Escolar Documentos
Profissional Documentos
Cultura Documentos
9science 2 Is Matter Around Us Pure
Enviado por
Abhishek Abh-iTítulo original
Direitos autorais
Formatos disponíveis
Compartilhar este documento
Compartilhar ou incorporar documento
Você considera este documento útil?
Este conteúdo é inapropriado?
Denunciar este documentoDireitos autorais:
Formatos disponíveis
9science 2 Is Matter Around Us Pure
Enviado por
Abhishek Abh-iDireitos autorais:
Formatos disponíveis
Cbse-spot.blogspot.
com
1
Class IX Chapter 2 Is Matter Around
Us Pure Science
Question 1:
What is meant by a pure substance?
Answer:
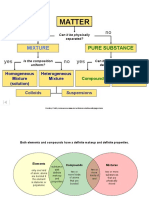
A pure substance is the one that consists of a single type of particles, i.e., all constituent
particles of the substance have the same chemical nature. Pure substances can be
classified as elements or compounds.
Question 2:
List the points of differences between homogeneous and heterogeneous mixtures.
Answer:
A homogeneous mixture is a mixture having a uniform composition throughout the
mixture. For example: salt in water, sugar in water, copper sulphate in water
A heterogeneous mixture is a mixture having a non-uniform composition throughout
the mixture. For example: sodium chloride and iron fillings, salt and sulphur, oil and
water
Cbse-spot.blogspot.com
2
Exercise Question 1:
Differentiate between homogeneous and heterogeneous mixtures with examples.
Answer:
A homogeneous mixture is a mixture having a uniform composition throughout the
mixture. For example, mixtures of salt in water, sugar in water, copper sulphate in
water, iodine in alcohol, alloy, and air have uniform compositions throughout the
mixtures.
On the other hand, a heterogeneous mixture is a mixture having a non-uniform
composition throughout the mixture. For example, composition of mixtures of sodium
chloride and iron fillings, salt and sulphur, oil and water, chalk powder in water, wheat
flour in water, milk and water are not uniform throughout the mixtures.
Question 2:
How are sol, solution and suspension different from each other?
Answer:
Sol is a heterogeneous mixture. In this mixture, the solute particles are so small that
they cannot be seen with the naked eye. Also, they seem to be spread uniformly
throughout the mixture. The Tyndall effect is observed in this mixture. For example:
milk of magnesia, mud
Cbse-spot.blogspot.com
3
Solution is a homogeneous mixture. In this mixture, the solute particles dissolve and
spread uniformly throughout the mixture. The Tyndall effect is not observed in this
mixture. For example: salt in water, sugar in water, iodine in alcohol, alloy
Suspensions are heterogeneous mixtures. In this mixture, the solute particles are
visible to the naked eye, and remain suspended throughout the bulk of the medium.
The Tyndall effect is observed in this mixture. For example: chalk powder and water,
wheat flour and water Question 3:
To make a saturated solution, 36 g of sodium chloride is dissolved in 100 g of water at
293 K. Find its concentration at this temperature.
Answer:
Mass of solute (sodium chloride) = 36 g (Given)
Mass of solvent (water) = 100 g (Given)
Then, mass of solution = Mass of solute + Mass of solvent
= (36 + 100) g
= 136 g
Therefore, concentration (mass by mass percentage) of the solution
Cbse-spot.blogspot.com
4
Question 1:
How will you separate a mixture containing kerosene and petrol (difference in their
boiling points is more than 25C), which are miscible with each other?
Answer:
A mixture of two miscible liquids having a difference in their boiling points more than
25C can be separated by the method of distillation. Thus, kerosene and petrol can be
separated by distillation.
Cbse-spot.blogspot.com
5
In this method, the mixture of kerosene and petrol is taken in a distillation flask with
a thermometer fitted in it. We also need a beaker, a water condenser, and a Bunsen
burner. The apparatus is arranged as shown in the above figure. Then, the mixture is
heated slowly. The thermometer should be watched simultaneously. Kerosene will
vaporize and condense in the water condenser. The condensed kerosene is collected
from the condenser outlet, whereas petrol is left behind in the distillation flask.
Question 2:
Name the technique to separate
(i) butter from curd
(ii) salt from sea-water
(iii) camphor from salt Answer:
(i) Butter can be separated from curd by centrifugation.
(ii) Salt can be separated from sea-water by evaporation.
