Escolar Documentos
Profissional Documentos
Cultura Documentos
Activation and Denaturation of Hydrolases in Dry and Humid Supercritical Carbon Dioxide (SC-CO2)
Enviado por
FENFOGDescrição original:
Direitos autorais
Formatos disponíveis
Compartilhar este documento
Compartilhar ou incorporar documento
Você considera este documento útil?
Este conteúdo é inapropriado?
Denunciar este documentoDireitos autorais:
Formatos disponíveis
Activation and Denaturation of Hydrolases in Dry and Humid Supercritical Carbon Dioxide (SC-CO2)
Enviado por
FENFOGDireitos autorais:
Formatos disponíveis
Journal of Supercritical Fluids 19 (2000) 79 86 www.elsevier.
com/locate/supu
Activation and denaturation of hydrolases in dry and humid supercritical carbon dioxide (SC-CO2)
Christoph Bauer *, Doris-J. Steinberger, Gerald Schlauer, Thomas Gamse, Rolf Marr
Uni6ersity of Technology Graz, Institute fu r Thermische Verfahrens -und Umwelttechnik, Inffeldgasse 25, A -8010 Graz, Austria
Abstract The effects of enzyme treatment with dry and humid supercritical carbon dioxide (SC-CO2) were investigated using hydrolases (EC 3.1.1.1 and EC 3.1.1.3). A crude and a puried preparation of esterase EP10 from Burkholderia gladioli was incubated in SC-CO2 for long term and repeated high pressure treatments. Concerning the crude preparation, incubation for 24 h in SC-CO2 at 150 bar and 35C had no effect on enzyme activity while incubation at 75C led to a distinct loss of residual activity. After 30 pressurization and depressurization steps at 35C and 150 bar, the crude enzyme preparation showed an activity increase. Using the puried enzyme preparation of esterase EP10 from B. gladioli, no signicant effects could be observed. Fluorescence spectra indicated no conformational change before and after treatment with SC-CO2. Treatment of a preparation of esterase from porcine liver with wet and dry SC-CO2 at 200 bar at different temperatures showed a signicant denaturing inuence of the dissolved water on residual activities of the enzyme at temperatures of more than 40C. Lipase from Candida rugosa and esterase from porcine liver were treated at 150 and 300 bar at a constant temperature of 40C and an incubation time of 22 h. During these treatments, different amounts of water were introduced into the SC-CO2. The results showed an increase of the water content in the treated enzyme preparation while the enzyme activity remained stable till the maximum amount of water soluble in this medium was injected into the SC-CO2. 2000 Elsevier Science B.V. All rights reserved.
Keywords: Supercritical carbon dioxide; Hydrolases; Stability; Activity; Water content
1. Introduction The application of enzymes as biocatalysts in supercritical carbon dioxide (SC-CO2) has been described in the scientic literature since the mid
* Corresponding author. Tel.: + 43-316-8737984; fax: + 43316-8737472. E -mail address: bauer@tvtut.tu-graz.ac.at (C. Bauer).
1980s by Randolph et al. [1], Hammond et al. [2] and Nakamura et al. [3], who demonstrated that enzymes are active and stable in SC-CO2. From these rst demonstrations, various studies have been reported on the inuence of several parameters on the catalytic activity and stability of enzymes in SC-CO2, the inuence of temperature, pressure and humidity of the SC-CO2 and pressurization and depressurization steps.
0896-8446/00/$ - see front matter 2000 Elsevier Science B.V. All rights reserved. PII: S 0 8 9 6 - 8 4 4 6 ( 0 0 ) 0 0 0 7 0 - X
80
C. Bauer et al. / J. of Supercritical Fluids 19 (2000) 7986
Enzymes need a specic amount of bound water to be active. This is of vital importance for biocatalysis in SC-CO2, which is a non aqueous medium. However, if the water content of the carbon dioxide is too high or water is a product in the reaction (for example, esterications), the humidity can increase to a value where the enzyme gets inactivated. SC-CO2 may dissolve 0.3 0.35% (w/w) water, depending on pressure and temperature. Kasche et al. [4] reported that a-chymotrypsin and trypsin were partly denatured by humid (3% (w/v)) SC-CO2 caused by partial unfolding of the enzyme structure during the depressurization step. The denaturation of these enzymes can be avoided when the depressurization is performed after incubation of the enzyme in dry SC-CO2. They also reported, that penicillin amidase is rapidly denatured during the depressurization step after incubation in both humid and dry SCCO2. Chulalaksananukul et al. [5] studied the thermostability of immobilized lipase from Mucor miehei at different water contents and temperatures in SC-CO2. They noted that the water content and the temperature level must be properly balanced for optimum enzyme stability and activity. After reaching a maximum value of catalytic activity, the enzyme activity decreased with increasing water content at all temperatures. By examining lipase catalyzed reactions is SC-CO2, Dumont et al. [6], Marty et al. [7] and Yoon et al. [8] came to the conclusion, that there is always a distinct optimum amount of dissolved water depending on the enzyme. Zagrobelny et al. [9] showed by uorescence spectroscopic studies with trypsin, that pressurization steps are the reason for a change in protein conformation. The inuence of purity of the enzyme preparation was examined in only one publication by Giebauf et al. [10] using two preparations of lipase from Aspergillus niger (3.4 and 1 U mg 1; triolein as substrate). No inuence on temperature stability in SC-CO2 and on stability against pressurization and depressurization steps was detected.
