Escolar Documentos
Profissional Documentos
Cultura Documentos
2012 Electron Transport
Enviado por
Georgios PlethoDescrição original:
Direitos autorais
Formatos disponíveis
Compartilhar este documento
Compartilhar ou incorporar documento
Você considera este documento útil?
Este conteúdo é inapropriado?
Denunciar este documentoDireitos autorais:
Formatos disponíveis
2012 Electron Transport
Enviado por
Georgios PlethoDireitos autorais:
Formatos disponíveis
1
Electron Transport
and
Oxidative Phosphorylation
General Biochemistry
2012
Glucose Oxidation (Glycogen)
1.Phase glycolysis (glycogenolysis)
2.Phase citric acid cycle
3.Phase electron transport and
oxidative phosphorylation
2
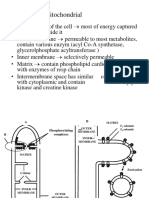
Electron Transport
Localization: inner mitoch. membrane
Cristae the density of cristae is related to
the respiratory activity of a cell
Energy: the oxidation of NADH and
FADH
2
will produce most of the ATP
generated by the oxidation of Glc
3
Mitochondrion
4
The Electron Transport Chain
Complex I (NADH-coenzyme Q reductase)
FMN (1x)
Fe-S centers (6-7x)
Complex II (succinate-coenzyme Q reductase)
Succinate dehydrogenase (1xdimer), FAD (2x)
Fe-S centers (9x)
Cyt b
560
The Electron Transport Chain
Complex III (coenzyme Q-cyt c reductase)
Cyt b (2x)
Fe-S centers (1x)
Cyt c
1
(1x)
Complex IV (cyt c oxidase)
Cyt a
Cu (2x)
Cyt a
3
5
Complex I
The largest enzyme of inner mitochondrial membrane,
26 subunits (850 kDa)
NADH FMN Fe-S CoQ
-0,32 -0,30 +0,04
FMN
All forms are stable
Ability to accept or donate 1 or 2 e
-
6
Fe-S centers
Fe in each center forms conjugative system (+2; +3)
Coenzyme Q
7
Complex II
5 subunits = dimer of succinate dehydrogenase,
3 other small hydrophobic subunits, (127 kDa)
succinate FAD Fe-S cyt b
560
CoQ
+0,030 -0,040 -0,080 +0,045
it is not proton pump because the free-energy
change of the catalyzed reaction is too small
important for electron enter (with relatively high
potential) into the electron transport chain
FAD
8
Complex III
10 subunits, (280 kDa)
CoQ cyt b
562
cyt b
566
Fe-S cyt c
1
cyt c
+0,045 +0,030 -0,030 +0,215 +0,235
Cytochromes: Hem Proteins
9
Cytochromes: Hem Proteins
Cytochrome c
10
Complex IV
Dimer protein, 6-13 subunits, (160-200 kDa)
cyt c cyt a Cu
A
Cu
B
cyt a
3
O
2
+0,235 +0,210 +0,245 +0,340 +0,385 +0,815
4 cyt c
2+
+ 4H
+
+ O
2
4 cyt c
3+
+ 2H
2
O
Complex IV Electron Transport
Fe
II
-O-O-Cu
I
Fe
III
-O
-
-O
-
-Cu
II
Fe
II
-OH-O
-
-Cu
II
Fe
IV
=O +
-
HO-Cu
II
Fe
III
-OH
-
+
-
HO-Cu
II
2H
2
O + Fe
III
+ Cu
II
11
Q Cycle
Redox Potential Changes
12
1978 Peter D. Mitchell Nobel Prize for Chemistry
Complex V
ATP-synthase
2 subunits
Generation of a proton
gradient permits ATP
synthesis
Changes by H
+
translocation
13
ATP Synthesis
F1 unit has 3 catalytic b subunits, which are
intrinsically identical but are not functionally
equivalent at any particular moment
L=loose binds the substrates loosely and is catalycally inactive
T=tight binds them tightly and is active
O=open has very low affinity for substrates
1997 (Paul D. Boyer, John E. Walker, Jens C. Skou) Nobel Prize for Chemistry
Uncoupling of Oxidative
Phosphorylation
14
Respiratory Control
Control by substrate availability
ADP, Pi, O2, NAD+, FAD+
Cytochrome oxidase
Regulatory enzyme
Control by substrate availability reduce form of cyt c (c
2+
)
[c2+]/[c3+] = ([NADH]/[NAD+]) x ([ADP][Pi]/[ATP])
