Escolar Documentos
Profissional Documentos
Cultura Documentos
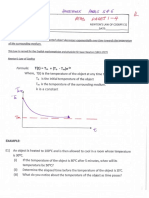
1991 Zhu and Sverjensky GCA
Enviado por
Victor ValdiviaDescrição original:
Direitos autorais
Formatos disponíveis
Compartilhar este documento
Compartilhar ou incorporar documento
Você considera este documento útil?
Este conteúdo é inapropriado?
Denunciar este documentoDireitos autorais:
Formatos disponíveis
1991 Zhu and Sverjensky GCA
Enviado por
Victor ValdiviaDireitos autorais:
Formatos disponíveis
GwElrimica et C~~rn~imim Acfa Vol. 55, pp.
18374858
Copyright 0 1991 Paganon Rw pk. Printed in U.S.A.
P~tioning of F-Cl-OH between minerals and hydrothermal fluids
CHEN ZHU and DIMITRI A. SVERJ ENSKY
Department of Earth and Planetary Sciences, The Johns Hopkins University, Baltimore, MD 212 18, USA
(Received September 20, 1990, accepted in revisedform April 9, 199 1)
Abstract-A thermodynamic analysis of F-Cl-OH partitioning between minerals and hydrothermal fluids
has resulted in the retrieval of standard-state Gibbs free energies for fluormuscovite, fluorphlogopite,
fluorannite, fluortremolite, fluortalc, hydroxya~tite, fluorapatite, chlorapatite, and chlorannite from hy-
drothexmal experimental studies. Standard-state entropies, heat capacities, and volumes are either derived
from experimental studies or estimated following HELCEXJN et al. ( 1978). The derived thermodynamic
properties are internally consistent and consistent with the thermodynamic properties of minerals and
aqueous species from BERMAN ( 1988,1990), SVERJENSKY et al. ( 199 la), SHUCK and HELGESON ( 1988 ) ,
and SHUCK et al. ( 1989 1, and therefore can be extrapolated over a wide range of temperatures and
pressures for apportion to geochemistry, igneous and rne~o~~c petrology, and ore deposits.
The derived standard-state thermodynamic properties for F and Cl endmember phases provide a basis
for predicting the fluoride and chloride concentrations of former aqueous fluids from the measured F
and Cl concentrations in minerals. Speciation and solubility calculations simulating F and Cl partitioning
between minerals and hydrothermal fluids in the systems NazO-K@-AlzO,-SiO&F-HzO and Na@-
K~~~~O~-SiO~H~-H~O, and sy&ems containing apatites, show that the partitioning is a strong function
of temperature, pressure, and 5uid composition. Increase of temperature favors partitioning of F into
fluids with respect to minerals, while it favors partitioning of Cl into an&e. The decrease of both pressure
and pH of fluids favors partitioning of Cl into annite with respect to fluids. In addition to predicting
fluoride and chloride concentrations in hydrothermal fluids, the results of this study enable mass transfer
calculations including both F-Cl-OH partitioning and metal complexing of halides during water-rock
intera~ons in a variety of geological systems.
INTRODUCTION
~u~RINE AN0 CHLORINE ARE common minor or tmce con-
stituents of micas, ~phi~l~, and apatites in which they
substitute for hydroxyl (DEER et al., 1966). Knowledge of
the thermodynamic properties of F and Cl endmember min-
erals is very important in assessing the influence of F and Cl
on mineral stability under metamorphic conditions (ABER-
CROMBIE et al., 1987; DUFFY and GREETED, 1979; PE
TERSEN et al., 1982; RICE, 1980a,b; VALLEY et al., 1982) and
in evaluating the effects of F and Cl on the evolution and
partitioning of ore metals into magmatic hydrothermal 5uids
( BURNHAM, 1979; CANDELA, 1986; CANDELA and HOLLAND,
1984, 1986; WEBSTER and HOLLOWAY, 1990, MA~NG,
1981; WYLLIE and TUTTLE, 1961, 1964). Knowledge of F-
Cl-OH partitioning between various minerals as a function
of temperature and pressure will also help to constrain the
F, Cl, and P budgets of the Earth (NASH, 1984; SMITH, 198 1) .
Fluorine and chlorine are also ubiquitous con~tuen~ of
hydrothemal fluids in the Earths crust. Their importance
in metal transport through the formation of aqueous metal
halide complexes ( HELGESON, 1964, 1969; Barnes, 1979;
White, 1981) and in metasomatism through ionization of
the acids HF and HCl (MEYER and HEMLEY, 1967) has led
to considerable effort to establish the fluoride and chloride
contents of hydrothermal fluids associated with magmatic,
metamorphic, and ore-forming processes. Most hydrothermal
fluids cannot be directly sampled, however. Those which can
be sampled, such as geothermal fluids, hot springs, and oil-
field brines, are limited to low temperature-low pressure re-
gimes (HENLEY, 1984, WHITE, 198 1). Moreover, in many
cases, it is often doubtful that collected samples truly represent
the in situ conditions at which minerals formed. The most
valuable line of evidence comes from studies of fluid inclu-
sions in minerals (e.g., ROEDDER, 1979). However, in many
situations such evidence is not definitive: analysis of freezing-
point depression data may yield data on salinity or chlorinity
of the fluids, but these data are ambiguous in multicomponent
systems; bulk crushing and ieaching techniques may give
constraints on the 5uoride and chloride contents of the fluids,
but these constraints have little value when there are multiple
generations of inclusions. Moreover, fluid inclusion studies,
so far, have hardly reported any estimation of fluoride con-
centrations in hydrothermal fluids. As a result, the lack of
knowledge of chloride and 5uoride inanitions in ancient
fluids greatly hampers, for instance, the characterization of
the compositions of aqueous metamorphic electrolyte solu-
tions (FERRY and BURT, 1982).
As an alternative and complementary method to the above
approaches, the fluoride and chloride ~ncen~tions of hy-
drothermal fluids can be predicted fmm the F and Cl con-
centrations of minerals (HOLLAND, 1956; MCINTIRE, 1963;
SVERJENSKY, 1984, 1985 ). Recent studies of F and Cl in
igneous systems (AGUE and BRIMHALL, 1988a,b, BAILEY,
1977; SPEER, 1984; Tu et al., 1979), in rne~o~~c rocks
( ABERCROMBIE et al., 1987; FERRY, 1989; GUIDOTTI, 1984;
KAPUSTIN, 1987; MORA and VALLEY, 1989; PETERSEN et
al., 1982; RICE, 1977a,b, 1980a,b; VALLEY et al., 1982;
YARDLEY, 1985), and in ore deposits (GUNOW et al., 1980;
KWAK and ASKINS, 198 1; MOLLING, 1989; MUNOZ, 1984;
WHITE et al., 1981) have provided abundant observational
evidence for F and Cl contents in minerals. Experimental
1837
1838 C. Zhu and D. A. Svejensky
studies of the partitioning of fluoride and chloride between
minerals and hydrothermal fluids ( DUFFV and GREENWOOD,
1979; KORZHINSKIY, 198 1; MUNOZ and EUGSTER, 1969;
MUNOZ and 1977; MUNOZ and SWEN-
SON, 1981; TROLL and GILBERT, 1972; VOLFINGER et al.,
1985; VOLFINGER and PASCAL, 1989; WESTRICH, 1978,
198 1) are by necessity limited to equilibrium reversals in the
pressure-temperature-compositional space that can be at-
tained in the laboratory. The theoretical work necessary to
maximize the use of available experimental data has lagged
behind. As a consequence, F and Cl analyses of natural min-
erals have been interpreted only qualitatively, in terms of
indicating fluoride and chloride concentrations of former
aqueous fluids. Even these qualitative indications could be
dangerous because the partitioning of F and Cl is very sen-
sitive to temperature, pressure, and fluid pH and composition
(see below).
In this paper, we present a theoretical study of the ther-
modynamics of F and Cl partitioning between minerals and
fluids, which provides a quantitative basis for predicting the
fluoride and chloride concentrations of former aqueous fluids
from the measured F and Cl concentrations in minerals.
Standard-state thermodynamic properties for fluoride and
chloride endmember minerals-fluorphlogopite, fluormus-
covite, fluorannite, fluortremohte, fluortalc, hydroxyapatite,
fluorapatite, chlorapatite, and chloranmte (see mineral ab-
breviations and formulae in Table 1 ), have been retrieved
and estimated from hydrothermal exchange experiments
(ibid). The thermodynamic data retrieved and estimated here
are internally consistent and consistent with the thermody-
namic database for minerals from BERMAN ( 1988, 1990)
and from SVERJENSKY et al. ( 199la); with the thermody-
namic database for aqueous species of HELGESON and KIRK-
HAM ( 1974a,b), HELGESON and IRKHAM ( 1976)) HELGE-
SON et al. ( 198 1) , SHOCK and HELGESON ( 1988 ), SHOCK et
al. (1989), and TANGER and HELGEZ~ON (1988); and with
thermodynamic properties for water of HAAR et al. ( 1984 ) .
Our set of thermodynamic data for F and Cl endmember
minerals is the first such set to be internally consistent and
consistent with the thermodynamic properties of aqueous
species.
This is important for two reasons. First, increasing numbers
of igneous and metamorphic petrologists, and economic
geologists routinely use the above thermodynamic data sets
in their research. However, interpretations of experimental
calibrations of F/OH and Cl/OH exchange have not always
used consistent thermodynamic properties for minerals (e.g.,
MUNOZ and LUDINGTON, 1974). Second, all applications of
the available experimental data on F/OH and Cl/OH par-
titioning have interpreted the experimental data in terms of
fugacities of HF, HCl, and HzO. The fugacities of HF and
HCl do not directly reflect the total fluoride and chloride
contents of the aqueous solutions. To calculate the total flu-
oride and chloride concentrations from these fugacity values,
we stiff first need to know the activities of aqueous HF and
HCl . By basing our retrieval on the thermodynamic prop
et-ties of aqueous HF o and HCl , it will be simpler to relate
measured concentrations in minerals to total concentrations
in hydrothermal fluids (see below). This approach also allows
Table 1. Mineral abbreviations and formulae
Mineral abv. FOlllIId~
Ab
NaAISi3Og
Albite
Andalusite
Annite
Anorthite
Hematite
K-feldspar
MaglXtitL?
MU.SCOVite
Paragonite
Phlogopite
Qua
Sillimanite
T.lC
Tremolite
Wollastonite
Fluorapatite
Hydraxyapatite
Chlarapatite
Fluorannite
Chlorannite
Fluomwscovite
Fluorphlogopite
Ftuortalc
Fluortopaz
Fluortremolite
And
AnIl
An
Hem
Kf
M%
MS
9
Phl
Qu
Sill
TC
Tr
WO
FAP
HAP
ClAp
FtUXl
ClZUlll
Fms
Fphl
FtLIlC
Ftopaz
FU
Al2SiO5
KFe3[AlSi3Olt$oH)2
CaAl2Si20S
Fe203
KAlSi3OS
Fe?04
KAl@lSi30lol(oH)2
NaAl~[AlSi30l~l(OH)~
KMg~[~Si~Otcl(OH)~
SiO2
Al2SiO5
MgS[Si40t01(OH)2
Ca$@sSis oU(OH)2
CaSiO3
Ca@4)3F
Cag(P04)3oH
Ca5(Po4)3Cl
KF~3[~Si301t$F)2
KFe3[AlSi30lt$C1)2
KAl2[~Si3010l(F)2
KMSs[AtSiSotcl0j)2
MSs[St4otcl(F)2
A12ISiO&F)2
&$SsSis 92(n2
one to incorporate the results of this study into existing spe-
ciation and mass transfer computer codes (e.g., EQ3/6.
WOLERY, 1983,1984; WOLERY et al., 1984), permitting mass
transfer calculations including both F-Cl-OH partitioning and
complexing of halides during water-rock interactions in a
variety of geological systems.
RECALCULATION OF HF AND HCL BUFFER SYSTEMS
Only those hydrothermal experiments in which activities of HF
and HCI are known can be used for retrieval of thermodynamic
properties for F and Cl endmember solid phases. The accuracy of
retrieved thermodynamic data for F and Cl endmember minerals
depends on calculated values of the thermodynamic activities of HF
and HCI imposed by buffer reactions. The calculation of activities
of HF and HCl , however, demands that internally consistent ther-
modynamic properties be used. In this section, we review the tradi-
tional buffer experiments used to control HF and HCl activities
and reevaluate them based on internally consistent thermodynamic
data for minerals and aqueous species.
HF Buffers
The anorthite-thtorite-silhmanitequartx ( AFSQ) and wollastonite-
fluoritequartz (WFQ) buffers invented by MUNOZ and EUGSTER
( 1969) have been used to study F-OH exchange reactions in mus-
covite, phlogopite, anmte, tremolite, and talc. The portlandite-fluorite
(PF) buffer has been used by KORZHINSK~Y ( 198 1) to study apatite.
These buffers can be represented by the following reactions:
CaA12S~208 + ZHF;,,
anorthite
= CaF2 + AlaSiOr + SiOr + Hz0 ( 1)
fluorite sillimanite quartz
Partitioning of F-Cl-OH in minerals 1839
CaSiOr + 2HF&, = CaFs + SiOs + HZ0 (2)
wollastonite fluorite quart.2
Ca(OH), + 2HF&, = CaFr + 2HrO. (3)
portlamlite fluorite
Expressions of the law of mass action for reactions ( 1) and (2) result
in
and for reaction (3) the corresponding equation is
log &uffa = 1% -$g
( )
(4)
(5)
where lo8 K&&I. is the equilibrium constant of either reaction ( 1 ),
( 2 ) , or ( 3)) assuming unit activities for all crystalline phases at the
pressure and temperature of interest. The symbols unr. and au,
represent activities of aqueous species HF and of water, respectively.
The standatd states for aqueous species are hypothetical, one-molal
solutions referenced to infinite dilution at the temperature and pres-
sure of interest. The standard-state for water is pure water at the P
andTofinterest.Thestandardstatsforsolidphasesarepurecrystals
at the P and T of interest. Values of log K&t_ at the experimental
temperatures and pressures were recalculated in the present study
using standard-state thermodynamic properties for the pertinent
minerals taken from BERMAN ( 1988) with the exceptions of fluorite
and portlandite which are from CODATA (GARVIN et al. 1987).
~epropertiesofaqueousHFweretakenfromSHoCKetal.(1989).
Figure 1 shows the dependence of log Kw on pressure and tem-
perature for reactions ( 1)-f 3).
All previous interpretations of HF buffers were based on the cui-
cuhted H20/HF fugacity ratios (DROLL and SECK, 1984; DUFFY
and GREENWOOD, 1979; KORZHI NSKI Y, 1981; MUNOZ and EUGS-
TER, 1969; MUNOZ and LUDI NGTGN, 1974,1977), such as for AFSQ
and WFQ:
1&2kb
0 -
0 200 400 600 800 1000
Temperature%
FI G. 1. Dependence of log K,, defined by anorthite-fluorite-
sillimanitequarti ( AFSQ) and wollastonite-Iluoritequartx ( WFQ)
or defined by portlandite-fluorite
on temperature and pms-
sure. The lines for 1 and 2 kb coincide.
where fu* and fnr are fugacities for water and HF, respectively.
However, to interpret experimental F-OH exchange reactions, such
as Eon. ( 12) below, the values of fugacity ratio
log
( 1
fare
f&F
are needed, which differ from the right-hand side of Eon. (6) by
containing an additional J;1* term in the numerator. Therefore, val-
ues of J;1r had to be calculated from the Eqn. (5) of MUNOZ and
EUGSTER ( 1969 ) :
where Y&, r&, and rup are fu8acity coefficients for pure water,
I& and HF, respectively. Pw is the total pressure of gas mixtures
in the systems. The caltntahions of fugacities of HF using Eon. (7)
depend on the assumption of ideal mixing of real O-H-F gas species.
They also require knowledge of fu,. This is the mason that MUNOZ
and EUGSTER ( 1969) and all others used an oxygen buffer in con-
junction with HF buffers in their experiments. However, it is much
simpler and more straightforward to define the HF buffers in terms
of ~e~~~arnic activities of aqueous I-IF o (or HCl o ) at the ex-
perimental P and F, as in Eqs. (4) and (5). MUNOZ and EUGSTER
( 1969) showed that HF is only a small fraction relative to I-Is0 in
the experimental systems. Raoults law is probably obeyed and the
activity of water in these experimental systems can be assumed to
be unity. Therefore, the recalculated buffers can be directly used in
in~~reting the F-OH exchange experiments. In our recalculation of
the HF buffers, the assumption of ideal-mixing of gas species is no
longer necessary. They also omit the requirement of knowledge of
& which eliminates any possible problems inherited from oxygen
buffer calibrations. In addition, it is also computationally advanta-
geous. Applications to relate the measured concentration of F ( or
Cl) in minerals to the total Iluoride (or chloride) inanition in
former aqueous fluids require speciation calculations of lluoride (or
chloride) in the aqueous phases. The results of this study can be
easily incorporated into existing speciation and mass transfer com-
puter codes (EQ3/6, WOLERY, 1983; 1984; WOLERY et al., 1984).
It should be noted that our assumption of a unit activity &e.ficient
for HF for HCl ) is ~e~~~a~~v eouivalent to the assumn-
tion of i&al-mixing of real F--H-O & ~~MUNOZ and EUGSTER
( 1969). Both assume that the Lewis and Bandall rule is obeyed.
