Escolar Documentos
Profissional Documentos
Cultura Documentos
Apch231 Edta
Enviado por
Tan Ze KaiDireitos autorais
Formatos disponíveis
Compartilhar este documento
Compartilhar ou incorporar documento
Você considera este documento útil?
Este conteúdo é inapropriado?
Denunciar este documentoDireitos autorais:
Formatos disponíveis
Apch231 Edta
Enviado por
Tan Ze KaiDireitos autorais:
Formatos disponíveis
APCH231: EDTA
Notes complied by Dr. C. Southway
30
APCH231: Chemical Analysis
Complexometric Titrations
EDTA
DTA forms stable complexes with most metal ions the exceptions being the
group 1 cations. The stability of the complexes can be attributed to the chelate
effect; when complexing a metal ion EDTA surrounds the metal cation, forming a
number of five-membered rings. Although ethylenediaminetetraacetic acid is already
shortened to EDTA, analytical chemist, shorten it even further to "Y".
Structure of EDTA
In a complexometric titration the analyte and titrant form a complex ion, and the
equilibrium constant is called the formation constant, Kf. Chelating (multidentate)
ligands form more stable complexes than do monodentate ligands, because the
entropy of complex formation favours the binding of one large ligand rather than
small ligands. Synthetic aminocarboyxlic acids such as EDTA have large
metal binding constants and are widely used in analytical chemistry.
E
Metal cation
APCH231: EDTA
Notes complied by Dr. C. Southway
31
Here are some stability constants for the formation of metal-EDTA complexes:
Metal ion K
MY
Metal ion K
MY
Mg
2+
4.9x10
8
Ni
2+
4.2x10
18
Ba
2+
5.8x10
7
Cu
2+
6.3x10
18
Ca
2+
5.0x10
10
Zn
2+
3.2x10
16
Mn
2+
6.2x10
13
Pb
2+
1.1x10
18
Questions
1. What reaction will occur if a solution containing Pb
2+
is added to a solution
containing MgY
2-
?
2. What reaction will occur if a solution containing Ba
2+
is added to a solution
containing CuY
2-
?
EDTA is the most widely used complexing agent for routine analysis of water
hardness and other applications. EDTA is a multidentate ligand that is represented
by the formula H
4
Y. Usually, EDTA titrations are conducted in alkaline
conditions under which EDTA will be present in different forms including H
4
Y,
H
3
Y
-
, H
2
Y
2-
, HY
3-
, and Y
4-
. Therefore, controlling the pH is one major factor that
affects complexation.
For EDTA to react with a metal ion, the hydrogens attached to the carboxylate
groups must be removed. In strongly basic solution, these hydrogens are removed
by reaction with hydroxide ion. In more acidic solutions, metal ions must be able
to displace the hydrogens if a complex is to be formed. Since metal ions differ
significantly in their ability to displace the hydrogens, the solution acidity can be
used to regulate the reactivity of EDTA toward metal ions. For example, most
metal ions react quantitatively with a stoichiometric amount of EDTA at pH 10,
but only a few, such as Fe
3+
and Hg
2+
, also react quantitatively at pH 2.
APCH231: EDTA
Notes complied by Dr. C. Southway
32
Effect of pH on the composition of EDTA
The strength and stability of EDTA complexes is pH dependent.
The total concentration of EDTA will be the sum of all equilibrium species
C
T
= [H
4
Y] + [H
3
Y
-
] + [H
2
Y
2-
] + [HY
3-
] + [Y
4-
]
where C
T
is the total molar concentration of uncomplexed EDTA.
The fraction of any species can be found if desired where:
o
= [H
4
Y]/C
T
1
= [H
3
Y
-
]/C
T
2
= [H
2
Y
2-]/C
T
3
= [HY
3-
]/C
T
4
= [Y
4-
]/C
T
The values may be estimated from a graph or calculated.
The value of
Y4-
depends only on the concentration of H
3
O
+
and the acid
dissociation constants for EDTA.
Now, assume a divalent metal reacts with EDTA, Y
4-
, the following equilibrium
will be observed in alkaline medium:
M
2+
+ Y
4-
MY
2-
Kf = [MY
2-
]
[M
2+
] [Y
4-
]
Y
4-
concentration is dependent on pH and, therefore, should be calculated as
follows:
4 =
K
1
K
2
K
3
K
4
[H
3
O
+
]
4
+K
1
[H
3
O
+
]
3
+K
1
K
2
[H
3
O
+
]
2
+k
1
K
2
K
3
[H
3
O
+
]+K
1
K
2
K
3
K
4
[Y
4-
] =
4
C
T
APCH231: EDTA
Notes complied by Dr. C. Southway
33
eing a weak acid, EDTA dissociates in solution and it is the unprotonated form
abbreviated as Y
4-
that is the species found in the complex. The fraction (
Y
)
of the total EDTA that is present as Y
4-
depends on the pH of the solution; as shown
in the table below.
pH
Y
pH
Y
1 1.9x10
-18
7 5.0x10
-4
2 3.3x10
-14
8 5.6x10
-3
3 2.6x10
-11
9 5.4x10
-2
4 3.8x10
-9
10 0.36
5 3.7x10
-7
11 0.85
6 2.3x10
-5
12 0.98
Note that Y
4-
is present at very low concentrations in acidic solution and does not
become the predominant species until the pH exceeds a value of 10.