Regulation by acceptor regulaion by ATP/ADP
Você também pode gostar
- Oksidasi BiologiDocumento62 páginasOksidasi BiologisenadaAinda não há avaliações
- Electron Transport ChainDocumento22 páginasElectron Transport Chainbluegreenalga100% (2)
- ELECTRON-TRANSPORT-CHAIN-STRYERDocumento47 páginasELECTRON-TRANSPORT-CHAIN-STRYERAngelikaOdimerAinda não há avaliações
- Biological Oxidation: Zhang Hai-Feng Department of Biochemistry, Basic Medical School of Zhengzhou UniversityDocumento102 páginasBiological Oxidation: Zhang Hai-Feng Department of Biochemistry, Basic Medical School of Zhengzhou Universityapi-19916399Ainda não há avaliações
- 09 (1) BIO462e-Trnsprt Oxid PhosDocumento22 páginas09 (1) BIO462e-Trnsprt Oxid PhosAs ShahirahAinda não há avaliações
- Electron Transport Chain NotesDocumento14 páginasElectron Transport Chain NotesseanAinda não há avaliações
- Electron Transport and Oxidative PhosphorylationDocumento34 páginasElectron Transport and Oxidative PhosphorylationAbeWatanabeAinda não há avaliações
- 5BBB0223 Mitochondrial Function & Dysfunction: Timothy Pullen With Thanks To: DR Clare Thornton, RVCDocumento63 páginas5BBB0223 Mitochondrial Function & Dysfunction: Timothy Pullen With Thanks To: DR Clare Thornton, RVCW BAinda não há avaliações
- In Relation To Lsm2101 Lec 2Documento11 páginasIn Relation To Lsm2101 Lec 2jojolim18Ainda não há avaliações
- Electron Transport and Oxidative PhosphorylationDocumento34 páginasElectron Transport and Oxidative Phosphorylationapi-321453350Ainda não há avaliações
- BiochemDocumento10 páginasBiochemHoàng LâmAinda não há avaliações
- Intermediary MetabolismDocumento91 páginasIntermediary MetabolismConrad Monterola100% (1)
- Respiratory Chain & Oxidative PhosphorylationDocumento57 páginasRespiratory Chain & Oxidative PhosphorylationHanifa AffianiAinda não há avaliações
- Electron Transport ChainDocumento20 páginasElectron Transport ChainAhmed JawdetAinda não há avaliações
- Electron Transport SystemDocumento58 páginasElectron Transport SystemSantosh KumarAinda não há avaliações
- Online Practice Tests, Live Classes, Tutoring, Study Guides Q&A, Premium Content and MoreDocumento27 páginasOnline Practice Tests, Live Classes, Tutoring, Study Guides Q&A, Premium Content and MoreYoAmoNYC100% (1)
- Online Practice Tests, Live Classes, Tutoring, Study Guides Q&A, Premium Content and MoreDocumento27 páginasOnline Practice Tests, Live Classes, Tutoring, Study Guides Q&A, Premium Content and MoreJSlinkNYAinda não há avaliações
- Online Practice Tests, Live Classes, Tutoring, Study Guides Q&A, Premium Content and MoreDocumento27 páginasOnline Practice Tests, Live Classes, Tutoring, Study Guides Q&A, Premium Content and MoreabctutorAinda não há avaliações
- Online Practice Tests, Live Classes, Tutoring, Study Guides Q&A, Premium Content and MoreDocumento27 páginasOnline Practice Tests, Live Classes, Tutoring, Study Guides Q&A, Premium Content and MoreabctutorAinda não há avaliações
- Lecture 03 PDFDocumento4 páginasLecture 03 PDFRana AmjadAinda não há avaliações
- Lecture 03 PDFDocumento4 páginasLecture 03 PDFMuhammad TariqAinda não há avaliações
- BiologyDocumento14 páginasBiologyHarsh SharmaAinda não há avaliações
- Bio12 SM 04 2Documento2 páginasBio12 SM 04 2Maya AwadAinda não há avaliações