However, the assumption of unit activity coefficients is only invoked
in the speciation calculations in this study. It is not necessary for
recalculation of HF and HCl buffers (see Eons. 4 and 5). In contrast
to the ~culations of HF bu&rs in terms of f&city ratios (Eqn. 6)
which rely on thermodynamic properties of pertinent gases, our re-
calculation of the HF buffers and, therefore, our retrieval are based
on the standard-state thermodynamic properties of aqueous HF
(and HCl ) and water.
The validity of our recalculation of HF buffers, the results of which
are displayed in Fig. 1, can be dernon~~ by comparing them to
experimental measurements for the WFQ buffer system (DROLL and
SECK, 1984). The solid curve in Fig. 2 represents the results of spe-
ciation calculations carried out in the system MgO-SiOs-CaO-HF-
Hz0 from 400 to 750C at 1 kbar incorporating the thermodynamic
properties for minerals cited above, together with standard free ener-
gies of aqueous species from SH& and HELGESON ( 1988) and
SHOCK et al. f 19893. The aoueous sneeiation model included the
species F-, HF, HF:, CaF, and iiF:- (Table 2). We assumed
unit activity coefficients for all neutral species including HF& and
unit activity for Hz0 (this assumption applies to all speciation cal-
culations throughout this study). The pH of the aqueous phases was
calculated Born the charge balance.
The results ofthe speciation cakulations (Table 2) show that above
55OC, I-IF&, accounts for more than 99% of total fluoride concen-
1840 C. Zhu and D. A. Sverjensky
4ooo .,--,-,-I-*
ceSiOs + 2HF&$) = CaFz +sio2 + Hro 1 kbar
wollastonite
a,
fluorite quartz
:
400 500 600 700 800 900 1000
TemperatureC
FIG. 2. Comparison of total dissolved fluoride for &ids in equi-
librium with the WFQ buffer from speciation calculation (solid curve)
with that from experimental measurements (symbols). Solid circles
represent experiments approaching equilibrium from F supematu-
ration; open circles from F undersaturation. Uncertainties associated
with F- analysis are not specified in DROLL and SECK ( 1984). The
dashed line was predicted from the log KS of the buffer reaction as-
suming all dissolved fluoride can he represented by HF&.
tration in the WFQ system. At lower temperatures (400-550C),
the species F- and CaF+ appear to account for about I- 16% and t -
7% of total d&oh& F, respectively. However, the complex HF; is
of the order of 10 molal and SiF:- is of the order of 10m20 molal
at all temperatures. The predicted total fluoride contents of the fluids
are in good agreement with the experimental data (Fig. 2).
These results allow us to further predict the total fluoride concen-
trations in the fluids by evaluation of reaction (2) atone at higher
temperatures. The predicted fluoride concentrations are represented
by the dashed line in Fig 2, and they agme well with the experimentally
determined fluoride concentrations. It suggests that ah the thermo-
dynamic properties used in the calculation of the WFQ buffer are
reliable over a wide range of temperatures. It also suggests that our
aqueous speciation model is adequate to represent the F speciation
and that HF&,) can be regarded as the dominant F species in the
system MgQ-SiOz-CaFz-HzO. The agreement of F-OH exchange re-
actions using both WFQ and AFSQ (see below) suggests that the
calculations for the AFSQ buffer are also valid.
HCl Buffers
Hydrothermal Cl-OH exchange experiments using the muscovite-
K-feldspar-quartz-HC-KC1 buffer have been conducted by MUNOZ
Table 2. Results of specie&m calculations for the WFQ-buffer system
at 1.0 kbar
ITT 1 Total F-
I 56 species
400
4.50
ppm molal(l03) F- HF HF2- CaF+ siF6
11 0.512 16 77 UO.l 7 <CO. 1
24 1.246 5 92 ro. 1 3 ao. 1
500 55 2.891 2 97 <co.1 1 ao. 1
508 62 3.270 1 98 no. 1 1 <CO. 1
525 80 4.206 2 98 no. 1 <o. 1 X0.1
550 111 5.813 1 99 a0.1 <o. 1 <co. 1
575 155 8.181 <o. 1 100 40.1 <o. 1 ao. 1
598 210 11.085 <o. 1 100 ro. 1 <o. 1 <co. 1
650 367 19.340 co. 1 100 ao. 1 <o. 1 ao. 1
688 551 29.011 <o. 1 100 <co. 1 <o. 1 x0.1
,750 997 52.472 co. 1 100 ao.1 co.1 ro. 1
Dissociation um%mits for CaF+ @SF-+ = Ca2+ + F-j were calculated using
thermodynamic properties from Sverjsnsky, Shock, and Helgeson (in prep.).
They are (at 400. 450, 5G0, 5.50. 600, 650, 700. 75OC and 1 kbw):
-4.20 -4.86 -5.48 -6.13 -6.79 -7.40 -7.88 -4.19
Table 3 Results of speciation calculations for the KMQ-buffer
l=c
FB 168 BHCIQ
Bspecies
Cl Hcl* KQ
445 4.05 -2.34 65.35 0.83 33.82
498 4.26 -1.08 60.42 1.21 38.37
540 4.49 -1.26 52.34 4.66 42.83
547 4.53 -0.94 49.67 5.80 44.52
-575 4.81 -0.72 61.97 9.53 28.50
Total molality of 2.0 Cl-; pressure of 1.0 kbar
and SWENSON ( 198 1) and VOLFINGER and PASCAL ( 1989) for annite,
muscovite, and phlogopite. When the total chloride concentrations
are fixed in the experiments, the activities of HCL are also fixed
and can he calculated consistent with the reaction,
0.5KA12[AISi~0,,,](OH)z t 3SiOr + KCI
muscovite quartz
= 1.5KAISi~0, + HCI. (8)
K-feldspar
Table 3 shows the results for speciation of a 2.0 molal chloride
fluid at temperatures and pressures relevant to the study of MUNOZ
and SWENSON ( 198 I). The~~ynamic properties for quartz are
from BERMAN ( 1988 ) and for K-bearing minerals, HCl, and KC1 D
from SVERJ ENSKY et al. ( 199Ia). The KMQ buffer system has heen
discussed in great detail in SVERJ ENSKY et al. (1991a).
KORZHINSKIY ( 1980, 198 1) used the Ag-AgC1 buffer, in conjunc-
tion with a number of oxygen buffers, to control the activity of HCl
for OH-Cl exchange reaction in annite and apatite. For the methods,
equations, and review of experimental work for, and existing proMems
with, the Ag-AgC1 buffer, the reader is referred to the papers of Eucis
TER et al. ( 1987) and CHOU ( 1987). However, data in the calibrations
of KORZHINSKIY ( 1980, 198 1) of the Ag-AgCl buffer are quite scat-
tered and some of them deviate greatly from other published data
(CHOU, 1987; CHOU and FRANTZ, 1977; EUGSTER et al., 1987;
FRANTZ and EUGSTER, 1973; FRANTZ and POPP, 1979; LUCE et al.,
1985). In addition, the likely presence of silver chloride complexes
(EUGSTER et al., 1987; SEWARD, 1976) and species such as
HAgCl,O ( RUAYA and SEWARD, 1987) in the fluids buffering HCI O
has not heen adequately addressed. Therefore, only those experimental
results of KORZHINSKIY ( 1980, 1981) that are in agreement with
other pub~sh~ data were used to retrieve p~limi~ ~e~~~arni~
properties in the present study. The calibrations of KORZHINSKIY
( 1980, 1981) of the Ag-AgCI buffer using the hematite-magnetite
oxygen buffer at 600C and 2 kbar are similar to those Of THOU and
FRANTZ( 1977)and LUCE~~al. ( 1985);and thecahbrationsofKOR-
ZHINSKIY ( 1980, 198 1) of Ag-AgCl buffer using the Ni-NiO oxygen
buffer at 6OOC, 7OOC, and 2 kbar agree with those of THOU and
FRANTZ ( 1977) and MOECHER and CHOU ( 1990). Cl-OH exchange
data using the Ag-AgCl buffer in conjunction with the above oxygen
buffers and at the above temperatures and pressure were used to
constrain a value of standard Gibbs free energy for chlorapatite. Be-
cause of problems with the experiments of KORZHINSKIY ( 198 1)
and of problems in Ag-AgCl buffer in general, as pointed out by
EUGSTER et al. ( 1987) and by CHOW (pen comm.), this value is at
best a preliminary quantity.
REVIEW OF SOLID SOLUTION MODEL
FOR (OH, F, Cl)
The effective ionic radius and electronegativities for F- and OH-
are very close. We therefore assumed ideal site mixing of F, OH in
ah minerals considered here except for talc. ABERCROMBIE et al.
(1987), KORZHINSKIY, (1981), MUNOZ and LUDINGTON (1974,
1977), and WESTRICH ( 1978, 1981) provided evidence to support
ideaI mixing of F-OH in museovite, phlogopite, annite, tremolite,
and apatite. Both experimental studies on talc ( DUFF~ and GREEN-
WOOD, 1979) and studies of natural talc ( ABERCROMBIE et al., 1987)
found that non-ideal mixing is evident. We adopted the regular ao-
lution model of ABERCROMBIE et al. ( 1987). The reason for the
Partitioning of F-Cl-OH in minerals 1841
deviation of F-OH mixing properties of talc from those of micas and
tremolite might lie in its crystal structure, which is not just a simple
interlayer-cationdeficient analogue of micas (EVANS and GUGGEN-
HEM, 1988).
Assuming ideal mixing of (F,OH ) on the hydroxyl site for biotite
does not imply ideal mixing across the hydroxyl site and octahedral
site. Ample experimental and observational data suggest strong cor-
relations between the (Mg,Fe) occupancy on the octahedral site and
(F,OH) occupancy on the hydroxyl site in biotite (JACOBS and PARRY,
1979; MUNOZ and LUDINGTON, 1974; MUNOZ, 1984; PARRY and
J ACOBS, 1975; SISSON, 1987; VALLEY et al., 1982). MUNOZ (1984)
used the reciprocal solid solution model of WOOD and NICHOLLS
( 1978) to take account of this cross-site interaction. Application of
the model can be easily achieved by using the thermodynamic prop
erties for fluorphlogopite and fluorannite obtained in the present
study. Similar cross-site effects on F-OH substitution are expected in
most minerals discussed in this study.
The radius of Cl- ( 1.8 1 A) is much laraer than that of OH- ( 1.35
A) and F- ( 1.30 A) (SHANNON, 1976). Non-ideal mixing of d-OH
and Cl-F is therefore more probable in all minerals. However, the
non-ideality cannot be evaluated before additional experimental ev-
idence and more highquality chemical analyses of natural samples
become available. Following MUNOZ and SWENSON ( 198 1 ), VOL-
FINGER et al. ( 1985), and VOLFINGER and PASCAL (1989), we also
assumed ideal mixing of OH-Cl. Any application of thermodynamic
data on Cl-OH partitioning to natural systems depends on this as-
sumption.
REGRESSION AND ESTIMATION OF C;, So, AND p
FOR F AND Cl ENDMEMBER MINERALS
The available experimental data to be used for the retrieval
of thermodynamic proper;ies for F and Cl endmember phases
are limited. Consequently, following approaches advocated
by BERMAN and BROWN ( 1985), BERMAN ( 1988), HELGE-
SON et al. ( 1978), HOLLAND and POWELL ( 1990), and Pow-
ELL and HOLLAND ( 1985 ) , we limit our retrieval to the stan-
dard Gibbs free energies of the endmember phases only. This
requires estimation procedures for the standard-state heat
capacities, entropies, and volumes. The validity of these es-
timates can be seen in the figures discussed below showing
the predicted temperature dependencies of the retrieval
curves.
Heat Capacity
The BERMAN and BROWN ( 1985) heat capacity function
for minerals adopted in this study is defined by
Cp = k, + k,T-. + k2T-2 + k,T-
(9)
where k, and k2 are constrained to be less than or equal to
zero. Equation (9) was used in the present study to regress
calorimetric measurements of high temperature heat contents
and low temperature heat capacities, resulting in values of
k,,, k,, k2, and k3 for fluorphlogopite, fluortopaz, fluorite,
fluorapatite, and hydroxyapatite (Table 4). Following BER-
MAN and BROWN ( 1985), the regression calculations were
weighted to emphasize the more accurate low temperature
adiabatic measurements, and the Cp functions from the high
temperature heat content measurements were constrained to
join smoothly with the low temperature heat capacity data.
Where heat capacity data were lacking, values of C, were
estimated assuming the standard molar heat capacities of re-
actions among oxides and silicates of the same or a similar
structural class to be zero ( HELGESON et al., 1978 ) according
to,
where C$,r,T,; represents the molar heat capacity of the mineral
to be estimated, and rii,, and ii;, represent stoichiometric coef-
ficients of the ith and ilh species in the rth reference reaction.
Using Eqn. ( lo), standard-state molar heat capacities for
fluormuscovite, fluorannite, fluortremolite, fluortalc, chlor-
apatite, and chlorannite were calculated based on the refer-
ence reactions summarized in Table 5. The estimated values
of C$ for each mineral were then regressed with Eqn. (9) as
described above yielding the coefficients listed in Table 4.
Volume and Volumetric Function Coefficients
Standard-state molar volumes at 25 C and 1 bar for F and
Cl endmember minerals are taken from the crystallographic
literature (Table 6). In the absence of good quality volume
data ( MUNOZ, 1984), the volume of fluormuscovite was as-
sumed to be the same as muscovite. X-ray diffraction data
of YODER and EUGSTER ( 1955) seem to support this as-
sumption, but their significance is doubtful because additional
phases of topaz, sanidine, and others present in their sample
may render interpretation ambiguous. However, MUNOZ
Table 4 Regression and estimation of Cp* coefficients for some minerals
Mineral
ko kt(x1.0-2) k2(xl.0-s) k3(xl.O-7) Ranges (T K) AADt Source of data
fluorphlogopite 156.24 -12.369 0.000 -1.758 256-296 0.29 Kelley et al. (1959)
400- 1609
fluorite 25.48 -1.529 0.000 -1.327 256-297 0.69 Todd (1949)
370-I 196 Naylor (1945)
fluortopaz 65.43 -3.184 -19.241 23.824 254-800 0.01 Barton et al. (1982)
fluorapatite 159.84 -10.837 0.000 -17.838 250-1582 0.40 Egan et al. (1951)
hydroxyapatite 181.36 -15.489 0.000 10.706 250-14719 0.31 Egan et al. (1950)
fluormuscovite 162.61 -15.573 0.000 2.643 273-967
fluorannite 177.54 -16.294 0.000 10.001 300-1000
fluortremolite 304.17 -22.690 -25.138 24.944 300-680
fluortalc 144.06 -11.393 0.000 -6.993 300-639
chlorannite 165.02 -11.377 -20.854 32.907 298-666
chloraoatite 158.67 -10.602 0.000 -16.497 298-1045
*Cp=cal.mol-1. K-1;
tAverage absolute percent deviation of tabulated Cp function from experimental measurements;
I non-weighted regression (250-1471 K) and Cp function good for 298-1471 K,
1842 C. Zhu and D. A. Svejensky
Table 5. Summaq of reference reactions for estimation of entropy and heat capacity
Miieralaame Formula Reference reaction for estimate
fluoriremoiite C;lzMgSSig OzfFfz CqMgSSig C&&t32 + KMg3~~Si3Olol(O~ = CazMggSig O&OH)2 + KMg3[~Si301~~)2
fluorannite
~cSWSi3O~ol~ Kt+#.ISiSO~ol(F)2 + KMg~~~S~~O~~1~OH)2=KFe~lA~Si~O~ol~OH~~ + KMgSWlSiSOto102
fluormuscovite
K&WSiSOR@&, K&[A1SiSOR$F)2 + KMg~~~S~~O~ol~OH)~=K~~~A1Si~O~ol~OH~~ + KMg3fAKii3OlolcF)2
fluoaatc
MgSlS~4O~o102 Mg3[Si40101~ + KMg~~~S~~O~~l~OH)~=Mg~~S~~O~offOH)2 + KMg3IAiSi3O~ol~)2
chloranoite
~e3[AISi3O~Ol(Cl)2 ~e3~AlSi30~01(~~)2 + Mg(OH)2 = ~e3~AISi30~01(0~2 + Mg(C02
chlorapatite
CyPO4)3CI Ca5tPO4)3CI + l/2 CaF2 = Cas(PO4)3F + 112 CaC12
cl> This reference reaction is the same reaction used by Tacker and Stormer (1989).
( 1984) reviewed crystallographic data which suggest that the
lack of significant volumetric change in muscovite with flu-
oride substitution might lie in the OH bond direction in
dioctahedral micas. Complete substitution of F for OH in
phlogopite, annite, tremolite, and humites (DUFFY and
GREENWOOD, 1979) reduces their molar volumes by 2.23 to
3.40 cm3/mol. Even if the volume of muscovite is reduced
by 3 cm3/mol, errors in estimated entropy are within an
uncertainty of 1% (see below). The molar volume for chlor-
an&e was estimated from volumes of FeC12 and Fe(OH)z
because both have layer structures simihir to the octahedral
layer in annite. Assuming that the difference between the
volumes of FeC& and Fe(OHb is the same as that between
chlorannite and annite leads to the equation
IGu, = I?Ll, + GL, - i/&ou,,.
In light of the paucity of thermal expansion and compression
data for F and Cl endmembers, volumetric coefficients of the
volume function of BERMAN ( 1988) for F and Q endmember
minerals are assumed to be the same as their hydroxyl coun-
terparts as a first approximation.
