Escolar Documentos
Profissional Documentos
Cultura Documentos
Vanda Pharmaceuticals v. Roxane Laboratories
Enviado por
PriorSmart0 notas0% acharam este documento útil (0 voto)
60 visualizações8 páginasOfficial Complaint for Patent Infringement in Civil Action No. 1:14-cv-00757-UNA: Vanda Pharmaceuticals Inc. v. Roxane Laboratories Inc. Filed in U.S. District Court for the District of Delaware, no judge yet assigned. See http://news.priorsmart.com/-lazN for more info.
Direitos autorais
© Public Domain
Formatos disponíveis
PDF, TXT ou leia online no Scribd
Compartilhar este documento
Compartilhar ou incorporar documento
Você considera este documento útil?
Este conteúdo é inapropriado?
Denunciar este documentoOfficial Complaint for Patent Infringement in Civil Action No. 1:14-cv-00757-UNA: Vanda Pharmaceuticals Inc. v. Roxane Laboratories Inc. Filed in U.S. District Court for the District of Delaware, no judge yet assigned. See http://news.priorsmart.com/-lazN for more info.
Direitos autorais:
Public Domain
Formatos disponíveis
Baixe no formato PDF, TXT ou leia online no Scribd
0 notas0% acharam este documento útil (0 voto)
60 visualizações8 páginasVanda Pharmaceuticals v. Roxane Laboratories
Enviado por
PriorSmartOfficial Complaint for Patent Infringement in Civil Action No. 1:14-cv-00757-UNA: Vanda Pharmaceuticals Inc. v. Roxane Laboratories Inc. Filed in U.S. District Court for the District of Delaware, no judge yet assigned. See http://news.priorsmart.com/-lazN for more info.
Direitos autorais:
Public Domain
Formatos disponíveis
Baixe no formato PDF, TXT ou leia online no Scribd
Você está na página 1de 8
IN THE UNITED STATES DISTRICT COURT
FOR THE DISTRICT OF DELAWARE
VANDA PHARMACEUTICALS INC.,
Plaintiff,
v.
ROXANE LABORATORIES, INC.,
Defendant.
)
)
)
)
)
)
)
)
)
C.A. No. 14-___
COMPLAINT FOR PATENT INFRINGEMENT
Plaintiff Vanda Pharmaceuticals Inc. (Vanda) for its Complaint against
Defendant Roxane Laboratories, Inc. (Roxane) alleges as follows:
I. THE PARTIES
1. Vanda is a Delaware corporation with its principal place of business at
2200 Pennsylvania Ave NW, Washington, DC 20037. Vanda is a pharmaceutical company that
focuses on the development and commercialization of new medicines to address unmet medical
needs, including FANAPT(iloperidone oral tablets), for the treatment of schizophrenia.
2. On information and belief, Roxane is a Nevada corporation, with a
principal place of business at 1809 Wilson Road, Columbus, Ohio 43228. On information and
belief, Roxane is in the business of manufacturing generic pharmaceutical drugs that it
distributes and sells in the State of Delaware and throughout the United States.
II. NATURE OF THE ACTION
3. This is an action arising under the patent laws of the United States (Title
35, United States Code, 100, et seq.) based upon Roxanes infringement of one or more of
claims of Vandas U.S. Patent No. 8,586,610 (the 610 patent), which relates to the field of
schizophrenia treatment.
2
4. On information and belief, Roxane filed an Abbreviated New Drug
Application (the Roxane ANDA) under 505(j) of the Federal Food, Drug, and Cosmetic Act
(the FFDCA), to obtain approval to commercially manufacture and sell generic iloperidone
tablets in their 1 mg, 2 mg, 4 mg, 6 mg, 8mg, 10 mg, and 12 mg strengths for the treatment of
schizophrenia.
5. Roxane has infringed one or more claims of the 610 patent under 35
U.S.C. 271(e)(2)(A) by virtue of its filing of the Roxane ANDA seeking FDA approval to
commercially manufacture, use, offer for sale, sell, distribute in, or import into the United States
generic FANAPT(iloperidone oral tablets) for the treatment of schizophrenia prior to the
expiration of the 610 patent, or any extensions thereof. Roxane will infringe one or more claims
of the 610 patent under 35 U.S.C. 271(a), (b), or (c) should it engage in the commercial
manufacture, use, offer for sale, sale, distribution in, or importation into the United States of
generic FANAPT(iloperidone oral tablets) for the treatment of schizophrenia prior to the
expiration of the 610 patent, or any extensions thereof.
