Escolar Documentos
Profissional Documentos
Cultura Documentos
Modelling Gibberella PB2002
Enviado por
phongquyenDescrição original:
Direitos autorais
Formatos disponíveis
Compartilhar este documento
Compartilhar ou incorporar documento
Você considera este documento útil?
Este conteúdo é inapropriado?
Denunciar este documentoDireitos autorais:
Formatos disponíveis
Modelling Gibberella PB2002
Enviado por
phongquyenDireitos autorais:
Formatos disponíveis
Process Biochemistry 37 (2002) 10331040
Modelling Gibberella fujikuroi growth and GA
3
production in
solid-state fermentation
Claudio Gelmi, Ricardo Perez-Correa*, Eduardo Agosin
Department of Chemical and Bioprocess Engineering, School of Engineering, Ponticia Uni6ersidad Catolica de Chile, Casilla 306,
Santiago 22, Chile
Received 5 August 2001; accepted 17 October 2001
Abstract
A simple differential equation model was developed to represent the growth and production of a secondary metabolite in
solid-state cultivation (SSC) under conditions of limited nitrogen. The model was used to interpret data obtained fromSSCs of
the fungus Gibberella fujikuroi, under different temperatures (25 and 31 C) and water activity conditions (0.985, 0.992, 0.999).
The model was calibrated in two steps. An innovative procedure to achieve good initial guesses for key parameters, such as
maintenancecoefcientsand death rateswasrst applied. Theseinitial guesseswerethen used in anon-linear optimisation routine
to get aminimumleast squares t for themodel. Themathematical model was ableto reproducethemeasured variables: biomass,
urea, starch, CO
2
, O
2
and GA
3
satisfactorily. Moreover, the model indicated that the fungus does not assimilate the nitrogen
source, urea, directly. The model will be useful in developing optimal feeding policies and on-line biomass estimators. 2002
Elsevier Science I reland Ltd. All rights reserved.
Keywords: Condence intervals; I nert support; Gibberellins; Parameter estimation; Secondary metabolite; Urea
Nomenclature
carbon dioxide (g/g.i.s.) CO
2
gibberellic acid (g/g.i.s.) GA
3
intermediary nitrogen (g/g.i.s.) N
I
starch (g/g.i.s.) S
oxygen (g/g.i.s.) O
2
urea (g/g.i.s.) U
X active biomass (g/g.i.s.)
measured biomass (g/g.i.s.) X
measu
www.elsevier.com/locate/procbio
1. Introduction
Scaling up and optimising the operation of a solid-
state cultivation (SSC) bioreactor can be largely sim-
plied if an accurate process model is available [1].
Unfortunately, building models for this kind of system
is difcult due to the absence of reliable on-line mea-
surements of biomass and nutrient concentrations. Fur-
thermore, SSC are highly complex dynamic systems
characterised by four interacting phases, in which the
phenomena involved arenot well understood and char-
acterised [2].
* Corresponding author. Tel.: +56-2-6864258; fax: +56-2-
6865803.
E-mail address: perez@ing.puc.cl (R. Perez-Correa).
0032-9592/02/$ - see front matter 2002 Elsevier Science I reland Ltd. All rights reserved.
PI I : S0032-9592(01)00314-4
C. Gelmi et al. / Process Biochemistry 37 (2002) 10331040 1034
Models able to predict biomass growth and metabo-
lite production accurately are necessary to optimise
SSC bioreactor operations. I n the specic case of sec-
ondary metabolite production, the changes in both
active biomass and limiting substrates should also be
described. I n this regard, the logistic growth equation
commonly used in SSC modelling presents limitations
for building reliable models. While this expression is
useful to describe the exponential and stationary
phases, it cannot provide a complete representation
during the death phase. Although changes in active
biomass can be described through an expression of a
death or inactivation kinetic rate [35], the logistic
model does not represent the production of secondary
metabolites well. This limitation would explain why
many authors reporting experimental results for
metaboliteproduction in SSC [6,7], havenot dealt with
modelling issues.
Environmental conditions, such as temperature (T)
and water activity (a
w
), also affect biomass growth and
metabolite production. Most published SSC models
have focused on describing the effect of T on biomass
growth, and different empirical mathematical expres-
sions have been proposed to represent this inuence
[4,5,8]. Other aspects, such astheeffect of a
w
[4,5,9] and
pH [5] on the SSC growth rate have received less
attention.
I n this paper, we describe changes in active biomass
and the production of a secondary metabolite in a
solid-state culture, modelling it for different T and
conditions of a
w
. Westudied thegrowth of thelamen-
tous fungus Gibberella fujikuroi and the production of
gibberellic acid (GA
3
) as an experimental case. To
simplify modelling, an inert non-porous solid support
was used, so solid degradation was not considered and
mass transfer effects were reduced.
The production of GA
3
by G. fujikuroi has been
thoroughly studied both in submerged [10,11] and
SSCs, with higher yields reported for the latter [12,13].