(iii) Camphor can be separated from salt by sublimation.
Question 3:
What type of mixtures is separated by the technique of crystallization?
Answer:
By the technique of crystallization, pure solids are separated from impurities. For
example, salt obtained from sea is separated from impurities; crystals of alum
(Phitkari) are separated from impure samples.
Cbse-spot.blogspot.com
6
Question 1:
Classify the following as chemical or physical changes:
Cutting of trees
Melting of butter in a pan
Rusting of almirah
Boiling of water to form steam
Passing of electric current through water, and water breaking down into hydrogen
and oxygen gas
Dissolving common salt in water
Making a fruit salad with raw fruits
Burning of paper and wood Answer:
Cbse-spot.blogspot.com
7
Cutting of trees Physical change
Melting of butter in a pan Physical change
Rusting of almirah Chemical change
Boiling of water to form steam Physical change
Passing of electric current through water, and water breaking down into hydrogen
and oxygen gas Chemical change
Dissolving common salt in water Physical change
Making a fruit salad with raw fruits Physical change
Burning of paper and wood Chemical change
Question 2:
Try segregating the things around you as pure substances or mixtures.
Answer:
Pure substance: Water, salt, sugar
Mixture: Salt water, soil, wood, air, cold drink, rubber, sponge, fog, milk, butter, clothes,
food
Question 1:
Which separation techniques will you apply for the separation of the following?
(a) Sodium chloride from its solution in water.
(b) Ammonium chloride from a mixture containing sodium chloride and ammonium
chloride.
(c) Small pieces of metal in the engine oil of a car.
(d) Different pigments from an extract of flower petals.
(e) Butter from curd.
Cbse-spot.blogspot.com
8
(f) Oil from water.
(g) Tea leaves from tea.
(h) Iron pins from sand.
(i) Wheat grains from husk.
(j) Fine mud particles suspended in water.
Answer:
(a) Sodium chloride from its solution in water Evaporation
(b) Ammonium chloride from a mixture containing sodium chloride and ammonium
chloride Sublimation
(c) Small pieces of metal in the engine oil of a car Centrifugation or filtration or
decantation
(d) Different pigments from an extract of flower petals Chromatography
(e) Butter from curd Centrifugation
(f) Oil from water Using separating funnel
(g) Tea leaves from tea Filtration
(h) Iron pins from sand Magnetic separation
(i) Wheat grains from husk Winnowing
(j) Fine mud particles suspended in water Centrifugation
Question 2:
Write the steps you would use for making tea. Use the words: solution, solvent, solute,
dissolve, soluble, insoluble, filtrate and residue.
Answer:
First, water is taken as a solvent in a saucer pan. This water (solvent) is allowed to
boil. During heating, milk and tea leaves are added to the solvent as solutes. They
form a solution. Then, the solution is poured through a strainer. The insoluble part of
Cbse-spot.blogspot.com
9
the solution remains on the strainer as residue. Sugar is added tothe filtrate, which
dissolves in the filtrate. The resulting solution is the required tea.
Question 4:
Explain the following giving examples:
(a) Saturated solution
(b) Pure substance
(c) Colloid
(d) Suspension Answer:
(a) Saturated solution
A saturated solution is a solution in which the maximum amount of solute has been
dissolved at a given temperature. The solution cannot dissolve beyond that amount of
solute at that temperature. Any more solute added will settle down at the bottom of
the container as a precipitate.
Suppose 500 g of a solvent can dissolve a maximum of 150 g of a particular solute at
40C. Then, the solution obtained by dissolving 150 g of that solute in 500 g of that
solvent at 300 K is said to be a saturated solution at 300 K.
Cbse-spot.blogspot.com
1
0
(b) Pure substance
A pure substance is a substance consisting of a single type of particles i.e., all
constituent particles of the substance have the same chemical properties.
For example, salt, sugar, water are pure substances.
(c) Colloid
A colloid is a heterogeneous mixture. The size of the solutes in this mixture is so small
that they cannot be seen individually with naked eyes, and seems to be distributed
uniformly throughout the mixture. The solute particles do not settle down when the
mixture is left undisturbed. This means that colloids are quite stable. Colloids cannot
be separated by the process of filtration. They can be separated by centrifugation.
Colloids show the Tyndall effect. For example, milk, butter, foam, fog, smoke, clouds.
