Escolar Documentos
Profissional Documentos
Cultura Documentos
Geology 228/378 Applied & Environmental Geophysics: Induced Polarization (IP) and Nuclear Magnetic Resonance (NMR)
Enviado por
Oscar BPDescrição original:
Título original
Direitos autorais
Formatos disponíveis
Compartilhar este documento
Compartilhar ou incorporar documento
Você considera este documento útil?
Este conteúdo é inapropriado?
Denunciar este documentoDireitos autorais:
Formatos disponíveis
Geology 228/378 Applied & Environmental Geophysics: Induced Polarization (IP) and Nuclear Magnetic Resonance (NMR)
Enviado por
Oscar BPDireitos autorais:
Formatos disponíveis
Geology 228/378
Applied & Environmental Geophysics
Lecture 8
Induced Polarization (IP) and
Nuclear Magnetic Resonance (NMR)
Induced Polarization (IP) and
Nuclear Magnetic Resonance (NMR)
1. Time domain IP and Frequency domain IP
2. Membrane and electrode polarization
3. Maxwell-Wagner effect
4. Statement on IP for environmental applications
5. Magnetic spin of hydrogen nuclei
6. Surface NMR: principles and applications
Induced Polarization
Induced polarization is an electromagnetic method that uses
electrodes with time-varying currents and voltages to map the
variation of electrical permittivity (dielectric constant) in the
Earth at low frequencies. Induced polarization is observed
when a steady current through two electrodes in the Earth is
shut off: the voltage does not return to zero instantaneously,
but rather decays slowly, indicating that charge has been
stored in the rocks. This charge, which accumulates mainly at
interfaces between clay minerals, is responsible for the IP
effect. This effect can be measured in either the time domain
by observing the rate of decay of voltage, or in the frequency
domain by measuring phase shifts between sinusoidal
currents and voltages. The IP method can probe to
subsurface depths of thousands of meters.
In nature, the induced polarization (IP) effect is seen primarily with
metallic sulfides, graphite, and clays. For this reason, IP surveys
have been used extensively in mineral exploration. Recently, IP has
been applied to hazardous waste landfill and groundwater
investigations to identify clay zones. As with electrical resistivity
surveys, vertical or horizontal profiles can be generated using IP. IP
can also be used in borehole logging.
Constraints: IP cannot be done over frozen ground or asphalt
because good contact with the ground is required, like DC
resitivitgy. IP is affected by changes in surface relief and lateral
changes in resistivity. The electrode array length is about 10 times
greater than investigation depth.
detection of disseminated
metallic minerals
discrimination of clay from silt
or sand where formation DC
resistivities are similar
Method: Induced polarization is the capacitance effect,
or chargeability, exhibited by electrically conductive
materials.
Time-domain IP is done by pulsing an electric current
into the earth at one or two second intervals through
metal electrodes. Disseminated conductive minerals in
the ground will discharge the stored electrical energy
during the pulse cycle. The decay rate of the discharge
is measured by the IP receiver. The decay voltage will
be zero if there are no polarizable materials present.
Generally, both IP and resistivity measurements are
taken simultaneously during the survey. Survey depth is
determined by electrode spacing. The final report
products are similar to those of resistivity surveys.
Polarizable Ground
Current injected into the ground causes some materials to become
polarized. There are two microscopic causes of this macroscopic
effect. The phenomenon is called induced polarization, and the
physical property that is measured is often called chargeability. The
figure below illustrates the phenomenon observed. Note how the
measured potential exhibits a delayed response when ground is
chargeable. Chargeable ground may take several seconds to return
to equilibrium after it has been polarized with a current source.
Rock / Soil System
Grain
Surface
Pore-
Solution
Electrical Transport = Flow + Storage
What is the Interfacial
Polarization?
The interface between
material with different
electrical properties results
in charge accumulation
under alternating electrical
field.
Accumulated charges result
in polarization (spatially
non-uniform charge
distribution).