Although many SSC studies using G. fujikuroi have
analysed theinuenceof substratefeeding policies [14],
environmental conditions [15,16], nutritional factors
[6,15], and different solid supports [6,7] on the culture,
few have included modelling of biomass growth and
metabolite production. Ebner et al. [17] modelled the
growth of this fungus in a batch SSC on a laboratory
scale, although the authors considered only one envi-
ronmental condition.
For this study, we developed a lumped parameter
differential equation model for SSC applying mass bal-
ances, which resulted in eight differential equations.
This paper describes the experimental setup and the
parameters estimation methodology in detail; analyses
environmental conditions inuence on both biomass
growth and metabolite production; and examines the
implications and applicability of the SSC model thus
developed.
2. Model development
The resulting model is a set of eight balance equa-
tions. The lumped parameter model describes the
changes in the biomass of G. fujikuroi growing on an
inert support (Amberlite I RA-900) in glass columns
[18]. The lag phase was not incorporated into the
model.
The main model assumptions are:
oxygen transfer resistance is negligible [19]
available nitrogen is the limiting substrate
the carbon source is not limiting [20]
T and a
w
remain constant throughout cultivation
model parameters remain constant throughout
cultivation.
2.1. Mass balances
2.1.1. Biomass growth
To describe respiration activity, the model also con-
siders active(X) and inactivebiomass. However, dueto
analytical limitations, only total biomass (X
measu
) was
measured periodically. The total biomass balance does
not consider lysis, resulting in
dX
measu
dt
= vX, (1)
where v represents the specic growth rate.
Assuming a rst order death rate, the change in the
active biomass is described by
dX
dt
= vXk
d
X. (2)
2.1.2. Urea and starch consumption
The consumption rate of the only nitrogen source,
urea, is given by
dU
dt
=k, (3)
where k is the conversion rate from urea to available
nitrogen for the microorganism, N
I
, which can be di-
rectly metabolised into active biomass. The following
expression represents the changes in available nitrogen
during cultivation:
dN
I
dt
=0.47kv
X
Y
X/N
I
, (4)
where 0.47 corresponds to the nitrogen content of the
urea.
Eq. (4) can be used to describe observed biomass
growth despite total urea depletion within the culture.
This observed growth cannot beattributed to theaccu-
mulation of either fat or carbohydrate, because total
biomass was calculated using the glucosamine method,
C. Gelmi et al. / Process Biochemistry 37 (2002) 10331040 1035
which measures chitin present in the cell wall. This
chitin increment can be explained either by biomass
growth or increased chitin content in the biomass.
However, laboratory observations (data not shown)
indicatethat thebiomass/chitin ratio for thefungus G.
fujikuroi remains relatively constant during the whole
culture. Moreover, respirometric gases behave consis-
tently with the biomass growth assumption. Hence, G.
fujikuroi seems to accumulatepart of thenitrogen from
urea conversion and use this nitrogen for biomass
growth when the external source has been depleted.
Similar behaviour has been reported for the fungus
Arthrobotrys oligospora [21] and the yeast Saccha-
romyces cere6isiae [22], which store intracellular nitro-
gen compounds that were later consumed upon
exhaustion of extracellular nitrogen to enable further
biomass growth. Therefore, our model considers that
the available nitrogen is the limiting substrate.
The behaviour of the carbon source, i.e. soluble
starch, is given by
dS
dt
=
vX
Y
X/S
m
s
X. (5)
Starch serves as a sourceof both carbon and energy.
Thesecond termof theequation, maintenance, refersto
the collection of energy required for the cells survival
or preservation of a certain state, which arenot directly
related to or coupled with the synthesis of more cells
[23]. Here, the contribution of product formation was
neglected.
2.1.3. Gibberellic acid
Since GA
3
is a secondary metabolite, its production
rate is proportional to the concentration of active
biomass [24]. An additional term is required to repre-
sent GA
3
degradation, which has been previously ob-
served [25]:
dGA
3
dt
=iXk
p
GA
3
. (6)
2.1.4. CO
2
production and O
2
consumption
CO
2
production has two components, oneassociated
with themicroorganisms growth and theother with its
maintenance:
dCO
2
dt
=v
X
Y
X/CO
2
+m
CO
2
X. (7)
Similarly, the O
2
consumption rate has two terms,
one associated with growth and the other with
maintenance.
dO
2
dt
=v
X
Y
X/O
2
+m
O
2
X. (8)
2.2. Constituti6e equations
2.2.1. Specic growth rate (v)
Usually the logistic equation is used to describe
biomass growth in solid-statecultivations [1]. However,
biomass growth cannot be related to limiting nutrients
usingthis equation. Thelogistic model, therefore, is not
appropriate to describe changes in active biomass and
secondary metabolites during the death phase. To do
so, theeffect of thelimitingnutrient on biomassgrowth
must be considered explicitly, as in the Monods rate
expression:
v=
v
max
N
I
(N
I
+k
n
)
. (9)
2.2.2. Specic production rate of GA
3
(i)
A substrate inhibition expression was used to model
theproduction rate, as successfully applied by Pastrana
et al. [26] to describe the production of GA
3
in sub-
merged fermentation. Here, we assume that available
nitrogen, rather than the carbon source, is the limiting
substrate. Furthermore, a simplied rate equation that
suitably represents experimental data was used:
i=
i
etam
1+K
i
N
I
. (10)
3. Materials and methods
3.1. Experimental setup
3.1.1. Microorganism
G. fujikuroi ATCC 12616, an asporogenic and hyper-
producing strain of GA
3
, was kept at 4 C and period-
ically subcultured in malt-yeast extract agar slant tubes
at 28 C.