We can make use of these -values in calculating conditional stability constants for
metal-EDTA complexes, which take into account the pH of the solution in which the
complex is formed. The stability constant, K
MY
, for the formation of the metal-EDTA
complex:
M
n+
+ Y
4-
MY
(n-4)+
is given by:
] ][ [
] [
4
) 4 (
+
+
=
Y M
MY
K
n
n
MY
Since [Y
4-
] =
4
x C
T
, then we can write:
T
n
n
MY
C M
MY
K
4
) 4 (
] [
] [
+
+
=
or:
MY
T
n
n
MY
K
C M
MY
K = =
+
+
] [
] [
) 4 (
4
where
MY
K is the conditional stability constant. It is this conditional stability constant
that should be used when considering equilibria related to EDTA.
B
I dont expect you to
do this. I am just
putting it here so that
you can see that it can
be done.
It is simply another
equilibrium expression.
APCH231: EDTA
Notes complied by Dr. C. Southway
34
Question
3. Calculate the fraction of EDTA present as Y
4-
in solution at the following pH:
a) pH 8. 0
b) pH 11.0.
Preparation of an EDTA Standard Solution
Primary standards of EDTA can not be prepared. EDTA solutions should be
standardized against ZnSO
4
or MgSO
4
of very high purity. Water used in EDTA
solution preparations should be free from polyvalent metal ions and preferably
distilled through all Pyrex glass. Calmagite is a suitable indicator. The titration is
conducted at a buffered solution at about pH 10.
Another important note concerns storage of standardized EDTA solutions where
these solutions should never be stored in soda based glass. Preferably,
polyethlene bottles should always be used.
Calculations Involved in EDTA Titrations
Titrimetric methods based on EDTA are simple and provide excellent results.
Usually, EDTA is the titrant except in case of a back titration. The sample
containing the metal ion to be determined is placed in a flask together with a
suitable indicator.
The relationship
M
1
V
1
(EDTA) = M
2
V
2
(metal ion)
can be used for calculation of the metal ion concentration.
It is a common practice to express the concentration of the metal ion in ppm units
where
1 ppm = 1 mg
L
since this is the unit, which is used by analytical laboratories for comparative
purposes.
The number of mgs of the metal ion present in 1 L of the solution gives the ppm
value.
APCH231: EDTA
Notes complied by Dr. C. Southway
35
Question
4. A 100.0 mL aliquot of city drinking water was treated with a small amount of
an ammonia ammonium chloride buffer to bring the pH to 10.
After the addition of Calmagite indicator the solution required 21.46 mL of
5.140 10
-3
M EDTA for titration. Calculate the hardness in terms of parts per
million calcium carbonate.
uring a titration with EDTA, the metal ion solution is in the flask and EDTA is
added from the burette. At any point in the titration we can calculate the value
of pM (= -log[M
n+
]). For example, consider the titration of 50.0 ml of 0.0050 M Ca
2+
solution with 0.0100 M EDTA at pH = 10.
Before the addition of EDTA:
Before any EDTA has been added, [Ca
2+
] = 0.0050 M and pCa = -log0.0050 = 2.30
After the addition of 10.0 ml EDTA:
Some of the Ca
2+
will have reacted with the EDTA and we need to find the new
[Ca
2+
]
Ca
2+
+ Y
4-
CaY
2-
Start 2.50x10
-4
mol 1.00x10
-4
mol
React 1.00x10
-4
mol 1.00x10
-4
mol
Form 1.00x10
-4
mol
Final 1.50x10
-4
mol 0 1.00x10
-4
mol
The new volume is 50.0 + 10.0 = 60.0 ml and so [Ca
2+
] = 0.0025 M and pCa = 2.6.
At the equivalence point
The equivalence point occurs after 2.50x10
-4
mol of EDTA has been added, which
corresponds to a volume of 25.00 ml.