Complex Conductivity
Storage
Flow
E i
t
D
J
D
=
=
E J
C
=
E E i J J J
D C
* *
) ( = + = + =
Value of the complex dielectric constant
" ' i + =
is the parameter responsible for the
observed phenomena in IP measurements
Nomenclature
*
*
*
1
i = =
+
= j
*
+
= j
*
=
= =
0
'
Flow
Storage
0.01
0.012
0.014
0.016
1.E-03 1.E+00 1.E+03 1.E+06
Frequency (Hz)
'
(
S
/
m
)
1.E-05
1.E-04
1.E-03
1.E-02
1.E-03 1.E+00 1.E+03 1.E+06
Frequency (Hz)
'
'
(
S
/
m
)
1.E+00
1.E+03
1.E+06
1.E+09
1.E-03 1.E+00 1.E+03 1.E+06
Frequency (Hz)
'
Complex Conductivity
Berea Sandstone 0.01M NaCl
Flow
Storage
dc
IP
Two microsopic effects that cause
ground to be chargeable
1)Membrane polarization
2)Electrode polarization
Electrode polarization
Electrode polarization occurs when pore
space is blocked by metallic particles.
Again charges accumulate when an
electric field is applied.
The result is two electrical double layers
which add to the voltage measured at
the surface.
Membrane polarization
Membrane polarization occurs when
pore space narrows to within several
boundary layer thicknesses.
Charges accumulate when an electric
field is applied.
Result is a net charge dipole which
adds to any voltage measured at the
surface.
Measured IP data
There are four types of commonly measured IP data.
Two in the time domain.
Two in the frequency domain
If "small" chargeabilities are assumed, the linear
relationship means measured data and chargeabilities
recovered by inversion have the same units.
Therefore, all types of data can be approximated by
J = d.
Two types of time domain data
1. Dimensionless, where M = (
s
) / (
), using parameters from the
adjacent waveform diagram. This form is difficult to measure directly, but
some instruments provide induced polarization measurements in units of
mV/V by measuring the decaying portion of this curve at several
positions and normalizing these measurements by dividing by the
primary voltage (
). The result is sometimes multiplied by 100 so the
apparent chargeability can be thought of as a percent. Also, several such
measurements (perhaps 10 or more) may be combined, or recorded
individually.
2. The most commonly measured form of time domain IP is the
area under the decay curve, specified by the following equation,
using parameters specified in the figure.
dt t M
t
t
s
m
) (
1
2
1
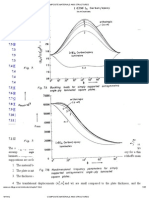
Two types of frequency domain data
1. Data with units known as percent frequency effect (PFE) require the response
to be measured at two frequencies. At higher frequencies, the ground has less
time to respond, therefore the signal is expected to be smaller. Below is the
equation providing PFE, and a figure illustrating how the data are gathered.
1
1 2
) (
a
a a
PPE
=
2. Data with units of phase are gathered by maintaining careful synchrony
between transmitted sine wave and the received signal. Then the phase
difference between the source and received signals is recorded as a
measure of chargeability. Units are usually milli-radians. The following figure
illustrates:
I P sur veys use non-pol ar i zat i on el ec t r odes
Rio Nuevo Landfill
A test was
conducted in1998 to
determine the
applicability of
geophysics for
locating buried
waste.
A series of test
boring showed high
correlation between
IP highs and waste.
A full 3D survey
was then
conducted which
detected not only
waste, but also
pockets of clay
fill.
Interpretation: some techniques & pitfalls
The following are a few comments on interpreting raw chargeability data, listed
in no particular order.
Chargeability measurements involve dynamic signals that are often 10 to 100
times smaller than signals required to obtain resistivity. Therefore results are
quite susceptible to noise of all types.
Recall that variations in pseudosections should be "smooth". Spikes or
outliers, especially at depth, are likely due to data errors. Stripes on the other
hand may be due to individual electrode placements.
Remember also that each value in the pseudosection is an apparent
chargeability, so intrinsic chargeability of the earth (i.e. the chargeability
structure) must be interpreted just as for resistivity surveys.
The effects of overburden are the same as those discussed in resistivity, but
remember that potentials measured are much smaller, so conductive
overburden is more difficult to deal with for chargeability.
Occasionally negative apparent chargeability values will be recorded. Intrinsic
chargeability can never be negative, but the apparent chargeability can be
negative.
