Escolar Documentos
Profissional Documentos
Cultura Documentos
Web - Chem.ucsb - Edu - Zhang - ZLM-chapter 10-Alcohol
Enviado por
Krisna PamungkasTítulo original
Direitos autorais
Formatos disponíveis
Compartilhar este documento
Compartilhar ou incorporar documento
Você considera este documento útil?
Este conteúdo é inapropriado?
Denunciar este documentoDireitos autorais:
Formatos disponíveis
Web - Chem.ucsb - Edu - Zhang - ZLM-chapter 10-Alcohol
Enviado por
Krisna PamungkasDireitos autorais:
Formatos disponíveis
LMZ/CHEM109A/ Winter 2010
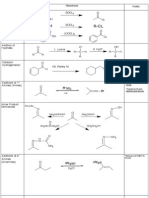
Chapter 10: Reactions of alcohols, amines, ethers, epoxides and sulfur-containing compounds I. Nucleophilic substitution reactions for the conversion of alcohols to other functional groups A. Introduction:
4. Reaction of alcohols (especially 1 or 2) with phosphorus halide (Sec. 11.8) R R PCl3, PBr3, or PCl5 (+ HOPX2 R' R' H X X= Br or Cl) OH H inversion for 1 and 2 C R' = H or alkyl or aryl (Note: For the synthesis of alkyl chloride, thionyl chloride generally gives better yields than PCl3 or PCl5.) Mechanism (for reaction with PBr3)?
B. Conversion of alcohol to alkyl halide (Reaction at C-O bond) (sec. 11.1): in-situ converting OH into good leaving groups 1. Conversion of alcohol to alkyl bromide by reaction with HBr (or NaBr, H2SO4) R* Br + H2O R* OH + HBr R* OH + HBr R* Br + H2O 1 S N SN2 inversion 3 racemic 1 mixture Mechanism?
e.g. 1
H C H3 C
OH PBr3 CH2CH3
C. Conversion of alcohols to tosylate (Reaction at O-H bond) (Sec. 10.3) 2. Conversion of alcohol to alkyl chloride by reaction with Lucas reagent (HCl, ZnCl2) ZnCl2 * ZnCl2 R* Cl + H2O R* OH + HCl R* OH + HCl R Cl + H2O 1 S N SN2 inversion 3 racemic 1 mixture Mechanism?
R R' H OH R' = H or alkyl or aryl R O R' Cl S CH3 H O O O S tosyl chloride (p-TosCl) O tosylate retension NEt or pyridine
3
CH3 + NEt3HCl (or pyridinum chloride)
e.g. 2 Me C H Et OH
O Cl S O
CH3 pyridine
D. Conversion of alcohol to other functional groups via halides or tosylates: Stereochemical consequences (sec/ 10.3)
R R' H OH PCl3or PBr3 R' R X + H2 O H inversion R CH3 R' (p-TosCl) H + HCl OTos retension Nu e.g. N3 SN2 R' Nu e.g. N3 SN 2 R' R H Nu
3. Reaction of alcohols (especially 1 or 2) with thionyl chloride (Sec. 11.9)
R R' H OH R' = H or alkyl or aryl SOCl2 R' R X H stereochemistry?? (+ SO2 + HCl)
inversion
double inversion : overall retention
O R Cl S R' H O O-H R Nu
Mechanism?
inversion H
retension + inversion : overall inversion
LMZ/CHEM109A/ Winter 2010
II. Elimination and rearrangement reactions of alcohols 1. Acid catalyzed dehydration of alcohol to alkene (Sec. 10.4) (3>2>1)
III. Oxidation of alcohols, aldehydes, and thiols A. Oxidation of alcohols and aldehydes (Sec. 10.5)
a. Oxidation of 1 alcohols to aldehydes or carboxylic acids
OH
[O], oxidation H R
[O], oxidation H
R C
N H H CrO3Cl aldehyde 1 alcohol (CrO , pyridine, HCl) 3
mechanism?