3.1.2. Fungal propagation and inoculum preparation
Homogenised hyphae (2 ml) were used to inoculate
thepropagation medium(100ml). I ts composition was:
80 g/l anhydrous glucose, 0.45 g/l magnesiumsulphate,
5 g/l of KH
2
PO
4
, 10 ml/l salts solution. Culture asks
wereincubated in ashaker (200rpm) at 28 C for 48h.
These were harvested after 40 h of cultivation, cen-
trifuged, and the mycelium then washed with sterile
salinewater. Theprocedurewas repeated twicewith the
resulting mycelial pellet then resuspended in 20 ml of
salinewater, beforeinoculation into Amberliteimpreg-
nated with nutrient solution (0.5% v/v).
3.1.3. Growth and GA
3
production
Amberlite I RA-900 (SI GMA), stabilised at pH 4.5,
was used as an inert support. TheAmberlitewas dried
at a humidity of 10%, and 5.1gweremixed with 5.9ml
of sterilenutrient solution. Themixturewasloaded into
C. Gelmi et al. / Process Biochemistry 37 (2002) 10331040 1036
specially designed glass columns (20 cm high and 2.5
cmin diameter) [18] to which 1.4 ml of mycelial solu-
tion resuspended in salinewater was added. Theappar-
ent density of the columns was 0.68 (g/cm
3
). For
control columns, 1.4ml sterilewater was added instead
of the mycelial solution.
3.1.4. Regulation of water acti6ity and temperature
Column T and a
w
was tightly controlled. A water
glycerol solution bubbling system was used to control
the humidity of the columns inlet air. I n total, 46
columns were fed continuously with sterile air and the
whole system was placed in a thermoregulated water
bath. Four different experimentswereperformed, in the
following physical conditions of T and a
w
: T=25 C/
a
w
=0.992; T=25 C/a
w
=0.999; T=31 C/a
w
=
0.985; T=31 C/a
w
=0.992.
3.1.5. Gas analyses
The on-line gas monitoring system measured the
owrate and gas composition of the outlet air stream
from four columns: three inoculated with microorgan-
isms and onean uninoculated control. Thegas owrate
for each column was adjusted using a manual valveset
at 35 ml/min and the valve was readjusted periodically
to hold the owrate constant. Data acquisition was
carried out with a programmable logic controller con-
nected to a personal computer (MI TAC 386SX), which
registered thetime, air owrateand percentageof CO
2
and O
2
for each column, every 20min. Theinformation
was processed usingEXCEL
, thus determininginstan-
taneous and accumulated curves for CO
2
and O
2
.
3.1.6. Analytical methods
Biomass was determined indirectly by the glu-
cosamine method [27]. Urea in the culture was tracked
with the urea S kit (Boehringer-Mannheim). Starch
content was measured using an orcinol-sulphuric
method [28]. GA
3
was quantied uorimetrically as
described by Kavanagh and Kuzel [29].
3.2. Mathematical techniques
3.2.1. Statistics
Algebraic equations to represent the experimental
curves (biomass, starch, GA
3
, accumulated CO
2
and
accumulated O
2
) were established. MATLAB
func-
tions nlint and nlpredci wereapplied to denethe95%
condence interval [20]. The 95% condence intervals
for the accumulated gas curves were used to compute
reasonable initial values for the parameters v
max
, k
d
,
m
CO
2
and m
O
2
.
3.2.2. Parameter estimation
Model parameters were tted to the curves obtained
in four independent experiments, as described above
[20]. Parameter estimation was divided in two stages.
First, reasonable initial values for v
max
, m
CO
2
, m
O
2
and
k
d
parameters were established by looking at the accu-
mulated gas curves, as described previously. Duringthe
second step, model parameters were calibrated all to-
gether using a non-restricted least square method.
Step 1: I nitial values
Thepossiblerangeof v
max
values was obtained from
thenatural logarithmic curves of accumulated CO
2
and
O
2
following Gelmi et al. [20]. I nitial values for the
respirometric parameters m
CO
2
and m
O
2
were obtained
using the procedure described below.
After the limiting substrate, N
I
, is depleted, the
growth termcan beneglected and thebiomass equation
simplied to
dX
dt
= k
d
X. (11)
Assuming constant k
d
this equation can be solved
analytically, yielding
X=X
0
e
k
d
(t t
0
)
, (12)
where t
0
and X
0
are integration constants.