Nuclear Magnetic Resonance (NMR)
NMR is the single geophysical tool directly
inquiring the information of the pore fluids
Fluid saturation
Fluid type
Fluid viscosity
Permeability
Proton Precession Magnetometer
A bottle of liquid (water, or other
fluid with a large number of
hydrogen nuclei) surrounded by a
suitable coil
Accuracy of 0.1 nT (nano-Tesla),
constrained by the polarization
time,
Proton Precession Magnetometer
A bottle of liquid (water, or other fluid with a large
number of hydrogen nuclei) surrounded by a suitable
coil
Accuracy of 0.1 nT (nano-Tesla, or gamma),
constrained by the polarization time,
bottle of liquid (water, or other fluid with a large number
of hydrogen nuclei) surrounded by a suitable coil
B
Proton Precession
Surface Nuclear Magnetic Resonance (NMR) Survey
Many atomic nuclei, including protons of the hydrogen atoms in water
molecules, have a magnetic moment . These nuclei can be described in
terms of a spinning charges particle. Generally speaking is aligned with the
local magnetic field B
0
of the earth. When another magnetic field is applied,
the axis of the spinning proton is deflected, owing to the torque applied.
When the second field is removed, the protons generated a relaxation
magnetic field as they become realigned along B
0
while precessing around
with the Larmor frequency:
0
= B
0,
where =0.267518 Hz/nT, the gyromagnetic ratio for hydrogen protons. In
SNMR surveys, the measurements use a circular or rectangular loop. An
alternating current with a frequency of
0
is passed through this loop for a
limited time , so that an excitation intensity (pulse moment) of q=I
0
is
achieved. After the current in the loop is shut off, a voltage is induced in the
loop by the relaxation of the protons. The initial amplitude of this induced
voltage is directly related to the water content; meanwhile, the relaxation time
T
2
is directly associated with the porosity or grain size.
The protons generated a relaxation magnetic field as they become realigned
along local Terrestrial geomagnetic field B
0
while precessing around B
0
after
the excitation current turning off, with the Larmor frequency,
0
= B
0,
= 0.267518 Hz/nT X 55000 nT = 14,713 Hz
In SNMR surveys, the measurements use a circular or rectangular loop. An
alternating current with a frequency is passed through this loop for a limited
time t, so that an excitation intensity (pulse moment) of q=I
0
is achieved.
After the current in the loop is shut off, a voltage is induced in the loop by the
relaxation of the protons. The initial amplitude of this induced voltage is
directly related to the water content; meanwhile, the relaxation time T
2
is
directly associated with the porosity or grain size.
Spin is a fundamental property of nature like electrical charge or mass.
Spin comes in multiples of 1/2 and can be + or -. Protons, electrons, and
neutrons possess spin. Individual unpaired electrons, protons, and
neutrons each possesses a spin of 1/2. In the deuterium atom (
2
H ), with
one unpaired electron, one unpaired proton, and one unpaired neutron,
the total electronic spin = 1/2 and the total nuclear spin = 1. Two or more
particles with spins having opposite signs can pair up to eliminate the
observable manifestations of spin. An example is helium. In nuclear
magnetic resonance, it is unpaired nuclear spins that are of importance.
deuterium helium
Properties of Spin
When placed in a magnetic field of strength B,
a particle with a net spin can absorb a photon,
of frequency f. The frequency f depends on the
gyromagnetic ratio, , of the particle.
f = B
For hydrogen, = 42.58 MHz / T.
Nuclei with Spin
The shell model for the nucleus tells us that nucleons, just like electrons,
fill orbitals. When the number of protons or neutrons equals 2, 8, 20, 28,
50, 82, and 126, orbitals are filled. Because nucleons have spin, just like
electrons do, their spin can pair up when the orbitals are being filled and
cancel out. Almost every element in the periodic table has an isotope with
a non zero nuclear spin. NMR can only be performed on isotopes whose
natural abundance is high enough to be detected. Some of the nuclei
routinely used in NMR are listed below.
Nuclei Unpaired Protons Unpaired Neutrons Net Spin (MHz/T)
1
H 1 0 1/2 42.58
2
H 1 1 1 6.54
31
P 1 0 1/2 17.25
23
Na 1 2 3/2 11.27
14
N 1 1 1 3.08
13
C 0 1 1/2 10.71
19
F 1 0 1/2 40.08
Energy Levels
To understand how particles with spin behave in a magnetic field, consider a
proton. This proton has the property called spin. Think of the spin of this
proton as a magnetic moment vector, causing the proton to behave like a tiny
magnet with a north and south pole.
When the proton is placed in an external magnetic field, the spin vector of the
particle aligns itself with the external field, just like a magnet would. There is a
low energy configuration or state where the poles are aligned N-S-N-S and a
high energy state N-N-S-S.