Ca
2+
+ Y
4-
CaY
2-
Start 2.50x10
-4
mol 2.50x10
-4
mol
React 2.50x10
-4
mol 2.50x10
-4
mol
Form 2.50x10
-4
mol
Final 0 0 2.50x10
-4
mol
D
APCH231: EDTA
Notes complied by Dr. C. Southway
36
How can we calculate the [Ca
2+
]? All the Ca
2+
has gone We have to remember
that the reaction is actually an equilibrium and some (very small amount) of the CaY
2-
will dissociate to give some Ca
2+
and we can find [Ca
2+
] by using:
T
CaY
C Ca
CaY
K
] [
] [
2
2
+
=
The only Ca
2+
in the solution will have come from the dissociation of the CaY
2-
and
this will also be the only source of uncomplexed EDTA; therefore:
[Ca
2+
] = C
T
At pH 10,
Y
= 0.36 and
CaY
K = 0.36 x 5.0x10
10
= 1.8x10
10
, so very little of the CaY
2-
will actually have dissociated and we have:
[CaY
2_
]
3 3
3
4
10 3 . 3
1
1000
0 . 75
10 50 . 2
= = dm mol x
dm
ml
x
ml
mol x
and
2 2
3
2
3
10
] [
10 3 . 3
] [
10 3 . 3
10 8 . 1
+
= =
Ca
x
C Ca
x
x
T
Then we can find:
[Ca
2+
] = 4.3x10
-7
mol dm
-3
and pCa = 6.37
After the equivalence point
After the equivalence point, all the Ca
2+
has been reacted and the only source of Ca
2+
is again the dissociation of the CaY
2-
complex. This time, however, we have excess
EDTA in the solution, and we can determine C
EDTA
and [CaY
2-
] from the
stoichiometric quantities. For example, after the addition of 50.0 ml of EDTA
solution:
Ca
2+
+ Y
4-
CaY
2-
Start 2.50x10
-4
mol 5.00x10
-4
mol
React 2.50x10
-4
mol 2.50x10
-4
mol
Form 2.50x10
-4
mol
Final 0 2.50x10
-4
mol 2.50x10
-4
mol
The total volume is 50.0 ml + 50.0 ml = 100.0 ml and we have:
3 3
3
4
10 50 2
1
1000
0 100
10 50 2
= = dm mol x .
dm
ml
x
ml .
mol x .
C
EDTA
APCH231: EDTA
Notes complied by Dr. C. Southway
37
3 3
3
4
2
10 50 2
1
1000
0 100
10 50 2
= = dm mol x .
dm
ml
x
ml .
mol x .
] CaY [
Since C
T
~ C
EDTA
, we can use:
EDTA
CaY
C Ca
CaY
K
] [
] [
2
2
+
=
to find:
[Ca
2+
] = 5.6x10
-11
mol dm
-3
and pCa = 10.26
Questions
5. A standard solution of EDTA (0.100 M) is being used to titrate 25.00 ml of a
0.100 M Zn
2+
solution (buffered at pH 8). Calculate the value of pZn after the
addition of:
a) 0 ml of EDTA solution.
b) 10.00 ml of EDTA solution
c) 25.00 ml of EDTA solution
d) 50.00 ml of EDTA solution.
6. For your answer to Q 5c) you assumed that [Zn
2+
] = C
EDTA
. Why are you justified in
making that assumption?
sing similar calculations to those shown above, it is possible to plot titration
curves under different conditions. For example, at different pH values or for
complexes with different stability constants. The effect of pH or value of stability
constant is only seen at and after the equivalence point before the equivalence point,
the value of
MY
K is not used in calculating pM because there is excess M
n+
and the
dissociation of the M-EDTA complex is unimportant. We can also predict how the
shape of the titration curve is affected by the concentrations of the reagents. As might
be expected, titrations are not very successful if the concentrations are too low the
steep part of the curve is smaller with lower concentrations.
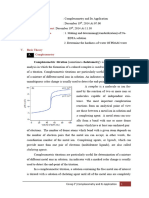
Questions
7. Look at the two titration curves (0.050 M Ca
2+
titrated with 0.050 M EDTA).
a) Which curve was obtained with pH = 10 and which with pH = 8?
U
APCH231: EDTA
Notes complied by Dr. C. Southway
38
b) What volume of Ca
2+
solution was used?
Titration Curve A
0.00
2.00
4.00
6.00
8.00
10.00
12.00
0 5 10 15 20 25 30 35 40 45 50
Vol EDTA added ( ml)
Titration Curve B
0.00
2.00
4.00
6.00
8.00
10.00
12.00
0 5 10 15 20 25 30 35 40 45 50
Vol EDTA added ( ml)
8. Look at the following two curves, for the titration of 25.00 ml of Pb
2+
solution
with EDTA at pH 6. One of the curves is for the titration of 0.050 M Pb
2+
with
0.050 M EDTA, the other is for 0.0005 M Pb
2+
with 0.0005 EDTA. Which is
which? Explain briefly.
