesB% connecting wires% switches% bulbs Aat least 0 per circuitB. D. /"in e#ectricit! hou#d never )e u ed direct#! for "n! of the e t!-e of inve ti("tion.
Link to Stage 5% !nit 0C metals conduct electricity. & step by step approach is recommended to ensure all pupils have a sound understanding.
8$min
9/m4
9odel and e'plain how common types of components% including cells AbatteriesB% affect current. Test e'planations by using them to make predictions and then evaluate these against evidence.
+nvestigate the flow of charge in a circuit. )mphasise that batteries produce charge which flows from one end to the other round a circuit. Students can suggest ways of increasing the rate of flow of charge Amore batteries% easier pathB. Let pupils test this e'planation by using a variable resistor to dim E brighten a bulb.
9)p0
Low voltage power supplies Ae.g. batteriesB Aat least 0 per circuitB connecting wires% switches% bulbs% variable resistor. D. /"in e#ectricit! hou#d never )e u ed direct#! for "n! of the e t!-e of inve ti("tion.
8$min
<1 1($5
Science Stage 9
14
%r"mework &ode 9/m6
'e"rnin( O)*ective 9easure current u in( "mmeter and voltage using voltmeters% including digital meters. Select ideas and produce plans for testing based on previous knowledge% understanding and research. :ecide which apparatus to use and assess any haCards in the laboratory% field or workplace. !se a range of materials and e#uipment and control risks. :raw conclusions. )valuate the methods used and refine for further investigations. 9easure current u in( "mmeter and voltage using voltmeters% including digital meters. Select ideas and produce plans for testing based on previous knowledge% understanding and research. :ecide which apparatus to use and assess any haCards in the laboratory% field or workplace.
Activitie +nvestigate the current in series and parallel circuits using a number of identical lamps. !se an ammeter to measure current in different parts of the circuit. /upils to make conclusion about the current in series circuits and parallel circuits.
+e ource Low voltage power supply Ae.g. batteriesB% connecting wires% switches% bulbs% ammeters. D. /"in e#ectricit! hou#d never )e u ed direct#! for "n! of the e t!-e of inve ti("tion.
&omment &pparently identical bulbs will have different brightness so it is worth e'changing them or selecting matching ones.
Time 8$min
9)p1
9)p7
9)o0 9)c1 9)c6 9/m6
+nvestigate the effect of adding various lengths of resistance wire% a variable resistor% lamps% and ammeters.
9)p1
Low voltage power supply Ae.g. batteriesB% connecting wires% switches% bulbs% ammeters% variable resistors% resistance wires of various lengths. D. /"in e#ectricit! hou#d never )e u ed direct#! for "n! of the e t!-e of inve ti("tion.
Students must be warned that short lengths of wire will get hot. Symbols for these components must be given.
8$min
9)p7
<1 1($5
Science Stage 9
11
%r"mework &ode 9)o0 9)c1 9)c6 9/m4
'e"rnin( O)*ective !se a range of materials and e#uipment and control risks. :raw conclusions. )valuate the methods used and refine for further investigations. 9odel and e'plain how common types of components% including cells AbatteriesB% affect current. Select ideas and produce plans for testing based on previous knowledge% understanding and research. :ecide which apparatus to use and assess any haCards in the laboratory% field or workplace. !se a range of materials and e#uipment and control risks. :raw conclusions.
Activitie
+e ource
&omment
Time
9)p1
!nderstand the effects of further components by finding out about mystery" components such as diodes% buCCers% motors and reed switches. /upils can establish which are one,way" devices but of course must be warned about any that may be broken by passing a current in the wrong direction.
Low voltage power supply Ae.g. batteriesB% connecting wires% switches% bulbs% diodes% buCCers% motors% reed switches% ammeters.
/upils could be challenged to protect a bo' from being opened by designing a buCCer alarm.
1$min
9)p7
9)o0 9)c1
<1 1($5
Science Stage 9
16
%r"mework &ode 9/m6
'e"rnin( O)*ective 9easure current u in( "mmeter and voltage using voltmeters% including digital meters. Select ideas and produce plans for testing based on previous knowledge% understanding and research. :ecide which apparatus to use and assess any haCards in the laboratory% field or workplace. !se a range of materials and e#uipment and control risks. :raw conclusions. )valuate the methods used and refine for further investigations.
Activitie !se a voltmeter to measure the voltage across a component. /upils should be shown that a voltmeter measures the voltage output of a cell% two cells% etc. +t can then be used to measure the voltage across any two points in a circuit. They should also try the putting the meter in series to show that the circuit then does not work". The voltage across a home,made cell can be detected using two different metals and a solution or simply a fruit. Students could investigate into the effect of different metals and different fruit E vegetables.
+e ource 3ruit e.g. apple% orange% vegetable e.g. potato% connecting clips% metal electrodes% voltmeters% low voltage power supply Ae.g. batteriesB% connecting wires% bulbs.
&omment
Time 8$min
9)p1
9)p7
9)o0 9)c1 9)c6
<1 1($5
Science Stage 9
18
Scheme of Work Science stage 9
Unit 2A: 9.0 Se1u"# +e-roduction "nd %#owerin( P#"nt
+n this unit% pupils build on their previous knowledge reproduction and plant growth to develop their knowledge of. Se'ual reproduction in flowering plants including pollination% fertilisation% seed formation and dispersal.
Scientific )n#uiry work focuses on=
Selecting ideas and plans for testing based upon previous knowledge% understanding and research. :eciding which measurements and observations are necessary% what e#uipment to use and assessing any haCards. !sing appropriate sampling techni#ues where re#uired to obtain reliable measurements. /resent results% describing correlations and drawing conclusions.
2ecommended <ocabulary for this unit=
/ollination fertiliCation dispersal pollen ovule gamete.
<1 1($5
Science Stage 9
15
%r"mework &ode 9.p4
'e"rnin( O)*ective !nderstand se'ual reproduction in flowering plants including pollination% fertilisation% seed formation and dispersal. 9ake observations and measurements.
Activitie 3or a selection of locally occurring flowering plants% identify the different parts of the plant% including leaf% stem% roots% flower. 2eview the functions of each part and e'plain that the flower is the part where se'ual reproduction takes place. :iscuss the difference between flowers from different plant. +dentify the positions and functions of the reproductive parts of a flowering plant. +nvestigate using secondary sources. /upils can investigate the structure of the flowers of locally occurring plants. :raw diagrams of a flower showing the male and female reproductive parts. +nclude ovules in the ovary.
+e ource Selection of locally occurring flowering plants. /hotos may be substituted for live specimens.
&omment 2evise previous knowledge of plant structures and functions from Stage 9% !nit 1&% Stage 7 !nit 4&% Stage 5 !nit 1&.
Time 4$ min
9)o4
9.p4
!nderstand se'ual reproduction in flowering plants including pollination% fertilisation% seed formation and dispersal.
Secondary sources% selection of flowers from locally occurring plants% ;and lenses.
9ale H anther% filament% pollen. 3emale H stigma% style% ovary% ovule.
8$min
9.p4
!nderstand se'ual reproduction in flowering plants including pollination% fertilisation% seed formation and dispersal. 9ake observations and measurements.
+dentify pollen as the male se' cell and the ovule as the female se' cell. +ntroduce gamete" as meaning se' cell. Fbserve pollen grains under the microscope.
9icroscopes% prepared slides of different pollen grains or e#uipment to make their own slides.
6$min
9)o4
<1 1($5
Science Stage 9
17
%r"mework &ode 9.p4
'e"rnin( O)*ective !nderstand se'ual reproduction in flowering plants including pollination% fertilisation% seed formation and dispersal.
Activitie +nvestigate e'amples of wind and insect pollinated flowers Alive% diagrams or photographsB and if possible a local flower showing the pollen and sticky stigma clearly. )'plain what is meant by pollination. :iscuss different ways pollen may travel from one flower to another. :iscuss the features of a wind pollinated and an insect pollinated flower. :iscuss the advantages and dis, advantages of self,pollination and cross,pollination. )'plain what is meant by fertilisation. Fbserve pollen tubes using a microscope. :raw diagrams to show how the pollen causes a tube to grow down the style and into the ovary to allow fertilisation.