Repeating the same procedure for the CO
2
balance,
we get the following result:
dCO
2
dt
=m
CO
2
X. (13)
Replacing Eq. (12) gives
dCO
2
dt
=m
CO
2
X
0
e
k
d
(t t
0
)
(14)
and nally integrating
CO
2
(t)=
CO
2
0
+
m
CO
2
X
0
k
d
m
CO
2
X
0
e
k
d
(t t
0
)
k
d
. (15)
The same applies to the O
2
consumption equation
O
2
(t)=
O
2
0
+
m
O
2
X
0
k
d
m
O
2
X
0
e
k
d
(t t
0
)
k
d
. (16)
The CO
2
0
, O
2
0
and X
0
terms correspond to the mea-
sured or estimated values at timet
0
. Model parameters
(k
d
, m
CO
2
, m
O
2
) were tted through a least squares
procedure within EXCEL
.
Step 2: Final calibration
A non-linear optimisation routine within MAT-
LAB
, fminsearch, was used in order to obtain least
square estimates for all model parameters. The esti-
mated initial values for parameters v
max
, m
CO
2
, m
O
2
and
k
d
werecalculated in Step 1. For theremainingparame-
ters, where possible, initial values were taken from
typical values in the literature. Measured values for
X
measu
, S, GA
3
, CO
2
and O
2
were compared with
simulated values obtained fromintegratingthedifferen-
tial equationsto computethesquareerrors. Each of the
error equation terms was divided by the maximum
C. Gelmi et al. / Process Biochemistry 37 (2002) 10331040 1037
value of this variable and the quantity of available
experimental data. The function ODE113 from MAT-
LAB
, a variable order AdamsBashforthMoulton
solver, was employed for this integration.
4. Results and discussion
4.1. Cur6e tting
I n most experiments, the mathematical model t
experimental data well, with model curves remaining
inside the datas condence interval most of the time.
I n the particular case of the culture at 25 C and
a
w
=0.999, thelag phasewas unusually long. Sincethe
model does not consider the lag phase, the simulation
in this case started at 20 h of cultivation.
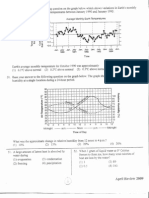
Fig. 1 presents data indicating how well changes
predicted by the model in total biomass, urea, GA
3
,
starch, CO
2
and O
2
matched experimental data for a
typical case (25 C and a
w
=0.992). Results under
other conditions arenot shown here. Thetotal biomass
curve fell outside the datas 95% condence interval
from 0 to 20 h and from 25 to 50 h (Fig. 1a). The
limitation involved in this kind of simplekinetic expres-
sion can be overcome using more elaborated models
such as neural nets [30]. Fig. 1b reveals for this condi-
tion GA
3
production starts at 40 h of cultivation. The
Fig. 1. Casestudy: experimental (dots) and simulated data (solid line). (a) Total biomass data (), dotted lineis a simulation of activebiomass.
(b) GA
3
production (). (c) Urea () and simulated intermediary. (d) Starch consumption (). (e) Accumulated CO
2
and O
2
consumed. I n all
cases, the solid line represents the variable simulated using the mathematical model and the shaded region represents a 95%condence interval.
C. Gelmi et al. / Process Biochemistry 37 (2002) 10331040 1038
Table 1
Estimated parameters
31 C 25 C Parameters
a
w
=0.999 a
w
=0.985 a
w
=0.992 a
w
=0.992
0.57 v
max
(1/h) 0.12 0.28 0.17
3.310
5
k
n
(gN
I
/g.i.s.) 2.310
4
1.110
3
1.810
4
0.020 0.044 0.031 0.029 k
d
(1/h)
k (g/h) 5.010
4
1.3310
4
1.5510
4
1.3210
4
14.9 7.2 20.8 9.6 Y
X/N
I
(gX/gN
I
)
0.24 0.05 Y
X/S
(gX/gS) 0.19 1.21
0.098 0.39 0.11 0.25 m
s
(gS/gX h)
1.61 0.22 Y
X/CO
2
(gX/gCO
2
) 0.98 0.58
0.11 0.31 0.11 0.25 m
CO
2
(gCO
2
/gX h)
8.9 0.91 Y
X/O
2
(gX/gO
2
) 4.6 2.11
0.05 0.25 0.06 0.13 m
O
2
(gO
2
/gX h)
6.510
4
i
etam
(gGA
3
/gX h) 1.910
4
3.810
3
1.010
3
2.9510
5
3.0710
4
7.8610
5
5.810
5
K
i
(1/gN
I
g.i.s.)
4.410
4
K
p
(1/h) 0 9.510
4
1.510
4
g.i.s.: grams of inert support.
modelled curve remains within the condence interval
up to 130 h. At this point, the model strongly deviates
frommeasured values, which continued to risequickly.