pyridinium chlorochromate (PCC)
e.g. Na2Cr2O7, H2O, R OH ethanol carboxylic acid Or Jone's reagent (CrO3, H3O+, acetone) OR KMnO4, H2O
[O] e.g. Na2Cr2O7, H2O, ethanol, OR CrO3, H3O+, acetone, (OR KMnO4, H2O)
b. Oxidation of 2 alcohols to ketones
OH
[O], oxidation R' e.g. PCC, CH2Cl2 OR Na2Cr2O7, H2O, ethanol OR CrO3, H3O+, acetone R
R C
[O] R' no further oxidation
- carbocation rearrangement may occur
H 2 alcohol
ketone
c. 3 alcohols cannot be oxidized
OH
[O], oxidation R' NO REACTION
R C
R" 3 alcohol
- SN2 reaction competes with E2 dehydration
mechanism:
2. Dehydration by POCl3 IV. Amines as organic bases (sec. 10.6) - amino (NH2- or NR2-) is a very poor leaving group; - amines does not undergo elimination or substitution reactions on the C-N bond.
- as base
LMZ/CHEM109A/ Winter 2010
V. Nucleophilic substitution reactions of ethers (ROR) A. RO- is poor leaving groups similar to OH-: need acid activation - reaction proceeds with neither pure SN2 or SN1. - regioselectivity:
B. SN1 or SN2 mechanism (PCl3 or TsCl not working)
D. basic conditions:
- regioselectivity controlled by sterics - stereochemistry: SN2
VI. Epoxide (one kind of ether) ring opening (sec. 10.8) A. Due to ring strain, much more reactive than normal ethers VII. Crown ethers - help dissolve metal cations - Na+ --- [15]-crown-5 - K+ -----[18]-crown-6 - Li+ ----[12]-crown-4
B. under acidic conditions-- opened by halide, even H2O and ROH
LMZ/CHEM109A/ Winter 2010
IX. Thiols, sulfide (sec. 10.11)
B. Organolithium compounds: prepared in alkanes - strongly basic (pKa >40), can be nucleophile as well - C-Li bond: polarized covalent bond (not ionic bond)
A. naming thiols - as the principle functional group, alkane + thiol (similar to alcohol case)
- naming priority: OH>SH>NH2, when treated as substituent, use 'mercapto'
C. Organomagnesium compounds -- Grignard reagents (in ethereal solvent) - strongly basic, but less reactive than corresponding lithium compounds. - C-Mg bond: polarized covalent bond, not ionic bond.
B. naming sulfide: (similar to ether)
C. Strong nucleophiles due to high polarizability (softness)
D. Reactions:
X. Organometallic compounds (sec. 10.12) A. compare:
Você também pode gostar
- Smith 4th Ed Chap 9 Alcohols, Ethers and Epoxides (Fall 2013)Documento47 páginasSmith 4th Ed Chap 9 Alcohols, Ethers and Epoxides (Fall 2013)Clifford PhilogeneAinda não há avaliações
- Summary of Important Organic ReactionsDocumento41 páginasSummary of Important Organic ReactionsKathyAinda não há avaliações
- FGIDocumento6 páginasFGIJulia MaramatAinda não há avaliações
- C10K Carbonyl Chemistry EmailDocumento37 páginasC10K Carbonyl Chemistry EmailMatthew яeject'z BennettAinda não há avaliações
- Haloalkanes and HaloarenesDocumento14 páginasHaloalkanes and HaloarenesHarshitha GowdaAinda não há avaliações
- 12 Chemistry Keypoints Revision Questions Chapter 10 PDFDocumento13 páginas12 Chemistry Keypoints Revision Questions Chapter 10 PDFSahil Kalra100% (1)
- A Review of Organic Reactions and Reagents For Chemistry 551Documento38 páginasA Review of Organic Reactions and Reagents For Chemistry 551Cris WRAinda não há avaliações
- Nucleophilic Substitution: The General Reaction and TerminologyDocumento23 páginasNucleophilic Substitution: The General Reaction and Terminologyapi-239546340Ainda não há avaliações