Low energy state High energy state
Transitions
This particle can undergo a transition between the two energy states by the
absorption of a photon. A particle in the lower energy state absorbs a photon
and ends up in the upper energy state. The energy of this photon must exactly
match the energy difference between the two states. The energy, E, of a
photon is related to its frequency, f , by Plank's constant (h =6.626x10-34 J s).
E = hf
In NMR and MRI, the quantity is called the resonance frequency and the
Larmor frequency.
Energy Level Diagrams
The energy of the two spin states can be represented by an energy level
diagram. We have seen that f= B and E = hf, therefore the energy of the
photon needed to cause a transition between the two spin states is
E = hB
When the energy of the photon matches the energy difference between the two
spin states an absorption of energy occurs.
In the NMR experiment, the frequency of the photon is in the radio frequency
(RF) range. In NMR spectroscopy, is between 60 and 800 MHz for hydrogen
nuclei. In clinical MRI, is typically between 15 and 80 MHz for hydrogen
imaging.
Boltzmann Statistics
When a group of spins is placed in a magnetic field, each spin aligns in one of the
two possible orientations.
At room temperature, the number of spins in the lower energy level, N+, slightly
outnumbers the number in the upper level, N-. Boltzmann statistics tells us that
N-/N+ = e
-E/kT
.
E is the energy difference between the spin states; k is Boltzmann's constant,
1.3805x10-23 J /Kelvin; and T is the temperature in Kelvin.
As the temperature decreases, so does the ratio N- /N+. As the temperature
increases, the ratio approaches one.
Summary: IP and NMR
Both need more physical insight and
high demand of skills
High potential
Surface NMR is still in infancy
NMR holding the promise for
environmental & groundwater research
Review Points:
1, SI unit system:
base units and derived units;
2, Site investigation:
purpose and approaches;
3, Material properties:
Mechanic, electric, and electromagnetic;
4, Seismic refraction (G228):
how to get 2-layer velocity structure (v1, v2, and layer thickness);
5, The Maxwell Equations (G278/378):
constitutive relations, governing equations, and Helmholtz eq.
6, DC resistivity:
different type of arrays and their K-factor;
7, Geomagnetism:
decomposition of the local geomagnetic vector;
features of near-surface anomalies caused by local sources;
8, IP and NMR:
types of IP measurements, chargeability, NMR Larmor frequency.
Você também pode gostar
- Induced Polarisation ThesisDocumento8 páginasInduced Polarisation Thesistjgyhvjef100% (1)
- Applied GeophysicsDocumento192 páginasApplied GeophysicsWorldbest ManyangAinda não há avaliações
- GPH 231Documento113 páginasGPH 231Adwaitesh Aaradhya100% (2)
- Applied Geophysics For The Hydrocarbon Exploration: Electrical and Electromagnetic MethodsDocumento26 páginasApplied Geophysics For The Hydrocarbon Exploration: Electrical and Electromagnetic MethodsbrunoAinda não há avaliações
- GPH 321-Survey DesignDocumento127 páginasGPH 321-Survey DesignAdarta MuhAinda não há avaliações
- Electrical Resisitivity and EM MethodsDocumento117 páginasElectrical Resisitivity and EM MethodsAbdul BasitAinda não há avaliações
- Induced-Polarisation Method in Mineral Exploration: Presented byDocumento19 páginasInduced-Polarisation Method in Mineral Exploration: Presented bySatish ChennamsettiAinda não há avaliações
- Electrode PolarizationDocumento66 páginasElectrode PolarizationasmitaAinda não há avaliações
- Lecture 5 Induced Polarization MethodDocumento19 páginasLecture 5 Induced Polarization MethodSiyad AbdulrahmanAinda não há avaliações
- 061cardimona Resistivity OverviewDocumento10 páginas061cardimona Resistivity OverviewgoomeyAinda não há avaliações