+e ource Secondary sources.
&omment
Time 7$min
9.p4
!nderstand se'ual reproduction in flowering plants including pollination% fertilisation% seed formation and dispersal. 9ake observations and measurements. +nterpret results using scientific knowledge and understanding. !nderstand se'ual reproduction in flowering plants including pollination% fertilisation% seed formation and dispersal. 9ake observations and measurements. +nterpret results using scientific knowledge and understanding
9icroscopes% fresh pollen grains% glucose soln% microscope slides and cover slips.
8$min
9)o4 9)c0 9.p4
9)o4 9)c0
& seed such as a large bean can be bisected to identify the parts and a test done for starch. Some can be grown one way up% some another to compare the outcome. :iscuss the variety of seeds and identify what part of different plants contains the seed e.g. cherry stones% orange pips% tomato seeds% wheat ears. 3ruits can be seen as the development of the ovary. Science Stage 9
Soaked broad bean seeds% hand lenses% +E@+ soln.
Seed structure does not have to be learned.
8$min
<1 1($5
19
%r"mework &ode 9.p4
'e"rnin( O)*ective !nderstand se'ual reproduction in flowering plants including pollination% fertilisation% seed formation and dispersal. 9ake observations and measurements. +nterpret results using scientific knowledge and understanding. :raw conclusions. )valuate the methods used and refine for further investigations. Compare results and methods used by others. /resent conclusions and evaluation of working methods in different ways. !nderstand se'ual reproduction in flowering plants including pollination% fertilisation% seed formation and dispersal. !nderstand se'ual reproduction in flowering plants including pollination% fertilisation% seed formation and dispersal
Activitie )'amine a wide range of fruits and discuss methods of dispersal. +nvestigate wind dispersal by making a paper model Atwo or more wings and a weighted centreB. .y adjusting the wing siCe% total mass% shape etc students aim to make it stay in the air for the longest possible time% after dropping it from a certain height. Students should be shown e'amples of seeds and predict the method of dispersal.
+e ource & range of fruits. /hotos may be substituted for live specimens.
&omment
Time 8$min
9)o4 9)c0 9)c1 9)c6 9)c8 9)c5
9.p4
2esearch the life cycle of a flowering plant and display on a hoop of paper. Students discuss the importance of seeds within the life cycle. +nvestigate reasons for the dispersal of plants. Compare the growth of plants which are crowded with those with plenty of space. The same amount of water% light and nutrients should be supplied to% for e'ample% cress seeds.
1$ min
9.p4
1$min
Scheme of Work Science stage 5
<1 1($5
Science Stage 9
0$
Scheme of Work Science stage 9
Unit 2,: 9.2 +e"ctivit! "nd +"te of +e"ction
+n this unit% pupils build on their previous knowledge of chemical reactions to develop their knowledge of The reactivity series of metals with o'ygen% water and dilute acids. :isplacement reactions. The effects of concentration% particle siCe% temperature and catalysts on the rate of a reaction.
Scientific )n#uiry work focuses on=
!sing preliminary work to decide how to carry out an investigation based upon previous knowledge% understanding and research. :eciding which apparatus to use and assess any haCards. Choosing whether to use evidence from first,hand e'perience of secondary sources. Fbtaining reliable results. :escribing patterns AcorrelationsB. :rawing conclusions and evaluating methods.
2ecommended <ocabulary for this unit=
2eactivity series displacement reaction concentration catalyst.
%r"mework &ode 9Cc0
'e"rnin( O)*ective :escribe the reactivity of metals with o'ygen% water and dilute acids. !se a range of materials and e#uipment and control risks. :escribe patterns seen in results. :escribe the reactivity of metals with o'ygen% water and dilute acids. -ive an e'planation of the effects of concentration% particle siCe temperature and catalysts on the rate of a reaction. !se a range of materials and e#uipment and control risks. :escribe patterns seen in results. :escribe the reactivity of metals with o'ygen% water and dilute acids. !se a range of materials and e#uipment and control risks. :escribe patterns seen in results.
Activitie Compare the rates of reaction of some metals with o'ygen. Small samples of a range of metals can be cleaned and left in air. They are checked at intervals for signs of o'idation. The same range of metals can each be heated in air and placed in a gas jar of o'ygen. *rite word e#uations for each reaction. Compare the rates of reaction of some metals with water. Small pieces of metal are cleaned and left in water to observe changes after the ne't few days. Some may only react when heated in steam% some will not react at all. The reaction of a small piece of sodium with water can be demonstrated by the teacher with the usual safety precautions. *rite word e#uations for each reaction. Compare the rates of reaction of some metals with dilute acid. Small pieces of metals ADFT including sodiumB are added to dilute hydrochloric acid and the reaction observed. The hydrogen gas made can be tested. *rite word e#uations for each reaction.
+e ource Copper% iron% magnesium%Cinc% o'ygen. Lab glassware and heating apparatus.
&omment Safety goggles must be used.
Time 6$min
9)o0 9)c1
9Cc0
9Cc8
Copper% iron% magnesium% Cinc. Lab glassware and heating apparatus. Sodium
Safety goggles must be used.
6$min
9)o0 9)c1 9Cc0
Copper% iron% magnesium% Cinc% dil ;Cl. Lab glassware.
Safety goggles must be used.
1$min
9)o0 9)c1
%r"mework &ode 9Cc0
'e"rnin( O)*ective :escribe the reactivity of metals with o'ygen% water and dilute acids. Look critically at sources of secondary data. :ecide whether to use evidence from first,hand e'perience or secondary sources. )'plore and understand the reactivity series. Look critically at sources of secondary data. :ecide whether to use evidence from first,hand e'perience or secondary sources. Select ideas and produce plans for testing based upon previous knowledge% understanding and research. :ecide which measurements and observations are necessary and what e#uipment to use. :ecide which apparatus to use and assess any haCards in the laboratory.
Activitie Construct a reactivity series for metals. /upils should collect their observations of reactions in a table and suggest an order of reactivity. This can be enhanced with research or supplied information about metals which have not been observed.
+e ource
&omment
Time 4$min
9)c4 9)p8
9Cc4 9)c4 9)p8
+nvestigate whether the reactivity series is the same with different acids and whether some acids and more reactive than others. /upils should plan their own investigations including a basic risk assessment and decide whether to use primary data andEor secondary data.
Copper% iron% magnesium% Cinc% dilute acids Asulphuric% nitric% ethanoicB.
Safety goggles must be used.
8$min
9)p1
9)p5
9)p7
%r"mework &ode 9)o1
'e"rnin( O)*ective 9ake sufficient observations and measurements to reduce error and make results more reliable. !se a range of materials and e#uipment and control risks. 9ake observations and measurements. Choose the best way to present results. :escribe patterns AcorrelationsB seen in results. +nterpret results using scientific knowledge and understanding. :raw conclusions. )valuate the methods used and refine for further investigations. )'plain results using scientific knowledge and understanding. Communicate this clearly to others.
Activitie See above.
+e ource See above.
&omment
Time
9)o0 9)o4 9)o1 9)c1 9)c0 9)c1 9)c6 9)c7
%r"mework &ode 9Cc1 9)p0
'e"rnin( O)*ective -ive e'amples of displacement reactions. Test e'planations by using them to make predictions and then evaluate these against evidence.
Activitie 9ake predictions about displacement reactions. /ut a steel rod or blade into copper sulfate solution and a copper" coin into iron sulfate solution. *hen the idea of displacement is clear% students can predict% and confirm% the results of the reaction between other metals and solutions. )'plain why the historical order of the discovery of metals is related to the reactivity series. :ifferent students can research the methods used for e'tracting named metals and the dates of their discovery. -roup results can be used to relate the difficulty of e'traction and history to position in the reactivity series. Show how rate of reaction depends on concentration of reactants. Carry out an investigation into the time taken for a 4cm length of magnesium ribbon to completely react in 06 cm4 of hydrochloric acid. varying the concentration of the acid. Show how rate of reaction depends on particle siCe. The time taken the same mass of magnesium powder and magnesium ribbon to completely react with hydrochloric acid can be compared.