- UmpolungDocumento28 páginasUmpolungmeauna100% (1)
- Chemitry NotesDocumento6 páginasChemitry NotesRahul SinghAinda não há avaliações
- Aldehydes and KetonesDocumento9 páginasAldehydes and KetonesCamille AdleAinda não há avaliações
- Hand-Out: Chemistry Chapter 4: Haloalkanes & HaloarenesDocumento11 páginasHand-Out: Chemistry Chapter 4: Haloalkanes & HaloarenesLuisgarciaBerlangaAinda não há avaliações
- Aldehydes and KetonesDocumento19 páginasAldehydes and KetonesVaibhav TarkasbandAinda não há avaliações
- Haloalkanes & HaloarenesDocumento10 páginasHaloalkanes & Haloarenesakshatshukla2021Ainda não há avaliações
- Useful Reactions PDFDocumento8 páginasUseful Reactions PDFagusrimbombanteAinda não há avaliações
- Hydroxyl at I OnDocumento60 páginasHydroxyl at I OngbgbkrishnaAinda não há avaliações
- CH 18Documento32 páginasCH 18Dimas MitraAinda não há avaliações
- Orgo Reaction SheetDocumento9 páginasOrgo Reaction SheetKyle Broflovski100% (1)
- 12 Chemistry Keypoints Revision Questions Chapter 12Documento20 páginas12 Chemistry Keypoints Revision Questions Chapter 12sangam patraAinda não há avaliações
- Epoxidation & Opening PDFDocumento84 páginasEpoxidation & Opening PDFRajkumar ChinnuAinda não há avaliações
- Halo Alkanes and ArenesDocumento8 páginasHalo Alkanes and ArenesRahul SharmaAinda não há avaliações
- Reagent TableDocumento10 páginasReagent Tablebluebeary22Ainda não há avaliações
- Amines: + HCL RC O N R' H R'NH + RC O CLDocumento7 páginasAmines: + HCL RC O N R' H R'NH + RC O CLCamille AdleAinda não há avaliações
- Pdf-Haloalkanes and HaloarenesDocumento159 páginasPdf-Haloalkanes and HaloarenesOmkar Singh Shekhawat100% (2)
- PDFDocumento12 páginasPDFJhonsonAinda não há avaliações
- Alcohol SummaryDocumento3 páginasAlcohol SummarydanielmahsaAinda não há avaliações
- Chapter 5 Alkyl HalidesDocumento32 páginasChapter 5 Alkyl HalidesMohd HanafiahAinda não há avaliações
- Chap 16 Aldehydes and KetonesDocumento88 páginasChap 16 Aldehydes and KetonesAna Liza DolomandingAinda não há avaliações
- 03 Hydro (Alkanes Theory 01)Documento16 páginas03 Hydro (Alkanes Theory 01)ayushAinda não há avaliações
- Barta2001Bis (Acetylacetonato) Zinc (II)Documento2 páginasBarta2001Bis (Acetylacetonato) Zinc (II)Horatiu MoldovanAinda não há avaliações
- Xii Ch10 Haloalkane and HaloarenesDocumento4 páginasXii Ch10 Haloalkane and HaloarenesYash RajAinda não há avaliações
- Crux and Reagents of Organic ChemDocumento4 páginasCrux and Reagents of Organic ChemBILL RUSSO100% (5)
- Carbon-Carbon Bond Formation: Comprehensive Organic Synthesis 1991, Vol. 2, 99Documento31 páginasCarbon-Carbon Bond Formation: Comprehensive Organic Synthesis 1991, Vol. 2, 99mmiliyasAinda não há avaliações
- Reaction MechanismDocumento41 páginasReaction MechanismVarsha Dange88% (8)
- Carbenoids 2Documento15 páginasCarbenoids 2costea0028Ainda não há avaliações
- Day 14 PDFDocumento85 páginasDay 14 PDFAman9692Ainda não há avaliações
- Reactions of Alcohol: Oxidation ReductionDocumento18 páginasReactions of Alcohol: Oxidation ReductioncikguhafidzuddinAinda não há avaliações
- Chapter 15: Alcohols, Diols, and Thiols 15.1: Sources of Alcohols (Please Read)Documento9 páginasChapter 15: Alcohols, Diols, and Thiols 15.1: Sources of Alcohols (Please Read)Rammohan VaidyanathanAinda não há avaliações