Only few studies deal with the fact that SCCO2 treatment leads to a signicant increase of enzyme activity. However, SC-CO2 is known to be a nonpolar solvent, which exhibits excellent solubility for many impurities of crude enzyme preparations like carbohydrates, fatty acids and triglycerides, while the protein itself is generally insoluble in SC-CO2. Kamihira et al. [11] reported an activity increase (21 and 35%) of a crude a-amylase mixed with Escherichia coli or bakers yeast at a weight ratio of 9:1 during a sterilization step at 200 atm and 35C for 2 h. Giebauf et al. [10] showed, that treatment of lipase from Pseudomonas species with SC-CO2 at 150 bar and 45C for 1 h led to an activity increase of 20%, and incubation of lipase from porcine pancreas at 150 bar and 75C for 24 h caused an activity increase of several hundred percent [12]. Up to the present, these purication effects have not been found at enzyme preparations from other sources. The aim of this paper is to investigate the inuence of SC-CO2 treatment on stability and the catalytic activity of hydrolases (esterases (EC 3.1.1.1) and lipases (EC 3.1.1.3)), which are used for esterications and transesterications in non aqueous media. The inuence of purity on long term stability at various temperatures and on stability against pressurization and depressurization steps was examined using a crude and a puried preparation of esterase EP10 from Burkholderia gladioli. Michor et al. [13] reported that esterase EP10 from B. gladioli proved to be a suitable catalyst for transesterication of D,Lmenthol for the production of enantiomerically pure L-menthyl acetat with high enantioselectivity. To examine the possibility that the enzyme becomes modied during processing, the samples were characterized by uorescence spectroscopy. The inuence of water on enzyme stability was studied by two experiments, treatment of esterase from porcine liver with wet and dry SCO2 at different temperatures. Treatment of esterase from porcine liver and lipase from C. rugosa with SC-CO2 at different pressures with different amounts of water at a temperature commonly used in biocatalysis with hydrolases. Esterase from porcine liver is applied for resolution of
C. Bauer et al. / J. of Supercritical Fluids 19 (2000) 7986
81
racemic esters by esterication and lipase from C. rugosa is the lipase of choice for resolution of esters of secondary alcohols. Esterase fromporcine liver was used instead from B. glad ioli because Michor et al. [14] have already demonstrated, that any amount of additional water in the SC-CO2 inactivates this hydrolase. Both esterase from porcine liver and lipase from C. rugosa proved to be stable and active in dry SC-CO2 as reported by Giebauf et al. [10] and Steinberger et al. [15].
2.2. Determination of enzyme acti6ity
Photometric assays were used for enzyme activity measurements before and after treatment with SC-CO2 (all absorbance measurements were performed with a Shimadzu UV 160A spectrophotometer equipped with a Lauda RM6 temperature control). The activity was measured after storage of the preparations in plastic tubes (closed with a screw cap) in a freezer at 18C for at least 24 h.
2. Materials and methods
2.1. Materials
All chemicals used, if not otherwise stated, were purchased from Merck (Darmstadt) and were of p.a. (pro analysi) quality. The carbon dioxide with a purity \ 99.94% (v/v) and a dewing point lower than 60C was purchased from Linde (Graz) and stored in a tank with a capacity of 3200 l. Esterase EP10 from B. gladi oli was a gift from the Department of Biotechnology, University of Technology, Graz. Two preparations were used, crude (0.2 U mg 1 lyophilisate, 2-nitrophenyl butyrate as substrate) and chromatographically (HIC, hydrophobic interaction chromatography) puried (0.4 U mg 1 lyophilisate, 2-nitrophenyl butyrate as substrate). Specication of esterase EP10, recombinant enzyme overpressed in E. coli BL21 [DE3]. A preparation of esterase from porcine liver (19 U mg 1 preparation, ethyl butyrate as substrate) was purchased from Sigma Aldrich (Vienna). Lipase AY 30 from C. rugosa (30 U mg 1 preparation, olive oil as substrate) was supplied by Amano (Nagoya). 2-nitrophenyl butyrate (o NPB) was supplied by Sigma Aldrich (Vienna). Thesit and 1,2-O -dilauryl-rac-glycero-3-glutaric acid-resorun ester (DGGR) were purchased from Boehringer Mannheim Biochemica (Mannheim). Coomassie Brilliant Blue G-250 was provided by Serva (Heidelberg). Celluloseacetate lters (not sterile, 0.22 mm) were supplied by Roth (Karlsruhe). Karl Fischer solutions were purchased from Merck (Darmstadt).