Entropy
Sided-site third law entropies at 25C and 1 bar for
fluorphlogopite, fluortopaz, hydroxyapatite, and fluorapatite
have been measured experimentally ( BARION et al., 1982;
EGAN et al., 1950, 1951a,b; KELLEY et al., 1959). Standard-
state entropies for F and Cl minerals for which there are no
calorimetric measurements were estimated using the volume
correction scheme of HELGESON et al. ( 1978, Eqn. 62) with
the exception of the entropy of hydroxyapatite, which was
retrieved from hydro~e~~ exchange experiments (see be-
low). The reference reactions for the entropy estimates are
the same as those for the C:! estimates (Table 5). Entropies
for hydroxides and chlorides used in the estimations that are
not available from BERMAN ( 1988) were taken from ROBIE
et al. ( 1979) or GARVIN et al. ( 1987) (Table 7). No correction
for ferrous ion in fluorannite or chlorannite was .made
(HELGESON et al., 1978) because the electronic configuration
of ferrous ions in annite, fluorannite, and chlorannite is be-
lieved to be the same in all three phases. Any correction ap-
plied should cancel. HELGESON et al. ( 1978) claim that this
method has an accurary of -i- 1%.
Estimation of the standard entropies of endmember Cl-
silicates is subject to significant uncertainties because of the
lack of experimentally measured values. The calorimetric
value for sodalite appears to differ bawdy from the value
required by phase equilibria studies (SHARP et al., 1989).
Consequently, we used two independent methods for esti-
mating values of So for chlorannite. Based on the estimated
volume of 172.47 cm3/mol for chlorannite cited above, we
estimated a value of S$,, of 107.44 cala mol- - K- using
the method of HELGESON et al.) ( 1978). In contrast, using
the method of HOLLAND ( 1989) for comparison, the entropy
for chlorannite can be calculated from
Table 6. Summary of the standard state thermodynamic properties
for some F.CI end-member minerals at 25C and 1 bar
flUOtllUXCOVite
fluorpblogopite
nuorannite
fluortremolite
fluorapatite
hy~xya~tite
chiorapatite
tlUOII&
cbloranrdte
Formula va Sa
c
Mlf
K~r~t3Otol~z 140.87<t 71.47 -1380100
KN3WSt3Wo102 146.37 <= 80.4 <s -1449550
~e3[AtSt3OiolOz 150.75<3 100.93 -1191550
c~gss$Q22cnz 270.4@ 136.30 -28 16000
Ca5PW3 F 157.532<? 92.70 <= - 15399268
Ca~~4)3(OH) 158.223<7 95.30<9 -1505187
Casm04hCI 164.025c7 95.58 -1486000
&!3[WtoI(F)2 133.30<4 63.11 _ 1366400
KFe?lAISi?Olo(CI)? 172.47<5 107.44 _ 1092500
A@
-1461027
-1530761
-1265485
-2917526
-1630843
-1~~9
-1576783
-1447477
-1166152
a: cm3 moie; h: caI moles K-; L: Cal mole?; <l> assumed to be the. same as OH-muucoviie:
a> R&ii et al. (1979): <3> cakatated from at1 parameters of Muaoz and Ludinaum 11974); <4>B,f@
and Greenwe& (1979); <5> ass,,,& Pc,,bmni~= Pti. + V*w2-V*ww; <6> Heigesat et al. (1978);
~1, Sudarsaun aad Ywng (1978X <8> Staadard state for P is from CODATA (Gawin et at.. 1987):
&retrieved fran experimenu (see text).
Partitioning of F-Cl-OH in minerals 1843
Table 7. solids at 25C and 1 bar used
in this study which are NOT from Berman (1988.1990)
Mineral Formula
fluorite CaF2
V a so h AGot AH? Cpb
24.54 16.38< -280968< -293500<
(H%s-
H%s)
portlandite Ca(OH)2 33.0s 19.93< -235459< -214507 36.57<2
whitlockite E-c~~(Po& -922818 -9792474 49347<
WOWz
21 .07<3
lawrencite Fe@)2 39.46<
Ms(Ct)z
40.81 21.42 16.98
CaW2
25.90c2 17.35<2
E: cm3 mole ; p: cal mole- K-l; E: cal mole? ; <I> Robie et al. (1979); <2> CODATA
(Gavin et al.. 1987): <3> calculted from cell parameters in J CPDS 13-89: <4> calculated from
dolubility product df Gregory et al. (1974);
+ (S - Vhawencite
+ magnetic term ( 11)
where values of (S - V)i for microcline, ferrosillite, and tri-
dymite, and the magnetic term for Fe2+ were taken from
HOLLAND ( 1989 ) , and ( S - I)i for lawrencite was calculated
from ROBIE et al. ( 1979). We calculated a value of 107.53
cal - mol- -K-i which is only 0.09 Cal- mol- - K- larger
than that estimated from the method of HELGESON et al.
(1978). We adopted the value of 107.44 obtained from
HELGESON et al. ( 1978) for consistency with the other esti-
mates. Together with the estimated heat capacities, our es-
timate of So for chlorannite is consistent with the temperature
dependence of the Cl-OH exchange experiments (see below).
RETRI EVAL OF AG&,,290 FOR F AND Cl ENDMEMBER
PHASES FROM HYDROTHERMAL EXPERI MENTS
Hydrothermal F-OH exchange reactions involving a (OH,
F) bearing mineral, (Y, for example, can be written as
a( OH)2 + 2HF&, = (Y( F)2 + 2H20
(12)
where (u( 0H12 and (r( F)2 stand for the hydroxyl and fluoride
endmember of the phase (Y, respectively. The law of mass
action states that
KT,P= (-j-)/(z)
(13)
where KTpp is the equilibrium constant of reaction ( 13 ) , and
II,(r), and @u(ou)2 denote the activities of components a(F),
and (Ye in phase (Y. The standard state for LX(F)~ and
a( OH)* are pure endmember solids at the temperature and
pressure of interest.
Assuming ideal mixing of F-OH in (Y, Eqn. ( 13) can be
written as
(14)
From Eqns. (4) and ( 5 ) , and assuming unit activity of Hz0
in the AFSQ and WFQ systems,
.
(15)
Expressions similar to Eqns. ( 12) and ( 13 ) for Cl-OH ex-
change reactions can also be derived. Values of log K have
been recalculated in the present study from the experimentally
measured mole fractions of F and Cl in (Y and the equilibrium
constants of the corresponding buffer reactions at the exper-
imental temperatures and pressures (Tables 8 and 9). Stan-
dard free energies of reactions, AG$, are related to these
equilibrium constants by the relation
AG; = -2.303RT log K.
(16)
The standard free energies of reaction can be calculated from
the apparent free energies of formation at the temperatures
and pressures of interest, AG$,i,P,r, of each phase or species
i in reactions such as Eqn. ( 12), which can be expressed as
W,/P,T = AG~,i,ltar,298 - S~lbar,298( T - 298)
+
s
T
298
CgidT - T 2r8 $$ dT +
s s
P
VPdP (17)
1
where AGy,i,ihr,298 denotes the standard Gibbs free energies
of formation of the ith phase or species from its elements at
298 K and 1 bar. Note that we adopted the HELGE~ON et al.
( 1978) convention for the apparent free energy function in
this study, which is different from the BERMAN ( 1988, 1990)
convention. The difference between the two conventions is
that HELGESON et al. ( 1978) incorporated the entropies of
the elements at 298.15 K and 1 bar in defining their Gibbs
free energy function,
AG:,iti,2,29a = ~&a,,298 - T. A%m,m (18)
where A$,ih,,298 is the entropy of formation from elements
for ith phase or species. In the BERMAN ( 1988, 1990) con-
vention,
AG?,,h,298 = ~&bar,298 - T. %.ar,298 (19)
where S&2gs is the third law entropy of the ith phase or
species.
Method of Retrieval and Estimate of Uncertainties
Using Eqn. ( 17) and the experimental and estimated values
of 9, c,, and V discussed above, values of AG~,,~,2g8 for
F and Cl endmember phases were retrieved from hydrother-
mal F-OH and Cl-OH exchange experiments. Several meth-
ods of retrieval have been summarized in the literature in
1844 C. Zhu and D. A. Svejensky
T&k 8. Recalfulation of Lag KS of F-OH exchange redions
Run ## T-C P (bar) starting buffer Log Kbuffcr FI F+OH L0g K 20
compn LOgK
murcovitez Ms+2HFn=Fms+2Hz0 <I >
MSE-7 462 2000 OH
MSE-8 462 2000 %2F
MSE-I O 500 2000 CXi
MSE-12 590 2000 MSE-6 612 2000 z
MSE-5 612 2000 2.9% F
MSE-3 640 2000 M
MSE-1 690 2000 w
pblogopite: Pbl+WF=Fphl+2HzO <2>
P-30fOH) 730 1000
P-33(F) 730 1000
a4
F
P-12 550 2000
P-16 700 2000
P-15 775 2000
P-17 700 2000
aanite: Ann+2HF=Fano+2HzO <3>
FAN-41 601 1000
FAN-42 601 1000
FAN-114 454 2000
FAN-47 550 2000
FAN-46 550 2000
FAN-79 551 2000
FAN-50 607 2000
FAN-116 625 2000
FAN-115 671 2000
FAN-103 680 2000
FAN-16 698 2000
FAN- 15 702 2000
FAN-19 697 2000
talc: TctWF=Ftc+WzO <4>
TCO-3R 750 2000
TC60-6R 747 1990
tremdite: Tr+2HHF=FtrtLHzO <5>
846 1550
799 1990
791 1980
846 1550
799 1970
800 1990
700 1970
614 2000
594 990
qatite: HAp+HF=FAp+H20 ~6s
709 500
710 500
1000
1000
713 600 1000
714 600 1000
B 675 1000
711 500 2000
712 500 2000
715 600 2000
716 600 2000
721 500 4000
722 500 4000
723 600 4000
CSI
F
M
F
cw
z
ai
F
0-i
F
M
F
cl+
F
aI
CH
a+
F
E
F
F
F
HAP
PF
PAP
PF
HAP
PF
FAP
PF
HAP
PF
FAP
PF
HAP
PF
FAP
PF
HAP
PF
FAP
PF
HAP
PF
3.80 0.05 1.24 0.18
3.80 0.16 2.36 0.21
3.26 0.11 1.44 0.20
2.15 0.19 0.89 0.21
1.91 0.19 0.65 0.21
1.91 0.22 0.81 0.22
1.63 0.21 0.48 0.22
1.16 0.31 0.46 0.25
2.72 0.76 3.72 0.48
2.72 0.79 3.87 0.42
4.37 0.60 4.72 0.15
2.74 0.71 3.52 0 17
2.09 0.75 3.05 0.19
1.07 0.95 3.62 0.73
2.04 0.48 I .97 0.42
2.04 0.52 2.11 0.42
5.75 0.03 2.73 1.19
2.61 0.38 2.19 0.37
2.61 0.36 2.11 0.38
4.36 0.06 1.97 0.92
3.68 0.09 1.67 0.64
1.78 0.40 1.42 0.22
1.33 0.43 1.08 0.21
1.25 0.51 1.28 0.42
1.08 0.59 1.40 0.36
1.05 0.51 1.08 0.42
2.77 0.12 1.04 0.49
2.30 0.43 1.70t 0.15
2.33 0.45 1.66$ 0.16
1.65 0.47 1.53 0.47
1.90 0.24 0.89 0.52
1.92 0.32 1.26 0.32
1.65 0.86 3.21 1.35
1.91 0.60 2.26 0.69
1.90 0.70 2.61 0.33
2.74 0.95 5.35 1.56
3.61 0.71 4.38 0.57
2.19 0.84 3.66 1.25
4.19v 0.69 4.53 0.22
4.19 0.80 4.79 0.35
3.55 0.56 3.66 0.16
3.55 0.66 3.84 0.20
3.19 0.40 3.02
4.10 0.64 4.35 0.19
4.10 0.69 4.45 0.22
3.41 0.59 3.57 0.17
3.41 0.66 3.70 0.20
4.04 0.59 4.20 0.17
4.04 0.80 4.64 0.35
3.32 0.55 3.40 0.15
724 600 4000 FAp PF 3.32 0.74 3.77 0.27
Expcrimestrl data from <I > Mona and Ludiigtot.~(1977); Q> and O> MWAQZ md Ludiitoa (1974):
<4> Duffy and (ircsnwood (1979): cS> Troll and Qilbai (1972); <6> Korzhiiiy (1981): B: Bigger (1967).
Y112 log K (PF buffer)=log (aH20/aH~->for all oumbwr below in this column: tlqFlc=l.46. I ny~~=2.27:
t?:I n7Ftc1.39, lr~~~=2.52 calculated from Abercrembit CL rl. (1987):
recent years (e.g., BERMAN et al., 1985; BERMAN, 1988 ; HAAS
and tremolite) were constrained simultaneously. This method
and RSHER, 1976; HELGESON et al., 1978; HOLLAND
is justifiable because only one or two narrow reversed brackets
and POWELL, 1990; POWELL and HOLLAND, 1985). In the
present study, we retrieved the values of AGy,lbar,29g graphi-
were available for most exchange reactions. Many experi-
cally from the log K versus Tplots. These AGj,lbar,298 values
mental runs clearly indicated the reaction directions, but ap
parently did not get close to equilibrium. This situation pre-
secured the log K versus T cur ves passing through all pertinent
reversed experimental brackets and stayed on the equilibrium
cludes using the regression method. The limited experimental
data also only permit one-parameter retrieval of
side of all half-brackets, which were bounded by two standard
deviations of log K and temperature measurements (see be-
AGyp b,z98. The entropies, heat capacities, and volumes for
the F and Cl endmember phases were as determined or es-
low). Internal consistency was achieved when multiple re-
actions containing common phases (e.g., apatite, phlogopite,
timated in the previous sections. More. elegant treatments
(BERMAN et al., 1985; BERMAN, 1988; HAAS and RSHER,
Partitioning of F-Cl-OH in minerals
1845
Table 9. Recalculation of log Ks of Cl-OH exchange reactions
Run # TC P (bar) starting buffer
-log aHClo 1%
log K 2
compn (Wl) GWKOH) alogK
annite: ann+2HCI =clann+2H~0 cl>
50A 445 1000 0.00 2 2.01 -1.65 0.71 0.32
4lA 498 1000 0.00 1.62 -1.65 -0.06 0.32
47B 498 1000 0.00
57A 540 1000 0.30 2
1.62 -1.54 0.16 0.70
1.05 -1.71 -1.32 0.37
57B 540 1000 0.75
55A 547 1000 0.00 z
1.05 -1.35 -0.60 0.30
0.95 -1.67 -1.45 0.76
55B 547 1000 1.40 0.95 -1.25 -0.61 0.24
51A 575 1000 0.00 z 0.53 -1.69 -2.32 0.27
apatite: HAptHCEClAp+H~O <2>
432 600 2000 HAP A&l-Agt 1.47 0.53 1.99 0.15
433 600 2000 ClAp AnCl-Agt 1.47 0.58 2.05 0.17
Experimental data from <I> Munoz and Swenson (1981); <2> Korzhinskiy (1981).
twith hematite-magnetite buffer
1976; HELGESON et al., 1978; HOLLAND and POWELL, 1990;
POWELL and HOLLAND, 1985) are not warranted here.
The uncertainties in log KS for the exchange reactions re-
sulting from the analytical uncertainties (F and Cl) were es-
timated following general error propagation equations (e.g.,
ANDERSON, 1977; SHOEMAKER et al., 1981). For mineral-
fluid F-OH exchange reactions, uncertainties in log KS re-
sulting from the analytical uncertainty of F (or Cl) can be
calculated from
(20)
where uxF is the standard deviation of F mole fraction in a
mineral and ulwK is the standard deviation in log K of ex-
change reactions resulting from ax,. No provision for un-
certainty from log Kbu,rcr is provided in Eqn. (20) because no
uncertainties of thermodynamic properties for minerals from
BERMAN ( 1988, 1990) and for aqueous species from SHOCK
et al. ( 1989) are provided.
For mineral ( A)-mineral( B) F-OH exchange reaction,
L
u8K=ln(10)
x ~(XF,p_XJ + (XF(YXJ. t21)
Implicitly buried in both equations is the assumption of
x,, = 1 - XF
in all minerals. MUNOZ ( 1984) summarized possible prob-
lems with this assumption, but to assess such problems we
need more accurate analytical data.
The results of the retrieval calculations are shown in Figs.
3-8, 11, 12, 15, and 16, and Table 6. Values of AGT and
AH; for F endmember silicates retrieved in this study from
phase equilibrium studies differ from those of calorimetric
measurements generally within OS%, which is similar to that
for hydroxyl endmember silicates (BERMAN, 1988).
Fluormicas
Standard molar Gibbs free energies of formation from the
elements at 1 bar and 298 K for fluormuscovite, fluorphlogo-
pite, and fluorannite were retrieved from the hydrothermal
experimental studies of MUNOZ and EUGSTER ( 1969) and
MUNOZ and LUDINGTON ( 1974,1977 ) . The recalculated ex-
perimental results are listed in Table 8 and represented by
the symbols in Figs. 3-5, whereas the retrieval calculations
are represented by the solid curves. Comparison of the re-
trieved AH: of - 1,530,76 1 cal/mol for fluorphlogopite with
the calorimetric value of ROBIE et al. ( 1979) of - 1,527,936
Cal/ mol (based on ALLEY et al., 1959) gives a difference of
-2825 cal/mol, or -0.2%, which is within the general range
of +0.5% in the differences of Bermans retrieved AH: values
and calorimetric values derived from HF solution calorim-
etry. The value of - 1,5 18,487 cal/ mol from molten salt cal-
orimetry ( WESTRICH and NAVROTSKY, 198 1 ), however, is
12,274 cal/mol more positive than our value.