- Lehninger Principles of Biochemistry PDFDocumento37 páginasLehninger Principles of Biochemistry PDFmonitamiftah100% (1)
- 1BMS1153 BL1-Energy MetabolismDocumento35 páginas1BMS1153 BL1-Energy Metabolismshakila786Ainda não há avaliações
- Electron Transport and Oxidative Phosphorylation: NADH-ubiquinone OxidoreductaseDocumento4 páginasElectron Transport and Oxidative Phosphorylation: NADH-ubiquinone OxidoreductaseAries DocAinda não há avaliações
- 5-Electron Transport Chain - MDDocumento31 páginas5-Electron Transport Chain - MDIbrahim BarhamAinda não há avaliações
- Electron TransportDocumento59 páginasElectron TransportEllexie JoycenolAinda não há avaliações
- Bioenergetics and Biological Oxidation PDFDocumento50 páginasBioenergetics and Biological Oxidation PDFGEROME ABENILLA100% (1)
- Ch. 9 Biological OxidationDocumento71 páginasCh. 9 Biological OxidationKrishna KanthAinda não há avaliações
- Bioemergetics Cellular RespDocumento59 páginasBioemergetics Cellular RespHassan Abib BasalAinda não há avaliações
- Cells and Sugars 09 Mitochondria and Ox Phos StudentDocumento21 páginasCells and Sugars 09 Mitochondria and Ox Phos StudenttyhbbhhAinda não há avaliações
- Biology: Energy & Equilibrium: RespirationDocumento28 páginasBiology: Energy & Equilibrium: RespirationTimothy HandokoAinda não há avaliações
- BIO 361 Exam 4 ReviewDocumento45 páginasBIO 361 Exam 4 ReviewNigel Zhang100% (1)
- Grade-11 General-Biology-1 Q2 Wk7 GLAKDocumento20 páginasGrade-11 General-Biology-1 Q2 Wk7 GLAKClarisse De GuiaAinda não há avaliações
- 2.5 (BIOCHEMISTRY) Electron Transport Chain and Oxidative PhosphorylationDocumento7 páginas2.5 (BIOCHEMISTRY) Electron Transport Chain and Oxidative Phosphorylationlovelots1234100% (2)
- Electron Transport Chain: Jump To Navigation Jump To SearchDocumento16 páginasElectron Transport Chain: Jump To Navigation Jump To SearchJennie KimAinda não há avaliações
- BBC2 K27,28RCDocumento42 páginasBBC2 K27,28RCMarieta RitongaAinda não há avaliações
- Molybdenum and Molybdenum-Containing EnzymesNo EverandMolybdenum and Molybdenum-Containing EnzymesMichael P. CoughlanAinda não há avaliações
- Fosforilasi OksidatifDocumento82 páginasFosforilasi OksidatifSanti WilujengAinda não há avaliações
- The Structure and Hydrolysis of ATPDocumento38 páginasThe Structure and Hydrolysis of ATPBellony SandersAinda não há avaliações
- Respiration in PlantsDocumento15 páginasRespiration in Plantsyahake5036Ainda não há avaliações
- Etc N Oxid PhoshphorylationDocumento74 páginasEtc N Oxid Phoshphorylationhassanainshahi13Ainda não há avaliações
- Electron Transport ChainDocumento37 páginasElectron Transport ChainFan SmithAinda não há avaliações
- Electron Transport Chain - 1Documento7 páginasElectron Transport Chain - 1Manash SarmahAinda não há avaliações
- Gayatree PaniDocumento23 páginasGayatree PaniGobindaSahuAinda não há avaliações
- Etc PDFDocumento14 páginasEtc PDFjamalAinda não há avaliações
- Electron Transport Chain - WikipediaDocumento53 páginasElectron Transport Chain - WikipediaLsaurusAinda não há avaliações
- Biological Inorganic Chemistry: Electron TransferDocumento14 páginasBiological Inorganic Chemistry: Electron TransferChris LittleAinda não há avaliações
- ATP SynthesisDocumento3 páginasATP SynthesisAmanyAinda não há avaliações
- Oxidative Phosphorylation 1Documento22 páginasOxidative Phosphorylation 1fatin nadia100% (1)