Titration Curve C
0.00
5.00
10.00
15.00
0 5 10 15 20 25 30 35 40 45 50
Vol EDTA added ( ml)
Titration Curve D
0.00
5.00
10.00
15.00
0 5 10 15 20 25 30 35 40 45 50
Vol EDTA added ( ml)
9. For some titrations with EDTA, it is necessary to use a NH
3
/NH
4
+
buffer.
However, the NH
3
can form complexes with many metals for example, Zn
2+
.
a) Would you expect the presence of NH
3
to have any effect on the value
of pZn? Briefly explain.
b) Would you expect the presence of NH
3
to affect the shape of the
titration curve? Briefly explain.
Indicators for EDTA Titrations
he endpoint for an EDTA titration is usually found by using a metallochromic
indicator this is a dye that can act as a complexing agent to complex the metal
being titrated. The colour of the indicator depends on whether it is attached to the
metal ion or free. At the beginning of a titration, some of the analyte metal ion is
complexed by the indicator.
T
p
Z
n
p
Z
n
APCH231: EDTA
Notes complied by Dr. C. Southway
39
M
n+
+ In
n-
M-In
As EDTA is added, the free metal ion concentration decreases but some metal ion
remains complexed by the indicator. If the correct indicator and conditions are
chosen, at the equivalence point the EDTA has complexed all of the free metal ion
and then removes the metal from the indicator complex (charges are removed for
simplicity):
MIn + EDTA MEDTA + In
Where MIn and In have different colours.
Among the very important indicators that are routinely used, you will encounter
two indicators namely Muroxide and Eriochrome Black T (solochrome Black).
However, there are many other indicators some of them are very important
because of their high selectivity towards specific metal ions.
Sharpness of the End Point
The end point is affected by two major factors, the stability constant of the metal
ion-EDTA complex and the concentrations of the metal ion and EDTA. As the
value of both factors is increased, sharper end points are achieved. If either of the
two factors has a low value, end points of less sharpness result. For very low
stability constants diffuse end points are observed and large errors should be
expected.
In order to get sharp endpoints, solutions are typically buffered at basic conditions,
i.e. higher pHs are desirable for sharp endpoints and formation of metal-EDTA
complexes.
Question
10. What would happen if the metal-indicator complex was so stable that the EDTA
could not remove the metal from the complex?
here are several strategies for successful titrations using EDTA. In a direct
titration the analyte is titrated with a standard solution of EDTA, using a suitable
indicator. EDTA forms stable complexes with all metal cations except those in Group
1 of the Periodic Table. If a mixture of metal ions is to be titrated, it may be possible
to determine each one individually by selecting the titration conditions carefully. For
T
APCH231: EDTA
Notes complied by Dr. C. Southway
40
example, by adjusting the pH or by adding a masking agent which protects one of the
metal ions from reacting with EDTA.
Questions
11. You need to determine the concentrations of Mg
2+
and Zn
2+
in a mixture.
a) Calculate the values of the conditional stability constants for MgY
2-
and ZnY
2-
at pH 10.
b) Calculate the values of the conditional stability constants for MgY
2-
and ZnY
2-
at pH 4.
c) A 25.00 ml aliquot of a mixture containing Mg
2+
and Zn
2+
is buffered
at pH 10 and titrated with 0.04875 M EDTA. The endpoint volume is
34.27 ml. A second 25.00 ml aliquot of the Mg/Zn mixture is buffered
at pH 4 and titrated with the same EDTA solution. This time the
endpoint volume is 22.56 ml. Calculate the molar concentrations of
Mg
2+
and Zn
2+
.
12. A 25.00 ml aliquot of a solution containing Cu
2+
and Fe
3
was titrated with 16.06
ml of 0.05083 M EDTA. A second 25.00 ml aliquot of the Cu/Fe mixture was
treated with NaF to form a stable iron-fluoride complex. This mixture was then
titrated with 0.05083 M EDTA and the endpoint volume was found to be 5.43 ml.
Calculate the molar concentrations of Cu
2+
and Fe
3+
.