Fluortremolite
The value of AGy,,h,298 for fluortremolite was retrieved
from the F-OH exchange reactions between tremolite and
phlogopite (Fig. 6a) and between tremolite and apatite (Fig.
6b) calibrated by WESTRICH ( 1978; 198 1 ), and from F-OH
3.0, . I . I . I . , I I . ,
2 kb
i
Y
$
-I
Muscovite + PIiF = Fluormuscovite + H20
200 300 400 500 600 700 BOO 900
Temperature72
FIG. 3. Calculated (curve) and experimental (symbols) equilibrium
constants for the F-OH exchange reaction in muscovite buffered by
AFSQ as a function of temperature. The curve was generated using
the thermodynamic properties for fluormuscovite in Tables 4 and 6.
Solid symbols indicate experiments approaching equilibrium from
the F rich endmember; open symbols from the OH-rich endmember
(Table 8). Error bars are two standard deviations calculated as in
text.
1846
C. Zhu and D. A. Svejensky
Y
::
-I
Phlogopite + PHP(aq) = Fluorphlogopite + H20
6.0 -
5.0 -
4.0 -
3.0 -
1 kb
2 kb
2.0 . . . . 1
400 500 600 700 600 900
Temperature%
RG .4. Calculated (curve) and experimental (symbols) equilibrium
constants for F-OH exchange reaction in phlogopite as a function of
temperature and pressure. Asterisk and open circle represent exper-
imental results without knowing reaction directions. Other symbols
as in Fig. 3.
exchange reactions between tremolite and fluids buffered by
WFQ and AFSQ studied by TROLL and GILBERT ( 1972)
(Fig. 7). Hydrothermal experiments buffered by wollastonite-
fluorite-quartz of TROLL and GILBERT ( 1972) did not reach
equilibrium due to reaction kinetics, yet resulted in broad
but well-reversed brackets (Fig. 7). The anorthite-fluorite-
sillimanitequartz buffer partly melted above 700C in the
experiments of TROLL and GILBERT ( 1972 ) , and hence their
AFSQ data above 700C could not be used in the retrieval.
All experimental results mentioned above are consistent with
each other within experimental uncertainties. Our value of
-2,8 16,000 cal/mol for AGy,1h,,29r for tremolite is 5070 cal/
mol more positive than the molten salt calorimetric value of
-2,821,070 cal/mol of GRAHAM and NAVROTSKY (1986).
However, our value is not only consistent with all phase
equilibrium experiments, but also consistent with F parti-
tioning coefficients between near-Mg endmember tremolite
5.c
4.0
Y
3.0
::
-I
2.0
1.0
Annite + PHF(aq) = Fluorannite + H20
0. AFSQlkb
lkb
2kb
0.0 I
200 300 400 500 600 700 600 900
Temperature%
FIG. 5. calculated (curve) and experimental (symbols) equilibrium
constants for the F-OH exchange reaction in annite as a function of
temperature and pressure. Note the excellent agreement between ex-
perimental data obtained using the AFSQ and WFQ buffers. Symbols
as in Fig. 3.
Y
$
-I
Y
0
-I
1 kb 8 0.5 kb
Tremolite + Flwrphlogopits = Fluortremolile + Phlogopite .
Data from Weotrich ( 1981)
600 600
Temperature%
0.0 1
KTr+FAp=lRFtr+HAp
4 kb
-1.0 -
-2.0 -
-3.0
400 606 600 1000 1200
Temperature%
Frc .6. calculated (curve) and experimental (symbols) equilibrium
constants for F-OH exchange reaction between tremolite-phlogopite
(Fig. 6a) and between tremohte and apatite (Fig. 6b) as a function
of temperature. Symbols as in Fig. 3.
and phlogopite in Grenville siliceous marbles (ZHU and
SVERIENSKY, in prep.). This agreement of experimental and
natural data also encompasses a wide range of temperature
(400-1000C) and pressure (OS-4 kbar).
Y
0
J
6.0
Tremolite + PHF(aq) = Fluortremolite + H20
5.0 -
4.0 -
3.0 -
2.0 -
.
1.0 - A
0
0.0 1
300 500 700 900
1
Temperature%
Ftcr .7. Calculated (curve) and experimental (symbols) equilibrium
constants for F-OH exchange reaction in tremolite as a function of
temperature and pressure. Symbols as in Fig. 3.
Partitioning of F-Cl-OH in minerals 1847
Fluortalc
A value of AGy,lbar,298 for fluortalc was retrieved from the
F-OH exchange experiments conducted by DUFFY and
GREENWOOD ( 1979 ) . One reversed bracket was achieved with
the WFQ buffer at 750C and 2 kbar (Fig. 8). The asym-
metrical regular solution model of ABERCROMBIE et al. ( 1987)
for (OH,F) talc solid solution is adopted. The estimated and
retrieved properties are listed in Table 4 and 6.
Fluorapatite, Hydroxyapatite, and Chlorapatite
Experimental determinations of the thermodynamic
properties of apatites (BELL et al., 1978; BIGGAR, 1967;
CLARK, 1955; DUFF, 1971,1972; E~~~etal., 1950,1951a,b;
FARR et al., 1962; GOTTSCHAL, 1958; JACQUES, 1963; KOR-
ZHINSKIY, 1981; LATIL and MAURY, 1977; MCCANN, 1968;
MORENO et al., 1968; SMIRNOVA et al., 1962; WESTRICH,
1978; WESTRICH and NAVROTSKY, 1981; WIER et al., 1970)
give conflicting values for the free energies of formation of
fluorapatite ( FAp), hydroxyapatite (HAP), and chlorapatite
( ClAp) as a result of different reference values, experimental
designs, and stoichiometry and crystallinity of the materials
used in the experiments (see discussions of MCCONNELL,
1973; VALYASKHO et al., 1968; TACKER and STORMER,
1989 ) . For example, solubility experiments on HAp are nu-
merous, but of doubtful value because HAp formed at low
temperatures is non-stoichiometric (TACKER and STORMER,
1989; VALYASHKO et al., 1968, and refs. therein). Reviews
of experimental work by VALYASHKO et al. ( 1968) and
VIEILLARD and TARDY ( 1984) are restricted to solubility data
near room temperature only. It is desirable for geological
applications, however, to retrieve a set of thermodynamic
properties for endmember apatites that are also consistent
with phase equilibrium studies at high temperatures and
pressures. TACKER and STORMER ( 1989) made a compre-
hensive review of the mixing properties for (OH-F) and (OH-
Cl) apatite solid solutions and retrieved a value of AG/o for
chlorapatite. Although TACKER and STORMER ( 1989) criti-
5
t
Talc + PHF(aq) = Fluortalc + H20
2 kb
01
I
300 500 700 900
FIG. 8. Calculated (curve) and experimental (symbols) equilibrium
constants for F-OH exchange reaction in talc as a function of tem-
perature. Experiments were conducted in the WFQ buffer. Symbols
as in Fig. 3.
tally evaluated the thermodynamic data they chose to use
for fluorapatite and hydroxyapatite, they did not ensure the
internal consistency of the data between the two phases. They
employed the enthalpies for HAP and FAp from ROBIE et
al. ( 1979) and from WESTRICH and NAVROTSKY ( 198 1 ),
respectively. In the present study, we have retrieved a set of
thermodynamic properties for FAp, HAp, and ClAp that are
internally consistent and also consistent with those for other
minerals and aqueous species discussed in this study over a
wide range of temperatures and pressures.
To introduce a new component, such as phosphate, into
the existing mineral database of BERMAN ( 1988, 1990) an
independent reference mineral must be used (BERMAN, 1988;
POWELL and HOLLAND, 1985; SVERIENSKY et al., 1991).
We chose fluorapatite because it is the best characterized
mineral in this group.
WESTRICH and NAVROTSKY ( 198 1) measured the enthalpy
of formation of fluorapatite with the reaction
/&aFz + /rCa,(PG4)2 = Ca004)3F
(22)
and obtained -17.469 + 1.6 kcal/mol at 985 K. From this
result we calculated the enthalpy of formation from the ele-
ments for fluorapatite to be - 1,630,787 cal/mol at 298 K
and 1 bar. We used the enthalpy of formation from the ele-
ments at 298 K and 1 bar for fluorite from CODATA ( GAR-
VIN et al., 1987), heat contents (H&s - H!&) for fluorite
and fluorapatite calculated from Cp functions given in Table
4 and for 8-Ca3(PG4h from Table 7. A value of
AHy,lbar,~98 of -922,818 cal/mol for /J-Ca,(PO,), was Cal-
culated based on the solubility measurements of GREGORY
et al. ( 1974) and an entropy of BCa,( P04)z from WAGMAN
et al. ( 1982). Our calculated value of AZ$y,,ti,,298 for &
Ca,( PO& is 5648 cal/mol more endothermic than the value
listed in WAGMAN et al. ( 1982). We prefer the value from
solubility measurements because GREGORY et al. ( 1974) used
well-characteIized crystals synthesized at similar temperatures
to those of WESTRICH and NAVROTSKY ( 198 1) .
The Gibbs free energy for fluorapatite we calculated agrees
with solubility products for FAp of FARR and ELMORE ( 1962)
and VALYASHKO et al. ( 1968), which were calculated from
reversed solubility measurements (FARR et al., 1962) and
with the solubility product of MCCANN ( 1968 ) , and is within
two standard deviations of the measurement of WESTRICH
and NAVROTSKY ( 198 1) (Fig. 9). We adopted a value be-
tween the value from FARR and ELMORE ( 1962) and the
recalculated value of WESTRICH and NAVROTSKY ( 198 1 ),
resulting in m,,ihr,298 = - 1,630,843 cal/mol for fluorapatite
(Table 6).
Our value agrees well with the solution calorimetric mea-
surements of GOTTSCHAL ( 1958) and is in reasonable agree-
ment with calorimetric measurements of SMIRNOVA et al.
( 1962), and with the recalculated value of VIEILLARD and
TARDY ( 1984) from the phase equilibrium study of DUFF
(1972), (see Fig. 10). However, our value is about 4 kcal/
mol more exothermic than that of TACKER and STORMER
( 1989) and is 17 kcal/mol more endothermic than the ca-
lorimetric value of JACQUES ( 1963), in which an impure
natural apatite (2.2% F rather than 3.77% F for pure end-
member) was used in experiments, and about 10 kcal/mol
1848
C. Zhu and D. A. Sverjensky
a
z
D
Y
$
-I
-56
-59 -
I . I I . I
_ QS(P04) tF =5&+ + 3 PO:. + F-
-62 .
10 20 30 40 50 60
Temperature%
FIG. 9. Comparison of the solubility product of fluorapatite cal-
culated from recalculated calorimetric measurements of WESTRICH
and NAVROT~KY ( 198 1) with those measured by the solubihty studies
of FARR and ELMORE ( 1962) and MCCANN ( 1968). The calorimetric
measurements agree with solubility measurements within experi-
mental error.
more endothermic than the value cited by ROBIE et al. ( 1979)
and WAGMAN et al. ( 1982).
A value of AGT,lbar,298 for hydroxyapatite was retrieved
from the hydrothermal exchange experiments using the port-
landite-fluorite buffer ( KORZHINSKIY, 198 1)) and from apa-
tite-phlogopite and apatite-tremolite exchange reactions
( WESTRICH, 1978). We used the data of KORZHINSKIY
( 198 1) at 500 and 6OOC, and 1,2, and 4 kbar only, because
of possible melting of the portlandite-fluorite buffer above
about 660C ( BIGGAR, 1967). The results from KORZHIN-
SKIY ( 198 1) were recalculated in Table 9 and are depicted
in Fig. 11. WESTRICH ( 1978) obtained one unequivocal re-
versal for apatite-tremolite and apatite-phlogopite exchange
reactions at 4 kbar and 1000C (Figs. 12 and 6b). BIGGAR
( 1967 ) studied the system Ca( 0H)&aFZ-Ca3( P04)2-H20
and found the equilibrium assemblage portlandite-fluorite-
(F,OH) apatite at 1 kbar, 675C, with X&, = 0.40. This
agrees remarkably well with Korzhinskiys data obtained in
hydrothermal systems using the portlandite-fluorite buffer
3 - 1600 -
g - 1610 -
8
s - 1620 -
:
: - 1630 -
e
Q - 1640 -
-1650
I -
-1660
FIG. 10. Comparison of values of AHj),rgg for fluorapatite from
different sources.
Y
$
J
Y
$
-I
Y
$
-I
6
I I I
HAp + HF(wf) = FAp + H20
7
1 ftb, PF buffer
6-
5-
4-
3-
Aecalcufated from
2 - A 6bw(1~7)
. l 0 KorzfMnskfy(l991)
1
I a I
6,
I
a I
HAp + HF(aq) = FAp + H20
Konhinskiy (1961)
Temperature%
FIG. 11. Calculated (curve) and experimental (symbols) equihb-
rium constants for F-OH exchange reactions in apatite buffered by
portlandite-fluorite as a function of temperature and pressure. Sym-
bols as in Fig. 3. The data of BICXAR ( 1967) were not used in the
retrieval.
(Fig. 11) , despite possible inaccuracy in Bigger composition
determination. The experimental brackets constrain the
A@~~298 and So
298 for hydroxyapatite to - 1,600,069 cal /
mol and 95.30 cal - mol- - K- , respectively. The retrieved
entropy is 2 cal higher than the experimentally measured
value from low-temperature calorimetry ( EGAN et al., 195 1 a),
in which a monoclinic hydroxyapatite was inferred to have
been used (TACKER and STORMER, 1989). Hydroxyapatite
undergoes a phase transition from a monoclinic to a hex-
Partitioning of F-Cl-OH in minerals 1849
-0.4 I I
l/Z Phl + FAp = l/Z Fphl + HAp
I
-0.6 -
4 kb
- 0. 8 -
-1.0 -
Y
$ -1.2 -
-I
-1.4 -
-1.6 - Westrich ( 1978) _
-1.6
400
I
600 600 1000 1200
Temperature%
FIG. 12. Calculated (curve) and experimental (symbols) equilib-
rium constants for F-OH exchange reaction between apatite-phlog-
opite as a function of temperature. Symbols as in Fig. 3.
agonal form at 211SC (VAN REES et al., 1973). The heat
content data of EGAN et al. ( 1950) are scarce around the
transition temperature and no thermal properties can be in-
ferred. From crystal structure considerations, it is probably
a second-order transition (TACKER and STORMER, 1989 ) .
We therefore based our entropy for hydroxyapatite on high-
temperature and high-pressure experiments to avoid the am-
biguity of the phase transition. This is practical because many
applications are in the high P,T range. Hence, our entropy
value together with the calculated free energy corresponds to
standard-state properties at 1 bar and 298 K for a hypothetical
hexagonal hydroxyapatite.
Comparison of measurements of the Gibbs free energy
and enthalpy for hydroxyapatite (Fig. 13) shows that values
obtained from different experimental methods and authors
vary up to 16 kcal/mol. However, our values is closest to
that of VALYASHKO et al. ( 1968) which was calculated from
CLARK ( 1955). Clarks study is probably the only reversed
solubility experiment and the only experiment at high tem-
peratures (90C) among the vast numbers of solubility mea-
surements. The considerable discrepancies shown for the rest
of the results in Fig. 13 can be attributed to systematic errors
- 1590
g - 1600
E
1
2
- 1810
sg - 1620
: - 1630
e
Q - 1640
A
A
n
0
.
l
X
. wammeta ,lW
- 1660
1
FI G. 13. Comparison of values of AJY& for hydroxyapatite from
different sources.
l
___________1__~____________. 2. 5 kca,
- 30
_~_________________
A
L--*---F-2.5 kcal
FIG. 14. Comparison of values of AHTJA, - M;,HAp from dif-
ferent sources.
in the experiments. This can be seen in Fig. 14 where the
differences between enthalpies of FAp and HAp from the
same author are plotted together. Most disparities are reduced
to within about 2.5 kcal/mol of our preferred value. The
deviation of the value of GOTTSCHAL ( 1958) for hydroxy-
apatite from the others may be attributed to the materials
5. 0 . I. I. I . I. I -
HAp + HCI = ClAp + H20
4. 0 - AgCI-Ag-Hem-Meg-HP0 buffer _
2 kb
y 3. 0 -
$
- I 2. 0 -
1. 0 -
. Recalculated from
o Korzhinskiy (1961)
(see Table 9)
0.0 . ' . ' . ' . ' .
0 200 400 600 800 1000 1200
Temperature%
- 1460
- 1480
J
- 1500'
I
FIG. 15. (a) Calculated (curve) and experimental (symbols) equi-
librium constants for Cl-OH exchange reaction in apatite buffered
by Ag-AgCl-hematite-magnetite as a function of temperature. Solid
symbols indicate experiments approaching equilibrium from the Cl-
rich endmember; open symbols from the OH-rich endmember (Table
9). Error bars as in Fig. 3. (b) Comparison of values of AGT,298 for
chlorapatite from different sources.
1850 C. Zhu and D. A. Sverjensky
used in the experiments. We should note here that ROBIE et
al. ( 1979 ) used the red triclinic phosphorus as reference state
(So = 5.46 cal*mol-SK-). WAGMAN et al. (1982) and
SHOCK and HELGESON ( 1988), however, used the white
phosphorus as reference state (9 = 9.82 calf mol- , K- ).
The choice of reference state matters in defining entropy of
formation for all phosphors-~ng phases or species.