+e ource Copper sulfate soln% iron sulfate soln% steel rod% copper coin% magnesium% Cinc% magnesium sulfate soln.
&omment <ery small #uantities can be used by carrying out the tests on a spotting tile. Safety goggles must be used.
Time 8$min
9Cc4 9)p1
)'plore and understand the reactivity series. :iscuss and e'plain the importance of #uestions% evidence and e'planations% using historical and contemporary e'amples.
6$min
9Cc8
-ive an e'planation of the effects of concentr"tion% particle siCe temperature and catalysts on the rate of a reaction.
Safety goggles must be used.
1$min
9Cc8
-ive an e'planation of the effects of concentration3 -"rtic#e i4e temperature and catalysts on the rate of a reaction.
Safety goggles must be used.
1$min
%r"mework &ode 9Cc8
'e"rnin( O)*ective -ive an e'planation of the effects of concentration% particle siCe temperature and c"t"#! t on the rate of a reaction. 9ake observations and measurements.
Activitie Show how rate of reaction depends on the presence of a catalyst. ;ydrogen pero'ide can be decomposed into water and o'ygen. Students observe the rate of the reaction before and after the addition of a small #uantity of manganeseA+<B o'ide. +nvestigate the use of a catalyst in industry and find out how it relate to energy% cost and pollution issues using secondary sources.
+e ource ;ydrogen pero'ide soln% 9anganeseA+<B o'ide Laboratory glassware.
&omment Safety goggles must be used.
Time 1$min
9)o4
9Cc8
-ive an e'planation of the effects of concentration% particle siCe temperature and catalysts on the rate of a reaction.
!se the kinetic theory to e'plain effects on rates of reaction. Through diagrams% use ideas about particle theory to e'plain the effects of the different variables on the speed of reactions% i.e. concentration increases the number of particles% temperature increases their speed and that increased lump siCe decreases the area for particles to approach one another.
& good conte't is found in recipes where cooking times vary for% e.g.% potatoes depending on area e'posed.
0$min
Scheme of Work Science stage 9
Unit 2&: 9.5 /oment 3 Pre
ure "nd 6en it!
+n this unit% pupils build on their previous knowledge of forces and movement to develop their knowledge of. Fbjects turning on a pivot and understand the principle of moments. /ressure as caused by the action of force on an area. /ressures in gases and li#uids A#ualitative onlyB. The densities of solids% li#uids and gases.
Scientific )n#uiry work focuses on=
:eciding which measurements and observations are necessary% which e#uipment to use and assess any haCards and control risks. 9aking sufficient observations and measurements to reduce error and make results more reliable. +nterpreting results using scientific knowledge and understanding. :rawing conclusions. Communicating clearly.
2ecommended <ocabulary for this unit=
Lever moment pivot density pressure.
%r"mework &ode 9/f1
'e"rnin( O)*ective @now that forces can cause objects to turn on a pivot and understand the principle of moments.
Activitie :escribe a lever as a simple machine which uses a pivot. :iscuss how to open a tin with a tight fitting lid. )'plain that they are using a lever with a force and a pivot. :emonstrate other common e'amples of a lever in action e.g. a wrench% wheel brace. +nvestigate% as a whole class activity% the effect of changing the distance between the force used and the pivot% and the siCe of the force on the effectiveness of a lever.
+e ource 3orcemeter% tin with tight fitting lid% lever.
&omment /upils to realise that the turning effect of the lever increases with the distance from the force and the pivot and the siCe of the force
Time 1$min
9/f1
@now that forces can cause objects to turn on a pivot and understand the principle of moments. Select ideas and produce plans for testing based upon previous knowledge% understanding and research. :ecide which measurements and observations are necessary and what e#uipment to use. 9ake observations and measurements.
!nderstand the principle of moments by looking at a see saw and introduce the idea of balancing. +nvestigate the rule of balancing using appropriate apparatus. :erive the principle of moments from their e'perimental results.
See,saw balances% weights E counters of e#ual masses Ae.g. 1$gB.
6$min
9)p1
9)p5
9)o4
%r"mework &ode 9)o1 9)c1 9)c0 9)c1 9/f0
'e"rnin( O)*ective Choose the best way to present results. :escribe patterns AcorrelationsB seen in results. +nterpret results using scientific knowledge and understanding. :raw conclusions. :etermine densities of solids% li#uids and gases.
Activitie See above.
+e ource See above.
&omment
Time
:etermine the density of a regular solid. /upils must appreciate that they need to know the mass and the volume of an object to calculate the density. +f pupils are not completely happy with the concept of volume it is helpful if solids can be matched" by blocks of 1 centimetre cubes. The mass of the solids can then be found. Students investigate the density of different siCed blocks of material.
*eighing balance% ruler Emeasuring tape% selection of solids of different materials but of similar siCe and shape e.g. cubes of one centimetre side.
)'plain that materials can only be compared if they have e#ual volumes so the mass of 1 cm4 or 1 m4 must be found in each case.
6$min
%r"mework &ode 9/f0 9)p1
'e"rnin( O)*ective :etermine densities of o#id 3 li#uids and gases. Select ideas and produce plans for testing based upon previous knowledge% understanding and research. :ecide which measurements and observations are necessary and what e#uipment to use. 9ake observations and measurements. Choose the best way to present results. :escribe patterns AcorrelationsB seen in results. +nterpret results using scientific knowledge and understanding. :raw conclusions.
Activitie :etermine the density of an irregular solid. :iscuss ways of finding the volume of an irregular solid and a solid that floats in water. +nvestigate the density of a number of different materials.
+e ource *eighing balance% ruler Emeasuring tape% and selection of solids of different materials. Fther re#uirements as re#uested by pupils from their investigation plan.
&omment
Time 6$min
9)p5
9)o4 9)o1 9)c1 9)c0 9)c1
%r"mework &ode 9/f0 9)p1
'e"rnin( O)*ective :etermine densities of solids% #i7uid and gases. Select ideas and produce plans for testing based upon previous knowledge% understanding and research. :ecide which measurements and observations are necessary and what e#uipment to use. 9ake observations and measurements. :raw conclusions. :etermine densities of solids% #i7uid and gases. Select ideas and produce plans for testing based upon previous knowledge% understanding and research. :ecide which measurements and observations are necessary and what e#uipment to use. 9ake observations and measurements. :raw conclusions. 'e"rnin( O)*ective
Activitie /upils discuss ways of finding the density of a li#uid and then find the density of water% salt water and other non,haCardous li#uids.
+e ource
&omment
Time 6$min
9)p5
9)o4 9)c1 9/f0 9)p1
:iscuss the problems of measuring the density of a gas. )'plain suitable ways of measuring the density of a gas. :emonstration of finding the density of carbon dio'ide.
;eat a sample of a metal carbonate and collect the gas produced in an upturned measuring cylinder.
The mass of the gas will be the difference in mass of the solid before and after heating.
8$min
9)p5
9)o4 9)c1 %r"mework &ode
Activitie
+e ource
&omment
Time
9/f1
)'plain that pressure is caused by the action of a force on an area. 9ake observations and measurements.
)'plain the pressure due to a solid. :iscuss appropriate e'amples of e'perience of pressure such as walking on snow% mud% dry sand. Students e'plain why knives and drawing pins are effective but only if used the right way round. Students can investigate pressure by pressing objects into plasticine% provided they are pressed with e#ual forces% shows that the smaller the area of contact the greater the pressure.
:ifferent objects% plasticine E modelling clay.
9)o4
!nits can be DEcm0 or DEm 0 or kDEm0 . Dote that although this may be regarded as an appropriate activity% pressure calculations will not be tested at checkpoint level.