13. A 50.00 ml aliquot of a solution containing Ca
2+
and Mg
2+
was buffered at pH 10
and titrated with 0.04865 M EDTA. The endpoint volume was 44.27 ml. A
second aliquot of the same mixture was made stongly basic by the addition of
NaOH this causes the Mg
2
to precipitate as Mg(OH)
2
. The solution was then
titrated with the 0.04865 M EDTA and the endpoint volume was found to be
34.26 ml. Calculate the molar concentrations of Ca
2+
and Mg
2+
.
ometimes a direct titration is not possible perhaps because a suitable indicator is
not available or because the analyte does not react with EDTA or does not react
quickly enough. In these cases, other strategies may be used. Sometimes a back
titration can be used if a suitable indicator is not available. A known quantity of
EDTA is added to the sample so that all of the analyte reacts with the EDTA and
some EDTA is left over. The amount of EDTA left over is then determined by
S
APCH231: EDTA
Notes complied by Dr. C. Southway
41
titration with (for example) a standard solution of Mg
2+
. It is important that the metal
used to back-titrate the EDTA does not form a more stable EDTA complex than the
analyte does.
Question
14. A 25.00 ml sample containing Pb
2+
was treated with 10.00 ml of 0.0367 M EDTA.
The excess EDTA was then back-titrated with 0.0461 M Mg
2+
solution; 2.37 ml
was required.
a) What was the concentration of the Pb
2+
in the original solution?
b) Would the procedure have worked if Cu
2+
had been used to back-titrate
the excess EDTA?
15. A 25.00 mL aliquot of a solution containing Hg
2+
in dilute nitric acid was treated
with 10.00 mL of 0.04882 MEDTA and the pH was adjusted to 10.0 with an
ammonia buffer. Two drops of a freshly prepared EBT indicator solution were
added and the excess EDTA back titrated with 0.01137 M Mg
2+
, requiring
24.66 mL to reach the endpoint. What is the molarity of Hg
2+
in the sample?
EDTA can also be used in indirect titrations the EDTA does not directly react with
the analyte, but reacts with some metal that can be related stoichiometrically to the
analyte.
Question
16. Sulfide ion was determined indirectly by titration with EDTA. A 25.00 ml
aliquot of a solution containing sulfide ions was added to 25.00 ml of a Cu
2+
solution. The CuS precipitate was filtered and washed with water. The pH of the
combined filtrate and washings was then adjusted by the addition of ammonia and
the solution was titrated with 0.04332 M EDTA, using a suitable indicator
(murexide). The endpoint volume was found to be 12.11 ml. Find the molarity of
the sulfide in the sample solution.
Chemical Masking and Demasking Agents
Some metal ions that interfere in EDTA titrations can be masked by addition of a
suitable masking agent. A masking agent decreases the concentration of a free metal
ion to a level where a particular interfering reaction will not occur.
APCH231: EDTA
Notes complied by Dr. C. Southway
42
For example, cyanide ion can be used to mask certain metals that interfere in the
titration of Ca
2+
or Mg
2+
with EDTA. In the absence of cyanide, transition metals such
as Zn
2+
, Cd
2+
and Hg
2+
interfere by reacting simultaneously with the EDTA. When
cyanide ion is added, these cations form stable complexes and are prevented from
reacting with the EDTA.
The titration is performed then, if desired, a demasking agent can be added to free
the previously masked metal ions so that they can be determined.
If desired, Cd and Zn ions can be demasked by addition of chloral hydrate or a
3:1 formaldehyde : acetic acid solution.
Effect of Auxillary Complexing Agents on Metal-Ion
Concentrations
Auxillary complexing agents, which compete with EDTA for the metal ion and
thereby limit the sharpness of the titration curve, are sometimes necessary to keep the
metal in solution.
Metals react most completely with EDTA in basic solution where many of them also
form insoluble hydroxides or basic oxides. Once formed, these insoluble products will
react only slowly with EDTA, making a titration impossible. To avoid this problem,
an auxiliary complexing agent may be added to react with the metal ion and prevent
its precipitation when the solution is made basic. The complex must be intermediate
in stability between the metal hydroxide and the metal-EDTA complex. In this way it
will form in preference to the hydroxide, but will give up the metal ion to the EDTA
titrant as it is added. Ammonia is especially useful for this purpose because it forms
soluble complexes with many transition metals, and when mixed with its conjugate
acid, ammonium ion, it forms a basic pH buffer.