Most hydrothermal experiments dealing with chlorapatite
(DUFF, 1972; EKSTROM, 1973; ELLIOT and YOUNG, 1967;
LATIL and MAURY, 1977; KORZHINSKIY, 1981; PRENER,
1967; RUSZALA and KOSTINER, 1975) provide little infor-
mation for extracting thermodynamic properties for chlor-
apatite, simply because they are not buffered experiments
and therefore activities of HCl * in the experimental systems
cannot be characterized. The only exceptions are the exper-
iments of KORZHINSKIY ( 198 1) using the Ag-AgCl buffer.
We have retrieved a Gibbs free energy value of - 1,486,OOO
cal/mol for chlorapatite from a reversed bracket in Kor-
zhinskiys experiments at 600% and 2 kbar using the he-
matite-magnetite oxygen buffer (Fig. 15a ) . Korzhinskiys re-
sults using the Ni-NiO oxygen buffer at 600 and 700C and
2 kbar agree with the retrieved value, but were not useful for
retrieval because of large uncertainties in the log xls resulting
from near Cl endmember apatite compositions in the exper-
iments. For reasons we have discussed above, the retrieved
Gibbs free energy value is at best a preliminary result. How-
ever, calculations of natural systems show that this result is
consistent with calculations from our chlorannite data and
consistent with mass transfer calculations during metaso-
matism (RIPPLE and FERRY, 199 1). Notably, our value is
9.2 kcal/md more than that of VALYASHKO et al. (1968),
1.5 kcal/mol more than that of VIEILLARD and TARDY
(1984), and 4.0 kcal/mol less than that of TACKER and
STORMER ( 1989) (Fig. 15b). Note that Tacker and Stormers
Gibbs free energy value reflects their choice of entropy of
119.226 cal * mol- - K- for chlorapatite.
Chlorapatite also undergoes a phase transition at about
2OOC ( PRENER, 1967). The exact nature of the transition
is not known and experimental data for the phase transition
are certainly needed. In light of the large uncertainties in our
preliminary entropy and enthalpy values, effects of this phase
transition cannot be incorporated.
Chlormicas
Hydrothermal Cl-OH exchange experiments have been
conducted by KORZHINSKIY ( 1980), MUNOZ and SWENSON
(19811, VOLFINGER et al. ( 1985), and VOLFINGER and PAS-
CAL ( 1989) for annite, muscovite, phlogopite, and biotites.
The experiments conducted using the muscovite-K-feldspar-
quartz-HCl-KC1 buffer ( MUNOZ and SWENSON, 198 1; VOL-
FINGER and PASCAL, 1989) can be used for retrieval of
A@,,w.z~~.
A value of AG$,lber,298 for chlorannite was retrieved from
the log KS recalculated from experimental data of MUNOZ
and SWENSON ( 198 1) (Table 9). MUNOZ and SWENSON
( 198 1) obtained one reversed bracket (Fig. 16). The data
point for reference at 6OOC and 2 kbar (Fii. 16) is from
VOLFINGER et al. ( 1985). The value of log aHan equal to
4.0 . I . , . 1 . I . I .
Annite + 2HCP(aq) = Chlorannite + H20
2.0 - A
0.0 -
-4.0 -
. Recalculated from
_6.0 _ z Munoz and Swenson (1981)
A voltinger et a~. (1985)
0 200 400 600 800 1000 1200
FIG. 16. Calculated (curves) and experimental f symbols) equilib-
rium constants for Cl-OH exchange reaction in annite as a function
of temperature and pressure. Symbols as in Fig. 15a. Experiments
of MUNOZ and SWENSON ( 198 1) were conducted in the KMQ buffer.
-3.68 is calculated from speciation of a 0.44 KC1 solution
with pH calculated from charge balance. This value and the
subsequent value for the log K is, at best, a tirst a~ro~mation.
Retrieval of AGy,lbar,298 for chlormuscovite and chlorphlo-
gopite from KMQ-buffered experiments of VO~FINGER and
PASCAL (1989) were unsuccessful. Similar exercises weK:
conducted as in the retrieval for chlorannite. Values of log
aHc10 for the experimental conditions were calculated from
the KMQ-buffer and experimental input of mha-. The re-
sultant log 1% define a very steep line between 400 and 6OPC,
which requires unreasonably small entropies for chlormus-
covite and chlorphlogopite (<20 calamol- -K-l). This
cannot be accounted for by errors in estimation of the heat
capacities. Our analysis suggests systematic errors in the ex-
periments themselves, which were apparently not reversed
( VOLFINGER and PASCAL, 1989 ) .
APPLICATION TO PREDICMNG AQUEOUS
PHASE ARGONS
The possibilities of using the trace element contents of
minerals to deduce their concentrations in fluids have long
been explored HOLLAND, 1956; MCINTIRE, 1963; SVERJEN-
SKY, 1984, 1985), although the paucity of data relevant to
the thermodynamics of trace element partitioning has not
permitted much progress. The thermodynamic properties of
F and Cl endmember minerals obtained in this study provide
a ~e~~~arnic basis for predicting the Buoride and chlo-
ride concentrations of fluids from the measured F and Cl
concentrations in minerals. Total concentrations of fluoride
and chloride in fluids can be predicted by considering both
F-Cl-OH equilibrium partitioning between fluids and min-
erals and speciation calculations taking into account cation
complexing with fluoride and chloride in the fluids. We have
made such calculations in some model systems presented
below. The solubility and speciation calculations were carried
out using the computer program EQ3 ( WOLERY, 1983 ) . All
thermodynamic properties for minerals were from BERMAN
(1988, 1990) and SVERJ ENSKY et al. (199la), for aqueous
Partitioning of F-Cl-OH in minerals 1851
600 I 1
Ms-Kf-Qtz-Ab-HF-H20 I
P)
2
5 600 -
3,
E
$%
>
$ 4 4oo
. 2
U=
ii .E 200
0
1
300 400 500 600
Temperature%
FIG. 17. Predicted total dissolved fluoride in 1 .O m chloride so-
lutions as a function of temperature and F content of muscovite.
Solutions are in equilibrium with the assemblage muscovitequartz-
albite-K-feldspar.
species from SHOCK and HELGESON ( 1988), SHOCK et al.
( 1989 ), and SVERJENSKY et aI. ( 199 1 b) . Activity coefficients
for all neutral species and activity of water were assumed to
be unity. Activity coefficients for all ionic species were cal-
culated from the extended Debye-Hiickel equation of
HELGESON et al. ( 198 1) using b, values from OELKERS and
HELGESON ( 1990).
System Na20-K20-A1203-SiO*-HF-H~O
We have demonstrated above that our speciation model
for the system MgG-Si02-CaF*-Hz0 agrees well with exper-
imental measurements. Systems that contain A&O3 may, in
addition to the species in Table 2, have important aluminum
fluoride species ( HASELTON et al., 1988; MUNOZ, 1974). To
study the speciation of such systems and retrieve thermo-
dynamic properties for aqueous fluoride species is beyond
the scope of this study. Instead, we try to demonstrate the
applicability of our retrieval calculations to some relatively
simple model systems. Therefore, the results presented below
may reflect minimum Iluoride concentrations in light of the
ignorance of Al-F complexing.
Speciation calculations have been made for 1.0 molal Cl
solutions in equilibrium with the assemblage muscovite-K-
feldsparquartz-albite with 0.1 and 0.01 mole fraction of
fluormuscovite. The fluoride species included are F-, HF,
HF;, SiF;, and NaF . *HF was the predominant F-bear-
ing species in all our calculations. F- and NaF were on the
order of IOF molal and constitute ~2% of the total fluoride.
Figure 17 shows the predicted total fluoride contents of the
fluids equilibrated with the above assemblage as a function
of temperature. Fluorine is increasingly partitioned into the
fluids with respect to muscovite with increasing temperature.
Dissociation constants for NaP were calculated using thermo-
dynamic properties from SVERJENSKY et al. ( 1991b). They are (at
400,450, 500, 550,600, 650, 700, and 750C and 2 kbar): -0.49;
-0.77; -1.06; -1.34; -1.65; -1.99; -2.37; -2.77.
Speciation calculations for the fluid compositions equili-
brated with the assemblage andalusite-muscovitequartz-pa-
ragonite give similar total fluoride concentrations (Fig. 18))
although the two assemblages define different pH values for
the fluids. This is obviously because fluoride is strongly as-
sociated with HF at high temperatures.
System Na20-K20-A1~O~SiO~-Fe0-HCI-H20
Speciation calculations have also been carried out in the
system Na20-Kz(rA1203-Si0*-Fe0-HC1-Hz0 at 400-750C
1 and 2 kbar, and 200-300C and the vapor saturation pres-
sures of water (Pm). The fluid compositions were constrained
with the mineral assemblage muscovitequartz-K-feldspar-
albite-annite or andalusitequartz-K-feldspar-albite-annite
and fixed activity of HCl . The activities of HCl in fluids
corresponding to equilibration with fixed Cl contents in annite
have been calculated by using properties for chIorannite in
Tables 4 and 6. Activities of all aqueous species and pH of
the solutions are fixed.
Figure 19 shows the predicted total Cl- molality in fluids
that are in equilibrium with the above mineral assemblages
and a range of Cl contents in annite. Fluids, in equilibrium
with muscovite-quartz-K-feldspar-albite-annite and with Cl
contents in annite From 140 to 1290 ppm at 1 kbar and 500C
contain 0.29 to 3.12 molal Cl-. Fluids in equilibrium with
andalusitequartz-K-feldspar-albite-annite and with Cl con-
tents in annite from 500 to 10,000 ppm at 2 kbar and 750C
contain 0.18 to 3.65 molal Cl-. Natural biotites contain Cl
ranging from less than 100 up to 11,000 ppm, although rarely
exceeding about 7600 ppm (AGUE and BRIMHALL, 1988a,b,
LEE, 1958; GUIDOTTI, 1984; GUNOW et al., 1980; JACOBS
and PARRY, 1979; MORA and VALLEY, 1989; MUNOZ, 1984;
PARRY and JACOBS, 1975). MORA and VALLEY ( 1989) re-
ported that the lower detection limit of Cl in biotite is 200
ppm in their electron microprobe study.
7oor .
I
Zkb,m , c, =I.0
xFrr. = cl:,
600
q And-Ms-Qtz-Pg
0 Kf-Ms-Otz-Ab
________-------
4.5 5.0
PH
5.5 6.0
PIG. 18. Predicted total dissolved fluoride in 1 .O m chloride so-
lutions as a function of temperature and mineral assemblages. So-
lutions are at equilibrium with the mineral assemblage muscovite-
quartz-albite-K-feldspar and the assemblage muscovitequartz-pa-
ragonite-andalusite, respectively, and with 0.1 mole fraction of fluor-
muscovite. It shows that total fluoride concentrations in fluids does
not change with different mineral assemblage.
1852
C. Zhu and D. A. Svejensky
4. 0 . I . I . I . I ' I
0 1 kb, 500C
Ms.Qtz-Kf-Ab-Ann-HWH20
q 750C 2kb
And-Qtz-Kf-Ab-Ann-HWH20
0.0 . "I . I. '
0 2000 4000 6000 6000 10000
Cl ppm in annite
TemperatuWC
FIG. 19. Predicted total dissolved chloride in aqueous solutions as
FI G. 2 1. Predicted total dissolved chloride as a function of tem-
a function of Cl contents in annite. The solutions are in equilibrium
perature for the mineral assemblages muscovitequartz-K-feldspar-
with the assemblage muscovitequartz-albite-K-feldspar-annite at 1
albiteannite and muscovitequartz-K-feldspar-pamgonite-annite at
kb and 500C and with the mineral assemblage andalusitequartz-
1 kb. The annite contains 0.001 mole fraction of chlorannite ( 140
albite-K-feldspar-annite at 2 kb and 750C. ppm Cl).
Figure 20 shows the predicted total Cl- concentrations in
the fluids equilibrated with an annite with 0.00 1 mole fraction
Cl (or 140 ppm) as a function of temperature and pressure.
We can see that the total Cl- contents of fluids in equilibrium
with this fixed annite composition are a strong function of
temperature. Fluids in equilibrium with muscovitequartz-
K-feldspar-albite-annite contain eO.7 molal Cl- for T
> 5OOC, but 0.7-3.7 molal for 400 < T < 500C at 2 kbar.
In addition to the strong temperature dependence below
SOOC, the Cl- contents of fluids depend strongly on pressure.
The Cl- contents of the fluids change from 1.6 to 3.7 molal
between 1 and 2 kbar at 400C. Furthermore, the Cl- con-
tents of fluids are also a function of the mineral assemblage;
i.e., the pHs of the fluids are buffered by the mineral assem-
blage. Figure 2 1 shows that the fluid in equilibrium with the
assemblage muscovite-quartz-K-feldspar-paragonite-annite
a .I#I.ZI.I.
MS-Qtz-Kf-Ab-Ann-HCLH20
Xcl.n= 0.001
6-
i oo 200 300 400 500 600 700 800
Temperature%
FIG. 20. Predicted total dissolved chloride as a function of tem-
perature and pressure for aqueous solutions in equilibrium with the
assemblage muscovitequartz-albite-K-feldspar-annite with mole
fraction of chlorannite of O.OOl( 140 ppm Cl).
&a= 0.001. lkb
0 Ms.Qtz-Kf-Ab-Ann
o Ms.Qtz-Kf-Pg.Ann
L
01
\5
300 400 500 600 700
has 2.5 molal Cl- at 1 kbar and 4OOC, which is about 1.0
molal higher than the fluid equilibrated with the assemblage
muscovitequartz-K-feldspar-albite-annite.
The dominant chloride species in our model of this system
are Cl-, NaCl , and KC1 . In systems including MgO and
CaO, Mg and Ca chlorides are also important (EUGSTER,
1982, 1986; EUGSTER and BAUMGARTNER, 1987; SVEFUEN-
SKY, 1987). Annite is used in calculations here because data
on the influence of octahedral Mg on Cl partitioning is lack-
ing. In light of the possible trend of Mg-Cl avoidance (Mu-
NOZ, 1984,199O; VOLFINGER et al., 1985), we have probably
calculated the minimum values of total molal Cl- compo-
sition with respect to Fe-Mg biotite.
Systems including Apatites
Apatites are the most abundant phosphate-bearing min-
erals in the crust (DEER et al., 1966; MCCONNELL, 1973).
They also occur in practically all geological environments:
in all igneous rocks from basic to acid, in metamorphic rocks,
sedimentary rocks, mantle xenoliths, meteorites, and lunar
rocks ( BUSHWALD, 1984; MCCONNELL, 1973; NASH, 1984).
The wide occurrence of apatite makes it a potentially useful
mineral for predicting the F and Cl contents of former fluids.
In the present study, we made some calculations in simple
model systems using the thermodynamic properties sum-
marized in Table 6.
Figure 22 shows an activity-activity diagram at 300C and
pressure of vapor saturation of water. Line a represents the
fluid composition of about 5.0 total molal Cl- defined in Fig.
20 at this temperature. With as low as 10m6 molal F- in
fluids, the apatite equilibrated with fluid would be predom-
inantly fluorapatite (&, > 0.9). This may explain why most
hydrothermal apatites are dominantly F rich (e.g., KELLY
and RYE, 1979).
We have also made speciation calculations in the system
Naz0-A120~-Si02-Ca0-P~O~-HF-HC1-H~0 at 2 kbar and
750C. The fluid compositions were buffered by the mineral
Partitioning of F-Cl-OH in minerals 1853
-4 -
-5 -
.HAP
-6 -
FAP .
-7 - +d %=9/j
-8, .
-8 -7 -6 -5 -4 -3 -2 -1 0
log (aHFVaH20)
FIG. 22. Predicted relative dominance fields for HAp, FAp, and
ClAp component in apatite solid solution at 300C and pressure of
vapor saturation of water (86 bar). The bold solid lines represent
equal mole fractions of neighbouring F, Cl, OH endmembers. The
thin solid lines represent relative mole fractions 9/ 1 as indicated.
The dash-and-dot-line (a) represents fluid composition defined in
Fig. 20 which has 5.0 total molal Cl- and is in equilibrium with an
annite with 140 ppm Cl and the mineral assemblage K-feldspar-albite-
muscovitequartz-annite.
assemblage anorthite-albite-quartz-andalusite and total Cl-
contents from 0.5 to 5.0. The fluoride content was chosen
to be 500 ppm and the phosphate contents of the fluids was
set arbitrarily to be 10m5 molal. The calculated mole fractions
of Fap, HAp, and ClAp are shown in Figure 23. We can see
that apatite would be F rich even in dominantly Cl rich fluids.
We also made speciation calculations in systems similar to
the above system, but without F. Figure 24 shows that high
mole fraction of chlorapatite can be obtained in fluorine-free
saline NaCl solutions.
Figure 25 shows the relative fields for hydroxyapatite, flu-
orapatite, and chlorapatite components in an apatite solid
solution up to 1OOOC and 5 kbar. It can be seen that at
temperatures from 25C up to about 4OOC, conditions cor-
1.0
0.8
0.6
0.4
0.2
An-Ab-And-Qtz-HF.HCI-H20
2 kbar, 750%
500 ppm F in fluids
0 1 2 3 4 5 6
Total molal chloride
FIG. 23. Predicted mole fraction of FAp, ClAp, and HAp in an
apatite solid solution as a function of chlorinity. Fluids are at equi-
librium with the assemblage anorthite-albite-andalusitequartz. Flu-
orine content of the fluids are set to be 500 ppm.