- Alcohol, Phenols and Ethers PDFDocumento13 páginasAlcohol, Phenols and Ethers PDFRahul JaiswalAinda não há avaliações
- Microsoft PowerPoint - Aldehydes and KetonesDocumento24 páginasMicrosoft PowerPoint - Aldehydes and KetonesAR LazagaAinda não há avaliações
- Chapter 13 Compound Contaning NitrogenDocumento27 páginasChapter 13 Compound Contaning NitrogenTanvi ShahAinda não há avaliações
- Aldehyde Ketone and AcidDocumento15 páginasAldehyde Ketone and AcidAbir DuttaAinda não há avaliações
- CBSE Class 12 Chem Notes Question Bank Haloalkanes and Haloarenes PDFDocumento18 páginasCBSE Class 12 Chem Notes Question Bank Haloalkanes and Haloarenes PDFhehe11Ainda não há avaliações
- Carboxylic AcidsDocumento26 páginasCarboxylic Acidsapi-3734333100% (1)
- Organic Chemistry IIDocumento8 páginasOrganic Chemistry IIRoberto SIlvaAinda não há avaliações
- 12 Chemistry Impq CH10 Haloalkanes and Haloarenes 02Documento47 páginas12 Chemistry Impq CH10 Haloalkanes and Haloarenes 02Swaroop SurendraAinda não há avaliações
- CHEM 215 F12 Chapter 13 Notes UMICHDocumento13 páginasCHEM 215 F12 Chapter 13 Notes UMICHRoxanne IlaganAinda não há avaliações
- chm207 chp5Documento92 páginaschm207 chp5Muhd Mirza HizamiAinda não há avaliações
- Water An An Ether: AlcoholDocumento35 páginasWater An An Ether: AlcoholEshita SharmaAinda não há avaliações
- Acids, Derivatives and NitrilesDocumento23 páginasAcids, Derivatives and NitrilesLuqman HakimAinda não há avaliações
- DerivativesDocumento58 páginasDerivativesravi_balaskarAinda não há avaliações
- Halo - NotesDocumento13 páginasHalo - NotesGuestAinda não há avaliações
- CLS Aipmt 19 20 XII Che Study Package 4 Level 1 Chapter 10Documento36 páginasCLS Aipmt 19 20 XII Che Study Package 4 Level 1 Chapter 10Utkarsh KumarAinda não há avaliações
- Lec - EnolDocumento46 páginasLec - EnolZamzam Siti MultazamAinda não há avaliações
- AlcoholsDocumento16 páginasAlcoholsNirvaniAinda não há avaliações
- Organic 6 CDocumento26 páginasOrganic 6 CDr.Rajarshi PatelAinda não há avaliações
- Preparation of Alkyl Halides, R-X Reaction of Alkanes With CL & BR (F Is Too Reactive, I Is Unreactive)Documento20 páginasPreparation of Alkyl Halides, R-X Reaction of Alkanes With CL & BR (F Is Too Reactive, I Is Unreactive)davidtomyAinda não há avaliações
- CH 7 S Cbse BDocumento13 páginasCH 7 S Cbse BjsjsjskakkakoAinda não há avaliações
- Handbook of Coordination Catalysis in Organic ChemistryNo EverandHandbook of Coordination Catalysis in Organic ChemistryAinda não há avaliações
- Coordination Chemistry—XIV: Plenary Lectures Presented at the XIVth International Conference on Coordination Chemistry Held at Toronto, Canada, 22—28 June 1972No EverandCoordination Chemistry—XIV: Plenary Lectures Presented at the XIVth International Conference on Coordination Chemistry Held at Toronto, Canada, 22—28 June 1972A. B. P. LeverAinda não há avaliações
- AkosidasiDocumento10 páginasAkosidasiKrisna PamungkasAinda não há avaliações
- Reaksi Lipid Dan VitaminDocumento4 páginasReaksi Lipid Dan VitaminKrisna PamungkasAinda não há avaliações
- YyyyyDocumento3 páginasYyyyyKrisna PamungkasAinda não há avaliações
- Would The World Be Better With My PresenceDocumento1 páginaWould The World Be Better With My PresenceKrisna PamungkasAinda não há avaliações