This deviation appeared in many other cases (not
shown) and cannot be explained using the developed
model. This is probably due to heterogeneous growth,
sincethesubstrateis not mixed during cultivation. The
changein urea (Fig. 1c) clearly shows zero-order kinet-
ics, with total degradation within 25 h. Fig. 1d repre-
sents a typical pattern for starch consumption, which
indicates clearly that carbon is not limiting. Fig. 1e
providesaccumulatedCO
2
productionandO
2
consump-
tion. Here, condence intervals grow wider over time,
since these are accumulated values and therefore inte-
grate measurement errors. However, condence inter-
vals remain narrow because gas measurements are
accurate. I n this case, the O
2
curve shows a better t
than theCO
2
curve, with themost signicant deviations
occurring in the2560 h interval. Weshould underline
that for therest of theexperiment, both curvesmatched
signicantly better.
4.2. Estimated parameters
Table 1 summarizes the parameters estimated using
the mathematical model for four cultivations.
When compared with theoretical predictions and
those reported by other authors [3,5,17,31], values for
almost all parameter estimates are within the expected
range. However, a theoretical valuefor Y
X/N
I
cannot be
determined, because there is no information about the
proposed intracellular intermediary accumulated by the
fungusG. fujikuroi. Moreover, other parameterssuch as
v
max
and Y
X/O
2
fell outside the expected range for
conditionsat 25 C and a
w
=0.999, wherethelagphase
was unusually long. I n this case, some difculties with
theparameter estimation procedurearose, probably due
to the existence of several local minimums. Hence, for
analysis, the set of parameters for this condition were
considered unreliable.
By observing the parameters at conditions of 25 C
a
w
=0.992, 31 C a
w
=0.985and 31 C a
w
=0.992, the
inuence of T and a
w
could be determined. I n the rst
place, T mainly affects themaintenancecoefcients, m
s
,
m
CO
2
, m
O
2
, and the yield coefcient, Y
X/O
2
. I n contrast,
k andk
d
werelesssensitiveto T variations. Secondly, a
w
strongly affects the yield coefcients Y
X/O
2
, Y
X/CO
2
and
Y
X/S
. Here, the least sensitive were k, m
CO
2
and k
n
.
Using a sensitivity analysis, the most important
parameter was found to be the yield coefcient, Y
X/N
I
.
This mainly affects the simulation of total biomass
(X
measu
), intermediarynitrogen(N
I
), starchconsumption
(S), CO
2
production and O
2
consumption. Moreover,
this parameter is more inuenced by T than a
w
. The
secondmost important parameter wasthedeathrate, k
d
,
which mainly affects active biomass (X) and GA
3
pro-
duction, and is more inuenced by a
w
than T. Finally,
variations in m
CO
2
and m
O
2
generated signicant devia-
tions in CO
2
production and O
2
consumption rates.
Nonetheless, thelimited number of experiments stud-
ied does not allow us to infer a mathematical relation-
ship between T and a
w
, on one hand, and growth and
production on theother. I t is possibleto note, however,
by means of our basic analysis that environmental
conditionshaveastrongimpact on growth and produc-
tionparameters. Thus, T mainlyaffectsbiomassgrowth,
whilea
w
affects GA
3
production in theanalysed range.
I t is important to keep these results in mind when the
model isapplied under different conditionsof T and a
w
.
For example, a distributed parameter model would be
unavoidable when dealing with large-scale solid-state
bioreactors, where T and a
w
proles are signicant.
C. Gelmi et al. / Process Biochemistry 37 (2002) 10331040 1039
5. Conclusions
Applying concepts developed for liquid fermentation
it was possible to build a differential equation growth
model for G. fujikuroi and production of GA
3
on batch
solid substrate cultivation. This model satisfactorily
reproduced the measured variables: biomass, urea,
starch, CO
2
, O
2
and GA
3
.
Themodel suggests that G. fujikuroi does not assimi-
late urea directly. Rather, a by-product of the urea is
proposed, which accumulates within thecell and is then
consumed by the fungus. However, laboratory work is
necessary to identify the intermediary nitrogen com-
pound proposed in our model.
The model developed established the basis for opti-
mising the cultivation of G. fujikuroi, by means of
appropriate fed-batch policies and optimal initial con-
centrations of nitrogen. This model will also contribute
to thedevelopment of on-lineestimators of biomass by
means of on-line measurements of CO
2
and O
2
. As
shown above T and a
w
have a signicant effect on
biomass growth, hencemoreexperiments at newT and
a
w
will be necessary.
Acknowledgements
This work was supported by project FONDECYT
1960360. Technical assistance from Andrea Chicurel,
Mauricio Gonzalez, Kathleen Pouliot, Lenka Torres
and Cristian Valenzuela is appreciated. Wearegrateful
to Lake Sagaris for careful English review of the
manuscript.
References
[1] Mitchell DA, Krieger N, Stuart DM, Pandey A. New develop-
ments in solid-statefermentation I I . Rational approaches to the
design, operation and scale-up of bioreactors. Proc Biochem
2000;35:121125.
[2] Perez-Correa J R, Agosin E. Solid substrate fermentation, au-
tomation of solid fermentation processes. I n: Flickinger M,
DrewS, editors. Encyclopedia of bioprocess technology: fermen-
tation, biocatalysis and bioseparation. New York: Wiley,
1999:242946.