III. JURISDICTION AND VENUE
6. This Court has subject matter jurisdiction over Vandas patent
infringement claims under 28 U.S.C. 1331, 1338(a), 2201, and 2202.
7. This Court has personal jurisdiction over Roxane by virtue of the fact that,
inter alia, Roxane has committed, induced, contributed to, aided, abetted, or participated in in the
commission of the tortious act of patent infringement that has led to foreseeable harm and injury
to Vanda, a Delaware corporation. This Court has personal jurisdiction over Roxane for the
additional reasons set forth below.
3
8. This Court has personal jurisdiction over Roxane, by virtue of, inter alia,
its having conducted business in Delaware, having availed itself of the rights and benefits of
Delaware law, and having engaged in substantial and continuing contacts with the State.
9. On information and belief, Roxane develops, manufactures, seeks
approval for, and sells FDA-approved generic pharmaceutical drugs, which are being marketed,
distributed, and sold in Delaware and in the United States. Thus, on information and belief,
Roxane does substantial business in Delaware, derives substantial revenue from Delaware, and
engages in other persistent courses of conduct in Delaware. These continuous and systematic
contacts, including, but not limited, to those described above and below, are more than sufficient
for this Court to exercise general personal jurisdiction over Roxane.
10. On information and belief, Roxane, following any FDA approval of the
Roxane ANDA, will sell the generic product that is the subject of the infringement claims in this
action in the State of Delaware and throughout the United States.
11. On information and belief, Roxane has previously availed itself of this
forum for purposes of litigating its patent disputes. For example, Roxane has submitted to the
jurisdiction of this Court by asserting counterclaims in other civil actions initiated in this
jurisdiction. Specifically, Roxane admitted jurisdiction (for purposes of the litigation) and filed
counterclaims in at least the following actions in this Court: Novartis Pharm. Corp. v. Roxane
Labs., Inc., 13-cv-1973 (D. Del.); Eisai Co. v. Roxane Labs., Inc., 13-cv-1284 (D. Del.); Glaxo
SmithKline LLC v. Roxane Labs., Inc., 11-cv-542 (D. Del.); Abbott Labs. v. Roxane Labs., Inc.,
10-cv-998 (D. Del.).
12. Venue is proper in this Court pursuant to 28 U.S.C. 1391 (b) and (c)
and 1400(b).
4
IV. THE PATENT-IN-SUIT U.S. PATENT NO. 8,586,610
13. The allegations of 1-12 are incorporated herein by reference.
14. Vanda is the owner of all rights, title and interest in the 610 patent,
entitled Methods for the Administration of Iloperidone. The United States Patent and
Trademark Office (USPTO) duly and legally issued the 610 patent on November 19, 2013, to
Curt D. Wolfgang and Mihael H. Polymeropoulos, which was assigned to Vanda. A true and
correct copy of the 610 patent is attached to this Complaint as Exhibit A.
15. The 610 patent covers methods of using iloperidone for the treatment of
schizophrenia in certain patients based on whether the patients are poor metabolizers of
FANAPT. The patients that are poor metabolizers of FANAPThave certain mutations of a
gene known as CYP2D6. The 610 patent covers the identification of patients that are poor
metabolizers by genotyping and making a specific dose reductionthe dosage must be halved
in those patients to avoid prolonged QTc as measured by an electrocardiogram (EKG).
Various studies have shown that patients with QTc prolongation may have an increased risk of
cardiovascular side effects, including serious arrhythmias, such as ventricular tachycardia,
ventricular fibrillation, and irregular heartbeats (torsades de pointes or TDP), which could lead to
cardiac death.