An-Ab-And-Ptz-HCCH20 2 kbar, 750C
al 0.8 -
.z
4
.E 0.6 -
5
.-
5
9 0.4 -
a,
8
0.2 -
0.0.
0 1 2 3 4 5 6
Total molal chloride
FIG. 24. Predicted mole fractions of ClAp and HAp in an apatite
solid solution as a function of chlorinity. Fluids are at equilibrium
with the assemblage anorthite-albite-andalusite-quartz. The system
is free of F.
responding to sedimentary, diagenetic, and hydrothermal vein
environments, carbonate-free apatites should be F rich. Sed-
imentary and diagenetic apatites are indeed invarably F rich
(MCCONNELL, 1973; VALYASHKO etal., 1968).Hydrother-
mal vein apatites are also F rich, for example, the fluorapatites
from the Tin-tungsten deposits at Panasquiera, Portugal
(KELLY and RYE, 1979). With increase of temperature and
pressure, however, the stability field for HAp steadily expands.
Both FAp and ClAp become less stable with respect to HAp
with increase of temperature. However, ClAp becomes more
stable relative to FAp with increase of temperature.
The above solubility and speciation calculations demon-
strate the following :
1. Increase of temperature favors partitioning of F- into fluid
with respect to muscovite while it favors partitioning of
Cl- into annite with respect to fluid.
2. The relationship between total molal Cl- in fluids and Cl
contents in annite is very sensitive to the pH of the fluid
or the mineral assemblage which buffers the fluid pH. This
sensitivity is the result of charged chloride species such as
Cl- being important in the fluid. In contrast, the rela-
tionship between total fluoride concentration in fluids and
F in muscovite is not sensitive to the pH of fluid or the
mineral assemblage which buffers the fluid pH, which re-
flect the predominance of the neutral species HF in our
speciation models. The latter proposition ignores the pos-
sible complexation between Al and F in hydrothermal
fluids (HASELTON et al., 1988; MUNOZ, 1974).
3. The partitioning of Cl between minerals and fluids is a
strong function of pressure while the partitioning of F
between mineral and fluids is not. This is because of the
difference between the dependences of HCl and HF
dissociation on pressure.
To summarize this section, fluoride and chloride concen-
trations of former aqueous phases can be predicted from the
F and Cl composition in minerals through the consideration
of thermodynamic partitioning of F-Cl-OH and speciation
1854
C. Zhu and D. A. Sverjensky
HAP
FAP
HAP
FAP
r
FAP
- HAp
- 10 . 5 0
HAP
FAP
HAP
FAP.
ClAp
Log (aHF/aH20)
FIG. 25. Predicted relative dominance fields for HAp, FAD, and ClAp components in apatite solid solution up to
1000C and 5 kb. The lines in the figure represent equal mole fractions of neighbouring F, Cl, and OH endmember
apatites. The triple junctions represent equal mole fractions of the three endmember components.
calculations taking account of the chloride and fluoride com-
plexes. The incorporation of F and Cl into minerals is a strong
function of temperature, pressure, pH, and fluid composition,
which can be characterized in our prelimina~ thermody-
namic treatment.
CONCLUDING REMARKS
A set of internally consistent thermodynamic properties
for F and Cl endmember minerals have been obtained in
this study. Because they are also consistent with the database
for minerals of BERMAN ( 1988, 1990) and SVERJENSKY et
al. ( 199 1 a), and that for aqueous species (SHOCK and
HELGESON, 1988; SHOCK et al., I989 ), these thermodynamic
properties can be applied up to 1000C and 5 kbar. Examples
of solubility and speciation calculations in this study dem-
onstrate that measured F and Cl contents in minerals can be
interpreted as total dissolved F and Cl in the former aqueous
phases. Such applictions depend on the speciation models
used for particular systems. Mass transfer calculations in-
cluding both F-Cl-OH partitioning and halide complexing of
metals should provide better constrained chemical modeling
of water-rock intem~ions in ok-foxing, me~mo~hic, dia-
genetic, and magmatic processes.
The thermodynamic data obtained for F,Cl rock-forming
minerals will also help to assess mineral stability under meta-
morphic and mantle conditions, calibrate geothermometers
(e.g., garnet-biotite geothe~ometer-~RRY and SPEAR,
1978; and apatite-biotite geothermometer-STORMER and
Partitioning of F-Cl-OH in minerals
1855
CARMICHAEL, 197 1; LUDINGTON, 1978), and evaluate the
evolution of magmatic systems.
Acknowledgments-We are especially grateful to Greg Dipple and
John Ferry for sharing unpublished data with us. Thanks are also
due to Robert Berman for his advice on heat capacity regression
studies, to I-Ming Chou for his advice on the Ag-AgCl buffer, and to
David Moecher and I-Ming Chou for providing us data prior to pub-
lication. This report is supported by NSF grant EAR-9005483. In
addition, acknowledgment is made to the donors of the Petroleum
Research Fund, administered by the American Chemical Society,
for partial support of this research. We also thank Greg Dipple, John
Ferry, Greg Symmes, and David Veblen for valuable discussions.
Constructive comments from Phillip Candela, James Munoz, Chris
Tacker, and Scott Wood helped to improve this paper.
Editorial handling: S. A. Wood
REFERENCES
ABERCROMBIE H. J., SK~PPEN G. B., and MARSHALL D. D. ( 1987)
F-OH substitution in natural tremolite, talc, and phlogopite. Con-
trib. Mineral. Petrol. 97, 305-3 12.
AGUE J. J. and BRIMHALL G. H. ( 1988a) Regional variations in bulk
chemistry, mineralogy, and the compositions of mafic and accessory
minerals in the batholiths of California. Geol. Sot. Amer. Bull.
100,891-911.
AGUE J. J. and BRIMHALL G. H. ( 1988b) Magmatic arc asymmetry
and distribution of anomalous plutonic belts in the batholiths of
California: Effects of assimilation, crustal thickness, and depth of
crystallization. Geol. Sot. Amer. Bull. 100,912-927.
ANDERSON G. M. ( 1977) Uncertainties in calculations involving
thermodynamic data. In Applications of Thermodynamics to Pe-
trology and Ore Deposits (ed. H. J. GREENWOOD), pp. 199-215.
BAILEY, J. C. ( 1977) Fluorine in granitic rocks and melts: A review.
Chem. Geol. 19, l-42.
BARTON M. D., HASELTON H. T., JR., HEMMINGWAY, B. S., KLEPPA
0. J., and ROBIE R. A. ( 1982) The thermodynamic properties of
fluor-topaz. Amer. Mineral. 67, 350-355.
BELL L. C., MIKA H., and KRUGER B. J. ( 1978) Synthetic hydroxy-
apatite solubility product and stoichiometry of dissolution. Arch.
Oral Biol. 23, 329-336.
BERMAN R. G. ( 1988) Internally-consistent thermodynamic data
for minerals in the system Naz~K,o-Cao-Mgo-Feo-Fe203-Alzo,-
SiOz-TiOz-HrO-CO2 : representation, estimation, and high tem-
perature extrapolation. J. Petrol. 29,445-522.
BERMAN R. G. ( 1990) Mixing properties of Ca-Mg-Fe-Mn garnets.
Amer. Mineral. 75, 328-344.
BERMAN R. G. and BROWN T. H. ( 1985) The heat capacity of min-
erals in the system KzO-Na,O-CaO-MgO-FeO-Fe*Oa-AlzOrSi02-
TiO*-H&-CO2 : representation, estimation, and high temperature
extrapolation. Contrib. Mineral. Petrol. 89, 168- 183.
BERMAN R. G., BROWN T. H., and GREENWOOD, H. J. ( 1985) An
internally consistent thermodynamic data base for minerals in the
system KzO-Naz0-Ca0-Mg0-Fe0-Fe~O~-Al~O~-SiO~-TiO~-H~O-
COz. Technical Report TR-377, Atomic Energy of Canada Lim-
ited.
BIGGAR G. M. ( 1967) Apatite compositions and phase relationships
on the ioin Ca(OH h-CaF,-Ca~(PO~h-H,O from 250 to 4000 bars.
Mineral. Mag.36, 539-564. _. .- -
BURNHAM C. W. ( 1979) Magmas and hydrothermal fluids. In Geo-
chemistry of Hydrothermal Ore Deposits, 2nd edn. (ed. H. L.
BARNES), Chap. 3, pp. 71-133. Wiley &Sons.
BUSHWALD V. F. ( 1984) Phosphate minerals in meteorites and lunar
rocks. In Phosphate Minerals (eds. J. 0. NRIAGU and P. B.
MOORE), Chap. 5, pp. 199-2 15. Springer-Verlag, Berlin.
CANDELA P. A. ( 1986) Toward a thermodynamic model for the
halogens in magmatic systems: An application to melt-vapor-apatite
equilibria. Chem. Geol. 57, 289-301.
CANDELA P. A. and HOLLAND H. D. ( 1984) The partitioning of
copper and molybdenum between silicate melts and aqueous fluids.
Geochim. Cosmochim. Acta 48,373-380.
CANDELA P. A. and HOLLAND H. D. ( 1986) A mass transfer model
for copper and molybdenum in magmatic hydrothermal systems:
The origin of porphyry-type deposits. Econ. Geol. 81, l-19.
CHOU I-MING ( 1987) Oxygen buffer and hydrogen sensor techniques
at elevated temperatures and pressures. In Hydrothermal Experi-
ment Techniques (4s. G. C. ULMER and H. L. BARNES), Chap.
3, pp. 6 l-99. J. Wiley & Sons.
CHOU, I-MING AND FRANTZ J. D. ( 1977) Recalibration of Ag + AgCl
acid buffer at elevated pressures and temperatures. Amer. J. Sci.
277, 1067-1072.
CLARK J. S. ( 1955) Solubility criteria for the existence of hydroxy-
apatite. Canadian J. Chem. 33, 1696-1700.
DEER W. A., HOWIE R. A., and ZUSSMAN J. ( 1966) An I ntroduction
to the Rock-forming Minerals. Longman.
DIPPLE G. and FERRY J. M. ( 199 1) Fluid flow and metasomatism
in ductile faults. (in prep.)
DROLL K. and SECK H. A. ( 1984) A new sampling technique for
fluid phases in hydrothermal experiments applied to determination
of the HF-fugacity of the WFQ-buffer. Contrib. Mineral. Petrol.
88,276-279.
DUFF E. J. ( 197 1) Orthophosphates. II. The transformation Brush-
ite + Iluorapatite and monetite + fluorapatite in aqueous potas-
sium fluoride solution. J. Chem. Sot. A, 33-38.
DUFF E. J. ( 1972) Orthophosphates-IX. Chlorapatite: Phase re-
lationships under aqueous conditions along the CasF(PO,h-
CaSOH ( P0,)&a5Cl( po4)r joins of the system CaO-CaCl&aF*-
PzOrHzO. J. Chem. Sot. London (A)34,921-926.
DUFFY C. J. and GREENWOOD H. J. ( 1979) Phase equilibria in the
system MgO-MgF2Si02-H20. Amer. Mineral. 64, 1156-l 174.
EGAN E. P., JR., WAKEFIELD Z. T. and ELMORE K. L. ( 1950) High-
temperature heat content of hydroxyapatite. Amer. Chem. Sot. J.
72,2418-2421.
E&AN E. P., JR., WAKEF~ELD Z. T., and ELMORE K. L. ( 195la)
Low-temperature heat capacity and entropy of hydroxyapatite.
Amer. Chem. Sot. J. 73,5579-5580.
EGAN E. P., JR., WAKEFIELD Z. T., and ELMORE K. L. ( 195 lb)
Thermodynamic properties of fluorapatite, 15 to 1600K. Amer.
Chem. Sot. J. 73,5581-5582.
EKSTROM T. K. ( 1973) Synthetic and natural chlorine-bearing apa-
tite. Contrib. Mineral Petrol. 38, 329-338.
ELLIOTT J. C. and YOUNG R. A. ( 1967) Conversion ofsingle crystals
of chlorapatite into single crystals of hydroxyapatite. Nature 214,
904-906.
EVANS B. W. and GUGGENHEIM S. ( 1988) Talc, pyrophyllite, and
related minerals. In Hydrous Phyllosillicates (exclusive of micas)
(ed. S. W. BAILEY ); Reviews in Mineralogy 19, Chap. 8, pp. 225-
294.
EUGSTER H. P. ( 1982) Rock-fluid equilibrium systems In High-
Pressure Researches in Geosciences (ed. W. SCHREYER), pp. 501-
5 18. E. Schweizerbartsche Verlagsbuchhandlung, Stuttgart.
EUGSTER H. P. ( 1986) Minerals in hot water. Amer. Mineral. 71,
655-673.
EUGSTER H. P. and BAUMGARTNER L. ( 1987) Mineral solubilities
and speciation in supercritical metamorphic fluids. In Thermo-
dynamic Modelling of Geological Materials: Minerals, Fluids, and
Melts (eds. I. S. E. CARMICHAEL and H. P. EUGSTER); Reviews
in Mineralogy 18, Chap. 10, pp. 367-398.
EUGSTER H. P., CHOU I-MING, and G. A. WILSON ( 1987) Mineral
solubility and speciation in supercritical chloride fluids. In Hydra
thermal Experimental Techniques (ed. G. C. ULMER and H. L.
BARNES), Chap. 1, pp. I-19. J. Wiley & Sons.
FARR T. D. and ELMORE K. L. ( 1962) System CaO-P205-HF-H20:
Thermodynamic properties. J. Phys. Chem. 66, 315-3 18.
FARR T. D., TAR~UT~ON G., and LEWIS H. T., JR. ( 1962) System
CaO-P20S-HF-Hz0 equilibrium at 25 and 50C. J. Phys. Chem.
66,318-321.
FERRY J. M. ( 1989 ) Contact metamorphism of roof pedant at Hope
1856
C. Zhu and D. A. Svejensky
Valley, Alpine County, California, USA. Contrib. Miners. Petrol. In Fluid-mineral E~il~bria in Hydrothe~l Systems: Rev. Econ.
101,402-417. Geol. I,Chap. 2, pp. 9-27.
FERRY J. M. and BURT D. M. ( 1982) Characterization of meta-
morphic fluid composition through mineral equilibria. Rev. Min-
eral. 10, 207-258.
FERRY J. M. and SPEAR F. S. ( 1978) Experimental calibration of
the partitioning of Fe and Mg between biotite and garnet. Contrib.
Mineral. Petrot. 66, 113-l 17.
FRANTZ J. D. and EUGSTER H. P. ( 1973) Acid-base buffers: Use of
Ag + AgCl in the experimental control of solution equilibria at
elevated pressures and temperatures. Amer. J . Sci. 273,268-286.
FRANTZ J. D. and POPP R. K. ( 1979) Mineral-solution equilibria-
I. An experimental study of complexing and the~~ynamic
properties of aqueous MgCIr in the system Mu-Sip-H~~HCl.
Geochim. Cosmochim. Acta 43, 1233-1239.
GARVIN D., PARKER V. B., and WHITE H. J., JR. ( 1987) CODATA
thermodynamic tables-selections for some compounds of calcium
and related mixtures: A Prototype Set of Tables. Hemisphere Public
CO.
HOLLAND H. D. ( 1956) The chemical composition ofvein minerals
and the nature of ore-forming fluids. Econ. Geol. 51,78 l-797.
HOLLAND T. J. B. ( 1989) Dependence of entropy on volume for
silicate and oxide miner& A review and a predictive model. Amer.
Mineral. 74,5- 13.
HOLLAND T. J. B. and POWELL R. ( 1985 ) An internally consistent
thermodynamic dataset with uncertainties and correlations: 2. Data
and results. J . Metam. Geol. 3, 343-370.
HOLLAND T. J. B. and POWELL R. ( 1990) An enlarged and updated
internally consistent thermodynamic dataset with uncertainties and
correkuions: The system K*~Na~~M~Mn~F~F~O~-
AI,OX-TiCh-Sifh-C-H,-O,. J. Metam. Geol. 8.89-124.
JAC&&D. C: and*PARiY W. T. ( 1979) Geochemistry of biotite in
the Santa Rita Porphyry copper deposit, New Mexico. Econ. Geol.
74,860-887.
G~~~CHAL A. J. ( 1958) Heats of formation of hydroxy-, fluor-,
and chlor-apatites. South African Chem. I nst. l&45-52.
GRAHAM C. M. and NAVROTSKY A. ( 1986) Thermochemistry of
the tremolite-edenite amphiboles using fluorine analogues, and
applications to amphibole-plalgioclasequartz equilibria. Contrib.
Mineral. Petral. 93, 18-32.
JACQUES J. K. ( 1963) The heats of formation of fluorapatite and
hydroxyapatite. 1. Chem. Sot. London 7,3820-3822.
KAFWSTIN Y. L. ( 1987) The composition of apatite from metamor-
phic rocks. Geochem. I nt. 24,(i), 45-5 1.
KELLEY K. K.. BARANY R.. KING E. G.. and CHRISTENSEN A. U.
( 1959) Some thermodynamic properties of fluorphlogopite mica.
US Bur, Mines Rept. Inv. 5436.
GREGORY T. M., MORENO E. C., PATEL J. M., and BROWN W. E.
( 1974) Solubility of ts_ca3(PO~)~ in the system Ca(OH)r-HsP04-
Hz0 at 5, 15,25, and 37C. J . Res. Eur. Stand. 78A, 667-674.
GUI DOTTI C. V. ( 1984) Micas in metamorphic rocks. In Micas (ed.
S. W. BAILEY ); Reviews in Mineralogy 13, Chap. 10, pp. 357-
456.