2.2.1. Esterase assay Ten microliter of substrate solution (84 ml o NPB dissolved in 916 ml ethanol) were added to 990 ml of an enzyme solution (100 mg esterase ml 1 in 0.1-M tris(hydroxymethyl)aminomethan/HCl, pH 7.0). The linear increase of absorbance at 420 nm after 7 min of incubation at 25C was used to determine the enzyme activity. Three independent activity measurements for each sample were performed and the standard deviation (S.D.) was calculated. 2.2.2. Lipase assay A hundred microliter of substrate solution (6 mg 1,2-O -dilauryl-rac-glycero-3-glutaric acid-resorun ester were dissolved in 12 ml of a 1:1 mixture of dioxan/Thesit) were added to 900 ml of the enzyme solution (2.2 mg lipase ml 1 dissolved in 0.1-M KH2PO4, pH 6.8). The linear increase of absorbance at 572 nm after 2 min of incubation was used to measure the activities. Three independent activity measurements for each sample were performed and the S.D. was calculated. 2.3. Measurement of water content
Water contents of the untreated and treated enzymes were determined by the Karl Fischer titration method at 55C. The device consisted of a ABU93 triburette automatically controlled by the Radiometer Copenhagen VIT 90 video titrator. Three independent measurements were performed and the S.D. was calculated.
82
C. Bauer et al. / J. of Supercritical Fluids 19 (2000) 7986
2.4. Fluorescence spectroscopy 2.4.1. Tryptophan uorescence Fluorescence spectra of the intrinsic uorescence of the clear solutions of the proteins (0.1 mg ml 1) after ltering through the cellulose acetate lters were recorded with a Perkin Elmer LS50B spectrouorimeter (instrument settings, excitation wavelength, 280 nm; emission wavelength, 300 420 nm; excitation/emission slits, 3 nm; scan speed, 400 nm min 1). The emission spectra recorded are the average of ten scans. The proteins were dissolved in the same buffer solution as used for activity measurements and the emission spectra were recorded at 30C. 2.5. Long term stability of crude and puried enzyme preparations of esterase EP10 from B. gladioli
Thirty milligram of enzyme preparation was balanced on a lter paper and the folded lter was put into a high-pressure reactor (140 ml) placed in a water bath (Fig. 1). The temperature inside the reactor was measured by means of a platinum sensor. CO2 was compressed by a piston pump up to the required pressure and the enzyme was incubated under these conditions for 24 h at 150 bar and 35 and 75C, respectively. After treatment, the enzyme was stored at 18C and the activity was measured. Fluorescence spectra were recorded of the preparation with treatment at 75C.
2.6. Pressurization and depressurization experiments with crude and puried enzyme preparations of esterase EP10 from B. gladioli
The same device, as described above, was used. The enzyme preparation (30 mg) was incubated for 1 h in SC-CO2 at 150 bar and 35C. Then the reactor was depressurized to atmospheric pressure (duration about 6 min) and again pressurized to 150 bar. This procedure was repeated 30 times. Afterwards, the enzyme was stored at 18C in a freezer, the activity was measured and uorescence spectra were recorded.
2.7. Inuence of temperature on acti6ity of esterase from porcine li6er in wet and dry SC -CO2
To check the inuence of dissolved water in SC-CO2 during enzyme incubation, two high pressure reactors (140 ml) as shown in Fig. 1 were used, one lled with dry SC-CO2, the other with dry SC-CO2 and 30 ml of water placed at the bottom of the reactor. The enzyme preparation (30 mg) was placed at the top of both reactors (to avoid a direct contact to the liquid, if water was inside). During the experiment at 200 bar and 1-h incubation time, the inuence of different temperatures (20100C) on enzyme stability in wet and dry CO2 was investigated. Before measuring enzyme activity, the enzyme was stored at 18C in a freezer after treatment.
2.8. Inuence of water on lipase from C. rugosa and esterase from porcine li6er
These experiments were carried out with enzyme preparations (400 mg) incubated in a high pressure reactor placed in a water bath at 150 and 300 bar at a constant temperature of 40C and an incubation time of 22 h (Fig. 2). During these treatments, different amounts of water (100, 200 and 300 ml) were injected through a HPLC valve to the system (total volume, 170 ml), while SCCO2 was pumped in a cycle by a gear pump. Water was given to the system in 50 ml portions, waiting about 30 min between the injections.
Fig. 1. Schematic illustration of the device, used for the long term experiments and pressurization/depressurization experiments, a, cryostat; b, piston pump; c, high pressure reactor; d, heater; e, wash bottle; PI, pressure indicator; TI, temperature indicator; V1, inlet valve; V2, outlet valve.