- Lecture-12 - Introduction & Theoritical Background - Electrical MethodDocumento22 páginasLecture-12 - Introduction & Theoritical Background - Electrical MethodNamwangala Rashid NatinduAinda não há avaliações
- Geophysics (Electric Method)Documento79 páginasGeophysics (Electric Method)Vaqas Ali Khan100% (1)
- 6 IP MethodDocumento21 páginas6 IP Method045 Johannes KevinAinda não há avaliações
- Geophysical Investigation: Electrical MethodsDocumento25 páginasGeophysical Investigation: Electrical Methodsghassen laouiniAinda não há avaliações
- Geo-Resistivity & IP Survey DiscussionDocumento4 páginasGeo-Resistivity & IP Survey DiscussionCris SosaAinda não há avaliações
- Elec Resist Lec Note 2018Documento20 páginasElec Resist Lec Note 2018Wubayehu DessalegnAinda não há avaliações
- Geofisika Hidrogeologi: Hidrologi Dan Pemetaan Geologi Pemboran IntiDocumento10 páginasGeofisika Hidrogeologi: Hidrologi Dan Pemetaan Geologi Pemboran IntialingAinda não há avaliações
- A Comparison of Electrodes in IP ArraysDocumento17 páginasA Comparison of Electrodes in IP ArraysLuis Alonso SAAinda não há avaliações
- Hydro GeophysicsDocumento50 páginasHydro GeophysicsKirti SaharanAinda não há avaliações
- Fam 1995bDocumento8 páginasFam 1995bAline RozaAinda não há avaliações
- Measurement and Application of Zeta-Potential: Branko SALOPEK, Dragan and Suzana FILIPOVIDocumento5 páginasMeasurement and Application of Zeta-Potential: Branko SALOPEK, Dragan and Suzana FILIPOVIAmber ClarkAinda não há avaliações
- Measurement and Application of Zeta-Potential: Branko SALOPEK, Dragan and Suzana FILIPOVIDocumento5 páginasMeasurement and Application of Zeta-Potential: Branko SALOPEK, Dragan and Suzana FILIPOVIgusuchihaAinda não há avaliações
- 2018-GE-27 Geophysics LabDocumento15 páginas2018-GE-27 Geophysics LabMuhammad Saad GorayaAinda não há avaliações
- A Fault Diagnosis Method For Substation Grounding Grid Based On The Square-Wave Frequency Domain ModelDocumento9 páginasA Fault Diagnosis Method For Substation Grounding Grid Based On The Square-Wave Frequency Domain Modelostojic007Ainda não há avaliações
- Interpretation of Schlumberger and Magnetotelluric Measurements: Examples From The Philippines and IcelandDocumento26 páginasInterpretation of Schlumberger and Magnetotelluric Measurements: Examples From The Philippines and IcelandGian Angga PratamaAinda não há avaliações
- Ves Bayelsa SHMBRG MethDocumento9 páginasVes Bayelsa SHMBRG MethWadadiAinda não há avaliações
- 3 Lagmanson PDFDocumento41 páginas3 Lagmanson PDFMiguel de OliveiraAinda não há avaliações
- Presentation DielectricDocumento25 páginasPresentation Dielectricjose mirandaAinda não há avaliações
- Presentation On Geophysical MethodsDocumento8 páginasPresentation On Geophysical MethodsZanuraAinda não há avaliações
- Lecture-12 - Introduction & Theoritical Background - Electrical MethodDocumento22 páginasLecture-12 - Introduction & Theoritical Background - Electrical MethodIkhsan Parinduri100% (1)
- Methodes: GeophysicsDocumento11 páginasMethodes: GeophysicsWayan TrimawiasaAinda não há avaliações
- Ultrasonic Sensor For Level MeasurementDocumento34 páginasUltrasonic Sensor For Level MeasurementAshish RawatAinda não há avaliações
- Lecture-12 - Introduction & Theoritical - Electrical MethodDocumento22 páginasLecture-12 - Introduction & Theoritical - Electrical MethodEzzadin Baban75% (4)
- Configuration and Specification of Equipments Used in DC Resistivity SurveyDocumento43 páginasConfiguration and Specification of Equipments Used in DC Resistivity SurveyAditya Kumar AnandAinda não há avaliações
- The Hydroelectric Problem of Porous Rocks: Thermodynamic Approach and Introduction of A Percolation ThresholdDocumento6 páginasThe Hydroelectric Problem of Porous Rocks: Thermodynamic Approach and Introduction of A Percolation ThresholdRaulia RenoAinda não há avaliações