- Glycolysis TCA ETCDocumento61 páginasGlycolysis TCA ETCLê Trà GiangAinda não há avaliações
- Bioenergetics: Mahpara Gondal Pharm D Ms Pharmaceutical Chemistry Rashid Latif College of PharmacyDocumento30 páginasBioenergetics: Mahpara Gondal Pharm D Ms Pharmaceutical Chemistry Rashid Latif College of PharmacyShafaqat Ghani Shafaqat GhaniAinda não há avaliações
- Biological OxidationDocumento51 páginasBiological OxidationGorav Sharma100% (2)
- ETC NotesDocumento3 páginasETC Notesaminahali04.aaAinda não há avaliações
- Chapter 4 - Intermediary MetabolismDocumento53 páginasChapter 4 - Intermediary MetabolismBaxxAinda não há avaliações
- Plan For Today: Finish Up With Electron Transport Chain and Oxidative PhosphorylationDocumento52 páginasPlan For Today: Finish Up With Electron Transport Chain and Oxidative PhosphorylationKalai KrishnamurthyAinda não há avaliações
- BotanyDocumento2 páginasBotanyIrish Diana De AldayAinda não há avaliações
- Oxidation and Reduction: PBL G5 Case 4Documento42 páginasOxidation and Reduction: PBL G5 Case 4sankarAinda não há avaliações
- Iron Metabolism: From Molecular Mechanisms to Clinical ConsequencesNo EverandIron Metabolism: From Molecular Mechanisms to Clinical ConsequencesNota: 5 de 5 estrelas5/5 (1)
- PRS Alb 1960-1972Documento582 páginasPRS Alb 1960-1972Georgios Pletho100% (1)
- An V Farmacie 2012 2013 EnglezaDocumento2 páginasAn V Farmacie 2012 2013 EnglezaGeorgios PlethoAinda não há avaliações
- General Biochemistry: 2nd Year of Study - FarmacyDocumento12 páginasGeneral Biochemistry: 2nd Year of Study - FarmacyGeorgios PlethoAinda não há avaliações
- Credited Exams March April 2015Documento2 páginasCredited Exams March April 2015Georgios PlethoAinda não há avaliações
- Porphyrins Synthesis and Breakdown of HemeDocumento42 páginasPorphyrins Synthesis and Breakdown of HemeGeorgios PlethoAinda não há avaliações
- 2012 Transport in OrganismDocumento22 páginas2012 Transport in OrganismGeorgios PlethoAinda não há avaliações
- 2012 Metabolic InterrelationshipsDocumento10 páginas2012 Metabolic InterrelationshipsGeorgios PlethoAinda não há avaliações
- Lipid Metabolism IDocumento35 páginasLipid Metabolism IGeorgios PlethoAinda não há avaliações
- Physiology Urinary SystemDocumento44 páginasPhysiology Urinary SystemGeorgios PlethoAinda não há avaliações
- CNS Stimulants and Psychotomimetic DrugsDocumento21 páginasCNS Stimulants and Psychotomimetic DrugsGeorgios PlethoAinda não há avaliações
- Encyclopedia GRRuMblianaDocumento264 páginasEncyclopedia GRRuMblianaGeorgios Pletho100% (1)
- 2045 General Biochemistry Exam QuestionsDocumento3 páginas2045 General Biochemistry Exam QuestionsGeorgios PlethoAinda não há avaliações
- Exam Questions 2012/2013Documento2 páginasExam Questions 2012/2013Georgios PlethoAinda não há avaliações
- Kaplan - Sefer Yetzirah, The Book of Creation in Theory and PracticeDocumento423 páginasKaplan - Sefer Yetzirah, The Book of Creation in Theory and PracticeGeorgios Pletho100% (26)
- Protein Structure - Lecture 3Documento18 páginasProtein Structure - Lecture 3Aakash HaiderAinda não há avaliações
- Carbohydrates Metabolism 3 Glycolysis ISUDocumento25 páginasCarbohydrates Metabolism 3 Glycolysis ISUsjs6r8wwv9Ainda não há avaliações
- SimMom IQDocumento3 páginasSimMom IQSiddharth JayaseelanAinda não há avaliações