References
1. Analytical Chemistry, Principles and Techniques, Larry G. Hargis.
2. iugaza.edu/users/abdellatif/.../Complexometric_titrations.htm -
Você também pode gostar
- Critical Evaluation of Equilibrium Constants Involving 8-Hydroxyquinoline and Its Metal Chelates: Critical Evaluation of Equilibrium Constants in Solution: Part B: Equilibrium Constants of Liquid-Liquid Distribution SystemsNo EverandCritical Evaluation of Equilibrium Constants Involving 8-Hydroxyquinoline and Its Metal Chelates: Critical Evaluation of Equilibrium Constants in Solution: Part B: Equilibrium Constants of Liquid-Liquid Distribution SystemsAinda não há avaliações
- Advanced Pharmaceutical analysisNo EverandAdvanced Pharmaceutical analysisNota: 4.5 de 5 estrelas4.5/5 (2)
- TitrationDocumento14 páginasTitrationManP13Ainda não há avaliações
- Complexometry: Pharmaceutical Analysis For Liquid and Semisolid PreparationDocumento32 páginasComplexometry: Pharmaceutical Analysis For Liquid and Semisolid PreparationaulianiAinda não há avaliações
- Complexometric and Precipitation TitrationsDocumento10 páginasComplexometric and Precipitation Titrationskhanny96100% (1)
- EDTADocumento29 páginasEDTA5P4RT4NII7Ainda não há avaliações
- EdtaDocumento13 páginasEdtaChongZY100% (1)
- Complexometric Lab Report Experiment 02Documento10 páginasComplexometric Lab Report Experiment 02PDPPPMAT0621 Ruhilin Binti NasserAinda não há avaliações
- Analytical 1.2.2Documento5 páginasAnalytical 1.2.2Seyram DavidAinda não há avaliações
- Practicum 8 Complexometric Titration Experiment Summary: ObjectivesDocumento4 páginasPracticum 8 Complexometric Titration Experiment Summary: ObjectivesMia RismaliaAinda não há avaliações
- Tittrations Based On Complex FormationDocumento5 páginasTittrations Based On Complex FormationNisaAinda não há avaliações
- Analytical Chemistry Notes IvDocumento6 páginasAnalytical Chemistry Notes IvJabez MatigaAinda não há avaliações
- General Chemistry IV: CHEM-224 Lecture 2: Titrimetric Methods of AnalysisDocumento36 páginasGeneral Chemistry IV: CHEM-224 Lecture 2: Titrimetric Methods of AnalysisGeorge chaupi NyondoAinda não há avaliações
- Complexometry Determination Hardness ofDocumento30 páginasComplexometry Determination Hardness ofMichael CarloAinda não há avaliações
- Complexometric TitrationDocumento64 páginasComplexometric TitrationToyeba RahiAinda não há avaliações
- Titrari EDTADocumento35 páginasTitrari EDTAAdrian BlidarAinda não há avaliações
- Ch9 2021 2Documento19 páginasCh9 2021 2muhiafrancis975Ainda não há avaliações
- Complexometric TitrationDocumento8 páginasComplexometric TitrationYUNITA DWIAinda não há avaliações
- EDTA Titrations: Metal Chelate ComplexesDocumento35 páginasEDTA Titrations: Metal Chelate ComplexesalphhabetaAinda não há avaliações
- Complexometric Reactions and TitrationDocumento6 páginasComplexometric Reactions and TitrationAndre YosiAinda não há avaliações
- Question Answer of ComplexometryDocumento19 páginasQuestion Answer of ComplexometryNahzim RahmatAinda não há avaliações
- Complexometric Titration With EDTADocumento8 páginasComplexometric Titration With EDTAManP13100% (1)
- EDTA Titration LabDocumento16 páginasEDTA Titration LabJosef Hilton67% (3)
- Complexometric Titration 1Documento14 páginasComplexometric Titration 1Girma Selale0% (1)
- Chap 3. Complexation ReactionTitrationDocumento51 páginasChap 3. Complexation ReactionTitrationlesangg1406Ainda não há avaliações
- Complexometric TitrationDocumento4 páginasComplexometric TitrationZaim Akmal ZainalAinda não há avaliações
- Complexometric EDTADocumento36 páginasComplexometric EDTANqobile Nqoerh MnguniAinda não há avaliações
- Quantitative Determination of Total Hardness in Drinking Water by Complexometric EDTA TitrationDocumento14 páginasQuantitative Determination of Total Hardness in Drinking Water by Complexometric EDTA TitrationJoza Juan100% (2)
- Complexometric TitrationDocumento4 páginasComplexometric TitrationWendell Kim LlanetaAinda não há avaliações
- EdtaDocumento6 páginasEdtasam310justAinda não há avaliações
- Ch13 Ch16 SuppDocumento24 páginasCh13 Ch16 SuppQuoc AnhAinda não há avaliações