C. Bauer et al. / J. of Supercritical Fluids 19 (2000) 7986
83
3. Results and discussions
3.1. Long term stability of crude and puried enzyme preparations of esterase EP10 from B. gladioli
Table 1 shows the residual activities of the crude and puried esterase preparations after treatment with SC-CO2 at 75 and 35C at a pressure of 150 bar for 24 h. While there is no signicant change in enzyme activity of the puried enzyme preparation, the activity of the crude esterase preparation did not change after high pressure treatment at 35C (103.8%) but decreased to 62.3%, of the untreated preparation after incubation at 75C. The treatment at 75C had the effect that the appearance of the crude enzyme preparation changed from yellow color and a fatty consistence to a caramel brown color and to rm pieces while the color and consistency of the puried enzyme preparation did not change remaining a white power. In contrast to lipase from A. niger examined by Giebauf et al. [10], the purity of the enzyme preparation of esterase EP10 from B. gladioli is an important factor for thermal stability in SC-CO2.
Fig. 2. Schematic illustration of the device with SC-CO2 cycle, a, cryostat; b, piston pump; c, high pressure reactor; d, heater; e, gear pump; f, wash bottle; g, HPLC valve; V1, inlet valve; V2, valve for the cycle; V3, outlet valve; PI, pressure indicator; TI, temperature indicator.
Table 1 Comparison between the residual activity of crude and puried esterase from B. gladioli after treatment with SC-CO2 at 150 bar and different temperatures for 24 ha Temperature (C) Residual activity (%), crude (mean 9 S.D.) 103.8 9 4.10 62.3 9 5.82 Residual activity (%), puried (mean 9 S.D.) 98.7 9 5.46 93.7 9 8.11
35 75
a
Value of the untreated enzyme was set to 100%.
3.2. Pressurization and depressurization experiments with crude and puried enzyme preparations of esterase EP10 from B. gladioli
Table 2 shows the enzyme activities after 30 pressurization and depressurization cycles with SC-CO2 at 35C and 150 bar. Each incubation step lasted 1 h. A temperature of 35C was chosen for this experiment to avoid thermal inactivation. The experiment had no effect on the puried enzyme preparation and the appearance of the white powder did not change at all. The 30 pressurization and depressurization cycles did not generate any activity loss. The enzyme activity of crude esterase, however, increased to 120.7% of the untreated preparation and the crude enzyme preparation became a bright yellow dry power. Repeated extraction had a greater purifying effect than the long-term extraction, because the preparation was contacted with fresh CO2 at the beginning of each pressurization cycle. Therefore, the
Table 2 Comparison between enzyme activity of crude and puried esterase from B. gladioli before and after 30 pressurization and depressurization cyclesa Residual activity (%), crude (mean 9 S.D.) 120.7 9 3.58
a
Residual activity (%), puried (mean 9 S.D.) 101.9 9 2.93
Value of the untreated enzyme was set to 100%.
After depressurization, the water content of the treated enzyme was measured immediately by Karl Fischer titration and the activity was measured after storage in a freezer.
84
C. Bauer et al. / J. of Supercritical Fluids 19 (2000) 7986
mass transfer of the impurities into the supercritical phase was enhanced.
3.3. Fluorescence spectroscopy
In the study of the intrinsic uorescence of esterase EP10 from B. gladioli treated with SCCO2, it was found out that no change of emission maxima occurred. The exposure to SC-CO2 (longTable 3 Fluorescence emission maxima of esterase from B. gladioli in Tris-buffer at pH 7.0a Treatment Untreated 24 h, 75C, 150 bar 30 pressurization/depressurization steps, 35C, 150 bar
a
Fig. 5. Residual activity of esterase from porcine liver after treatment with various amounts of water dissolved in the SC-CO2.
A (nm) 340.0 341.0 339.5
B (nm) 331.5 331.0 330.0
A, crude preparation; B, puried preparation.
term treatment of pressurization and depressurization steps, see Table 3) causes, therefore, no larger protein conformational change. So, the activity decrease of the crude preparation at 75C might be caused by chemical modications on protein reactive groups by impurities in the preparation.
3.4. Inuence of temperature on acti6ity of esterase from porcine li6er in wet and dry SC -CO2
Incubation of the enzyme at different temperatures showed clear differences between wet (30 ml water inside the reactor) and dry SC-CO2 at temperatures of more than 40C (see Fig. 3). Working with dry carbon dioxide, only a slight loss of enzyme activity up to a temperature of 80C was observed. At 100C, the activity of the esterase sharply decreased to 76.6%. The results with wet carbon dioxide show this decrease to a residual activity of 71.3% already at 65C, remaining at this level at higher temperatures.
Fig. 3. Residual activity of esterase from porcine liver after treatment with dry and wet SC-CO2 at 200 bar at different temperatures.
3.5. Inuence of water on lipase from C. rugosa and esterase from porcine li6er
Figs. 4 and 5 show the residual enzyme activities after SC-CO2 treatment at 150 and 300 bar at 40C with increasing amounts of water for lipase from C. rugosa and esterase from porcine liver. The added water led to a signicant change in the water content of the preparation of both enzymes
Fig. 4. Residual activity of lipase from C. rugosa after treatment with various amounts of water dissolved in the SC-CO2.