8$min
9/f4 9)c7
)'plain pressures in gases and #i7uid A#ualitative onlyB. )'plain results using scientific knowledge and understanding. Communicate this clearly to others.
:emonstrate pressure in a li#uid. !se a plastic bottle which has holes in the sides at different heights. *hen filled% water is forced out sideways% the lower the hole the greater the pressure. /upils e'plain the pressure of a li#uid in terms of a particle model.
/lastic bottle prepared as suggested.
2eference can be made to dams and deep sea divers. Link to Stage 7 !nit 1..
8$min
%r"mework &ode 9/f4 9)c7
'e"rnin( O)*ective )'plain pressures in (" e and li#uids A#ualitative onlyB. )'plain results using scientific knowledge and understanding. Communicate this clearly to others.
Activitie :emonstrate pressure in a gas. & container of at least three litres is connected to a vacuum or suction pump and compressed by the pressure of the atmosphere. <ernatively a heat,proof container containing a little water% can be heated and then have the top screwed on tightly causing the same effect as it cools. .lowing up balloons or tyres or heating tins with a lid on causes an increase of pressure which pupils should e'plain using a particle model.
+e ource 3le'ible container e.g. plastic bottle% vacuum or suction pump% balloons% tin with lid as suggested.
&omment &ir pressure is relatively large so good demonstrations are possible. 3or either method safety screens and safety goggles should be used. Link to Stage 7 !nit 1..
Time 8$min
Scheme of Work Science stage 9
Unit 3A: 9.8 .co#o(!
+n this unit% pupils build on their previous knowledge of organisms in their environment to develop their knowledge of Constructing keys to identify plants and animals. 3ood chains% food webs and energy flow including the role of decomposers. ;ow living things are adapted to their habitats. ;ow characteristics are inherited. Selective breeding. The work of :arwin on natural selection and other scientists studying the natural world.
Scientific )n#uiry work focuses on=
:iscussing the way that scientists work today and how they worked in the past% including reference to e'perimentation% evidence and creative thought. The importance of #uestions% evidence and e'planations.
2ecommended <ocabulary for this unit=
Consumer producer trophic level primary secondary tertiary herbivore carnivore genetic material adaptation variation habitat.
Dote= This unit is delivered in less time than units 4. and 4C. This is to ensure e#ual time is allocated to biology% chemistry and physics learning objectives in term 0.
%r"mework &ode 9.v1
'e"rnin( O)*ective !se and construct keys to identify plants and animals.
Activitie Collecting leaves or seeds can provide samples for constructing a key but pupils will need to have worked with a provided key first. /upils make keys to identify class members and discuss whether it will work ne't week or ne't year. !sing provided keys% pupils identify plants and animals in the local environment Aalternatively% use provided keys to identify pictures% photosB. 2eview work on food chains from stage 5 and discuss need to find out% by observation or from secondary sources% which organism is eaten by which to be able to make a food chain. )'plain the terms producer% primary consumer% secondary consumer% tertiary consumer% herbivore% carnivore. 9ake simple food chains from plants and animal identified by their key.
+e ource Sample key.
&omment .ring out the point that some #uestions are less useful than others% e.g. length or mass% depth of colour etc. Link to Stage 5% !nit 4&.
Time 6$ min
9.v1 9.e4 9.e0
!se and construct keys to identify plants and animals. )'plain and model food chains% food webs and energy flow. 2esearch the work of scientists studying the natural world.
Charts and keys for identification.
1$ min
%r"mework &ode 9.e4
'e"rnin( O)*ective )'plain and model food chains% food webs and energy flow.
Activitie )'plain that that most food chains are interlinked as food,webs. /upils identify food chains within an e'ample of a food web% preferably of local species. :iscuss the effect of the removal of one type of organism on the other organisms in the food web. )'plain that food chains and webs show biomass and not individuals. +ntroduce the idea of energy flowing along the food chain and so flowing through the food web. +ntroduce the term trophic level". /upils redesign the layout of their food webs to show energy transfer through trophic levels.
+e ource <arious food webs.
&omment Consolidate understanding by e'amining various e'amples of food webs.
Time 6$min
9.e1
)'plain the role of decomposers.
)'plain the role of decomposers. :emonstrate the breakdown of bread Aor fruitB as moulds are allowed to grow on it in a sealed container. :iscuss where decomposers fit in the food web. :iscuss the importance of decomposers in food webs% in terms of recycling material such as minerals.
/repared sample of mouldy bread Aor fruitB in sealed container as e'ample.
0$min
%r"mework &ode 9.e1
'e"rnin( O)*ective )'plain the ways in which living things are adapted to their habitats. Secondary sources can be used.
Activitie /upils chooseAfrom a listB an animal and a plant and research and report on how they are adapted to be able to survive% by finding food and shelter and avoiding predators in their habitat.
+e ource
&omment Link to Stage 5% !nit 4&. & choice chamber used for wood lice E maggots etc will show preference for dark% damp conditions. & simple knowledge of genetic materials being located in the nucleus of most cells and being joined with other genes during fertiliCation is all that is re#uired. Link to Stage 7% !nit 4&
Time 1$min
9.v0
!nderstand that organisms inherit characteristics from their parents through genetic material that is carried in the cell nuclei.
:iscuss inherited characteristics by giving e'amples of similarities and differences between parents and offspring. )'plain that characteristics are passed on in genes% the genetic material is stored in the nucleus of the cell and discuss how this genetic material is passed on from one generation to another.
1$min
9.v4 9)p4
:escribe how selective breeding can lead to new varieties. :iscuss the way that scientists work today and how they worked in the past% including reference to e'perimentation and evidence.
2esearch selective breeding using secondary sources giving at least one e'ample to write an account about how to selectively breed a certain characteristic in an organism e.g. of a flower grower trying to achieve a flower of a certain colour% or similarly for a vegetable with a desirable property.
Secondary sources.
1$min
%r"mework &ode 9.v1
'e"rnin( O)*ective :iscuss the work of :arwin in developing the scientific theory of natural selection. :iscuss and e'plain the importance of #uestions% evidence and e'planations% using historical and contemporary e'amples. :iscuss the way that scientists work today and how they worked in the past% including reference to e'perimentation and evidence.
Activitie :iscuss how selective breeding may also occur in nature Anatural selectionB. +nvestigate :arwin"s work and how he used evidence to come up with his theory of natural selection.
+e ource Secondary sources.
&omment
Time 1$min
9)p1
9)p4
Scheme of Work Science stage 9
Unit 3,: 9.9 &hemic"# "nd Therm"# .ner(!
+n this unit% pupils build on their previous knowledge of chemical reaction and energy transfers to develop their knowledge of )ndothermic processes and e'othermic reactions. The thermal AheatB energy transfer processes of conduction% convection and radiation. Cooling by evaporation.
Scientific )n#uiry work focuses on=
+deas and plans for testing based upon previous knowledge% understanding and research :eciding measurements and observations to make% e#uipment to use% assessing haCards and controlling risks Fbtaining and presenting reliable results :escribing correlations )'plaining results and drawing conclusions using scientific knowledge and understanding )valuating e'perimental methods.
2ecommended <ocabulary for this unit=
)'othermic endothermic thermal AheatB energy transfer conduction convection radiation evaporation. Dote= This unit should be delivered in more time than units 4& and 4C. This is to ensure e#ual time is allocated to biology% chemistry and physics learning objectives in term 0.
%r"mework &ode 9Cc1
'e"rnin( O)*ective )'plore and e'plain the idea of endothermic processes and e'othermic reactions. 9ake sufficient observations and measurements to reduce error and make results more reliable. !se a range of materials and e#uipment and control risks. 9ake observations and measurements. Choose the best way to present results. :escribe patterns AcorrelationsB seen in results. +nterpret results using scientific knowledge and understanding. :raw conclusions.