KELLY W. L. and RYE R. 0. ( 1979) Geologic, 8uid inclusion, and
stable isotope studies of Tin-Tungsten Deposits of Panasqueira,
Portugal. Econ. Geol. 74,172 1- 1822.
KORZHINSK~Y M. A. ( 1980) A study of the Ag-AgCl buffer at low
values of uu2. In Outlines of Physicochemical Petrology, Vol. 9,
pp. 41-51. Nauka, Moscow.
GIJ NOW A. J., ~UDINGTON S. D., and MUNOZ J. L. ( 1980) Fluorine
in micas from the Henderson molybdenite deposit, Colorado. &on.
Geol. 75, 1127-1137.
KORZHINSKIY M. A. ( 198 1) Apatite solid solutions as indicators of
the fu8acity of HCl and HF in hydrothe~~ f l ui ds. Geochem. Zntl.
3,45-60.
HAAR L.,GALUGHER J. S., and KELL G. S.(1984) NBS/NRC
Steam Tables. Hemisphere Publishing Co.
HAAS J. L., JR. and RSHER J. R. ( 1976) Simul~eous evaluation
and correlation of the~~ynamic data. Amer. J Sci. 276,525-
545.
KWAK T. A. P. and ASKINS P. W. ( 198 1) Geology and Genesis of
the F-Sn-W (-Be-Zn) skam (Wrigghte) at Moina, Tasmania. Econ.
Geol. 76,439-467.
HASELTON H. T., JR., CYGAN G. L., and DLANGELO W. M. ( 1988 )
Chemistry of aqueous solutions coexisting with fluoride buffers in
the system KrO-AI&H@-FzO_r ( 1 kbar, 400-7OOC). Econ.
Geol. 83, 163-173.
HELGESON H. C. ( 1964) Comp~exing and Hydrother~l Ore De-
posits. MacMillan.
HELGESON H. C. ( 1969) Thermodynamics of hydrothermal systems
at elevated temperatures and pressures. Amer. J . Sci. 267, 729-
894.
HEU;ESON H. C. and KIRKHAM D. H. (1974a) Theoretical prediction
of the the~~ynamic behavior of aqueous electrolytes at hi8b
pressures and temperatures: 1. Summary of the thermodynamic/
electrostatic properties of the solvent. Amer. J . Sci. 274, 1089-
1198.
LATIL C. and MAURY R. ( 1977) Contribution a letude des bhange
dions OH-, Cl- et F- et de leur fixation darts les apatites hydro-
thermales. B&i. Sot. fi. Mineral. Cristollagr. 100,246-250.
LEE D. E. ( 1958) A chlorine-rich biotite from Lemhi County, Idaho.
Amer. Mineral. 43, 107- 111.
LUCE R. W., CYGAN G. L., HEMLEY J. J., and DANGELO W. M.
( 1985) Some mineral stability in the system CaO-MgO-Siq-H@-
HCl. Geochim. Cosm~him. Acta 49,525-538.
LUDINGTON S. ( 1978) The biotite-apatite geothermometer revisited.
Amer. Mineral. 63,551-553.
MANNING D. A. C. ( 198 1) The effect of fluorine on liquidus phase
relationships in the system Qz-AbOr with excess water at 1 kb.
Contrib. Mineral. Petrol. 76,X%-2 15.
MCCANN H. G. f 1968) The solubility of fluom~tite and its rela-
tionships to that of calcium fluorite. Arch. Oral Biol. 13, 987-
1001.
HELGE.WN H. C. and KIRKHAM D. H. ( 1974b) Theoretical prediction
of the the~~ynamic behavior of aqueous electrolytes at h&h
pressures and temperatures: II. Debye-Huckel parameters for ac-
tivity coefficients and relative partial molal properties. Amer. J .
Sci. 274, 1199- 126 1.
MCCONNELL D. ( 1973) Apatite: I ts crystal Chemistry, Mineralogy,
Utilization, and Geologic and Biologic Occurrences. Springer-Ver-
lag.
MC&TIRE W. L. ( 1963) Trace element partition coefficients-a re-
view of theory and applications to geology. Geochim. Cosmochim.
Acta 27, 1209-1264.
HELOE~~N H. C. and KI RKHAM D. H. ( 1976) Theoretical prediction
of the thermodynamic behavior of aqueous electroiytes at high
pressures and temperatures: III, Equation of state for aqueous spe-
ties at infinite dilution. Amer. J . Sci. 276, 1089-l 198.
HELGESON H. C., DELANY J. M., Nnsmrr H. W., and BIRD D. K.
( 1978) Summary and critique of the thermodynamic properties
of the rock-forming minerals. Amer. J . Sci. 278-A.
HELGE~ON H. C., KIRKHAM D. H., and FLOWERS G. C. ( 198 1)
Theoretical prediction of the the~~yn~ic behavior of aqueous
electrolytes at high pressures and temperatures: IV. Calculations
of activity coefficients, osmotic coefficients, and apparent molal
and standard and relative partial molal properties to 600C and
5 kb Amer. J . Sci. 281, 1249-1516.
MEYER C. and HEMLEY J. J. ( 1967) Wall rock alteration. In Geo-
chemistry of Hydrothermal Ore Deposits, 1st edn. (ed. H. L.
BARNES), pp. 166-235. Hoit, Rinehart and Winston.
MOECHER D. P. and CHOU I-M. ( 1990) Experimental investi~tion
of andradite and hedenbergite equilibria employing the hydrogen
sensor technique, with revised estimate of A,GL,298 for andradite
and hedenbergite. Amer. Mineral. 75, 1327- 134 1.
MOLLING P. A. ( 1989) Application of reaction progress variable to
hydrothermal alteration associated with the deposition of the
Questa Moly~enite Deposit, NM. Ph.D. dissertation, The Johns
Hopkins University.
HENLEY R. W. ( 1984) Chemical structure of geothermal systems.
MOLLING P. A. and SVER~ENSKY D. A. ( 1989) Thermodynamic
analysis of ore fluids at the Questa MO-porphyry deposits (abstr.)
Geol. Sot. Amer. Abstr. 21, 151.
MORA C. I. and VALLEY J. W., ( 1989) Halogen-rich scapolite and
Partitioning of F-Cl-OH in minerals 1857
biotite: Implications for metamorphic fluid-rock interaction. Amer.
Mineral. 14, 72 l-737.
MORENO E. C., GREGORY T. M., and BROWN W. E. ( 1968) Prep
aration and solubility of hydroxyapatite. J. Res. Bur. Stand. 72A,
773-782.
MUNOZ J. L. ( 1974) Measurements of quenched fluoride in synthetic
hydrothermal fluids (abstr.). Geol. Sot. Amer. Abstr. Prog. 4,882.
MUNOZ J. L. ( 1984) F-OH and Cl-OH exchange in micas with ap-
plications to Hydrothermal Ore Deposits. In Micas (ed. S. W.
BAILEY ); Reviews in Mineralogy 13, Chap. 1 I, pp. 469-493.
MUNOZ J. L. ( 1990) F and Cl contents of hydrothermal biotites: A
reevaluation ( abstr.) . Geol. Sot. Amer. Abstr. Prog. 22, A 135.
MUNOZ J. L. and EUGSTER H. P. ( 1969) Experimental control of
fluorine reactions in hydrothermal systems. Amer. Mineral. 54,
943-959.
MUNOZ J. L. and LUDINGTON S. D. ( 1974) Fluorine-hydroxyl ex-
change in biotite. Amer. J. Sci. 274, 396-4 13.
MUNOZ J. L. and LUDINGTON S. D. ( 1977) Fluorine-hydroxyl ex-
change in synthetic muscovite and its application to muscovite-
biotite assemblages. Amer. Mineral. 62, 304-308.
Munoz J. L. and SWENSON A. ( I98 I ) Chloride-hydroxyl exchange
in biotite and estimation of relative HCI/HF activities in hydro-
thermal fluids. Econ. Geol. 76,22 12-222 1.
NASH W. P. ( 1984) Phosphate minerals in terrestrial igneous and
metamorphic rocks. In Phosphate Minerals (eds. J. 0. NRIAGU
and P. B. MOORE), Chap. 6, pp. 2 15-242. Springer-Verlag, Berlin.
NAYLOR B. F. ( 1945) Heat contents at high temperatures of mag-
nesium and calcium fluorides. J. Amer. Chem. Sot. 67, I 50- 152.
OELKERS E. H. and HELCESON H. C. ( 1990) Triple ion anions and
polynuclear complexing in supercritical electrolyte solutions. Geo-
chim. Cosmochim. Acta 54,727-738.
PARRY W. T. and J ACOB D. C. ( 1975) Fluorine and chlorine in
biotite from Basin and Range plutons. Econ. Geol. 70, 554-558.
PETERSEN E. U., ESSENE E. J., PEACOR D. R., and VALLEY J. W.
( 1982) Fluorine endmember micas and amphiboles. Amer. Min-
eral. 67, 538-544.
POWELL R. and HOLLAND T. J. B. ( 1985) An internally consistent
thermodynamic dataset with uncertainties and correlations: 1
Methods and a worked example. J. Metam. Geol. 3, 327-342.
PRENER J. S. ( 1967) The growth and crystallographic properties of
calcium fluor- and chlorapatite crystals. J. Electrochem. Sot. 114,
77-83.
RICE J. M. ( 1977a) Progressive metamorphism of impure dolomite
limestone in the Marysville aureole, Montana. Amer. J. Sci. 277,
I -24.
RICE J. M. (1977b) Contact metamorphism of impure dolomite
limestone in the Boulder aureole, Montana. Contrib. Mineral. Pe-
trol. 59, 237-259.
RICE J. M. (1980a) Phase equilibria involving humite minerals in
impure dolomite limestones: Part I. Calculated stability of chno-
humite. Contrib. Mineral. Petrol. 71,219-235.
RICE J. M. ( 1980b) Phase eauihbria involvine humite minerals in
impure dolomitelimestones: Part II. Calculated stability of chon-
drodite and norbergite. Contrib. Mineral. Petrol. 75, 205-223.
ROBIE R. A., HEMMINGWAY B. S., and FISHER R. ( 1979) Ther-
modynamic properties of minerals and related substances at 298.15
K and 1 bar ( I O5 pascals) pressuire and high temperatures. US
Geol. SW-V. Bull. 1452.
ROEDDER E. ( 1979) Fluid inclusions as samples of ore fluids. In
Geochemistry of Hydrothermal Ore Deposits, 2nd ed. (ed. H. L.
BARNES), Chap. 14, pp. 684-731. J. Wiley & Sons.
RUAYA J. R. and SEWARD T. M. ( 1987) The ion-pair constant and
other thermodynamic properties of HCI up to 350C. Geochim.
Cosmochim. Acta 51, 121-130.
RUSZALA F. and KOSTINER E. ( 1975 ) Preparation and character-
ization of single crystals in the apatite system Ca,,( PO&( CI,OHh.
J. Crystal Growth 30,93-95.
SEWARD T. M. ( 1976) The stability of chloride complexes of silver
in hydrothermal solutions up to 350C. Geochim. Cosmochim.
Acta40, 1329-1341.
SHANNON R. D. ( 1976) Revised effective ionic radii and systematic
studies of interatomic distances in halides and chalcogenides. Acta
Cryst. A32, 75 l-767.
SHARP Z. D., HELFFRICH G. R., BOHLEN S. R., and ESSENE E. J.
( 1989) The stability of sodalite in the system NaAlSiOd-NaCl.
Geochim. Cosmochim. Acta 53, 1943-1954.
SHUCK E. L. and HELGESON H. C. ( 1988) Calculation of the ther-
modynamic and transport properties of aqueous species at high
pressures and temperatures: Correlation algorithms for ionic species
and equation of the state predictions to 5 kb and IooOC. Geochim.
Cosmochim. Acta 52,2009-2036.
SHUCK E. L., HELGESON H. C., and SVERJENSKY D. A. (1989)
Calculation of the thermodynamic and transport properties of
aqueous species at high pressures and temperatures: Standard par-
tial molal properties for inorganic neutral species. Geochim. Cos-
mochim. Acta 53,2 157-2 184.
SHOEMAKER D. P., GARLAND C. W., STEINFELD J. I., and NIBLER
J. W. ( 1981) Experiments in Physical Chemistry, 4th edn.
McGraw-Hill.
SISXBN V. B. ( 1987) Halogen chemistry as indicator of metamorphic
fluid interaction with the Ponder Pluton, Coast Plutonic Complex,
British Columbia, Canada. Contrib. Mineral. Petrol. 95, 123-13 1.
SMIRNOVA Z. G., ILLARIONOVA V. V., and VOLTIO~ICH S. I. ( 1962)
Heats of formation of fluorapatite, hydroxyapatite, and tricalcium
phosphate. Zh. Neorgun. Khimii 7( 8) (Russian calorimetric data
cited by VALYASHKO et al., 1968).
SMITH J. V. ( 198 1) Halogen and phosphorus storage in the Earth.
Nature 289,762-765.
SPEER J. A. ( 1984) Micas in igneous rocks. In Micas (ed. S. W.
BAILEY ); Reviews in Mineralogy 13, Chap. 9, pp. 299-349. Min-
eralogical Society of America.
STORMER J. C. and CARMICHAEL S. E. ( 1971) Fluorine-hydroxyl
exchange in apatite and biotite: A potential igneous geothermom-
eter. Contrib. Mineral. Petrol. 31, 12 I- 13 1.
SUDARSANAN D. A. and YOUNG R. A. ( 1978) Structural interactions
of F, Cl, OH in apatites. Crystullogr. Actu B34, 1401-1407.
SVERJENSKY D. A. ( 1984) Prediction of Gibbs free energies of calcite-
type carbonates and the equilibrium distribution of trace elements
between carbonate and aqueous solutions Geochim. Cosmochim.
Acta 48, 1127-I 134.
SVERJENSKY D. A. ( 1985) The distribution of divalent trace elements
between sulfides, oxides, silicates, and hydrothermal solutions: I
Thermodynamic basis. Geochim. Cosmochim. Acta 49,853-864.
SVERJENSKY D. A. ( 1987) Calculation of the thermodynamic prop
erties of aqueous species and the solubilities of minerals in super-
critical electrolyte solutions. In Thermodynamic Modelling of
Geological Materials: Minerals, Fluids, and Melts. (e&. I. S. E.
CARMICHAEL and H. P. EUGSTER H. L.); Reviews in Mineralogy
18, pp. 177-209. Mineral Sot. Am.
SVERJENSKY D. A., HEMLEY J. J., and DANGELO W. M. ( 1991a)
Thermodynamic assessment of hydrothermal alkali feldspar-mica-
aluminosilicate equilibria. Geochim. Cosmochim. Acta 55, 989-
1004.
SVERJENSKY D. A., SHOCK E. L., and HELGESON H. C. ( 1991b)
Prediction of the thermodynamic properties of inorganic aqueous
metal complexes to 1000 and 5 kb (in prep.).
TACKER R. C. and STORMER J. C., JR. ( 1989) A thermodynamic
model of apatite solid solutions, applicable to high-temperature
geologic problems. Amer. Mineral. 74, 877-888.
TANGER J. C. and HELGESON H. C. ( 1988) Calculation of the ther-
modynamic and transport properties of aqueous species at high
pressures and temperatures: Standard partial molal properties of
ions and electrolytes. Amer. J. Sci. 288, 19-98.
TODD S. S. ( 1949) Heat capacities at low temperatures and entropies
of magnesium and calcium fluorides. J. Amer. Chem. Sot. 71,
4115-4116.
TROLL G. and GILBERT M. C. ( 1972) Fluorine-hydroxyl substitution
in tremolite. Amer. Mineral. 57, 1386-1403.
TU G. Z. et al. ( 1979) The Geochemistry of Granites in Southeast
China. Scientific Publ. Co.
VALLEY J. W., PETERSEN E. U., ESSENE E. J., and BOWMAN J. R.
( 1982) Fluorphlogopite and fluortremohte in Adirondack marbles
and calculated C-O-H-F fluid compositions. Amer. Mineral. 67,
545-557.
VALYASHKO V. M., KOGARKO L. N., and KHODAKOVSKIY I. L.
( 1968) Stability of fluorapatite, chlorapatite, and hydroxyapatite
1858 C. Zhu and D. A. Svejensky
in aqueous solutions at different temperatures. Geochem. In?. 5,
21-30.
VAN REES H. B., MENGEOT M., and KOST~NER E. ( 1973) Mono-
clinic-hexagonal transition in hydroxyapatite and deuterohydrox-
yapatite. Materials Rex Bull. 8, 1307-l 320.
VIEILLARD P. and TARDY Y. ( 1984) Thermochemical properties of
phosphates. In Phosphate Minerals (eds. J. 0. NRIAGU and P. B.
MOORE), Chap. 4, pp. 17 I- 199. Springer-Verlag, Berlin.
VOLFINGER M. and PASCAL MARIE-LOLA ( 1989) Partitioning of
chlorine between muscovite and HCl-buffered solutions from 400
to 600C at 2 kbar. European J . Mineral. 1, 791-800.
VOLFINGER M., ROBERT J. L., VIELZEUF D., and NEIVA A. M. R.
( 1985) Structural control of the chlorine content of OH-bearing
silicates (micas and amphiboles). Geochim. Cosmochim. Acta 49,
37-48.
WAGMAN D. D., EVANS W. H., PARKER V. B., SCHUMM R. H.,
HALOW I., BAILEY S. M., CHURNEY K. L., and NUTTALL R. L.