- Instructor's Presentation-Lipids and LipoproteinsDocumento43 páginasInstructor's Presentation-Lipids and Lipoproteinsjomel rondinaAinda não há avaliações
- Properties of An Extracellular Protease of Bacillus Megaterium DSM 319 As Depilating Aid of HidesDocumento6 páginasProperties of An Extracellular Protease of Bacillus Megaterium DSM 319 As Depilating Aid of HidesSarah Fitriani MuzwarAinda não há avaliações
- Biology NotesDocumento3 páginasBiology Notespenelope stieglitzAinda não há avaliações
- Blood Clotting CascadeDocumento10 páginasBlood Clotting CascadeFitriyana WinarnoAinda não há avaliações
- Overview of The Coagulation SystemDocumento9 páginasOverview of The Coagulation SystemaksinuAinda não há avaliações
- CD MoleculesDocumento25 páginasCD MoleculesunknownxemAinda não há avaliações
- Protein VVDocumento3 páginasProtein VVSara khanAinda não há avaliações
- Enzymes Practice QuestionDocumento1 páginaEnzymes Practice QuestionZhering RodulfoAinda não há avaliações
- Assignment "Proteome Analysis and Its Applications" Paper Code: BIOTECH/04/SC/28 Paper Name: Genomics and ProteomicsDocumento10 páginasAssignment "Proteome Analysis and Its Applications" Paper Code: BIOTECH/04/SC/28 Paper Name: Genomics and ProteomicsLalruatdiki CAinda não há avaliações
- CURS 12 - Coagularea Intravasculara Diseminata (DR - Manuela Crisan-16.10)Documento71 páginasCURS 12 - Coagularea Intravasculara Diseminata (DR - Manuela Crisan-16.10)veronicaAinda não há avaliações
- Glycolysis CrosswordDocumento1 páginaGlycolysis Crosswordapi-405259496Ainda não há avaliações
- Amino Acids and Their Structure: Cooh-Ch-Nh2 - RDocumento12 páginasAmino Acids and Their Structure: Cooh-Ch-Nh2 - RRica NorcioAinda não há avaliações
- Perhitungan IodimetriDocumento3 páginasPerhitungan IodimetriMariahAinda não há avaliações
- GlycolysisDocumento8 páginasGlycolysisdani2703Ainda não há avaliações
- Protein: What Is ADocumento2 páginasProtein: What Is AFilip KesteliAinda não há avaliações
- BCDB Qualifying Exam Student X May 22 & 23, 2018Documento19 páginasBCDB Qualifying Exam Student X May 22 & 23, 2018Kemi' RedlipsAinda não há avaliações
- B.SC., Biochemistry Sylubus Sem-IDocumento4 páginasB.SC., Biochemistry Sylubus Sem-IHimakiran BabuAinda não há avaliações
- Potential Adverse Effects of Long-Term Consumption of Fatty AcidsDocumento11 páginasPotential Adverse Effects of Long-Term Consumption of Fatty Acidstaner_soysurenAinda não há avaliações
- Price List ApotekDocumento9 páginasPrice List ApotekMelinda ProductionAinda não há avaliações
- Cell Biology Resume PDFDocumento0 páginaCell Biology Resume PDFRaja Novi AriskaAinda não há avaliações
- Kyu Nutrition 1 - 230615 - 124944Documento35 páginasKyu Nutrition 1 - 230615 - 124944Zacharia muraciaAinda não há avaliações
- Biochemistry MITDocumento5 páginasBiochemistry MITAngga RyanAinda não há avaliações
- 4 +basic+nutritionDocumento71 páginas4 +basic+nutritionJasmine CorreosAinda não há avaliações
- Harpers 20Documento9 páginasHarpers 20Dewi RatnasariAinda não há avaliações
- Bile Acid SequestrantsDocumento4 páginasBile Acid SequestrantsFebrian IsharyadiAinda não há avaliações
- FA13 Cell Biology FinalDocumento113 páginasFA13 Cell Biology FinalSana Savana Aman R100% (2)
- Isolation of Casein and AlbuminDocumento3 páginasIsolation of Casein and AlbuminsunshinesparkleAinda não há avaliações