C. Bauer et al. / J. of Supercritical Fluids 19 (2000) 7986
85
(see Figs. 6 and 7) but had no effect on the activity over a wide range. Even at a water content of more than 170% of the untreated preparation at 300 bar and 200 ml added water, the enzyme preparation of lipase from C. rugosa remained stable and active (residual activity, 91.6%). In contrast to previous studies about enzyme stability in SC-CO2, no optimum amount of water could be detected. Both preparations remained stable and active (more than 85% residual activity at 150 bar and more than 90% at 300 bar) at all amounts of water added, that did not exceed the maximum amount of water soluble in the SC-CO2. For the used system volume (170 ml), this is 270 ml at 150 bar and 40C and 350 ml for 300 bar. Injection of 300 ml water at 150 bar led to a signicant loss of enzyme activity (residual activity, around 40%), because of denaturing effects of the undissolved water during the depressuriza-
tion step. Both enzymes are well suited for biocatalysis with production of high amounts of water. High pressure should be used to maximize the solubility of water in SC-CO2 at a given temperature.
4. Conclusion The results show that SC-CO2 treatment leads in certain cases to an increase of enzymatic activity when crude preparations are used. Purity was proved to be a factor, that inuences temperature stability, because impurities decreased the stability of the examined esterase during incubation in SC-CO2 at high temperatures. The water content of the SC-CO2 had a signicant inuence on activity and stability only at temperatures of more than 40C, the temperature level normally used for biocatalysis with hydrolases. So both enzymes are t for use for biocatalysis in SC-CO2 with high accumulation of water like esterication reactions. The purication effect, shown in this paper, might be another benet of the use of supercritical carbon dioxide in biocatalysis, which is still restricted to research on laboratory scale.
Acknowledgements This work was supported by a grant of the Austrian Science Fund FWF (project no. 13269CHE). Fluorescence spectra were recorded at the Department of Biochemistry at the Karl-Franzens University, Graz. Special thanks to Professor Schwab (Department of Biotechnology, University of Technology, Graz) and his co-workers for donating us esterase from B. gladioli.
Fig. 6. Water content of lipase from C. rugosa after treatment with various amounts of water dissolved in the SC-CO2.
References
[1] T.W. Randolph, H.W. Blanch, J.W. Prausnitz, C.R. Wilke, Enzymatic catalysis in a supercritical uid, Biotechnol. Lett. 7 (1985) 325 328. [2] D.A. Hammond, M. Karel, A.M. Klibanov, Enzymatic reactions in supercritical gases, Appl. Biochem. Biotechnol. 11 (1985) 393 400.
Fig. 7. Water content of esterase from porcine liver after treatment with various amounts of water dissolved in the SC-CO2.
86
C. Bauer et al. / J. of Supercritical Fluids 19 (2000) 7986 [10] A. Giebauf, W. Magor, D.-J. Steinberger, R. Marr, A study of hydrolases stability in supercritical carbon dioxide (SC-CO2), Enzyme Microb. Technol. 24 (1999) 577 583. [11] M. Kamihira, M. Taniguchi, T. Kobayashi, Sterilization of micro-organisms with supercritical carbon dioxide, Agric. Biol. Chem. 51 (2) (1987) 407 412. [12] A. Gieauf, T. Gamse, A simple process for increasing the specic activity of porcine pancreatic lipase by supercritical carbon dioxide treatment, J. Mol. Catal. B: Enzymatic 9 (2000) 57 64. [13] H. Michor, R. Marr, T. Gamse, T. Schilling, E. Klingsbichel, H. Schwab, Enzymatic catalysis in supercritical carbon dioxide: Comparison of different lipases and a novel esterase, Biotechnol. Lett. 18 (1996) 79 84. [14] H. Michor, R. Marr, T. Gamse, Enzymatic catalysis in supercritical carbon dioxide: effect of water activity, Proceedings of the Third International Symposium in High Pressure Chemical Engineering, In: R. Rohr, C. Trepp (Eds.), High Pressure Chemical Engineering, Netherlands, 1996, pp. 115 120. [15] D.-J. Steinberger, T. Gamse, R. Marr, Enzyme inactivation and prepurication effects of supercritical carbon dioxide (SC-CO2), Proceedings of the Fifth Conference on Supercritical Fluids and their Applications, Garda, 1999, pp. 339 346.