(d) Suspension
Suspensions are heterogeneous mixtures. The solute particles in this mixture remain
suspended throughout the bulk of the medium. The particles can be seen with naked
eyes. Suspension shows the Tyndall effect. The solute particles settle down when the
mixture is left undisturbed. This means that suspensions are unstable. Suspensions
can be separated by the method of filtration. For example, mixtures of chalk powder
and water, wheat flour and water.
Question 5:
Classify each of the following as a homogeneous or heterogeneous mixture.
Soda water, wood, air, soil, vinegar, filtered tea Answer:
Homogeneous mixtures: Soda water, air, vinegar
Heterogeneous mixtures: Wood, soil, filtered tea
Question 6:
How would you confirm that a colourless liquid given to you is pure water?
Answer:
Cbse-spot.blogspot.com
1
1
Every liquid has a characteristic boiling point. Pure water has a boiling point of 100C
(373 K) at 1 atmospheric pressure. If the given colourless liquid boils at even slightly
above or below 100C, then the given liquid is not pure water. It must boil at sharp
100C. Thus, by observing the boiling point, we can confirm whether a given colourless
liquid is pure water or not.
Question 7:
Which of the following materials fall in the category of a pure substance?
(a) Ice
(b) Milk (c)
Iron
(d) Hydrochloric Acid
(e) Calcium oxide
(f) Mercury
(g) Brick
(h) Wood
(i) Air
Answer:
The following materials fall in the category of a pure substance:
(a) Ice
(c) Iron
(d) Hydrochloric acid
(e) Calcium oxide
(f) Mercury
Question 8:
Identify the solutions among the following mixtures:
(a) Soil
Cbse-spot.blogspot.com
1
2
(b) Sea water
(c) Air
(d) Coal
(e) Soda water Answer:
The following mixtures are solutions:
(b) Sea water (c)
Air (e)
Soda water
Question 9:
Which of the following will show the Tyndall effect?
(a) Salt solution
(b) Milk
(c) Copper sulphate solution (d) Starch solution Answer:
Milk and starch solution will show the Tyndall effect.
Question 10:
Classify the following into elements, compounds and mixtures:
(a) Sodium
(b) Soil
(c) Sugar solution
(d) Silver
(e) Calcium carbonate
(f) Tin
(g) Silicon
Cbse-spot.blogspot.com
1
3
(h) Coal (i) Air
(j) Soap
(k) Methane
(l) Carbon dioxide
(m) Blood Answer:
Elements
(a) Sodium
(d) Silver
(f) Tin
(g) Silicon
Compounds
(e) Calcium carbonate
(k) Methane
(l) Carbon dioxide
Mixtures
(b) Soil
(c) Sugar solution
(h) Coal
(i) Air
(j) Soap
(m) Blood
Question 11:
Which of the following are chemical changes?
(a) Growth of a plant
(b) Rusting of iron
(c) Mixing of iron fillings and sand
(d) Cooking of food
Cbse-spot.blogspot.com
1
4
(e) Digestion of food (f) Freezing of water (g) Burning of candle Answer:
The following changes are chemical changes:
(a) Growth of a plant
(b) Rusting of iron
(d) Cooking of food
(e) Digestion of food
(g) Burning of candle
Você também pode gostar
- Manual of Formulas - Recipes, Methods & Secret ProcessesNo EverandManual of Formulas - Recipes, Methods & Secret ProcessesNota: 4.5 de 5 estrelas4.5/5 (2)
- Introductory Chemistry 4th Edition Russo Test BankDocumento14 páginasIntroductory Chemistry 4th Edition Russo Test BankJohnWhitextnzm100% (16)
- EDU & GOV LINKSDocumento3 páginasEDU & GOV LINKSDominikAnđelićAinda não há avaliações
- Classification of MatterDocumento30 páginasClassification of MattershasagailAinda não há avaliações
- W1 Q1 Day 1-5 Grade 6 Science ModuleDocumento33 páginasW1 Q1 Day 1-5 Grade 6 Science ModuleMichael Edward De Villa100% (1)