16. On May 6, 2009, FDA approved Vandas new drug application 22-192 for
FANAPT(iloperidone oral tablets) in their 1 mg, 2 mg, 4 mg, 6 mg, 8mg, 10 mg, and 12 mg
strengths under 505(b) of the FFDCA, 21 U.S.C. 355(b), for the treatment of schizophrenia
(FANAPTNDA). Novartis Pharmaceutical Corp. (Novartis) now owns the FANAPT
NDA.
5
17. The prescribing information for FANAPT (FANAPT Label),
instructs physicians to (1) determine whether the patient is a poor CYP2D6 metabolizer using
available laboratory tests,
1
and (2) administer either the target dose if the patient is a normal
CYP2D6 metabolizer or a halved dosage if the patient is a poor CYP2D6 metabolizer.
18. Thus, the use of FANAPT(iloperidone oral tablets) and any generic
iloperidone for the treatment of schizophrenia is covered by the 610 patent, and Vanda has the
right to enforce the 610 patent.
COUNT I
(INFRINGEMENT OF THE 610 PATENT)
19. The allegations of 1-18 are incorporated herein by reference.
20. On information and belief, Roxane filed the Roxane ANDA under 505(j)
of the FFDCA to obtain approval to commercially manufacture, use, offer to sell, and sell a
generic version of FANAPT(iloperidone oral tablets) for the treatment of schizophrenia before
the expiration of the 610 patent, and any extensions thereof.
21. On information and belief, Novartis received a letter (Roxane Notice
Letter) dated October 17, 2013, stating that Roxane had filed the Roxane ANDA seeking
approval to manufacture, use, offer to sell, and sell a generic version of FANAPT(iloperidone
oral tablets) in their 1 mg, 2 mg, 4 mg, 6 mg, 8mg, 10 mg, and 12 mg strengths for the treatment
of schizophrenia before the expiration of RE198, and therefore necessarily before the expiration
of the 610 patent.
22. On information and belief, the Roxane ANDA essentially copies the
FANAPTLabel as required by FDA, see 21 C.F.R. 314.94(a)(iv), and therefore instructs
1
There are numerous commercially available genotyping assays (offered by Roche,
Illumina, etc.).
6
physicians to (1) determine whether the patient is a poor CYP2D6 metabolizer using available
laboratory tests, and (2) administer either the target dose if the patient is a normal CYP2D6
metabolizer or a halved dosage if the patient is a poor CYP2D6 metabolizer.
23. Roxane has infringed the 610 patent under 35 U.S.C. 271(e)(2)(A) by
virtue of its submission of the Roxane ANDA to FDA for generic FANAPT(iloperidone oral
tablets) in their 1 mg, 2 mg, 4 mg, 6 mg, 8mg, 10 mg, and 12 mg strengths, for the treatment of
schizophrenia, which are covered by one or more claims of the 610 patent.
24. Roxanes participation in, contribution to, inducement of, aiding or
abetting the submission of the Roxane ANDA to FDA constitutes direct, contributory, or induced
infringement of one or more claims of the 610 patent under 35 U.S.C. 271(e)(2)(A).
25. The commercial manufacture, use, offer to sell, sale, distribution, or
importation of products under the Roxane ANDA would infringe directly or contribute to or
induce the infringement of one or more claims of the 610 patent.
26. Vanda seeks entry of an order pursuant to 35 U.S.C. 271(e)(4), including
an order of this Court that the effective date of any FDA approval of the Roxane ANDA be a
date that is not earlier than the expiration of the 610 patent, or any later expiration of exclusivity
for the 610 patent to which Vanda becomes entitled.
27. Vanda will be irreparably harmed if Roxane is not enjoined from
infringing or actively inducing or contributing to infringement of one or more claims of the 610
patent. Pursuant to 35 U.S.C. 283, Vanda is entitled to a permanent injunction against further
infringement. Vanda does not have an adequate remedy at law.
28. To the extent Roxane commercializes its product, Vanda will also be
entitled to damages under 35 U.S.C. 284.
7
PRAYER FOR RELIEF
WHEREFORE, Vanda respectfully requests that this Court enter judgment in its
favor against Roxane and grant the following relief:
A. an adjudication that Roxane has infringed directly, contributed to, or
induced the infringement of one or more claims of the 610 patent under 35 U.S.C.