[3] K. Nakamura, Y. Min Chi, Y. Yamada, T. Yano, Lipase activity and stability in supercritical carbon dioxide, Chem. Eng. Commun. 45 (1986) 207212. [4] V. Kasche, R. Schlothauer, G. Brunner, Enzyme denaturation in supercritical CO2: stabilization effects of S S bonds during depressurization step, Biotechnol. Lett. 10 (1988) 569 574. [5] W. Chulalaksananukul, J.-S. Condoret, D. Combes, Geranyl acetate synthesis by lipase-catalyzed transesterication in supercritical carbon dioxide, Enzyme Microb. Technol. 15 (1993) 691698. [6] T. Dumont, D. Barth, M. Perrut, Continuous synthesis of ethyl myristate by enzymatic reaction in supercritical carbon dioxide, Proceedings of the Second International Symposium on Supercritical Fluids, Boston (1991) 150 153. [7] A. Marty, V. Dossat, J.-S. Conderet, Continuous operation of lipase-catalyzed reactions in nonaqueous solvents: inuence of the production of the hydrophilic compound, Biotechnol. Bioeng. 56 (1997) 232238. [8] S.H. Yoon, O. Miyawaki, K.-H. Park, K. Nakamura, Transesterication between triolein and ethylbehenate by immobilized lipase in supercritical carbon dioxide, J. Ferment. Bioeng. 82 (1996) 334340. [9] J. Zagrobelny, F.V. Bright, In situ studies of protein conformation in supercritical uids: trypsin in carbon dioxide, Biotechnol. Prog. 8 (1992) 421423.
Você também pode gostar
- The Subtle Art of Not Giving a F*ck: A Counterintuitive Approach to Living a Good LifeNo EverandThe Subtle Art of Not Giving a F*ck: A Counterintuitive Approach to Living a Good LifeNota: 4 de 5 estrelas4/5 (5794)
- Manganase Zinc FerritaDocumento11 páginasManganase Zinc FerritaFENFOGAinda não há avaliações
- The Little Book of Hygge: Danish Secrets to Happy LivingNo EverandThe Little Book of Hygge: Danish Secrets to Happy LivingNota: 3.5 de 5 estrelas3.5/5 (399)
- Application of Electrocoagulation Process For Dairy WastewaterDocumento9 páginasApplication of Electrocoagulation Process For Dairy WastewaterFENFOGAinda não há avaliações
- A Heartbreaking Work Of Staggering Genius: A Memoir Based on a True StoryNo EverandA Heartbreaking Work Of Staggering Genius: A Memoir Based on a True StoryNota: 3.5 de 5 estrelas3.5/5 (231)
- The Distinct 01X-MainDocumento9 páginasThe Distinct 01X-MainFENFOGAinda não há avaliações
- Hidden Figures: The American Dream and the Untold Story of the Black Women Mathematicians Who Helped Win the Space RaceNo EverandHidden Figures: The American Dream and the Untold Story of the Black Women Mathematicians Who Helped Win the Space RaceNota: 4 de 5 estrelas4/5 (894)
- Emissions AthmosfericDocumento15 páginasEmissions AthmosfericFENFOGAinda não há avaliações
- The Yellow House: A Memoir (2019 National Book Award Winner)No EverandThe Yellow House: A Memoir (2019 National Book Award Winner)Nota: 4 de 5 estrelas4/5 (98)
- The INFLUENCE of NATURAL ORGANIC MATTER and AGING On SUSPENSION STABILITY in Guideline Toxicity Testing of Silver Zinc Oxide and Titanium Dioxide Nanopartcles With Daphania MagnaDocumento11 páginasThe INFLUENCE of NATURAL ORGANIC MATTER and AGING On SUSPENSION STABILITY in Guideline Toxicity Testing of Silver Zinc Oxide and Titanium Dioxide Nanopartcles With Daphania MagnaFENFOGAinda não há avaliações
- Investigation of MG (OH) AgentDocumento10 páginasInvestigation of MG (OH) AgentFENFOGAinda não há avaliações
- Elon Musk: Tesla, SpaceX, and the Quest for a Fantastic FutureNo EverandElon Musk: Tesla, SpaceX, and the Quest for a Fantastic FutureNota: 4.5 de 5 estrelas4.5/5 (474)
- The Distinct 01X-MainDocumento9 páginasThe Distinct 01X-MainFENFOGAinda não há avaliações
- Never Split the Difference: Negotiating As If Your Life Depended On ItNo EverandNever Split the Difference: Negotiating As If Your Life Depended On ItNota: 4.5 de 5 estrelas4.5/5 (838)
- Electrochemical Treatment and Operating Cost AnalysisDocumento11 páginasElectrochemical Treatment and Operating Cost AnalysisFENFOGAinda não há avaliações
- Eletro FloculaçãoDocumento6 páginasEletro FloculaçãoFENFOGAinda não há avaliações
- Devil in the Grove: Thurgood Marshall, the Groveland Boys, and the Dawn of a New AmericaNo EverandDevil in the Grove: Thurgood Marshall, the Groveland Boys, and the Dawn of a New AmericaNota: 4.5 de 5 estrelas4.5/5 (265)