[3] Sangsurasak P, Mitchell DA. I ncorporation of death kinetics
into a 2-dimensional dynamic heat transfer model for solid-state
fermentation. J ChemTech Biotechnol 1995;64:25360.
[4] Smits J P, van Sonsbeek HM, Tramper J , Knol W, Geelhoed W,
Peeters M, et al. Modelling fungal solid-state fermentation: the
role of inactivation kinetics. Bioproc Eng 1999;20:391404.
[5] Mitchell DA, Stuart DM. Solid-state fermentation, microbial
growth kinetics. I n: Flickinger M, DrewS, editors. Encyclopedia
of bioprocess technology: fermentation, biocatalysis and
bioseparation. New York: Wiley, 1999:240729.
[6] Lu ZX, XieZC, Kumakura M. Production of gibberellic acid in
Gibberella fujikuroi adhered onto polymeric brous carriers. Proc
Biochem1995;30(7):6615.
[7] Tomasini A, Fajardo C, Barrios-Gonzalez J . Gibberellic acid
production using different solid-state fermentation systems.
World J Microbiol Biotechnol 1997;13:2036.
[8] Saucedo-Castaneda G, Gutierrez-Rojas M, Bacquet G, Raim-
bault M, Viniegra-Gonzalez G. Heat transfer simulation in solid
substrate fermentation. Biotechnol Bioeng 1990;35:8028.
[9] Gervais P, Molin P, Grajek W, Bensoussan M. I nuence of the
water activity of a solid substrate on the growth rate and
sporogenesis of lamentous fungi. Biotechnol Bioeng
1988;31:45763.
[10] Borrow A, J efferys EG, Kessell RHJ , Lloyd EC, Lloyd PB,
Nixon I S. The metabolism of Gibberella fujikuroi in stirred
culture. Can J Microbiol 1961;7:22775.
[11] BorrowA, Brown S, J efferysEG, Kessell RHJ , Lloyd EC, Lloyd
PB, et al. The kinetics of metabolism of Gibberella fujikuroi in
stirred culture. Can J Microbiol 1964;10:40743.
[12] Kumar PKR, Lonsane BK. Gibberellic acid by solid-state fer-
mentation: consistent and improved yields. Biotechnol Bioeng
1987;30:26771.
[13] Agosin E, Maureira M, Biffani V, Perez F. Production of
gibberellins by solid substrate cultivation of Gibberella fujikuroi.
I n: Roussos S, Lonsane BK, Raimbault M, Viniegra-Gonzalez
G, editors. Advances in solid-state fermentation. Dordrecht:
Kluwer, 1997:35566.
[14] Kumar PKR, Lonsane BK. Batch and fed-batch solid-state
fermentations: kinetics of cell growth, hydrolytic enzymes pro-
duction, and gibberellic acid production. Proc Biochem
1988;April:437.
[15] Kumar PKR, Lonsane BK. Solid-state fermentation: physical
and nutritional factors inuencing gibberellic acid production.
Appl Microbiol Biotechnol 1990;34:1458.
[16] Qian XM, Du Preez J C, Kilian SG. Factors affecting gibberellic
acid production by Fusarium moniliforme in solid-state cultiva-
tion on starch. World J Microbiol Biotechnol 1994;10:939.
[17] Ebner A, Solar I , Acuna G, Perez-Correa R, Agosin E. Fungal
biomass estimation in batch solid substrate cultivation using
asymptotic observation. I n: Wise D, editor. Proceedings of the
third I nternational Symposiumon the I nternational Society for
Environmental Biotechnology, Global Environmental Biotech-
nology. Dordrecht: Kluwer, 1997:21120.
[18] Raimbault M, Alazard D. Culture method to study fungal
growth in solid fermentation. Eur J Appl Microbiol Biotechnol
1980;9:199209.
[19] Thibault J , Pouliot K, Agosin E, Perez-Correa R. Reassessment
of the estimation of dissolved oxygen concentration prole and
K
l
a in solid-state fermentation. Proc Biochem2000;36:918.
[20] Gelmi C, Perez-Correa R, Agosin E. Solid substrate cultivation
of Gibberella fujikuroi on an inert support. Proc Biochem
2000;35:122733.
[21] Rosen S, Sjollema K, Veenhuis M, Tunlid A. A cytoplasmic
lectin produced by the fungus Arthrobotrys oligospora functions
as a storage protein during saprophytic and parasitic growth.
Microbiology 1997;143:2593604.
[22] Kitamoto A, Yoshizawa K, Ohsumi Y, Anraku Y. Dynamic
aspects of vacuolar and cytosolic amino acid pools of Saccha-
romyces cere6isiae. J Bacteriol 1988;170(6):26836.
[23] Bailey J , Ollis D. Biochemical engineering fundamentals. New
York: McGraw-Hill, 1986.
[24] Blanch HW. I nvited review microbial growth kinetics. Chem
Eng Commun 1981;8:181211.