- PW2 - Hardness of WaterDocumento5 páginasPW2 - Hardness of Waters2ne228Ainda não há avaliações
- Complexometric TitrationDocumento25 páginasComplexometric Titrationpumeananda100% (1)
- Complejos InorgánicosDocumento2 páginasComplejos InorgánicosKthryn93Ainda não há avaliações
- Experiment 8 Chelatometric Analysis of The Complex For Cobalt Using EDTADocumento6 páginasExperiment 8 Chelatometric Analysis of The Complex For Cobalt Using EDTAJosef HiltonAinda não há avaliações
- Complexmetric TitrationDocumento17 páginasComplexmetric TitrationAnonymous oC3F7cxlLH100% (1)
- Expt 7Documento6 páginasExpt 7yayAinda não há avaliações
- Reading: Chapter 11, Quantitative Chemical Analysis, 8 Edition, Daniel C. Harris (7 Edition: Chapter 12)Documento7 páginasReading: Chapter 11, Quantitative Chemical Analysis, 8 Edition, Daniel C. Harris (7 Edition: Chapter 12)TYSON PETRO JONATHANAinda não há avaliações
- Lecture 11Documento3 páginasLecture 11prakash mishraAinda não há avaliações
- Coordination Assignment 2Documento6 páginasCoordination Assignment 2sara noorAinda não há avaliações
- ATQ7Documento3 páginasATQ7Joeco Abay-abayAinda não há avaliações
- Chem 26.1 Quantitative Determination of Total Hardness in Drinking Water by Complexometric EDTA TitrationDocumento4 páginasChem 26.1 Quantitative Determination of Total Hardness in Drinking Water by Complexometric EDTA TitrationBuiHopeAinda não há avaliações
- Expt. 6 Determination of The Hardness of A Given Water Sample by Complexometric TitrationDocumento8 páginasExpt. 6 Determination of The Hardness of A Given Water Sample by Complexometric TitrationManoj KhanalAinda não há avaliações
- Chem HW Key 222 Fall 2013 Exam 4 KeyDocumento8 páginasChem HW Key 222 Fall 2013 Exam 4 Keychem28dcaAinda não há avaliações
- Determination of Aluminium by Back TitrationDocumento5 páginasDetermination of Aluminium by Back TitrationKojo Eghan75% (12)
- Experiment: Determination of The Total Hardness (Permanent and Temporary)Documento1 páginaExperiment: Determination of The Total Hardness (Permanent and Temporary)Subhash DhungelAinda não há avaliações
- EdtaDocumento7 páginasEdtaRega Wahyu AnggrainiAinda não há avaliações
- Ionic Equilibrium NotesDocumento38 páginasIonic Equilibrium Notesumang jainAinda não há avaliações
- Chemistry Olympiad: Final Competition 2018Documento31 páginasChemistry Olympiad: Final Competition 2018Quoc AnhAinda não há avaliações
- Oxalic Acid and Sodium OxalateDocumento13 páginasOxalic Acid and Sodium Oxalateken34500775% (4)
- Section 08 Complex Formation Tit RationsDocumento19 páginasSection 08 Complex Formation Tit RationsHandugan Quinlog NoelAinda não há avaliações
- KGB Complexometric TitrationDocumento36 páginasKGB Complexometric TitrationpharmaprvAinda não há avaliações
- Chem 26.1 Formal Report 2Documento7 páginasChem 26.1 Formal Report 2Jo Fernandez100% (1)
- Chemistry 232 Water Hardness: EDTA Titrimetric Method: PurposeDocumento4 páginasChemistry 232 Water Hardness: EDTA Titrimetric Method: PurposekuochsochinAinda não há avaliações
- EDTA Titration of Calcium and MagnesiumDocumento3 páginasEDTA Titration of Calcium and MagnesiumAnonymous NxpnI6jC67% (3)
- Practice Makes Perfect in Chemistry: Acids, Bases, and SaltsNo EverandPractice Makes Perfect in Chemistry: Acids, Bases, and SaltsAinda não há avaliações
- Selected Constants: Oxidation–Reduction Potentials of Inorganic Substances in Aqueous SolutionNo EverandSelected Constants: Oxidation–Reduction Potentials of Inorganic Substances in Aqueous SolutionAinda não há avaliações
- Practice Makes Perfect in Chemistry: Acids, Bases, and Salts with AnswersNo EverandPractice Makes Perfect in Chemistry: Acids, Bases, and Salts with AnswersAinda não há avaliações
- Practice Makes Perfect in Chemistry: Oxidation-Reduction with AnswersNo EverandPractice Makes Perfect in Chemistry: Oxidation-Reduction with AnswersAinda não há avaliações
- Practice Makes Perfect in Chemistry: Oxidation-ReductionNo EverandPractice Makes Perfect in Chemistry: Oxidation-ReductionNota: 5 de 5 estrelas5/5 (1)