Activitie +nvestigate the reactions of magnesium sodium hydro'ide solution potassium hydrogencarbonate sodium hydrogencarbonate with acid measuring the temperature of the li#uid before and after adding the reactants. Students to classify the reactions on the basis of the temperature change. :iscuss e'othermic and endothermic reactions.
+e ource 9agnesium% sodium hydro'ide solution% potassium hydrogencarbonate% sodium hydrogencarbonate. :il acid. Lab glassware% thermometers.
&omment Spend time on discussing the planning process to develop skills of independent working as preparation for +-CS).
Time 7$min
9)o1
9)o0 9)o4 9)o1 9)c1 9)c0 9)c1
%r"mework &ode 9Cc1
'e"rnin( O)*ective )'plore and e'plain the idea of endothermic processes and e'othermic reactions. 9ake sufficient observations and measurements to reduce error and make results more reliable. !se a range of materials and e#uipment and control risks. 9ake observations and measurements. Choose the best way to present results. :escribe patterns AcorrelationsB seen in results. +nterpret results using scientific knowledge and understanding. :raw conclusions. )'plain results using scientific knowledge and understanding. Communicate this clearly to others.
Activitie +nvestigate the process of burning to be able to draw conclusions about the process. !sing dry bread or wooden splints% will give results for energy release. .urning a candle allows collection of the products and test for carbon dio'ide and water. !se secondary sources to find out that o'ygen is a reactant. *rite word e#uations.
+e ource
&omment
Time 5$min
9)o1
9)o0 9)o4 9)o1 9)c1 9)c0 9)c1 9)c7
%r"mework &ode 9Cc1
'e"rnin( O)*ective )'plore and e'plain the idea of endothermic processes and e'othermic reactions. !se a range of materials and e#uipment and control risks.
Activitie .y discussion identify the need for heat% fuel and o'ygen to start E maintain a fire. /upils should use this information to suggest ways of stopping different types of fire. /upils produce a poster on fire prevention in the home and Eor work environment. Compare the energy released by different fuels by heating e#ual volumes of water using a known mass of each fuel. Compare changes in temperature.
+e ource
&omment
Time 8$min
9)o0
9Cc1
)'plore and e'plain the idea of endothermic processes and e'othermic reactions. Select ideas and produce plans for testing based upon previous knowledge% understanding and research. :ecide which measurements and observations are necessary and what e#uipment to use. :ecide which apparatus to use and assess any haCards in the laboratory. 9ake sufficient observations and measurements to reduce error and make results more reliable. !se a range of materials and e#uipment and control risks. 9ake observations and measurements.
<arious fuels dependent on pupils plans% lab heating e#uipment% thermometers% lab glassware AheatproofB.
/upils should design as accurate an investigation as possible. /lans should be checked for safe procedures before pupils start the practical work.
7$min
9)p1
9)p5
9)p7
9)o1
9)o0 9)o4
%r"mework &ode 9)o1 9)c1 9)c0 9)c1 9)c6 9)c7
'e"rnin( O)*ective Choose the best way to present results. :escribe patterns AcorrelationsB seen in results. +nterpret results using scientific knowledge and understanding. :raw conclusions. )valuate the methods used and refine for further investigations. )'plain results using scientific knowledge and understanding. Communicate this clearly to others. )'plore and e'plain the idea of endothermic processes and e'othermic reactions. !se a range of materials and e#uipment and control risks. :ecide whether to use evidence from first,hand e'perience or secondary sources.
Activitie See above.
+e ource See above.
&omment
Time
9Cc1
9)o0 9)p8
Students can research and report on which they consider to be the best fuel for a certain purpose such as cookingAcampingB or transport. They should take into account factors such as convenience% cost% pollution% availability etc.
1$min
%r"mework &ode 9Cc1
'e"rnin( O)*ective )'plore and e'plain the idea of endothermic processes and e'othermic reactions. Select ideas and produce plans for testing based upon previous knowledge% understanding and research. :ecide which measurements and observations are necessary and what e#uipment to use. :ecide which apparatus to use and assess any haCards in the laboratory. 9ake sufficient observations and measurements to reduce error and make results more reliable. !se a range of materials and e#uipment and control risks. 9ake observations and measurements. Choose the best way to present results. :escribe patterns AcorrelationsB seen in results. +nterpret results using scientific knowledge and understanding.
Activitie +nvestigate the endothermic process of dissolving ammonium chloride. /redict what will happen if more ammonium chloride is dissolved or if less water is used. :esign and carry out investigation to test their prediction
+e ource &mmonium chloride% water% thermometers% lab glassware.
&omment Safety goggles must be worn. +nvestigation can also be done with ammonium sulfate. $.6 to 4.$ g of ammonium chloride in 1$ cm4 of water provides a suitable temperature decrease.
Time 7$min
9)p1
9)p5
9)p7
9)o1
9)o0 9)o4 9)o1 9)c1 9)c0
%r"mework &ode 9)c1 9)c6 9)c7
'e"rnin( O)*ective :raw conclusions. )valuate the methods used and refine for further investigations. )'plain results using scientific knowledge and understanding. Communicate this clearly to others. )'plore and e'plain the idea of endothermic processes and e'othermic reactions.
Activitie See above.
+e ource See above.
&omment
Time
9Cc1
The process of respiration can be reviewed to identify it as an e'othermic reaction% suggest getting hot when running. /hotosynthesis can be reviewed as an endothermic reaction that converts carbon dio'ide and water into glucose and o'ygen.
Link to Stage 9% !nit 1& and Stage 7% !nit 0&. 2espiration can be represented by the word e#uation glucose ? o'ygen >carbon dio'ide ?water. /hotosynthesis can be represented by the word e#uation carbon dio'ide ? water >glucose ? o'ygen. Small pieces of cloth Aor tissuesB. Timers. -ive a particle e'planation of the process.
1$min
9Cc1
)'plore and e'plain the idea of endothermic processes and e'othermic reactions. )'plain cooling by evaporation.
:iscuss why melting ice and evaporation are endothermic processes. +nvestigate which conditions aid the rate of evaporation using tissues or small pieces of cloth% dampened. 3actors which can be tested are temperature and moving air.
8$min
9/e4
%r"mework &ode 9/e0
'e"rnin( O)*ective +dentify and e'plain the thermalAheatB energy transfer processes of conduction3 convection and radiation. :ecide which apparatus to use and assess any haCards in the laboratory. 9ake observations and measurements. +nterpret results using scientific knowledge and understanding. +dentify and e'plain the thermal AheatB energy transfer processes of conduction% convection and radiation.
Activitie 2ods of different metals can be heated to find out which is the best thermal conductor. +t is important to heat the ends of the rods e#ually% perhaps by supporting on a non, combustible mat on a tripod and heating them all at the same time. The heat energy can be detected by a pin attached by wa' to the far end of the rod% it is released when the wa' melts. :iscuss everyday uses of conduction of heat energy. +nclude the use of bad conductors AinsulatorsB. Convection currents can be demonstrated in li#uid Awarming coloured crystals placed in a beaker of still waterB and air Ausing a candle in a bo' with two chimneys and placing a smoking taper above. :iscuss everyday uses of convection of heat energy e.g. solar heating panels.
+e ource 9etal rods Lab heating e#uipment *a' :rawing pins.
&omment :istinguish between heat and temperature. -ive a particle e'planation of the process. Safety goggles must be worn for heating.
Time 8$min
9)p7
9)o4 9)c0
9/e0
-ive a particle e'planation of the process.
8$min
%r"mework &ode 9/e0
'e"rnin( O)*ective +dentify and e'plain the thermal AheatB energy transfer processes of conduction% convection and r"di"tion.
Activitie Thermal radiation Ainfra,redB comes from all hot objects but pupils can investigate which surfaces emit E absorb heat the best. Fne way is to fill a metal container Aradiation cubeB with hot water. :ifferent surfaces% black% dull% white% shiny% give off more or less radiation. This can be detected by placing the hand 0,4 cm away from the surface. The rate of heat loss can also be estimated by measuring the rate of temperature loss. :iscuss everyday uses of heat transfer by radiation and also ways of preventing it.