[25] Perez F, Vecchiola A, Pinto M, Agosin E. Gibberellic acid
decomposition and its loss of biological activity in aqueous
solutions. Phytochemistry 1996;41:6759.
[26] Pastrana LM, Gonzalez MP, Torrado A, Murado MA. A fed-
batch culturemodel for improved production of gibberellic acid
froma waste medium. Biotechnol Lett 1995;17(3):2638.
C. Gelmi et al. / Process Biochemistry 37 (2002) 10331040 1040
[27] Vignon C, Plassard C, Mousain D, Salsac L. Assay of fungal
chitin and estimation of mycorrhizal infection. Physiol Vegetale
1986;24(2):2017.
[28] Tollier MT, Robin J P. Adaptation dela methodea` lorcinol-sul-
furique au dosage automatique des glucides neutres totaux:
conditions dapplication aux extraits dorigine vegetale. Ann
Technol Agric 1979;28(1):115.
[29] Kavanagh F, Kuzel NR. Fluorometric determination of gibbere-
llic and gibberellenic acids in fermentation products, commercial
formulations, and puried materials. Agric Food Chem
1958;6(6):45963.
[30] Acuna G, Giral H, Agosin E, J orquera H, Perez-Correa R,
Ferret P, et al. A neural network estimation for biomass concen-
tration of Gibberella fujikuroi growing on solid substrate. Bio-
technol Tech 1998;12(7):5159.
[31] Gutierrez-Rojas M, Auria R, Benet J C, Revah S. A mathemati-
cal model for solid-state fermentation of mycelial fungi on inert
support. ChemEng J 1995;60:18998.
Você também pode gostar
- Jurnal TFDocumento6 páginasJurnal TFDhian ZhahirahAinda não há avaliações
- Lei 2001Documento17 páginasLei 2001andres granadosAinda não há avaliações
- Paper 1Documento6 páginasPaper 1bagherilAinda não há avaliações
- A Simple Metabolic Ux Balance Analysis of Biomass and Bioethanol Production in Saccharomyces Cerevisiae Fed-Batch CulturesDocumento11 páginasA Simple Metabolic Ux Balance Analysis of Biomass and Bioethanol Production in Saccharomyces Cerevisiae Fed-Batch Culturesmuhammad azizul hakimAinda não há avaliações
- A Growth Kinetic Model of Cultures On Cheese Whey As SubstrateDocumento6 páginasA Growth Kinetic Model of Cultures On Cheese Whey As Substratewp_dessyAinda não há avaliações
- International Journal of EngineeringDocumento10 páginasInternational Journal of EngineeringChokri ChakiirAinda não há avaliações
- 43 BAB Optimization Microalgae Growth PhotobioreactorsDocumento8 páginas43 BAB Optimization Microalgae Growth PhotobioreactorsMasih KarimiAinda não há avaliações
- Yaka Boy Lu 2015Documento13 páginasYaka Boy Lu 2015Arash HedayatiAinda não há avaliações
- Advances in Biofeedstocks and Biofuels, Volume 2: Production Technologies for BiofuelsNo EverandAdvances in Biofeedstocks and Biofuels, Volume 2: Production Technologies for BiofuelsLalit Kumar SinghAinda não há avaliações
- Iijme 2019 01 23 1 PDFDocumento14 páginasIijme 2019 01 23 1 PDFInternational Journal of Application or Innovation in Engineering & ManagementAinda não há avaliações
- TG-FTIR For Kinetic Evaluation and Evolved Gas Analysis of Cellulose With Different StructuresDocumento8 páginasTG-FTIR For Kinetic Evaluation and Evolved Gas Analysis of Cellulose With Different Structures一二三四Ainda não há avaliações
- Biomass Yield in Flat PanelDocumento12 páginasBiomass Yield in Flat PanelShailendra Singh KhichiAinda não há avaliações
- Introduction to Renewable Biomaterials: First Principles and ConceptsNo EverandIntroduction to Renewable Biomaterials: First Principles and ConceptsAli S. AyoubAinda não há avaliações
- Effects of Dilution Rate and Water Reuse On Biomass and Lipid ProductionDocumento9 páginasEffects of Dilution Rate and Water Reuse On Biomass and Lipid ProductionPNAinda não há avaliações
- Mass and Energy BalanceDocumento9 páginasMass and Energy Balancerussell_mahmood100% (1)
- Silva 2012Documento7 páginasSilva 2012Brian Oro BeltránAinda não há avaliações
- 1 s2.0 S2589014X23002372 MainDocumento11 páginas1 s2.0 S2589014X23002372 Mainjuan.ponceAinda não há avaliações
- Ba VariableDocumento8 páginasBa VariableDavid SantosAinda não há avaliações
- Citric Acid Production Stirred TankDocumento9 páginasCitric Acid Production Stirred TankKarliiux MedinaAinda não há avaliações
- Algal Research 2014 - Microalgae To Biofuels Lifecycle Assessment - Multiple Pathway EvaluationDocumento7 páginasAlgal Research 2014 - Microalgae To Biofuels Lifecycle Assessment - Multiple Pathway EvaluationNojus DekerisAinda não há avaliações