271(e)(2)(A), by submitting to FDA the Roxane ANDA to obtain approval for the commercial
manufacture, use, offer for sale, sale, distribution in, or importation into the United States of
generic FANAPT(iloperidone oral tablets) for the treatment of schizophrenia before the
expiration of the 610 patent;
B. an order pursuant to 35 U.S.C. 271(e)(4)(A) providing that the effective
date of any FDA approval of the Roxane ANDA for generic FANAPT(iloperidone oral
tablets) be a date that is not earlier than the date of the expiration of the 610 patent or any later
period of exclusivity to which Vanda is or may become entitled;
C. a permanent injunction enjoining Roxane, their officers, agents, servants,
employees, attorneys, affiliates, divisions, subsidiaries, and those persons in active concert or
participation with any of them from infringing the 610 patent, or contributing to or inducing
anyone to do the same, including the manufacture, use, offer to sell, sale, distribution, or
importation of any current or future versions of the product described in the Roxane ANDA;
D. an order enjoining Roxane, its officers, agents, servants, employees,
attorneys, affiliates, divisions, subsidiaries, and those persons in active concert or participation
with any of them from infringing the 610 patent, contributing to, or inducing anyone to do the
same, including the manufacture, use, offer to sell, sale, distribution, or importation of any
8
current or future versions of the product described in the Roxane ANDA while the litigation is
pending;
E. a judgment declaring that the manufacture, use, offer to sell, sale,
distribution, or importation of the products described in the Roxane ANDA would constitute
infringement of one or more claims of the 610 patent, or inducement of or contribution to such
conduct, by Roxane pursuant to 35 U.S.C. 271 (a), (b), or (c);
F. an assessment of pre-judgment and post-judgment interest and costs
against Roxane, together with an award of such interest and costs, in accordance with 35 U.S.C.
284; and
G. such other and further relief as this Court may deem just and proper.
OF COUNSEL:
Nicholas Groombridge
Eric Alan Stone
Josephine Young
PAUL, WEISS, RIFKIND, WHARTON
& GARRISON LLP
1285 Avenue of the Americas
New York, NY 10019
(212) 373-3000
MORRIS, NICHOLS, ARSHT & TUNNELL LLP
/s/ Karen Jacobs
Jack B. Blumenfeld (#1014)
Karen Jacobs (#2881)
1201 North Market Street
P.O. Box 1347
Wilmington, DE 19899
(302) 658-9200
jblumenfeld@mnat.com
kjacobs@mnat.com
Attorneys for Plaintiff
Vanda Pharmaceuticals Inc.
June 16, 2014
8310250
Você também pode gostar
- Novartis Pharmaceuticals Et. Al. v. Roxane LaboratoriesDocumento8 páginasNovartis Pharmaceuticals Et. Al. v. Roxane LaboratoriesPatent LitigationAinda não há avaliações
- Horizon V Actavis VIMOVODocumento26 páginasHorizon V Actavis VIMOVOiphawkAinda não há avaliações
- Celgene Et. Al. v. Par PharmaceuticalDocumento94 páginasCelgene Et. Al. v. Par PharmaceuticalPriorSmartAinda não há avaliações
- Alcon Pharmaceuticals Et. Al. v. Apotex Et. Al.Documento13 páginasAlcon Pharmaceuticals Et. Al. v. Apotex Et. Al.PriorSmartAinda não há avaliações
- Horizon Pharma Et. Al. v. Watson Laboratories, Inc. - Florida Et. Al.Documento14 páginasHorizon Pharma Et. Al. v. Watson Laboratories, Inc. - Florida Et. Al.Patent LitigationAinda não há avaliações