- ChemistryDocumento417 páginasChemistryKetsya Lenak100% (2)
- Chemistry at Home - A Collection of Experiments and Formulas for the Chemistry EnthusiastNo EverandChemistry at Home - A Collection of Experiments and Formulas for the Chemistry EnthusiastAinda não há avaliações
- Science 6 (Q1W1)Documento35 páginasScience 6 (Q1W1)Abigail Kaye DegamoAinda não há avaliações
- O Level Biology NotesDocumento221 páginasO Level Biology Notesemma100% (1)
- Grade 4 Science FinalDocumento28 páginasGrade 4 Science FinalLuna PagsuyuinAinda não há avaliações
- 7E Lesson Plan ChemistryDocumento2 páginas7E Lesson Plan ChemistryGervy Racoma Valeroso100% (1)
- Science 6-Q1-Lesson 1-Week 1 To 4Documento5 páginasScience 6-Q1-Lesson 1-Week 1 To 4Khryz Tin100% (3)
- O Level Biology NotesDocumento221 páginasO Level Biology NotesKhaled Elshamanhory89% (18)
- The Big Chemistry Book on Solutions - Chemistry for 4th Graders | Children's Chemistry BooksNo EverandThe Big Chemistry Book on Solutions - Chemistry for 4th Graders | Children's Chemistry BooksAinda não há avaliações
- General Chemistry 1: First Quarter-Module 1: The Nature of MatterDocumento26 páginasGeneral Chemistry 1: First Quarter-Module 1: The Nature of MatterAlessandra Gabrielle GarezAinda não há avaliações
- 6 DK L3 Xxas 17 Mo ZEZ2 T75Documento13 páginas6 DK L3 Xxas 17 Mo ZEZ2 T75SURYA PRAKASHAinda não há avaliações
- In Text Questions and AnswersDocumento8 páginasIn Text Questions and AnswersVinod MalikAinda não há avaliações
- Chapter 2 - Is Matter Around Us PureDocumento9 páginasChapter 2 - Is Matter Around Us PurepillapandurangapatruduAinda não há avaliações
- Is Matter Around Us PureDocumento7 páginasIs Matter Around Us PureAzimAinda não há avaliações
- Is Matter Around Us Pure: Question and Answers: Q1.Explain The Following Giving ExamplesDocumento4 páginasIs Matter Around Us Pure: Question and Answers: Q1.Explain The Following Giving ExamplesSai Uday Shankar BommakantiAinda não há avaliações
- NCERT Changes Around Us 9th ClassDocumento5 páginasNCERT Changes Around Us 9th ClassDaily Current affairsAinda não há avaliações
- Class 9 Cbse EnglishDocumento8 páginasClass 9 Cbse EnglishRoben SinghAinda não há avaliações
- Wa0029.Documento5 páginasWa0029.ElvinAinda não há avaliações
- Q.A. of Ch-2 Class-IXDocumento7 páginasQ.A. of Ch-2 Class-IXPrathaviraj Bhadoria 7 DAinda não há avaliações
- Is Matter Around Us Pure: Assess YourselfDocumento10 páginasIs Matter Around Us Pure: Assess YourselfAdil KhanAinda não há avaliações
- CHEM CLASS 9 NCERT Is Matter Around Us Pure Inayat V1.2Documento8 páginasCHEM CLASS 9 NCERT Is Matter Around Us Pure Inayat V1.2Gaurav ThakareAinda não há avaliações
- Physical Nature of MatterDocumento46 páginasPhysical Nature of MatterChandra ReddyAinda não há avaliações
- 5ad9ad59e4b0330dfab77595 PDFDocumento18 páginas5ad9ad59e4b0330dfab77595 PDFMehtab AnsariAinda não há avaliações
- 9 Science Ncert ch2 PDFDocumento10 páginas9 Science Ncert ch2 PDFSeenu SAinda não há avaliações
- Science Chap 2 Que AnsDocumento5 páginasScience Chap 2 Que AnsKhushi Kaur aroraAinda não há avaliações
- 9 Science Imp ch2 3Documento9 páginas9 Science Imp ch2 3Lipsa DasAinda não há avaliações
- Class 9 Science - Chapter 2 Is Matter Around Us Pure PDFDocumento6 páginasClass 9 Science - Chapter 2 Is Matter Around Us Pure PDFGaurav SethiAinda não há avaliações
- CBSE Class 9 Science Chapter 2 - Is Matter Around Us Pure Important Questions 2023-24Documento13 páginasCBSE Class 9 Science Chapter 2 - Is Matter Around Us Pure Important Questions 2023-24IX17-41 Suvayu ChatterjeeAinda não há avaliações