+e ource 2adiation cube ;ot water% thermometer.
&omment )'plain that the heat is not carried by particles in this case but by a type of ray similar to light which can travel through space. /upils should not touch the metal cube.
Time 8$min
9/e0
+dentify and e'plain the thermal AheatB energy transfer processes of conduction% convection and radiation.
& vacuum flask Aa broken one to reveal the inner layersB can be demonstrated as it has ways of preventing conduction% convection% radiation and evaporation which students can identify. )nsure that students understand that the flask can be used for keeping things hot or cold.
<acuum flask Aa broken one to reveal the inner layersB.
Students have great difficulty in distinguishing the different processes so as much practice as possible is necessary.
4$min
Scheme of Work Science stage 9
Unit 3&: 9.9 The .ner(! &ri i "nd :um"n Inf#uence
+n this unit% pupils build on their previous knowledge of energy and the environment to develop their knowledge of 3actors affecting the siCe of populations. Some effects of human influences on the environment. The world"s energy needs.
Scientific )n#uiry work focuses on=
Looking critically at sources of secondary data. :eciding which apparatus to use and assess any haCards in the laboratory% field or workplace. !sing appropriate sampling techni#ues where re#uired. The importance of #uestions% evidence and e'planations% using historical and contemporary e'amples.
2ecommended <ocabulary for this unit=
)nvironment solar hydroelectric nuclear fuel fossil fuel renewable and non,renewable sources.
%r"mework &ode 9.e6 9)c4
'e"rnin( O)*ective :escribe factors affecting the siCe of populations. Look critically at sources of secondary data.
Activitie Students investigate changes in population of species using secondary data e.g. changes over a year% changes over time. /lot a population E time graph. Start with chosen values of two species e.g. fo' and rabbit or lion and antelope. Suggest on the graph what happens when the population of rabbits E antelopes increases.
+e ource Secondary sources.
&omment Link to Stage 9% !nit 4& The graph shows a repeating curve for each of predator and prey% the line for the prey has higher peaks and reaches its highest point before that for the predator.
Time 4$min
9.e6 9)c4 9.e8
:escribe factors affecting the siCe of populations. Look critically at sources of secondary data. :escribe and investigate some effects of human influences on the environment. Look critically at sources of secondary data.
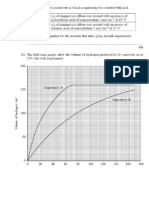
+nvestigate graphs of the global population over time and consider reasons for% and disadvantages of% the rate of increase. +nvestigate the problems of obtaining enough living space. .y researching the reasons for and effects of deforestation using secondary sources. +nclude information on how species might have been affected by destruction of habitat. /resent findings and possible solutions. +nvestigate the availability of clean water resources and why water should be conserved using secondary sources. /resent findings and possible solutions to a shortage of clean water.
;uman population Etime graphs.
4$min
Secondary sources.
6$min
9)c4
9.e8
:escribe and investigate some effects of human influences on the environment. Look critically at sources of secondary data.
Secondary sources.
9)c4
&s e'tension work pupils could do fieldwork at a stream site. +ndicator species found can show the relative cleanliness of the water.
6$min
%r"mework &ode 9/e1
'e"rnin( O)*ective !se knowledge of energy sources including fossil fuels and renewable energy resources to consider the world"s energy needs% including research from secondary sources. Look critically at sources of secondary data. :escribe and investigate some effects of human influences on the environment. !se knowledge of energy sources including fossil fuels and renewable energy resources to consider the world"s energy needs% including research from secondary sources. Look critically at sources of secondary data.
Activitie )'plain why the world"s energy needs is increasing. :iscuss why the world"s energy needs is increasing. 2elate to the increase in population and the increased use of modern technology.
+e ource Secondary sources.
&omment
Time 6$min
9)c4 9.e8
2esearch the origins and e'traction and reserves of the main fossil fuels% coal% oil and gas. )'plain why fossil fuels are non, renewable fuels. )'plain why there might be an energy crisis. +nvestigate the problem of atmospheric pollution caused by the combustion of fossil fuels e.g. the effects of acid ArainB on limestone or chalk AbuildingsB.
+t should be emphasised that the original source is the Sun.
5$min
9/e1
9)c4
Fther problems are cost of e'traction and transportation and damage to the environment.
%r"mework &ode 9.e8
'e"rnin( O)*ective :escribe and investigate some effects of human influences on the environment. !se knowledge of energy sources including fossil fuels and renewable energy resources to consider the world"s energy needs% including research from secondary sources. Look critically at sources of secondary data.
Activitie 2esearch different renewable fuels. :iscuss whether nuclear fuels" are renewable or non,renewable. :iscuss the advantages and disadvantages of using nuclear fuels" to generate electricity. !se secondary sources to find out about different renewable energy resources. &ssess the advantages and disadvantages of each energy resource and how the energy resource is used to generate electricity. /resent findings to the whole class as a poster% /ower/oint presentation or a hand,out.
+e ource
&omment & careful definition of renewable is necessary i.e. a source that can be replaced in a shorter time than it is used. 2enewable energy sources should include solar% waves% rivers% tides and wind.
Time 5$min
9/e1
9)c4
9.e8
:escribe and investigate some effects of human influences on the environment. !se knowledge of energy sources including fossil fuels and renewable energy resources to consider the world"s energy needs% including research from secondary sources. Look critically at sources of secondary data.
9/e1
:iscuss and list the problems of using all kinds of energy sources. These might include suitability of site% climate% cost of converting to electrical energy% pollution of any kind and destruction of habitats. Consider the energy sources most used in your region and whether there would be benefit in changing to other energy resources.