( 1982) The NBS tables of chemical thermodynamic properties:
Selected values for inorganic and Cl and C2 organic substances
in SI units. Amer. Chem. Sot.
WEBSTER J. D. and HOLLOWAY J. R. ( 1990) Partitioning of F and
Cl between magmatic hydrothermal fluids and highly evolved
granite magmas. In Ore-bearing Granite Systems; Petrogenesis and
Mineralizing Processes (eds. H. J. STEIN and J. C. HANNAH);
GSA Special Paper 246. pp. 2 l-34.
WESTRICH H. R. (1978) Fluorine-hvdroxvl exchanee eouilibria in
. , _ .
several hydrous minerals. Ph.D. thesis, Arizona State University.
WESTRICH H. R. ( 198 1) F-OH exchange equilibria between mica-
amphibole pairs. Contrib. Mineral. Petrol. 78, 3 18-323.
WE~TRICH H. R. and NAVROTSKY A. ( 198 1) Some thermodynamic
properties of fluorapatite, fluorpargasite, and fluorphlogopite. Amer.
J . Sci. 281, 1091-l 103.
WHITE W. H., BXXSTROM A. A., KAMILLI R. J., GANSTER
M. W., SMITH R. P., RANTA D. E., and STEININGER R. C. ( 198 1)
Character and origin of Climax-type molybdenum deposits. Econ.
Geol. (75th arm. vol.), 270-3 16.
WIER D. R., CHIEN S. H., and BLACK C. A. ( 1970) Solubility of
hydroxyapatite. SoilSci. 11, 107-I 12.
WOLERY T. J. ( 1983) EQ3/NR-A computer program for geo-
chemical aqueous speciation solubility calculations: Users guide
and documentation: UCRL-534 14. Lawrence Livermore Natl.
Lab., Univ. Calif., Livermore.
WOLERY T. J. ( 1984) EQ6-A computer program for reaction-path
modelling of aqueous geochemical systems: Users guide and doc-
umentation: UCRL51. Lawrence Livermore Natl. Lab., Univ.
Calif., Livermore.
WOLERY T. J., SHERWOOD D. J., J ACKSON K. J ., DELANY J. M.,
and PIJIGDOMENECH I. ( 1984) EQ3/6: Status and applications.
UCRL91884. Lawrence Livermore Natl. Lab., Univ. Calif., Liv-
ermore.
WORD B. J. and NICHOLLS J. ( 1978) The thermodynamic properties
of reciprocal solid solutions. Contrib. Mineral. Petrol. 66, 389-
400.
WYLLIE P. J. and TUTTLE 0. F. ( 1961) Experimental investigation
of silicate system containing two volatile components: Part II. The
effects of NH, and HF, in addition to Hz0 on the melting tem-
peratures of albite and granite. Amer. J . Sci. 259, 128- 143.
WYLUE P. J. and TUTTLE 0. F. ( 1964) Experimental investigation
of silicate system containing two volatile components: Part III.
The effects of SOS, P,O5, HCl, and LirO, in addition to Hz0 on
the melting temperatures of albite and granite. Amer. J . Sci. 262,
930-939.
YARDLEY B. W. D. ( 1985) Apatite composition and the fugacities
of HF and HCl in metamorphic fluids. Mineral. Mag. 49,77-79.
YODER H. S. and EUGSTER H. P. ( 1955) Synthetic and natural mus-
covites. Geochim. Cosmochim. Acta 8. 225-280.
Você também pode gostar
- Compañía de Minas Buenaventura S.A.ADocumento34 páginasCompañía de Minas Buenaventura S.A.AVictor ValdiviaAinda não há avaliações
- Skarn Barton (95 - MexIgnMetall - AGSD20 PDFDocumento38 páginasSkarn Barton (95 - MexIgnMetall - AGSD20 PDFVictor ValdiviaAinda não há avaliações
- Barley Tectonic Controls On MagmatichydrothermalDocumento59 páginasBarley Tectonic Controls On MagmatichydrothermalVictor ValdiviaAinda não há avaliações
- Rio Maranon Minerals Inc. NR - Cancha 2010-10-25Documento2 páginasRio Maranon Minerals Inc. NR - Cancha 2010-10-25Victor ValdiviaAinda não há avaliações
- Uvp Catalog 2015 PDFDocumento36 páginasUvp Catalog 2015 PDFVictor ValdiviaAinda não há avaliações
- Vasyukova 2013 Cathodoluminescence Properties of Quartz Eyes From Porphyry-Type Deposits PDFDocumento12 páginasVasyukova 2013 Cathodoluminescence Properties of Quartz Eyes From Porphyry-Type Deposits PDFVictor ValdiviaAinda não há avaliações
- Type and Origens of Quartz in PorphyryDocumento225 páginasType and Origens of Quartz in PorphyryVictor ValdiviaAinda não há avaliações
- Barley Tectonic Controls On MagmatichydrothermalDocumento59 páginasBarley Tectonic Controls On MagmatichydrothermalVictor ValdiviaAinda não há avaliações
- Altered Volcanic RocksDocumento288 páginasAltered Volcanic RocksDavid Katemaunzanga100% (12)
- Bernaolaetal SEG2019 CarhuacayanDocumento4 páginasBernaolaetal SEG2019 CarhuacayanVictor ValdiviaAinda não há avaliações
- Using Scenarios To Investigate The Long-Term Future of Copper Mining and Guide Exploration Targeting StrategiesDocumento26 páginasUsing Scenarios To Investigate The Long-Term Future of Copper Mining and Guide Exploration Targeting StrategiesVictor ValdiviaAinda não há avaliações
- Uvp Catalog 2015 PDFDocumento36 páginasUvp Catalog 2015 PDFVictor ValdiviaAinda não há avaliações
- Diversity of Primary CL TexturesDocumento16 páginasDiversity of Primary CL TexturesVictor ValdiviaAinda não há avaliações
- Yerington - Emplacement Rates - 2017 - Schopa - PDFDocumento20 páginasYerington - Emplacement Rates - 2017 - Schopa - PDFVictor ValdiviaAinda não há avaliações
- Vasyukova 2013 Cathodoluminescence Properties of Quartz Eyes From Porphyry-Type Deposits PDFDocumento12 páginasVasyukova 2013 Cathodoluminescence Properties of Quartz Eyes From Porphyry-Type Deposits PDFVictor ValdiviaAinda não há avaliações
- Garwin - Et - Al - 2005 Au-Cu Cenozoic Magmatic Arcs SE Asia-West Pacific - AppendixDocumento32 páginasGarwin - Et - Al - 2005 Au-Cu Cenozoic Magmatic Arcs SE Asia-West Pacific - AppendixherryadiAinda não há avaliações
- Examples of 3D Potenial Field Inversion Low Latitudes and Remanence PDFDocumento29 páginasExamples of 3D Potenial Field Inversion Low Latitudes and Remanence PDFVictor ValdiviaAinda não há avaliações
- Geological, Alteration and Mineralization Characteristics of Ali Javad Porphyry Cu-Au Deposit, Arasbaran Zone, NW IranDocumento17 páginasGeological, Alteration and Mineralization Characteristics of Ali Javad Porphyry Cu-Au Deposit, Arasbaran Zone, NW IranVictor ValdiviaAinda não há avaliações
- Application of Automated Detection Techniques in Magnetic Data For The Detection of Cu Au Porphyries in Coverd TerrainsDocumento21 páginasApplication of Automated Detection Techniques in Magnetic Data For The Detection of Cu Au Porphyries in Coverd TerrainsVictor ValdiviaAinda não há avaliações
- Yerington - Emplacement Rates - 2017 - Schopa - PDFDocumento20 páginasYerington - Emplacement Rates - 2017 - Schopa - PDFVictor ValdiviaAinda não há avaliações
- PIMS - BouzariDocumento1 páginaPIMS - BouzariVictor ValdiviaAinda não há avaliações
- 2011 Porphyry Cu-Au-Mo-epithermal Ag-Pb-Zn-distal Hydrothermal Au Deposits in The Dexing Area, Jiangxi Province, East China - A Linked Ore System PDFDocumento14 páginas2011 Porphyry Cu-Au-Mo-epithermal Ag-Pb-Zn-distal Hydrothermal Au Deposits in The Dexing Area, Jiangxi Province, East China - A Linked Ore System PDFVictor ValdiviaAinda não há avaliações
- The Beaver River Structure: A Cross-Strike Discontinuity of Possible Crustal Dimensions in The Southern Mackenzie Fold Belt, Yukon and Northwest Territories, CanadaDocumento11 páginasThe Beaver River Structure: A Cross-Strike Discontinuity of Possible Crustal Dimensions in The Southern Mackenzie Fold Belt, Yukon and Northwest Territories, CanadaVictor ValdiviaAinda não há avaliações
- Application of Automated Detection Techniques in Magnetic Data For The Detection of Cu Au Porphyries in Coverd TerrainsDocumento21 páginasApplication of Automated Detection Techniques in Magnetic Data For The Detection of Cu Au Porphyries in Coverd TerrainsVictor ValdiviaAinda não há avaliações
- CAFP - LDEE - Cantor - Hart - Iscaycruz - SEG2018 - 44x41 PDFDocumento1 páginaCAFP - LDEE - Cantor - Hart - Iscaycruz - SEG2018 - 44x41 PDFVictor ValdiviaAinda não há avaliações
- 4 PDFDocumento62 páginas4 PDFjoseAinda não há avaliações
- ArticuloDocumento20 páginasArticuloVictor ValdiviaAinda não há avaliações
- Report PDFDocumento136 páginasReport PDFditapermatasariAinda não há avaliações
- Mineralogy and Petrology OF Deposits: SkarnDocumento15 páginasMineralogy and Petrology OF Deposits: Skarnmanael pixelAinda não há avaliações
- Foster 2009 - Skarn - and - Porphyry - Deposits - EITH2009 PDFDocumento29 páginasFoster 2009 - Skarn - and - Porphyry - Deposits - EITH2009 PDFVictor ValdiviaAinda não há avaliações
- Never Split the Difference: Negotiating As If Your Life Depended On ItNo EverandNever Split the Difference: Negotiating As If Your Life Depended On ItNota: 4.5 de 5 estrelas4.5/5 (838)
- Elon Musk: Tesla, SpaceX, and the Quest for a Fantastic FutureNo EverandElon Musk: Tesla, SpaceX, and the Quest for a Fantastic FutureNota: 4.5 de 5 estrelas4.5/5 (474)
- The Subtle Art of Not Giving a F*ck: A Counterintuitive Approach to Living a Good LifeNo EverandThe Subtle Art of Not Giving a F*ck: A Counterintuitive Approach to Living a Good LifeNota: 4 de 5 estrelas4/5 (5783)
- Hidden Figures: The American Dream and the Untold Story of the Black Women Mathematicians Who Helped Win the Space RaceNo EverandHidden Figures: The American Dream and the Untold Story of the Black Women Mathematicians Who Helped Win the Space RaceNota: 4 de 5 estrelas4/5 (890)
- The Yellow House: A Memoir (2019 National Book Award Winner)No EverandThe Yellow House: A Memoir (2019 National Book Award Winner)Nota: 4 de 5 estrelas4/5 (98)
- The Little Book of Hygge: Danish Secrets to Happy LivingNo EverandThe Little Book of Hygge: Danish Secrets to Happy LivingNota: 3.5 de 5 estrelas3.5/5 (399)
- A Heartbreaking Work Of Staggering Genius: A Memoir Based on a True StoryNo EverandA Heartbreaking Work Of Staggering Genius: A Memoir Based on a True StoryNota: 3.5 de 5 estrelas3.5/5 (231)
- Team of Rivals: The Political Genius of Abraham LincolnNo EverandTeam of Rivals: The Political Genius of Abraham LincolnNota: 4.5 de 5 estrelas4.5/5 (234)
- Devil in the Grove: Thurgood Marshall, the Groveland Boys, and the Dawn of a New AmericaNo EverandDevil in the Grove: Thurgood Marshall, the Groveland Boys, and the Dawn of a New AmericaNota: 4.5 de 5 estrelas4.5/5 (265)
- The Hard Thing About Hard Things: Building a Business When There Are No Easy AnswersNo EverandThe Hard Thing About Hard Things: Building a Business When There Are No Easy AnswersNota: 4.5 de 5 estrelas4.5/5 (344)
- The Emperor of All Maladies: A Biography of CancerNo EverandThe Emperor of All Maladies: A Biography of CancerNota: 4.5 de 5 estrelas4.5/5 (271)
- The World Is Flat 3.0: A Brief History of the Twenty-first CenturyNo EverandThe World Is Flat 3.0: A Brief History of the Twenty-first CenturyNota: 3.5 de 5 estrelas3.5/5 (2219)
- The Unwinding: An Inner History of the New AmericaNo EverandThe Unwinding: An Inner History of the New AmericaNota: 4 de 5 estrelas4/5 (45)
- The Gifts of Imperfection: Let Go of Who You Think You're Supposed to Be and Embrace Who You AreNo EverandThe Gifts of Imperfection: Let Go of Who You Think You're Supposed to Be and Embrace Who You AreNota: 4 de 5 estrelas4/5 (1090)
- The Sympathizer: A Novel (Pulitzer Prize for Fiction)No EverandThe Sympathizer: A Novel (Pulitzer Prize for Fiction)Nota: 4.5 de 5 estrelas4.5/5 (119)
- Efficient Wood Fireplaces for Medium HomesDocumento4 páginasEfficient Wood Fireplaces for Medium HomesDANAinda não há avaliações
- Natural Circulation in BoilerDocumento9 páginasNatural Circulation in BoilerSajjad AhmedAinda não há avaliações
- Fluidization of Bulk SolidsDocumento77 páginasFluidization of Bulk SolidsManfredHeyde100% (1)
- Achiever DLP Unit Test 3 SoDocumento11 páginasAchiever DLP Unit Test 3 SoChetan LaxkarAinda não há avaliações
- Cooling MixturesDocumento3 páginasCooling MixturesSumanna ReddyAinda não há avaliações
- 3030 eDocumento58 páginas3030 ecesar luis gonzalez rodriguezAinda não há avaliações
- Asian Architecture Project 1: Case Study PaperDocumento20 páginasAsian Architecture Project 1: Case Study PaperjayaxeeAinda não há avaliações
- Coil Heat CalculationDocumento2 páginasCoil Heat CalculationTarmana Antt100% (1)
- (2010) Hongxi 2010Documento10 páginas(2010) Hongxi 2010Husnain AliAinda não há avaliações
- MAE 589 Heat Transfer Syllabus (Spring 2015)Documento8 páginasMAE 589 Heat Transfer Syllabus (Spring 2015)Pratik SankheAinda não há avaliações
- Calculation of Natural Gas Isentropic ExponentDocumento8 páginasCalculation of Natural Gas Isentropic ExponentsekharsamyAinda não há avaliações
- Newtons Law of CoolingDocumento6 páginasNewtons Law of CoolingSlumpSKD1Ainda não há avaliações
- Enclosure for Microface and Hiromatic for AC manualDocumento12 páginasEnclosure for Microface and Hiromatic for AC manualmskAinda não há avaliações
- Thermal Properties of MatterDocumento11 páginasThermal Properties of MatterRaja HassnainAinda não há avaliações
- Heat Sink Optimization for Electronics CoolingDocumento4 páginasHeat Sink Optimization for Electronics CoolingsaqibAinda não há avaliações
- Sand Casting Lit ReDocumento77 páginasSand Casting Lit ReIxora MyAinda não há avaliações
- Energies 13 01986Documento13 páginasEnergies 13 01986dieva ameliaAinda não há avaliações
- Mere423 03Documento3 páginasMere423 03Delia GantiaAinda não há avaliações
- Basic Civil and Mechanical Engineering UNIT V Presentation PDFDocumento70 páginasBasic Civil and Mechanical Engineering UNIT V Presentation PDFA.R. Pradeep KumarAinda não há avaliações
- Chiller and AHU NotedDocumento126 páginasChiller and AHU Notedkeruks100% (2)
- Tables and Index Thermodynamics Cengel 7E-2Documento118 páginasTables and Index Thermodynamics Cengel 7E-2tomtom9649Ainda não há avaliações
- Natural Convection Heat Transfer and Air Flow in Rectangular Enclosures With A Wavy WallDocumento11 páginasNatural Convection Heat Transfer and Air Flow in Rectangular Enclosures With A Wavy Wallnaru_saAinda não há avaliações
- Plan-J Physics Frm4Documento13 páginasPlan-J Physics Frm4Shairah FauziAinda não há avaliações
- Phase EquilibriaDocumento21 páginasPhase EquilibriasuperchellyAinda não há avaliações
- Synthesis and Structural Modification of PolyanilineDocumento1 páginaSynthesis and Structural Modification of PolyanilineChax EdrallagAinda não há avaliações
- Chapter 5 Thermochemistry - b5Documento70 páginasChapter 5 Thermochemistry - b5kikianitaAinda não há avaliações
- Rittal Enclosure and Process CoolingDocumento98 páginasRittal Enclosure and Process CoolingVadivelu DhanakotiAinda não há avaliações
- Course DescriptionDocumento54 páginasCourse DescriptionMesafint lisanuAinda não há avaliações
- BITS Pilani: Module 4: Design of Separation Systems Lecture-14Documento18 páginasBITS Pilani: Module 4: Design of Separation Systems Lecture-14pulkitAinda não há avaliações
- High Power Density Air-Cooled Microchannel Heat ExchangerDocumento8 páginasHigh Power Density Air-Cooled Microchannel Heat ExchangerAnkit LonareAinda não há avaliações