- Is Matter Around Us Pure RevisionDocumento18 páginasIs Matter Around Us Pure RevisionRaghvendra ShrivastavaAinda não há avaliações
- Class 9 CHAPTER 2 Is Matter Around Us Pure (H)Documento4 páginasClass 9 CHAPTER 2 Is Matter Around Us Pure (H)Game WorldAinda não há avaliações
- Class9 TH Chapter 2Documento7 páginasClass9 TH Chapter 2knramya18Ainda não há avaliações
- MLP HLP IX ch2-1Documento6 páginasMLP HLP IX ch2-1shalabh1976Ainda não há avaliações
- Is Matter Around Us PureDocumento5 páginasIs Matter Around Us PureMECH27DHYAN PATELAinda não há avaliações
- Judge purity of substancesDocumento35 páginasJudge purity of substancesNanc JoyAinda não há avaliações
- Is Matter Around Us Pure: Periodic TestDocumento17 páginasIs Matter Around Us Pure: Periodic TestMintu KhanAinda não há avaliações
- Solubility and Separation TechniquesDocumento10 páginasSolubility and Separation TechniquesSunil KumarAinda não há avaliações
- Is Matter Around Us Pure Paper 1Documento5 páginasIs Matter Around Us Pure Paper 1MECH27DHYAN PATELAinda não há avaliações
- 9 Science NcertSolutions Chapter 2 Exercises 1Documento7 páginas9 Science NcertSolutions Chapter 2 Exercises 1Kundan KumarAinda não há avaliações
- Classifying Matter and Separating MixturesDocumento24 páginasClassifying Matter and Separating MixturesRaghava VadavadagiAinda não há avaliações
- IS MATTER AROUND US PUREDocumento45 páginasIS MATTER AROUND US PUREsabirafrinAinda não há avaliações
- Class IX Chemistry Chapter 07Documento9 páginasClass IX Chemistry Chapter 07Sam FisherAinda não há avaliações
- GR 11 DRI September 12Documento37 páginasGR 11 DRI September 12Hind HammadAinda não há avaliações
- Chapter 1 - 2Documento5 páginasChapter 1 - 2Sureshkumar DevanAinda não há avaliações
- Ncert Sol For Cbse Class 9 Sci Chapter 2 Is Matter Around Us Pure PDFDocumento10 páginasNcert Sol For Cbse Class 9 Sci Chapter 2 Is Matter Around Us Pure PDFAbhishek PandeyAinda não há avaliações
- CH 2 Class 9 CbseDocumento13 páginasCH 2 Class 9 CbseekamAinda não há avaliações
- CHEMISTRY Ch2 Solved Assignment Class 9 CBSEDocumento5 páginasCHEMISTRY Ch2 Solved Assignment Class 9 CBSEgurdeepsarora8738100% (1)
- 1st Two Chap. of Class9 ChemistryDocumento7 páginas1st Two Chap. of Class9 ChemistryAbhinandan AngraAinda não há avaliações
- Ncert Science Class 9Documento24 páginasNcert Science Class 9suneel kumar rathoreAinda não há avaliações
- Is Matter Around Us PureDocumento24 páginasIs Matter Around Us PureNisha SinghAinda não há avaliações
- Purification of Benzoic AcidDocumento5 páginasPurification of Benzoic AcidHope CariñoAinda não há avaliações
- Chap 2Documento17 páginasChap 2Abhijeet BhagavatulaAinda não há avaliações
- Class 6 CH 5 SolvedDocumento8 páginasClass 6 CH 5 SolvedsayogitaAinda não há avaliações
- Chapter - 2: Is Matter Around Us PureDocumento24 páginasChapter - 2: Is Matter Around Us Pureuma mishraAinda não há avaliações
- Saturated Solution FINALDocumento16 páginasSaturated Solution FINALaahanatanayaAinda não há avaliações
- Is Matter Around Us PureDocumento7 páginasIs Matter Around Us PureVikashAinda não há avaliações
- SolutionsDocumento54 páginasSolutionsPhett MaestroAinda não há avaliações
- Example of Chemical ChangeDocumento7 páginasExample of Chemical ChangeRamel OñateAinda não há avaliações
- Unit 3Documento6 páginasUnit 3sabrina enadaAinda não há avaliações
- LAB 2-CHM01aLDocumento5 páginasLAB 2-CHM01aLNova Jane EdradAinda não há avaliações