- AstraZeneca AB v. Sun Pharma Global FZE Et. Al.Documento14 páginasAstraZeneca AB v. Sun Pharma Global FZE Et. Al.PriorSmartAinda não há avaliações
- Depomed v. Impax Laboratories Et. Al.Documento20 páginasDepomed v. Impax Laboratories Et. Al.PriorSmartAinda não há avaliações
- Janssen Products Et. Al. v. Lupin Et. Al.Documento14 páginasJanssen Products Et. Al. v. Lupin Et. Al.PriorSmartAinda não há avaliações
- Senju Pharmaceutical Et. Al. v. Aurobindo Pharma Et. Al.Documento12 páginasSenju Pharmaceutical Et. Al. v. Aurobindo Pharma Et. Al.PriorSmartAinda não há avaliações
- Astrazeneca Ab v. Aurobindo Pharma Et. Al.Documento8 páginasAstrazeneca Ab v. Aurobindo Pharma Et. Al.PriorSmartAinda não há avaliações
- Forest Laboratories Et. Al. v. Par PharmaceuticalDocumento9 páginasForest Laboratories Et. Al. v. Par PharmaceuticalPriorSmartAinda não há avaliações
- Roche Palo Alto Et. Al. v. Dr. Reddy's Laboratories Et. Al.Documento6 páginasRoche Palo Alto Et. Al. v. Dr. Reddy's Laboratories Et. Al.PriorSmartAinda não há avaliações
- Forest Laboratories Et. Al. v. Apotex Et. Al.Documento11 páginasForest Laboratories Et. Al. v. Apotex Et. Al.PriorSmartAinda não há avaliações
- Vnda Teva Hetlioz 13 Dec 22Documento73 páginasVnda Teva Hetlioz 13 Dec 22XDL1Ainda não há avaliações
- Salix Pharmaceuticals, Inc. Et. Al.Documento9 páginasSalix Pharmaceuticals, Inc. Et. Al.PriorSmartAinda não há avaliações
- Warner Chilcott Company Et. Al. v. Watson Pharmaceuticals Et. Al.Documento12 páginasWarner Chilcott Company Et. Al. v. Watson Pharmaceuticals Et. Al.PriorSmartAinda não há avaliações
- Galderma Laboratories Et. Al. v. Watson Pharmaceuticals Et. Al.Documento15 páginasGalderma Laboratories Et. Al. v. Watson Pharmaceuticals Et. Al.PriorSmartAinda não há avaliações
- Otsuka Pharmaceutical v. Par PharmaceuticalDocumento10 páginasOtsuka Pharmaceutical v. Par PharmaceuticalPriorSmartAinda não há avaliações
- Cadence Pharmaceuticals Et. Al. v. SandozDocumento9 páginasCadence Pharmaceuticals Et. Al. v. SandozPriorSmartAinda não há avaliações
- UnpublishedDocumento17 páginasUnpublishedScribd Government DocsAinda não há avaliações
- Senju Pharmaceutical Et. Al. v. Actavis Et. Al.Documento13 páginasSenju Pharmaceutical Et. Al. v. Actavis Et. Al.PriorSmartAinda não há avaliações
- Roxane Laboratories v. Camber Pharmaceuticals Et. Al.Documento8 páginasRoxane Laboratories v. Camber Pharmaceuticals Et. Al.PriorSmartAinda não há avaliações
- Nautilus Neurosciences Et. Al. v. Wockhardt Et. Al.Documento33 páginasNautilus Neurosciences Et. Al. v. Wockhardt Et. Al.PriorSmartAinda não há avaliações
- Apotex Et. Al. v. Pfizer Et. Al.Documento8 páginasApotex Et. Al. v. Pfizer Et. Al.PriorSmartAinda não há avaliações
- Enoxaparin FDA 2010Documento45 páginasEnoxaparin FDA 2010Imam Nur Alif KhusnudinAinda não há avaliações
- A. Action RequestedDocumento19 páginasA. Action RequestedNeeraj MundaAinda não há avaliações
- Shire Et. Al. v. Apotex Et. Al.Documento15 páginasShire Et. Al. v. Apotex Et. Al.Patent LitigationAinda não há avaliações
- Sanofi-Aventis Et. Al. v. Strides Et. Al.Documento22 páginasSanofi-Aventis Et. Al. v. Strides Et. Al.PriorSmartAinda não há avaliações
- Shire Et. Al. v. Mylan Pharmaceuticals Et. Al.Documento13 páginasShire Et. Al. v. Mylan Pharmaceuticals Et. Al.PriorSmartAinda não há avaliações