- AkosidasiDocumento10 páginasAkosidasiKrisna PamungkasAinda não há avaliações
- Math ProjectDocumento22 páginasMath ProjectKrisna PamungkasAinda não há avaliações
- Vib Spec PaperDocumento8 páginasVib Spec PaperKrisna PamungkasAinda não há avaliações
- Math ProjectDocumento22 páginasMath ProjectKrisna PamungkasAinda não há avaliações
- A Dynamic Reaction Cell For InductivelyDocumento12 páginasA Dynamic Reaction Cell For InductivelyKrisna PamungkasAinda não há avaliações
- Bt0210 - Molecular Biology LaboratorymanualDocumento29 páginasBt0210 - Molecular Biology LaboratorymanualVeerendra Singh NagoriaAinda não há avaliações
- Hydrogen Peroxide Test in Milk: AnalyserDocumento1 páginaHydrogen Peroxide Test in Milk: AnalyserKrisna PamungkasAinda não há avaliações
- Msds NaclDocumento6 páginasMsds NaclNur Oktri Mulya DewiAinda não há avaliações
- Chapter 4 A1 Poster Example 2Documento3 páginasChapter 4 A1 Poster Example 2Krisna PamungkasAinda não há avaliações
- A Comparative Pharmacokinetic Study of Aspirin Suppositories and Aspirin Nanoparticles Loaded Suppositories 2167 065X.1000105Documento5 páginasA Comparative Pharmacokinetic Study of Aspirin Suppositories and Aspirin Nanoparticles Loaded Suppositories 2167 065X.1000105Krisna PamungkasAinda não há avaliações
- KrisnaDocumento5 páginasKrisnaKrisna PamungkasAinda não há avaliações
- Pharmaceuticals 05 01346 v2Documento26 páginasPharmaceuticals 05 01346 v2Krisna PamungkasAinda não há avaliações
- Reaksi SaliwanoffDocumento9 páginasReaksi SaliwanoffKrisna PamungkasAinda não há avaliações
- Acetanilide Green ChemDocumento4 páginasAcetanilide Green Chemconker4Ainda não há avaliações
- KrisnaDocumento5 páginasKrisnaKrisna PamungkasAinda não há avaliações
- 212 Lec Notes 31 SstudmonosacrxnsDocumento50 páginas212 Lec Notes 31 SstudmonosacrxnsKrisna PamungkasAinda não há avaliações
- Acid ExtractionDocumento18 páginasAcid ExtractionKrisna PamungkasAinda não há avaliações
- Web - Chem.ucsb - Edu - Zhang - ZLM-chapter 10-AlcoholDocumento4 páginasWeb - Chem.ucsb - Edu - Zhang - ZLM-chapter 10-AlcoholKrisna PamungkasAinda não há avaliações
- Termokimia 1Documento8 páginasTermokimia 1Krisna PamungkasAinda não há avaliações
- Lecture 3Documento110 páginasLecture 3Krisna PamungkasAinda não há avaliações
- ProQuestDocuments 2014-03-24Documento16 páginasProQuestDocuments 2014-03-24Krisna PamungkasAinda não há avaliações
- ProQuestDocuments 2014 03 24Documento15 páginasProQuestDocuments 2014 03 24Krisna PamungkasAinda não há avaliações
- Jurnal Organic IIDocumento13 páginasJurnal Organic IIKrisna PamungkasAinda não há avaliações
- Van't HoffDocumento6 páginasVan't HoffKrisna PamungkasAinda não há avaliações
- Msds TolueneDocumento6 páginasMsds Toluenenirmal_subudhi100% (1)
- CIPAC - Numbers of A.IDocumento30 páginasCIPAC - Numbers of A.IAmera MasuodAinda não há avaliações
- Antox 71 E Plus: Safety Data SheetDocumento15 páginasAntox 71 E Plus: Safety Data SheetMohamed AdelAinda não há avaliações
- Lime (Material) - WikipediaDocumento4 páginasLime (Material) - Wikipediaz zzzkzmmzAinda não há avaliações
- 2C-B Synthesis Without LAHDocumento1 página2C-B Synthesis Without LAHFermin GamboaAinda não há avaliações
- 18th GroupDocumento12 páginas18th GroupSai Sasivardhan GampaAinda não há avaliações