- The ContrainsDocumento7 páginasThe ContrainsFENFOGAinda não há avaliações
- Mechanistic Investigation Into Antibacterial Behaviour of Suspensions of ZnO Nanoparticles Against E CliDocumento13 páginasMechanistic Investigation Into Antibacterial Behaviour of Suspensions of ZnO Nanoparticles Against E CliFENFOGAinda não há avaliações
- Wate Manegenent 1 s2.0 S0956053X1400052X MainDocumento6 páginasWate Manegenent 1 s2.0 S0956053X1400052X MainFENFOGAinda não há avaliações
- The Emperor of All Maladies: A Biography of CancerNo EverandThe Emperor of All Maladies: A Biography of CancerNota: 4.5 de 5 estrelas4.5/5 (271)
- Green Synthetic Approach For Starch Capped Silver NanoparticlesDocumento10 páginasGreen Synthetic Approach For Starch Capped Silver NanoparticlesFENFOGAinda não há avaliações
- Silver Nanoparticles As Potential Antibacterial AgentsDocumento20 páginasSilver Nanoparticles As Potential Antibacterial AgentsFENFOGAinda não há avaliações
- The Hard Thing About Hard Things: Building a Business When There Are No Easy AnswersNo EverandThe Hard Thing About Hard Things: Building a Business When There Are No Easy AnswersNota: 4.5 de 5 estrelas4.5/5 (344)
- Review On Zinc ParticleDocumento15 páginasReview On Zinc ParticleFENFOGAinda não há avaliações
- Team of Rivals: The Political Genius of Abraham LincolnNo EverandTeam of Rivals: The Political Genius of Abraham LincolnNota: 4.5 de 5 estrelas4.5/5 (234)
- Biological Synthesis of Silver Nanoparticles and Evaluation of Antibacterial and Antifugal Properties of Silver and Copper NanoparticlesDocumento7 páginasBiological Synthesis of Silver Nanoparticles and Evaluation of Antibacterial and Antifugal Properties of Silver and Copper NanoparticlesFENFOGAinda não há avaliações
- Viscoelastic Analysis of Com-Plex Polymer Melt Flows Using The EXtended PomÔÇôPom Model - J.N.N.F.M 2002Documento26 páginasViscoelastic Analysis of Com-Plex Polymer Melt Flows Using The EXtended PomÔÇôPom Model - J.N.N.F.M 2002FENFOGAinda não há avaliações
- The Use of AntibacterialDocumento9 páginasThe Use of AntibacterialFENFOGAinda não há avaliações
- Modelling Film Formation and Degradation of Semi-Transparent Exterior Wood Coatings - 2007Documento12 páginasModelling Film Formation and Degradation of Semi-Transparent Exterior Wood Coatings - 2007FENFOGAinda não há avaliações
- Development of High Temperature-Resistant Hydrosoluble PaintDocumento1 páginaDevelopment of High Temperature-Resistant Hydrosoluble PaintFENFOGAinda não há avaliações
- The Unwinding: An Inner History of the New AmericaNo EverandThe Unwinding: An Inner History of the New AmericaNota: 4 de 5 estrelas4/5 (45)
- Reator CSTRDocumento5 páginasReator CSTRThiago SobreiraAinda não há avaliações
- The World Is Flat 3.0: A Brief History of the Twenty-first CenturyNo EverandThe World Is Flat 3.0: A Brief History of the Twenty-first CenturyNota: 3.5 de 5 estrelas3.5/5 (2219)
- Electrochemical Oxidation of A Textile Industry EffluentDocumento2 páginasElectrochemical Oxidation of A Textile Industry EffluentFENFOGAinda não há avaliações
- A Review of The Rheology of Filled Viscoelastic Systems - R.R 2003Documento36 páginasA Review of The Rheology of Filled Viscoelastic Systems - R.R 2003FENFOGAinda não há avaliações
- A New and Simple Procedure For The Evaluation of The Association of Surfactants To ProteinsDocumento8 páginasA New and Simple Procedure For The Evaluation of The Association of Surfactants To ProteinsFENFOGAinda não há avaliações
- The Gifts of Imperfection: Let Go of Who You Think You're Supposed to Be and Embrace Who You AreNo EverandThe Gifts of Imperfection: Let Go of Who You Think You're Supposed to Be and Embrace Who You AreNota: 4 de 5 estrelas4/5 (1090)
- Laparoscopic MyomectomyDocumento2 páginasLaparoscopic MyomectomyclarissasveznaAinda não há avaliações
- Karakterisasi Koleksi Plasma Nutfah Tomat Lokal Dan IntroduksiDocumento7 páginasKarakterisasi Koleksi Plasma Nutfah Tomat Lokal Dan IntroduksiAlexanderAinda não há avaliações
- Methods, Method Verification and ValidationDocumento14 páginasMethods, Method Verification and Validationjljimenez1969100% (16)
- Optical Illusions and Vision Test AnswersDocumento8 páginasOptical Illusions and Vision Test AnswersZ'karia Al Ayi-zAinda não há avaliações