- Published United States Court of Appeals For The Fourth CircuitDocumento14 páginasPublished United States Court of Appeals For The Fourth CircuitScribd Government DocsAinda não há avaliações
- Purdue Pharma Et. Al. v. Impax LaboratoriesDocumento32 páginasPurdue Pharma Et. Al. v. Impax LaboratoriesPriorSmartAinda não há avaliações
- Avanir Pharmaceuticals, Inc., Et Al. v. Actavis South Atlantic LLC, Et Al., Consol. C.A. No. 11-704-LPS (D. Del. Apr. 30, 2014)Documento65 páginasAvanir Pharmaceuticals, Inc., Et Al. v. Actavis South Atlantic LLC, Et Al., Consol. C.A. No. 11-704-LPS (D. Del. Apr. 30, 2014)YCSTBlogAinda não há avaliações
- Helsinn Healthcare Et. Al. v. Accord Healthcare Et. Al.Documento8 páginasHelsinn Healthcare Et. Al. v. Accord Healthcare Et. Al.PriorSmartAinda não há avaliações
- Sanofi Et. Al. v. Alembic Pharmaceuticals Et. Al.Documento14 páginasSanofi Et. Al. v. Alembic Pharmaceuticals Et. Al.PriorSmartAinda não há avaliações
- AbbVie Generics LawsuitDocumento248 páginasAbbVie Generics LawsuitRobert GarciaAinda não há avaliações
- Bristol-Myers Squibb Company v. Apotex Et. Al.Documento9 páginasBristol-Myers Squibb Company v. Apotex Et. Al.PriorSmartAinda não há avaliações
- Novartis Pharmaceuticals Et. Al. v. Par PharmaceuticalDocumento7 páginasNovartis Pharmaceuticals Et. Al. v. Par PharmaceuticalPriorSmartAinda não há avaliações
- Counsel For Plaintiffs: Kirkland KirklandDocumento32 páginasCounsel For Plaintiffs: Kirkland KirklandXDL1Ainda não há avaliações
- RisperdalDocumento8 páginasRisperdalefuchs160Ainda não há avaliações
- United States Court of Appeals, Fourth CircuitDocumento9 páginasUnited States Court of Appeals, Fourth CircuitScribd Government DocsAinda não há avaliações
- Astrazeneca Ab Et. Al. v. Mylan Pharmaceuticals Et. Al.Documento26 páginasAstrazeneca Ab Et. Al. v. Mylan Pharmaceuticals Et. Al.Patent LitigationAinda não há avaliações
- ComplaintDocumento8 páginasComplaintpauloverhauserAinda não há avaliações
- Novartis Pharmaceuticals Et. Al. v. Mylan Et. Al.Documento12 páginasNovartis Pharmaceuticals Et. Al. v. Mylan Et. Al.PriorSmart100% (1)
- FDA Safety AlertsDocumento166 páginasFDA Safety AlertsChris HartoyoAinda não há avaliações
- United States v. Articles of Drug, Etc. Appeal of The Lannett Company, Inc, 585 F.2d 575, 3rd Cir. (1978)Documento17 páginasUnited States v. Articles of Drug, Etc. Appeal of The Lannett Company, Inc, 585 F.2d 575, 3rd Cir. (1978)Scribd Government DocsAinda não há avaliações
- Celgene Et. Al. v. IntellipharmaceuticsDocumento94 páginasCelgene Et. Al. v. IntellipharmaceuticsPriorSmartAinda não há avaliações
- Bristol-Myers Squibb Company v. Dr. Reddy's Laboratories Et. Al.Documento72 páginasBristol-Myers Squibb Company v. Dr. Reddy's Laboratories Et. Al.PriorSmartAinda não há avaliações
- Horizon Pharma Et. Al. v. Watson Laboratories, Inc. - Florida Et. Al.Documento78 páginasHorizon Pharma Et. Al. v. Watson Laboratories, Inc. - Florida Et. Al.Patent LitigationAinda não há avaliações
- Cadence Pharmaceuticals Et. Al. v. SandozDocumento37 páginasCadence Pharmaceuticals Et. Al. v. SandozPriorSmartAinda não há avaliações
- Drug Information Bulletin 42 05Documento4 páginasDrug Information Bulletin 42 05amritaryaaligarghAinda não há avaliações