- PPE1 - CHEM4B - Group 5 - AssignmentDocumento15 páginasPPE1 - CHEM4B - Group 5 - AssignmentJaymel ZamoraAinda não há avaliações
- Sci4 ST2 Q3Documento2 páginasSci4 ST2 Q3MAY ANNE SITJARAinda não há avaliações
- Realtime RadiographyDocumento13 páginasRealtime RadiographySivirahulAinda não há avaliações
- Transport Phenomena in Multiphase Flows (Fluid Mechanics and Its Applications Volume 112) - Roberto Mauri (Springer, 2015)Documento459 páginasTransport Phenomena in Multiphase Flows (Fluid Mechanics and Its Applications Volume 112) - Roberto Mauri (Springer, 2015)Irfan Mahmood100% (1)
- Organic Chemistry Chart PDFDocumento3 páginasOrganic Chemistry Chart PDFJayjayjay 5100% (2)
- Inorganic Organic Composites For Water and Wastewater TreatmentDocumento329 páginasInorganic Organic Composites For Water and Wastewater TreatmentjoeylayhoonAinda não há avaliações
- Atomic Absorption Spectrophotometry New PDFDocumento69 páginasAtomic Absorption Spectrophotometry New PDFMonicia AttasihAinda não há avaliações
- PTQ Efficient Crude BlendingDocumento7 páginasPTQ Efficient Crude BlendingArpit SharmaAinda não há avaliações
- Structure of The AtomDocumento18 páginasStructure of The Atom9B13PAVITHRAN.DAinda não há avaliações
- Chapter 4: Biocatalysis (Pyq) PSPM II 2016/2017Documento20 páginasChapter 4: Biocatalysis (Pyq) PSPM II 2016/2017JULITA AISYAH BINTI KHALID MoeAinda não há avaliações
- Plane Wall Lecture Week 7 HTDocumento17 páginasPlane Wall Lecture Week 7 HTToMemAinda não há avaliações
- No.454 From AE (Dated 20.11.2019)Documento9 páginasNo.454 From AE (Dated 20.11.2019)Venkat Raghav VasireddyAinda não há avaliações
- Liquid SulfurDocumento20 páginasLiquid SulfurMarcin MaruchaAinda não há avaliações
- Relaxation Processes in Glass: IMI-NFG's MITT Course OnDocumento17 páginasRelaxation Processes in Glass: IMI-NFG's MITT Course Onitamar_jrAinda não há avaliações
- Tutorial 9Documento14 páginasTutorial 9Abdimajid MohamedAinda não há avaliações
- Revision Notes Class 12 Chemistry Chapter 9 - Coordination Compounds Notes in 30 MinutesDocumento42 páginasRevision Notes Class 12 Chemistry Chapter 9 - Coordination Compounds Notes in 30 Minutessangee chandranAinda não há avaliações
- Comparison of The Solubilization Effect of Micronized PoloxamersDocumento1 páginaComparison of The Solubilization Effect of Micronized Poloxamerssaeedazadi1352Ainda não há avaliações
- A High Energy Density Asymmetric Supercapacitor From Nano-Architectured Ni (OH) /carbon Nanotube ElectrodesDocumento7 páginasA High Energy Density Asymmetric Supercapacitor From Nano-Architectured Ni (OH) /carbon Nanotube ElectrodesyatinAinda não há avaliações
- (2019) Chemical Bonding and Bonding Models of Main-Group Compounds - Lili ZhaoDocumento65 páginas(2019) Chemical Bonding and Bonding Models of Main-Group Compounds - Lili ZhaoWalter Sperandio SampaioAinda não há avaliações
- Heat Load EstimationDocumento4 páginasHeat Load Estimationdhanu_lagwankarAinda não há avaliações
- Electrical Transport MechanismsDocumento106 páginasElectrical Transport MechanismslindaAinda não há avaliações
- ESO201 IIT AssignmentDocumento4 páginasESO201 IIT AssignmentSunitAinda não há avaliações
- Naproxen Patch TransdermalDocumento9 páginasNaproxen Patch Transdermalnurhayati novitaAinda não há avaliações
- ME2-CO Carbon Monoxide Sensor Manual: Zhengzhou Winsen Electronics Technology Co., LTDDocumento3 páginasME2-CO Carbon Monoxide Sensor Manual: Zhengzhou Winsen Electronics Technology Co., LTDNguyen Vu Hoang ThachAinda não há avaliações
- ME 346 (S3) Tutorial 1 - SolutionsDocumento18 páginasME 346 (S3) Tutorial 1 - SolutionsdivyanshuAinda não há avaliações
- Pressure Drop in Packed ColumnsDocumento21 páginasPressure Drop in Packed ColumnsMohamad Samer KansouAinda não há avaliações
- Activity 1 2Documento8 páginasActivity 1 2Junard AsentistaAinda não há avaliações
- EnzymesDocumento17 páginasEnzymesakshaymoga0% (1)
- Cantor Alloys1Documento36 páginasCantor Alloys1Duygu GökkuşAinda não há avaliações
- Solid State Physics Chapter 1Documento9 páginasSolid State Physics Chapter 1Sergio NuñezAinda não há avaliações