- Is Matter Around Us PureDocumento23 páginasIs Matter Around Us PureAryan AgarwalAinda não há avaliações
- Bijendra Public School: Class: 6 Subject: Science Chapter - 5 Separation of SubstancesDocumento3 páginasBijendra Public School: Class: 6 Subject: Science Chapter - 5 Separation of Substancessmitha_gururajAinda não há avaliações
- Is Matter Around Us PureDocumento16 páginasIs Matter Around Us PureGiridhar RagavasimhanAinda não há avaliações
- 2ismatterarounduspure 150801135536 Lva1 App6891Documento24 páginas2ismatterarounduspure 150801135536 Lva1 App6891wildflower.0709.486Ainda não há avaliações
- Oil and Water Won't Mix and Other Mixture Separation Techniques - Chemistry Book for Kids 8-10 | Children's Chemistry BooksNo EverandOil and Water Won't Mix and Other Mixture Separation Techniques - Chemistry Book for Kids 8-10 | Children's Chemistry BooksAinda não há avaliações
- 10 Usp Social Science FA 03Documento2 páginas10 Usp Social Science FA 03Abhishek Abh-iAinda não há avaliações
- CSC 4to10Documento7 páginasCSC 4to10Abhishek Abh-iAinda não há avaliações
- 10 Social Science History The Making of A Global World Key 1 HindiDocumento1 página10 Social Science History The Making of A Global World Key 1 HindiAbhishek Abh-iAinda não há avaliações
- Time DistanceDocumento6 páginasTime DistanceAbhishek Abh-iAinda não há avaliações
- 2007 QuestionsDocumento51 páginas2007 QuestionsAbhishek Abh-iAinda não há avaliações
- NTSE 2013 (MAT) : Sample PapersDocumento25 páginasNTSE 2013 (MAT) : Sample PapersAbhishek Abh-iAinda não há avaliações
- FCGKJ La QDR Izos'K Izfr KSFXRK Ijh (KK I"KznDocumento1 páginaFCGKJ La QDR Izos'K Izfr KSFXRK Ijh (KK I"KznAbhishek Abh-iAinda não há avaliações
- HRD Department ' Admn - Office New No.4, (Old No.33) Sardar Patel Road, Guindy, Chennai - 600 032 Our Web SiteDocumento1 páginaHRD Department ' Admn - Office New No.4, (Old No.33) Sardar Patel Road, Guindy, Chennai - 600 032 Our Web SiteAbhishek Abh-iAinda não há avaliações
- Unit 2Documento11 páginasUnit 2Abhishek Abh-iAinda não há avaliações
- Join Indian Navy as SSC OfficerDocumento1 páginaJoin Indian Navy as SSC OfficerBtech EnggAinda não há avaliações
- Ncert/Cbse Physics Class 11 Textbook: Scaler Quantities: Vector QuantitiesDocumento1 páginaNcert/Cbse Physics Class 11 Textbook: Scaler Quantities: Vector QuantitiesAbhishek Abh-iAinda não há avaliações
- ST.T FormDocumento9 páginasST.T FormPatiala BsnlAinda não há avaliações
- Chapter 9Documento5 páginasChapter 9Abhishek Abh-iAinda não há avaliações
- 09 Impq Maths Sa 1 1 Number SystemDocumento3 páginas09 Impq Maths Sa 1 1 Number SystemAbhishek Abh-iAinda não há avaliações
- VMC and LMF OnlineDocumento63 páginasVMC and LMF OnlineAbhishek Abh-iAinda não há avaliações
- IISER Admission Test Question Paper 1Documento2 páginasIISER Admission Test Question Paper 1Abhishek Abh-iAinda não há avaliações
- Indian Navy invites online applications for 137th batch of Artificer ApprenticeDocumento1 páginaIndian Navy invites online applications for 137th batch of Artificer ApprenticeArjun PeralasseryAinda não há avaliações
- 09 Impq Maths Sa 1 1 Number SystemDocumento3 páginas09 Impq Maths Sa 1 1 Number SystemAbhishek Abh-iAinda não há avaliações
- Chapter 2 FederalismDocumento7 páginasChapter 2 FederalismAbhishek Abh-iAinda não há avaliações
- CBSE JEE Main correction window open until June 27Documento1 páginaCBSE JEE Main correction window open until June 27Abhishek Abh-iAinda não há avaliações