- Lilly V Par ComplaintDocumento12 páginasLilly V Par ComplaintpauloverhauserAinda não há avaliações
- Alza Et. Al. v. SandozDocumento13 páginasAlza Et. Al. v. SandozPatent LitigationAinda não há avaliações
- Apotex Et. Al. v. Lupin Pharmaceuticals Et. Al.Documento6 páginasApotex Et. Al. v. Lupin Pharmaceuticals Et. Al.PriorSmartAinda não há avaliações
- MOMENTA PHARMACEUTICALS V AMPHASTAR PHARMACEUTICALSDocumento54 páginasMOMENTA PHARMACEUTICALS V AMPHASTAR PHARMACEUTICALSIlyaAinda não há avaliações
- Novartis Et. Al. v. Glenmark Pharmaceuticals Et. Al.Documento9 páginasNovartis Et. Al. v. Glenmark Pharmaceuticals Et. Al.PriorSmartAinda não há avaliações
- Duchesnay Et. Al. v. Actavis Laboratories FL Et. Al.Documento17 páginasDuchesnay Et. Al. v. Actavis Laboratories FL Et. Al.Patent LitigationAinda não há avaliações
- Pfizer Et. Al. v. Inventia Healthcare PrivateDocumento12 páginasPfizer Et. Al. v. Inventia Healthcare PrivatePriorSmartAinda não há avaliações
- Herbal Supplements: Efficacy, Toxicity, Interactions with Western Drugs, and Effects on Clinical Laboratory TestsNo EverandHerbal Supplements: Efficacy, Toxicity, Interactions with Western Drugs, and Effects on Clinical Laboratory TestsAinda não há avaliações
- Drug Repositioning: Bringing New Life to Shelved Assets and Existing DrugsNo EverandDrug Repositioning: Bringing New Life to Shelved Assets and Existing DrugsAinda não há avaliações
- Advance Products & Systems v. CCI Piping SystemsDocumento5 páginasAdvance Products & Systems v. CCI Piping SystemsPriorSmartAinda não há avaliações
- Richmond v. Creative IndustriesDocumento17 páginasRichmond v. Creative IndustriesPriorSmartAinda não há avaliações
- VIA Technologies Et. Al. v. ASUS Computer International Et. Al.Documento18 páginasVIA Technologies Et. Al. v. ASUS Computer International Et. Al.PriorSmartAinda não há avaliações
- Perrie v. PerrieDocumento18 páginasPerrie v. PerriePriorSmartAinda não há avaliações
- ATEN International v. Uniclass Technology Et. Al.Documento14 páginasATEN International v. Uniclass Technology Et. Al.PriorSmartAinda não há avaliações
- Dok Solution v. FKA Distributung Et. Al.Documento99 páginasDok Solution v. FKA Distributung Et. Al.PriorSmartAinda não há avaliações
- Mcs Industries v. Hds TradingDocumento5 páginasMcs Industries v. Hds TradingPriorSmartAinda não há avaliações
- Multiplayer Network Innovations v. IgtDocumento8 páginasMultiplayer Network Innovations v. IgtPriorSmartAinda não há avaliações
- Shenzhen Liown Electronics v. Luminara Worldwide Et. Al.Documento10 páginasShenzhen Liown Electronics v. Luminara Worldwide Et. Al.PriorSmartAinda não há avaliações
- Faith HealingDocumento23 páginasFaith Healingcreamyfrappe0% (1)
- Vol 2, Issue 1Documento56 páginasVol 2, Issue 1Asfandyar Sheikh100% (1)
- Anaphy (Lab) ReviewerDocumento3 páginasAnaphy (Lab) ReviewerAgatha Cristie AndradaAinda não há avaliações
- Behavior Neurobiology of Alcohol AddictionDocumento722 páginasBehavior Neurobiology of Alcohol AddictionDassaev Fritz100% (1)
- Digestive SystemDocumento28 páginasDigestive Systemtessacruz1186Ainda não há avaliações
- Electronic Surgical Logbook For Orthopedic Residents AcceptanceDocumento7 páginasElectronic Surgical Logbook For Orthopedic Residents AcceptanceKhalil ur RehmanAinda não há avaliações