6$min
9)c4
Você também pode gostar
- Scheme of Work Science Stage 7.v1Documento45 páginasScheme of Work Science Stage 7.v1gkawsar22100% (2)
- Scheme of Work Science Stage 7.v1Documento44 páginasScheme of Work Science Stage 7.v1Swati Rathod71% (7)
- Scheme of Work Science Stage 9.v1Documento51 páginasScheme of Work Science Stage 9.v1Sue Adames de Velasco50% (2)
- Scheme of Work Science Stage 8.v1Documento51 páginasScheme of Work Science Stage 8.v1gkawsar22100% (2)
- Scheme of Work Science Stage 7.v1Documento44 páginasScheme of Work Science Stage 7.v1Sue Adames de VelascoAinda não há avaliações
- Scheme of Work Science Stage 6v1Documento22 páginasScheme of Work Science Stage 6v1vjahnaviAinda não há avaliações
- Scheme of Work Science Stage 8.v1Documento50 páginasScheme of Work Science Stage 8.v1Sue Adames de Velasco100% (2)
- 1113 Lower Secondary Science Curriculum Framework 2018 - v2 - tcm143-498591Documento16 páginas1113 Lower Secondary Science Curriculum Framework 2018 - v2 - tcm143-498591elena100% (2)
- Scheme of Work Maths Stage 9Documento16 páginasScheme of Work Maths Stage 9Sue Adames de Velasco50% (2)
- Scheme of Work Science Stage 4 - 2018 - tcm142-354171Documento38 páginasScheme of Work Science Stage 4 - 2018 - tcm142-354171R Raj Gurung100% (2)
- Scheme of Work Science Stage 8 v1Documento50 páginasScheme of Work Science Stage 8 v1Ibnul Mubarok100% (3)
- 0653 Scheme of Work (For Examination From 2019)Documento115 páginas0653 Scheme of Work (For Examination From 2019)Azalia Delgado Vera50% (2)
- Checkpoint ScienceDocumento8 páginasCheckpoint ScienceNiyi OmodaraAinda não há avaliações
- Scheme of Work Maths Stage 7Documento14 páginasScheme of Work Maths Stage 7Arumugam PalaniapanAinda não há avaliações
- Maths Stage6 SOW v1 July11 tcm142-354185Documento61 páginasMaths Stage6 SOW v1 July11 tcm142-354185R Raj GurungAinda não há avaliações
- Scheme of Work Science Stage 9 - 2018 - tcm143-353968Documento82 páginasScheme of Work Science Stage 9 - 2018 - tcm143-353968Arjun SrinivasanAinda não há avaliações
- Scheme of Work Maths Stage 7Documento18 páginasScheme of Work Maths Stage 7Sue Adames de Velasco100% (7)
- Cambridge Lower Secondary Science 0893 Curriculum FrameworkDocumento1 páginaCambridge Lower Secondary Science 0893 Curriculum FrameworkdeboAinda não há avaliações
- 0097 Stage 5 Primary Science Scheme of Work Tcm142-595394Documento69 páginas0097 Stage 5 Primary Science Scheme of Work Tcm142-595394Romana Miriam OlivettiAinda não há avaliações
- Cambridge Lower Secondary Science Curriculum Outline PDFDocumento2 páginasCambridge Lower Secondary Science Curriculum Outline PDFThe DevilzAinda não há avaliações
- 0893 Lower Secondary Science Stage 8 Scheme of Work - tcm143-595696Documento90 páginas0893 Lower Secondary Science Stage 8 Scheme of Work - tcm143-595696manojAinda não há avaliações
- Maths Stage5 SOW v1 July11 tcm142-353884Documento69 páginasMaths Stage5 SOW v1 July11 tcm142-353884R Raj GurungAinda não há avaliações
- Scheme of Work Science Stage 6 - 2018 - tcm142-354057Documento42 páginasScheme of Work Science Stage 6 - 2018 - tcm142-354057R Raj GurungAinda não há avaliações
- Physics: Revising For Your Physics ExamsDocumento10 páginasPhysics: Revising For Your Physics ExamsKamrul Hasan SagarAinda não há avaliações
- Pakistan International School Jeddah - English Section: Sr. No. Subjects Publisher Isbn Book TitleDocumento1 páginaPakistan International School Jeddah - English Section: Sr. No. Subjects Publisher Isbn Book TitleAhmad NaumanAinda não há avaliações
- 0893 Lower Secondary Science Stage 7 Scheme of Work - tcm143-595695Documento100 páginas0893 Lower Secondary Science Stage 7 Scheme of Work - tcm143-595695Long100% (2)
- Scheme of Work Science Stage 2v1Documento16 páginasScheme of Work Science Stage 2v1Trish Hoppy100% (1)
- Textbook Checkpoint Complete Biology Cambridge Secondary 1Documento260 páginasTextbook Checkpoint Complete Biology Cambridge Secondary 1Vrushali Sukhi100% (2)
- 0893 Lower Secondary Science Stage 7 Scheme of Work - tcm143-595695Documento103 páginas0893 Lower Secondary Science Stage 7 Scheme of Work - tcm143-595695manoj0% (1)
- Scheme of Work Science Stage 7Documento90 páginasScheme of Work Science Stage 7Arjun Srinivasan100% (1)
- IGCSE Chemistry SOW 2016Documento85 páginasIGCSE Chemistry SOW 2016ashathtAinda não há avaliações
- 0893 Lower Secondary Science Teacher Guide 2020 - tcm143-595694Documento48 páginas0893 Lower Secondary Science Teacher Guide 2020 - tcm143-595694Abdulkarim kiplelAinda não há avaliações
- Long-Term Planning Template: Cambridge Lower Secondary Science Stage 8Documento3 páginasLong-Term Planning Template: Cambridge Lower Secondary Science Stage 8marycperez100% (1)
- Scheme of Work Science Stage 5 - 2018 - tcm142-529974Documento42 páginasScheme of Work Science Stage 5 - 2018 - tcm142-529974R Raj Gurung83% (6)
- 9780198390206Documento6 páginas9780198390206rshamsulkamar0% (2)
- IGCSE Biology Workbook Answers: 1 Characteristics and Classification of Living ThingsDocumento28 páginasIGCSE Biology Workbook Answers: 1 Characteristics and Classification of Living ThingsJessica SamanthaAinda não há avaliações
- Cambridge Primary Science Scheme of Work Learning Objectives Stage 2Documento4 páginasCambridge Primary Science Scheme of Work Learning Objectives Stage 2Carolyne AchiengAinda não há avaliações
- Scheme of Work Science Stage 3 - 2018 - tcm142-354137Documento35 páginasScheme of Work Science Stage 3 - 2018 - tcm142-354137R Raj Gurung100% (1)
- Scheme of Work Maths Stage 8Documento18 páginasScheme of Work Maths Stage 8Sue Adames de VelascoAinda não há avaliações
- Cambridge Lower Secondary Science Teacher Guide 0893 2021Documento43 páginasCambridge Lower Secondary Science Teacher Guide 0893 2021Ghada Shohieb0% (1)
- Complete Science For Cambridge Lower Secondary PDFDocumento2 páginasComplete Science For Cambridge Lower Secondary PDFSultan_Alattaf60% (10)
- Checkpoint Science Challenge 7 - Part 01Documento9 páginasCheckpoint Science Challenge 7 - Part 01KhAinda não há avaliações
- IGCSE Coordinated Science Keyword Definitions Master Copy 20-3-12Documento13 páginasIGCSE Coordinated Science Keyword Definitions Master Copy 20-3-12Dhingra shelly100% (1)
- Cambridge Lower Secondary Science Curriculum Outline PDFDocumento2 páginasCambridge Lower Secondary Science Curriculum Outline PDFPhuc Dao50% (2)
- Scheme of Work Maths Stage 9Documento16 páginasScheme of Work Maths Stage 9Wisdom PhanganAinda não há avaliações
- Biology Workbook Answers 3rd EditionDocumento2 páginasBiology Workbook Answers 3rd Editionali ashraf50% (2)
- Learner Guide For Cambridge IGCSE Physics 0972Documento48 páginasLearner Guide For Cambridge IGCSE Physics 0972HassanKMAinda não há avaliações
- Cambridge IGCSE ANSWERS Chemistry Study and Revision Guide Third EditionDocumento11 páginasCambridge IGCSE ANSWERS Chemistry Study and Revision Guide Third EditionNermine Abdel GelilAinda não há avaliações
- Cambridge Checkpoint Science Challenge Book 8Documento22 páginasCambridge Checkpoint Science Challenge Book 8Rashmi TharakaAinda não há avaliações
- Cambridge Checkpoint Science Skills Builder 9Documento17 páginasCambridge Checkpoint Science Skills Builder 9JohnWang56% (9)
- Teachers Guide Lower Secondary Science PDFDocumento141 páginasTeachers Guide Lower Secondary Science PDFUbaid UllahAinda não há avaliações