- Voltammetry at A Microdisk ElectrodeDocumento16 páginasVoltammetry at A Microdisk ElectrodeFelipe Cepeda SilvaAinda não há avaliações
- Electrostatic Accelerated Electrons Within Information Horizons Exert Bidirectional Propellant-Less ThrustDocumento34 páginasElectrostatic Accelerated Electrons Within Information Horizons Exert Bidirectional Propellant-Less ThrustGherghe BogdanAinda não há avaliações
- Introduction To Induced Polarization SurveyingDocumento17 páginasIntroduction To Induced Polarization Surveyingrojara2008Ainda não há avaliações
- Notes Geophysical MethodsDocumento16 páginasNotes Geophysical MethodsApocalyptic TsunamiAinda não há avaliações
- AC ResistivityDocumento6 páginasAC ResistivityJulio TedescoAinda não há avaliações
- MethodologyDocumento10 páginasMethodologyola pedensAinda não há avaliações
- Prashant Edc NotesDocumento141 páginasPrashant Edc NotesAnurag ChughAinda não há avaliações
- Geophysical Field Theory and Method, Part B: Electromagnetic Fields INo EverandGeophysical Field Theory and Method, Part B: Electromagnetic Fields IAinda não há avaliações
- Archaeology TechniquesDocumento7 páginasArchaeology TechniquesvanpatoAinda não há avaliações
- Active Method "Electrical Resistivity" and "D.C Resistivity" Are Used Synonymously Measure Earth ResistiviDocumento26 páginasActive Method "Electrical Resistivity" and "D.C Resistivity" Are Used Synonymously Measure Earth ResistiviZeeshan ahmed100% (1)
- Geophysics - Intro To Resistivity SurveyDocumento9 páginasGeophysics - Intro To Resistivity SurveyMuhammad RashidAinda não há avaliações
- Self Potential MethodDocumento8 páginasSelf Potential MethodnamilaAinda não há avaliações
- Electromagnetic Method Assignment PresentationDocumento23 páginasElectromagnetic Method Assignment PresentationAndrew GwazaAinda não há avaliações
- Fundamentals of Electrochemistry: CHEM 7234 CHEM 720Documento56 páginasFundamentals of Electrochemistry: CHEM 7234 CHEM 720Marcelo CalegaroAinda não há avaliações
- Kamlah-Etal Spie2001 4333Documento7 páginasKamlah-Etal Spie2001 4333Mani PrakashAinda não há avaliações
- Field Practicals of Geo-Physical ExplorationDocumento37 páginasField Practicals of Geo-Physical ExplorationAdnan AliAinda não há avaliações
- Geophysics Exploration - Chapter 6Documento43 páginasGeophysics Exploration - Chapter 6Abraham SileshiAinda não há avaliações
- Applied PV Engineering - Lecture 3&4 NewDocumento40 páginasApplied PV Engineering - Lecture 3&4 Newnasir siyarAinda não há avaliações
- MRS: A New Geophysical Technique For Groundwater Work: Hydrogeophysics Hydrogeophysics SPECIALSECTION: HydrogeophysicsDocumento6 páginasMRS: A New Geophysical Technique For Groundwater Work: Hydrogeophysics Hydrogeophysics SPECIALSECTION: Hydrogeophysicsjaspalsingh3237Ainda não há avaliações
- Engineering Economics by J Tiong PDFDocumento31 páginasEngineering Economics by J Tiong PDFRainier RamosAinda não há avaliações
- Measurement and Application of Zeta PotentialDocumento5 páginasMeasurement and Application of Zeta PotentialSiêu Nhân Kem XôiAinda não há avaliações
- Subject Name: Electromagnetic Theory Subject Code: Ee 2202 Branch: Eee Semester: Iii Unit - Iii ContentsDocumento19 páginasSubject Name: Electromagnetic Theory Subject Code: Ee 2202 Branch: Eee Semester: Iii Unit - Iii ContentsShafiq RahmanAinda não há avaliações
- Electrical ResistivityDocumento58 páginasElectrical Resistivityعبد الرحمن القيسيAinda não há avaliações
- Electrical Resistivity Tests On RocksDocumento29 páginasElectrical Resistivity Tests On Rocksanshu832Ainda não há avaliações
- Principles of Electric Methods in Surface and Borehole GeophysicsNo EverandPrinciples of Electric Methods in Surface and Borehole GeophysicsNota: 3 de 5 estrelas3/5 (1)
- Springs TextDocumento0 páginaSprings Texter_wenAinda não há avaliações