- IISER Admission Test Question Paper 2Documento2 páginasIISER Admission Test Question Paper 2Abhishek Abh-iAinda não há avaliações
- IISER Admission Test Question Paper 3Documento2 páginasIISER Admission Test Question Paper 3Abhishek Abh-iAinda não há avaliações
- 2011 BITSAT Solved PaperDocumento23 páginas2011 BITSAT Solved PaperAbhishek Abh-iAinda não há avaliações
- IISER Admission Test Question Paper 4Documento3 páginasIISER Admission Test Question Paper 4Abhishek Abh-iAinda não há avaliações
- 2014 Fifa World CupDocumento5 páginas2014 Fifa World CupAbhishek Abh-iAinda não há avaliações
- Matter Test Study Guide KeyDocumento26 páginasMatter Test Study Guide Keyapi-278594802Ainda não há avaliações
- Spencer - Progress Its Law and CausesDocumento7 páginasSpencer - Progress Its Law and CausesJohannes2010Ainda não há avaliações
- DLL - Science 6 - Q1 - W2Documento4 páginasDLL - Science 6 - Q1 - W2Geoffrey Tolentino-UnidaAinda não há avaliações
- MS SCI PS Unit 1 Chapter 2 Nature of MatterDocumento30 páginasMS SCI PS Unit 1 Chapter 2 Nature of Mattermohamed shaabanAinda não há avaliações
- Chemguide - Answers: WWW - Chemguide.co - UkDocumento1 páginaChemguide - Answers: WWW - Chemguide.co - UkcusgakungaAinda não há avaliações
- Science Performance Task: Andrea Felize Parenia Grade 7 - BeigeDocumento17 páginasScience Performance Task: Andrea Felize Parenia Grade 7 - BeigeAndrea Felize PareniaAinda não há avaliações
- Assignment of MELCS For Video ProductionDocumento5 páginasAssignment of MELCS For Video ProductionNic's VlogsAinda não há avaliações
- Identifying Homogeneous and Heterogeneous MixturesDocumento4 páginasIdentifying Homogeneous and Heterogeneous MixturesROMAR ALLAPITAN ENORAinda não há avaliações
- Intro Cre1Documento3 páginasIntro Cre1Kai ChernAinda não há avaliações
- 1st Quarter Test in Science 6 With Tos and Key To CorrectionDocumento7 páginas1st Quarter Test in Science 6 With Tos and Key To CorrectionYolly MillaresAinda não há avaliações
- 806676Documento56 páginas806676veronicaAinda não há avaliações
- Mixtures ReviewDocumento3 páginasMixtures Reviewapi-301425989Ainda não há avaliações
- Regional Planning All - Part Conc PDFDocumento111 páginasRegional Planning All - Part Conc PDFPERIKALA TARUNAinda não há avaliações
- EDITED - Science4 - Q1 - Module 5Documento44 páginasEDITED - Science4 - Q1 - Module 5checheAinda não há avaliações
- LAUREN's Science 9 Chemistry Unit TestDocumento9 páginasLAUREN's Science 9 Chemistry Unit TestLauren NovakAinda não há avaliações
- G6 Science Module 1 - Week 1 - Q1 - MixturesDocumento14 páginasG6 Science Module 1 - Week 1 - Q1 - MixturesAnonymousAinda não há avaliações
- Anton Popovic - ShiftsDocumento11 páginasAnton Popovic - Shiftsaleynasumer2001Ainda não há avaliações
- A Cross-Curricular Reading and Science Activity: This Set Includes: Passage About Mixtures QuestionsDocumento4 páginasA Cross-Curricular Reading and Science Activity: This Set Includes: Passage About Mixtures QuestionsMarilyn Castro LaquindanumAinda não há avaliações
- Science Reviewer: A. Mixture: Chemical MixturesDocumento2 páginasScience Reviewer: A. Mixture: Chemical Mixturesbhang racho24Ainda não há avaliações
- Homeschool Weekly Plan Grade 8Documento7 páginasHomeschool Weekly Plan Grade 8Amina Angel Marie PatagnanAinda não há avaliações
- 1 TV Eskwela: Insert Soa Program Id 2 TV Eskwela: MSC Up For 5 Secs and Fade UnderDocumento15 páginas1 TV Eskwela: Insert Soa Program Id 2 TV Eskwela: MSC Up For 5 Secs and Fade UnderCristoper Bodiongan100% (1)