- Cambridge Secondary 1 Science Curriculum FrameworkDocumento14 páginasCambridge Secondary 1 Science Curriculum Frameworkapi-217350410100% (3)
- 8 States of MatterDocumento4 páginas8 States of Matterrashmi_harryAinda não há avaliações
- Complete Biology For Cambridge Secondary 1 Student Book For Cambridge Checkpoint and Beyond Cie CheckpointDocumento3 páginasComplete Biology For Cambridge Secondary 1 Student Book For Cambridge Checkpoint and Beyond Cie CheckpointHira Majid57% (7)
- Scheme of Work: Cambridge IGCSE / Cambridge IGCSE (9-1) First Language English 0500 / 0990Documento55 páginasScheme of Work: Cambridge IGCSE / Cambridge IGCSE (9-1) First Language English 0500 / 0990Amalia ZabalaAinda não há avaliações
- Checkpoint Revision Science Checklist 2018Documento12 páginasCheckpoint Revision Science Checklist 2018Hoàng Anh Bùi100% (1)
- Cambridge Checkpoint Science Challenge Workbook 9Documento20 páginasCambridge Checkpoint Science Challenge Workbook 9Natasha Natasha29% (7)
- Science Workbook 1 AnswersDocumento26 páginasScience Workbook 1 AnswersUzair Hussain81% (21)
- Relative Atomic MassDocumento8 páginasRelative Atomic Massgkawsar22Ainda não há avaliações
- Qualifying Test Result (2014-15) Subject: Chemistry: Bangladesh School MuscatDocumento2 páginasQualifying Test Result (2014-15) Subject: Chemistry: Bangladesh School Muscatgkawsar22Ainda não há avaliações
- Acid & BaseDocumento3 páginasAcid & Basegkawsar22Ainda não há avaliações
- Types of ExtractionDocumento5 páginasTypes of ExtractionMohammad NafaiAinda não há avaliações
- Periodic TableDocumento3 páginasPeriodic Tablegkawsar22Ainda não há avaliações
- Report of Preparatory Mockigcse SCDocumento55 páginasReport of Preparatory Mockigcse SCgkawsar22Ainda não há avaliações
- Test Nichrome Wire Wire Wire ColourDocumento2 páginasTest Nichrome Wire Wire Wire Colourgkawsar22Ainda não há avaliações
- Crude OilDocumento4 páginasCrude Oilgkawsar22Ainda não há avaliações
- Energy From ChemicalsDocumento4 páginasEnergy From Chemicalsgkawsar22Ainda não há avaliações
- AtomsDocumento4 páginasAtomsgkawsar22Ainda não há avaliações
- BondingDocumento5 páginasBondinggkawsar22Ainda não há avaliações
- Q 1Documento36 páginasQ 1gkawsar22Ainda não há avaliações
- ElectrolysisDocumento7 páginasElectrolysisgkawsar22Ainda não há avaliações
- Acid & BaseDocumento3 páginasAcid & Basegkawsar22Ainda não há avaliações
- A Guide To Practical QuestionsDocumento10 páginasA Guide To Practical Questionsgkawsar22Ainda não há avaliações
- HeinemannIGCSE Chemistry Chapter1Documento3 páginasHeinemannIGCSE Chemistry Chapter1gkawsar22Ainda não há avaliações
- RadiationDocumento3 páginasRadiationgkawsar22Ainda não há avaliações
- Acids QuestionsDocumento21 páginasAcids QuestionseeenusAinda não há avaliações
- Activity 13.3: How Does Concentration Affect Reaction Rate?Documento2 páginasActivity 13.3: How Does Concentration Affect Reaction Rate?gkawsar22Ainda não há avaliações
- The Aqueous Chemistry of CationsDocumento11 páginasThe Aqueous Chemistry of Cationsgkawsar22Ainda não há avaliações
- Seperatting and AnalysisDocumento3 páginasSeperatting and Analysisgkawsar22Ainda não há avaliações
- Rate of ReactionDocumento3 páginasRate of Reactiongkawsar22Ainda não há avaliações
- Q 2Documento25 páginasQ 2gkawsar22Ainda não há avaliações
- Heinemann IGCSEChemistry Teachers CDSample Activity 51Documento1 páginaHeinemann IGCSEChemistry Teachers CDSample Activity 51gkawsar22Ainda não há avaliações
- 0906 O Level Grade BoundariesDocumento2 páginas0906 O Level Grade Boundariesryuzaki589Ainda não há avaliações
- Making SaltsDocumento1 páginaMaking Saltsgkawsar22Ainda não há avaliações
- Oxygen and OxidesDocumento5 páginasOxygen and Oxidesgkawsar22Ainda não há avaliações
- Introducing Energy Changes in ReactionsDocumento2 páginasIntroducing Energy Changes in Reactionsgkawsar22Ainda não há avaliações
- Kinetic Theory of DiffusionDocumento1 páginaKinetic Theory of Diffusiongkawsar22Ainda não há avaliações
- Introducing Reversible ReactionsDocumento3 páginasIntroducing Reversible Reactionsgkawsar22Ainda não há avaliações
- Zombie Retreat2 WalkthroughDocumento6 páginasZombie Retreat2 WalkthroughVĩnh Khang Nguyễn DiệpAinda não há avaliações
- Nepalese FoodDocumento3 páginasNepalese FoodsonjekukAinda não há avaliações
- Module Kbat Bi Fasa 1 2018 PDFDocumento81 páginasModule Kbat Bi Fasa 1 2018 PDFpazaAinda não há avaliações
- Judge Beef FactsheetDocumento30 páginasJudge Beef FactsheetAugust Ridlof RiwuAinda não há avaliações
- Culinary TourismDocumento2 páginasCulinary TourismTófalvi JózsefAinda não há avaliações
- MeringueDocumento2 páginasMeringueVikas SinghAinda não há avaliações
- Part1 AV3Documento3 páginasPart1 AV3Phương Trinh Nguyễn NgọcAinda não há avaliações
- Food Insecurity in Pakistan 2009 SDPI 2009Documento129 páginasFood Insecurity in Pakistan 2009 SDPI 2009Iqra AkramAinda não há avaliações
- Neolithic TheoriesDocumento6 páginasNeolithic Theories8002 SNEHA MEENAAinda não há avaliações
- Definition, Composition, Standards and Processing of CreamDocumento15 páginasDefinition, Composition, Standards and Processing of CreamRonak Rawat100% (3)
- A Report On Business Policy, Ethics and Strategy of Grameen Danone Food Ltd. (GDFL)Documento23 páginasA Report On Business Policy, Ethics and Strategy of Grameen Danone Food Ltd. (GDFL)Shamim HossainAinda não há avaliações
- Utility Pump Station Design SpreadsheetDocumento16 páginasUtility Pump Station Design SpreadsheetFarhat Khan100% (4)
- VLORA-Lsta E Re X-Mas 2018Documento25 páginasVLORA-Lsta E Re X-Mas 2018eraldaAinda não há avaliações
- Europe 9N 10D ItineraryDocumento2 páginasEurope 9N 10D ItineraryAneesh KalekarAinda não há avaliações
- ChemicalDocumento2 páginasChemicaldalton2004Ainda não há avaliações
- PDF Study On Mcdonalds and Burger King - CompressDocumento45 páginasPDF Study On Mcdonalds and Burger King - CompressMharck Atienza100% (1)
- Amul Ice CreamDocumento18 páginasAmul Ice CreamIshu BhaliaAinda não há avaliações
- Speakout Grammar Extra Upper Intermediate Unit 8Documento2 páginasSpeakout Grammar Extra Upper Intermediate Unit 8xacobeoAinda não há avaliações
- Chap 11Documento8 páginasChap 11Emmanuel MariaAinda não há avaliações
- CXA - 006efs LISTA DE ESPECIFICACIONES DEL CODEX RELATIVAS A LOS ADITIVOS ALIMENTARIOS PDFDocumento98 páginasCXA - 006efs LISTA DE ESPECIFICACIONES DEL CODEX RELATIVAS A LOS ADITIVOS ALIMENTARIOS PDFLezly SantanaAinda não há avaliações
- Narmada District ProfileDocumento22 páginasNarmada District ProfileAmit GajjarAinda não há avaliações
- Self Care Recipe BookDocumento14 páginasSelf Care Recipe BookKopal Agarwal100% (1)
- ColitisDocumento2 páginasColitisyuvi087Ainda não há avaliações
- Phrasal VerbsDocumento3 páginasPhrasal Verbsapi-241242931Ainda não há avaliações
- 5 Forces & Vrine Apple and Starbucks CasesDocumento17 páginas5 Forces & Vrine Apple and Starbucks CasesIg Na0% (1)
- Instrumen Saringan Membaca Tahun 3Documento13 páginasInstrumen Saringan Membaca Tahun 3cikgudillaAinda não há avaliações
- Medical Protozoology. 1Documento55 páginasMedical Protozoology. 1miniwhiteyAinda não há avaliações
- STORYWORKS-Stone Soup-PlayDocumento6 páginasSTORYWORKS-Stone Soup-PlayReuben DylanAinda não há avaliações
- Nutrients of LumpiaDocumento3 páginasNutrients of LumpiaBevelyn NaulAinda não há avaliações
- WLBC Dec CalDocumento1 páginaWLBC Dec CalkristymadimikeAinda não há avaliações