- ME 012 Engineering Dynamics: Rectilinear Kinematics: Erratic Motion (Chapter 12, Section3)Documento16 páginasME 012 Engineering Dynamics: Rectilinear Kinematics: Erratic Motion (Chapter 12, Section3)masijaAinda não há avaliações
- COMBIN7 (LegacyElement)Documento7 páginasCOMBIN7 (LegacyElement)Feby Philip AbrahamAinda não há avaliações
- A New Multi-Axial Failure Criterion For Concrete: M. Como R. LucianoDocumento7 páginasA New Multi-Axial Failure Criterion For Concrete: M. Como R. LucianoRico PadillaAinda não há avaliações
- SX022a-En-EU-Example - Calculation of Effective Section Properties For A Cold-Formed Lipped Channel Section in BendingDocumento10 páginasSX022a-En-EU-Example - Calculation of Effective Section Properties For A Cold-Formed Lipped Channel Section in BendingWAinda não há avaliações
- Ed 9222 June 2011 AutDocumento4 páginasEd 9222 June 2011 AutAshokAinda não há avaliações
- High Tensile C Purlin: Steel Grade Equivalent To ASTM 446 Grade DDocumento2 páginasHigh Tensile C Purlin: Steel Grade Equivalent To ASTM 446 Grade DRodolfo ZazuetaAinda não há avaliações
- 356184Documento316 páginas356184alfonxxlAinda não há avaliações
- Module IDocumento27 páginasModule IManikandan SriramAinda não há avaliações
- Different Magnetic Interaction MechanismsDocumento5 páginasDifferent Magnetic Interaction MechanismsJoshuaAinda não há avaliações
- Buckling of Thin PlatesDocumento14 páginasBuckling of Thin PlatesOSCARDELTAAinda não há avaliações
- Strength of MaterialsDocumento264 páginasStrength of MaterialsDenisa TodoracheAinda não há avaliações
- Applied Thermal Engineering: SciencedirectDocumento11 páginasApplied Thermal Engineering: SciencedirectDedi AfandiAinda não há avaliações
- Fluid Mechanics PDFDocumento39 páginasFluid Mechanics PDFAgustin Jr., Reynold P.Ainda não há avaliações
- @StudyTime - Channel 12 - Sound (Ex) PDFDocumento10 páginas@StudyTime - Channel 12 - Sound (Ex) PDFSipra PaulAinda não há avaliações
- Final Report of Fire Pump Sets Selection - Sinha Knit Industries Ltd.Documento19 páginasFinal Report of Fire Pump Sets Selection - Sinha Knit Industries Ltd.Musfiqul AzadAinda não há avaliações
- 4403 Course Outline 2017 Without ColourDocumento3 páginas4403 Course Outline 2017 Without ColourirqoviAinda não há avaliações
- Numerical Study of An Asymmetrically Heated Rectangular Duct With Suspended CylindersDocumento7 páginasNumerical Study of An Asymmetrically Heated Rectangular Duct With Suspended CylindersGauravAinda não há avaliações
- Bearden On Maxwell's EquationsDocumento10 páginasBearden On Maxwell's Equationspaulsub63Ainda não há avaliações
- Gravimetry Is The Measurement of The Strength of A Gravitational FieldDocumento30 páginasGravimetry Is The Measurement of The Strength of A Gravitational FieldMahaManthraAinda não há avaliações
- Olp Igcse Mechanics CT 4 - New - Batch 1Documento5 páginasOlp Igcse Mechanics CT 4 - New - Batch 1Shabbir H. KhanAinda não há avaliações
- Learning Activity Sheet (Las) No. 1Documento4 páginasLearning Activity Sheet (Las) No. 1warren bascon100% (1)
- A21VGDocumento32 páginasA21VGcunvip163.comAinda não há avaliações
- Thermodynamics 2 (TRDMIA2)Documento24 páginasThermodynamics 2 (TRDMIA2)Njabulo MdlaloseAinda não há avaliações
- Circular Column Design Based On ACI 318-19: Input Data & Design SummaryDocumento5 páginasCircular Column Design Based On ACI 318-19: Input Data & Design SummaryJEEVENDRANAinda não há avaliações
- Composite Materials and StructuresDocumento35 páginasComposite Materials and StructuresGundoju SreenivasAinda não há avaliações
- Final ExamDocumento5 páginasFinal ExamNarcisa RudnicAinda não há avaliações
- Tips and Tricks From Joe Flow: Sample Preparation: Shaken or Stirred?Documento3 páginasTips and Tricks From Joe Flow: Sample Preparation: Shaken or Stirred?Dan MihailAinda não há avaliações
- Fluid Mechanics by S K MondalDocumento179 páginasFluid Mechanics by S K MondalAghu AkzAinda não há avaliações