- The Sympathizer: A Novel (Pulitzer Prize for Fiction)No EverandThe Sympathizer: A Novel (Pulitzer Prize for Fiction)Nota: 4.5 de 5 estrelas4.5/5 (119)
- FCPS SCERETS Rabia Ali Final - UPDATED Version 2014Documento9 páginasFCPS SCERETS Rabia Ali Final - UPDATED Version 2014Akas Rehman100% (4)
- Artificial Immunity Vs Natural ImmunityDocumento87 páginasArtificial Immunity Vs Natural ImmunityromeoqfacebookAinda não há avaliações
- Neural Network: Sudipta RoyDocumento25 páginasNeural Network: Sudipta RoymanAinda não há avaliações
- Effect of Gender On Short Term Memory FinishedDocumento27 páginasEffect of Gender On Short Term Memory Finishedhafsah286Ainda não há avaliações
- Respiration Course ObjectivesDocumento8 páginasRespiration Course Objectivesjoshy220996Ainda não há avaliações
- Cucumber Seed Germination: Effect and After-Effect of Temperature TreatmentsDocumento7 páginasCucumber Seed Germination: Effect and After-Effect of Temperature Treatmentsprotap uddin PolenAinda não há avaliações
- The Ecology of Photosintesis PathwaysDocumento5 páginasThe Ecology of Photosintesis PathwaysRicardo RicoAinda não há avaliações
- AP Bio Lab 1Documento5 páginasAP Bio Lab 1Kiran ShilaAinda não há avaliações
- Application of Edible Films and Coatings On MeatsDocumento2 páginasApplication of Edible Films and Coatings On MeatsAnasZeidAinda não há avaliações
- Exampro GCSE Biology: B2.5 InheritanceDocumento35 páginasExampro GCSE Biology: B2.5 InheritanceMeera ParaAinda não há avaliações
- Gen Bio 1 Module 1Documento30 páginasGen Bio 1 Module 1Louise Gabriel Lozada100% (1)
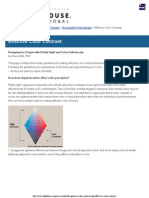
- Lighthouse International - Effective Color ContrastDocumento5 páginasLighthouse International - Effective Color ContrastVaishnavi JayakumarAinda não há avaliações
- Noise PollutionDocumento8 páginasNoise PollutiongoodthoughtsAinda não há avaliações
- Bio-Diversity Uses Threats and ConservationDocumento32 páginasBio-Diversity Uses Threats and ConservationRoshin VargheseAinda não há avaliações
- Specimen Cold Chain RequirementsDocumento6 páginasSpecimen Cold Chain RequirementsNikkae Angob0% (1)
- Chapter 1 of Blue MindDocumento23 páginasChapter 1 of Blue MindSouthern California Public Radio100% (1)
- Jhean Steffan Martines de CamargoDocumento2 páginasJhean Steffan Martines de CamargoJheanAinda não há avaliações
- Vol2 Final 19092016 PDFDocumento649 páginasVol2 Final 19092016 PDFshankarAinda não há avaliações
- Wbtet 2012Documento7 páginasWbtet 2012Partha BatabyalAinda não há avaliações
- Intergumentary System 10Documento40 páginasIntergumentary System 10Mike BasantaAinda não há avaliações
- Case Study On Dengue FeverDocumento76 páginasCase Study On Dengue FeverMary Rose Silva Gargar0% (1)
- Rankem SolventsDocumento6 páginasRankem SolventsAmmar MohsinAinda não há avaliações
- LAS 1 Cell TheoryDocumento12 páginasLAS 1 Cell TheoryJeremie CataggatanAinda não há avaliações
- Eosinophilic GastroenteritisDocumento7 páginasEosinophilic GastroenteritisSotir LakoAinda não há avaliações
- Post-Harvest Multiple Choice QuestionsDocumento8 páginasPost-Harvest Multiple Choice QuestionsErnestina OwireduAinda não há avaliações
- Plasma Gel Maker for Wrinkle Correction & Scar FillingDocumento1 páginaPlasma Gel Maker for Wrinkle Correction & Scar FillingPradeep AggarwalAinda não há avaliações
- Is That a Fact?: Frauds, Quacks, and the Real Science of Everyday LifeNo EverandIs That a Fact?: Frauds, Quacks, and the Real Science of Everyday LifeNota: 4.5 de 5 estrelas4.5/5 (3)
- Science Goes Viral: Captivating Accounts of Science in Everyday LifeNo EverandScience Goes Viral: Captivating Accounts of Science in Everyday LifeNota: 5 de 5 estrelas5/5 (1)
- Organic Chemistry for Schools: Advanced Level and Senior High SchoolNo EverandOrganic Chemistry for Schools: Advanced Level and Senior High SchoolAinda não há avaliações
- Napoleon's Buttons: 17 Molecules That Changed HistoryNo EverandNapoleon's Buttons: 17 Molecules That Changed HistoryNota: 4 de 5 estrelas4/5 (25)
- Chemistry for Breakfast: The Amazing Science of Everyday LifeNo EverandChemistry for Breakfast: The Amazing Science of Everyday LifeNota: 4.5 de 5 estrelas4.5/5 (90)
- The Periodic Table: A Very Short IntroductionNo EverandThe Periodic Table: A Very Short IntroductionNota: 4.5 de 5 estrelas4.5/5 (3)