- 1.1.4 Blood EvidenceDocumento3 páginas1.1.4 Blood EvidenceIlliana EddyAinda não há avaliações
- R 141bDocumento8 páginasR 141bLUISALBERTO06011985Ainda não há avaliações
- Immuno Cheat Sheet 1Documento2 páginasImmuno Cheat Sheet 1yolo51Ainda não há avaliações
- Problems and Remedies For MarsDocumento4 páginasProblems and Remedies For MarsSunil RupaniAinda não há avaliações
- Drug Interactions Results - MICROMEDEX - MAYODocumento10 páginasDrug Interactions Results - MICROMEDEX - MAYOMARIA JULIANA RENGIFO LARAAinda não há avaliações
- Politeknik Ungku Omar: Kementerian Pengajian Tinggi MalaysiaDocumento9 páginasPoliteknik Ungku Omar: Kementerian Pengajian Tinggi Malaysiaazmir100% (3)
- نسخة نسخة الاشعةDocumento10 páginasنسخة نسخة الاشعةDina MohamedAinda não há avaliações
- Antibiotics Chart 1Documento7 páginasAntibiotics Chart 1Vee MendAinda não há avaliações
- Guidelines For The Safe Use of UltrasoundDocumento5 páginasGuidelines For The Safe Use of UltrasoundLiCruzAinda não há avaliações
- What Is Permanent Make-Up?: First in Looks That LastDocumento4 páginasWhat Is Permanent Make-Up?: First in Looks That LastNatural Enhancement0% (1)
- Predisposing FactorsDocumento2 páginasPredisposing Factors-Lauяeиs© TolosaAinda não há avaliações
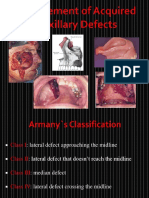
- Management of Acquired Maxillary Defects Partially Edentlous PDFDocumento129 páginasManagement of Acquired Maxillary Defects Partially Edentlous PDFRamy Khalifa AshmawyAinda não há avaliações
- Speck - X-Ray Contrast Media - Overview, Use and Pharmaceutical AspectsDocumento134 páginasSpeck - X-Ray Contrast Media - Overview, Use and Pharmaceutical AspectsSimona Mariana DutuAinda não há avaliações
- Analisis Berkaitan Penderaan Emosi Terhadap Kanak-Kanak Dari Sudut PerundanganDocumento27 páginasAnalisis Berkaitan Penderaan Emosi Terhadap Kanak-Kanak Dari Sudut PerundanganJamuna BatumalaiAinda não há avaliações
- Report of Mangalore VisitDocumento6 páginasReport of Mangalore VisitAmal DominicAinda não há avaliações
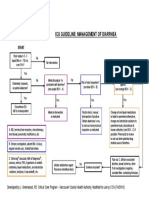
- Icu Guideline: Management of Diarrhea: StartDocumento1 páginaIcu Guideline: Management of Diarrhea: StartGracia VionaAinda não há avaliações
- Downward Movement - Left Hand Only: (Figure 3-7Documento20 páginasDownward Movement - Left Hand Only: (Figure 3-7mamun31Ainda não há avaliações
- Anaesthetic Management of A Case With Severe Mitral Stenosis Posted For Emergency Caesarean SectionDocumento2 páginasAnaesthetic Management of A Case With Severe Mitral Stenosis Posted For Emergency Caesarean SectionInternational Journal of Innovative Science and Research TechnologyAinda não há avaliações
- Twin Block Appliance ThesisDocumento6 páginasTwin Block Appliance Thesisjessicahillnewyork100% (2)
- Daftar Obat Alkes Trolley EmergencyDocumento15 páginasDaftar Obat Alkes Trolley EmergencydevitaAinda não há avaliações
- ER Technician Competency ProfileDocumento6 páginasER Technician Competency Profileplanedude86Ainda não há avaliações
- LMC ICD-10 PowerPointDocumento77 páginasLMC ICD-10 PowerPointNicholas HenryAinda não há avaliações
- Benign Diseases of ThyroidDocumento70 páginasBenign Diseases of ThyroidMounica MekalaAinda não há avaliações
- Bioethics Animal Paper PresentationDocumento14 páginasBioethics Animal Paper Presentationtimar iaAinda não há avaliações