- Chapter 13 Excretion in Humans - 2023-2024Documento41 páginasChapter 13 Excretion in Humans - 2023-2024buhAinda não há avaliações
- Markscheme SL Paper3Documento78 páginasMarkscheme SL Paper3Boshra NouriAinda não há avaliações
- Crystic 625PA: Isophthalic Resin For Large StructuresDocumento2 páginasCrystic 625PA: Isophthalic Resin For Large StructuresEldiyar AzamatovAinda não há avaliações
- Sodium Dichromate Dihydrate PDFDocumento6 páginasSodium Dichromate Dihydrate PDFhamMhamAinda não há avaliações
- Material Safety Data SheetDocumento2 páginasMaterial Safety Data SheetWinengku PamartajatiAinda não há avaliações
- ChemicalsDocumento11 páginasChemicalsNovahus1 asiamericaAinda não há avaliações
- MSDS TolueneDocumento6 páginasMSDS TolueneSamuel AgusAinda não há avaliações
- United States Patent (19) : D. Suresh, Macedonia Robert CDocumento5 páginasUnited States Patent (19) : D. Suresh, Macedonia Robert CKhabib FirmansyahAinda não há avaliações
- Fusion-Bonded Epoxy-Coated Structural Steel H-Piles and Sheet PilingDocumento5 páginasFusion-Bonded Epoxy-Coated Structural Steel H-Piles and Sheet PilingSama UmateAinda não há avaliações
- Prokaryotic CellDocumento3 páginasProkaryotic CellWareesha BatoolAinda não há avaliações
- Class Notes Mondal IsomerismDocumento49 páginasClass Notes Mondal IsomerismDeepanshu 1459Ainda não há avaliações
- New Ecojet-P j2 MsdsDocumento15 páginasNew Ecojet-P j2 Msdsholiday fotoserviceAinda não há avaliações
- Mind Map of Organic Complete - 1Documento1 páginaMind Map of Organic Complete - 1Shalini 沙鹿 ShaluAinda não há avaliações
- Tutorial 4: UEMK 4253 Petroleum Chemistry and PetrochemicalsDocumento13 páginasTutorial 4: UEMK 4253 Petroleum Chemistry and PetrochemicalsFong Cai YingAinda não há avaliações
- SPM Clone Sijil Pelajaran Malaysia Chemistry Paper 2 - Topic: Chemical Formulae & EquationDocumento3 páginasSPM Clone Sijil Pelajaran Malaysia Chemistry Paper 2 - Topic: Chemical Formulae & EquationHamidah MarsidiAinda não há avaliações
- Alcohols and HaloalkanesDocumento30 páginasAlcohols and HaloalkanesBObAinda não há avaliações
- Cat 5 Reaction Rate InvestigationDocumento6 páginasCat 5 Reaction Rate Investigationapi-461267688Ainda não há avaliações
- Lubricants: Solid Lubrication With Mos: A ReviewDocumento35 páginasLubricants: Solid Lubrication With Mos: A ReviewRadu George MotocAinda não há avaliações
- 3rd and 4thDocumento16 páginas3rd and 4thmohsinAinda não há avaliações
- Hydrogen Peroxide Oxidation of Tertiary AminesDocumento4 páginasHydrogen Peroxide Oxidation of Tertiary AminesRadja LintangAinda não há avaliações
- Research Paper On Bottom AshDocumento8 páginasResearch Paper On Bottom Ashaflbtrnpf100% (1)
- Electron Microscopy Methods and Protocols 2nd Edition Methods in Molecular Biology Vol 369Documento626 páginasElectron Microscopy Methods and Protocols 2nd Edition Methods in Molecular Biology Vol 369mintillaAinda não há avaliações
- Non-Stoichiometric Defects: Hari Prakash Sahu Id-Mu20Mch042 MSC 2Nd SemDocumento8 páginasNon-Stoichiometric Defects: Hari Prakash Sahu Id-Mu20Mch042 MSC 2Nd SemomiiAinda não há avaliações
- Chemistry 106 Sample Questions CH 4Documento5 páginasChemistry 106 Sample Questions CH 4Khai NguyenAinda não há avaliações
- Periodic FlashCardsDocumento20 páginasPeriodic FlashCardsAnonymous BPFIMnCdAinda não há avaliações