- Processes: in Situ Bio-Methanation Modelling of A Randomly Packed Gas Stirred Tank Reactor (GSTR)Documento13 páginasProcesses: in Situ Bio-Methanation Modelling of A Randomly Packed Gas Stirred Tank Reactor (GSTR)Giorgio VilardiAinda não há avaliações
- 5 PDFDocumento5 páginas5 PDFTuấn TrầnAinda não há avaliações
- ADM Pada MikroalgaDocumento7 páginasADM Pada MikroalgaYahdini QorninAinda não há avaliações
- Advances in Biofeedstocks and Biofuels, Volume 1: Biofeedstocks and Their ProcessingNo EverandAdvances in Biofeedstocks and Biofuels, Volume 1: Biofeedstocks and Their ProcessingLalit Kumar SinghAinda não há avaliações
- Raganati 2015Documento36 páginasRaganati 2015Stiven Loaiza CastroAinda não há avaliações
- Akesson01b PDFDocumento6 páginasAkesson01b PDFajayterdal8471Ainda não há avaliações
- Thermodynamics of Microbial Growth and MetabolismDocumento17 páginasThermodynamics of Microbial Growth and MetabolismJeimy MaciasAinda não há avaliações
- Mathematical DescriptionDocumento6 páginasMathematical DescriptionEleazar EscamillaAinda não há avaliações
- Comparacion de Metodos de Analisis para La Determinacion de Parametros CineticosDocumento7 páginasComparacion de Metodos de Analisis para La Determinacion de Parametros CineticosJulio NarvaezAinda não há avaliações
- Hydrogen Production From Glycerol Using Microbial Electrolysis Cell PDFDocumento4 páginasHydrogen Production From Glycerol Using Microbial Electrolysis Cell PDFesatjournalsAinda não há avaliações
- V LyubenovaDocumento5 páginasV LyubenovaMARKASGEORGEAinda não há avaliações
- Life Cycle Analysis of The Production of Biodiesel From MicroalgaeDocumento15 páginasLife Cycle Analysis of The Production of Biodiesel From MicroalgaeHema RawindranAinda não há avaliações
- Dynamic Photosynthetic Response of The Microalga Scenedesmus Obtusiusculus To Light Intensity Perturbations (Cabello Et Al)Documento8 páginasDynamic Photosynthetic Response of The Microalga Scenedesmus Obtusiusculus To Light Intensity Perturbations (Cabello Et Al)Aline DassolerAinda não há avaliações
- 8.3 Fed Batch Reactors 8.3.1 Variable Volume Fermentation (VARVOL and Varvold)Documento34 páginas8.3 Fed Batch Reactors 8.3.1 Variable Volume Fermentation (VARVOL and Varvold)Hana HamidAinda não há avaliações
- Zeiler 1995Documento6 páginasZeiler 1995arig alfath 18Ainda não há avaliações
- CineticaDocumento12 páginasCineticaIva Maicol Saavedra BecerraAinda não há avaliações
- 1 s2.0 S036031992304051X MainDocumento15 páginas1 s2.0 S036031992304051X Mainpedro.george.hysysAinda não há avaliações
- Energy Integration of Bioethanol Production Process From Algae Biomass: Comparison of SSF, SSCF and Acid HydrolysisDocumento6 páginasEnergy Integration of Bioethanol Production Process From Algae Biomass: Comparison of SSF, SSCF and Acid HydrolysisDavinson Aguirre RodriguezAinda não há avaliações
- Cheng 2017Documento14 páginasCheng 2017Insanul KholikAinda não há avaliações
- Jamie L Ifkovits, Robert F Padera and Jason A Burdick - Biodegradable and Radically Polymerized Elastomers With Enhanced Processing CapabilitiesDocumento8 páginasJamie L Ifkovits, Robert F Padera and Jason A Burdick - Biodegradable and Radically Polymerized Elastomers With Enhanced Processing CapabilitiesHutsDMAinda não há avaliações
- Fermentor Scale UpDocumento18 páginasFermentor Scale UpbabuvividAinda não há avaliações
- Scenedesmus Obliquus Metabolomics Effect of Photoperiods and CellDocumento13 páginasScenedesmus Obliquus Metabolomics Effect of Photoperiods and Cellrafael wadniparAinda não há avaliações
- 1 PBDocumento10 páginas1 PBEdUar NuÑezAinda não há avaliações
- Bio Reactor Design Via SpreadsheetDocumento7 páginasBio Reactor Design Via SpreadsheetDanilo Dorini100% (1)
- Continuous Lactose Fermentation by Clostridium AcetobutylicumDocumento7 páginasContinuous Lactose Fermentation by Clostridium AcetobutylicumAngelYussef UribeVasquezAinda não há avaliações
- Identification of The Growth Model Parameters For A Culture ofDocumento6 páginasIdentification of The Growth Model Parameters For A Culture ofgabriela07Ainda não há avaliações
- Model-2 PHB Azohydromonas LataDocumento11 páginasModel-2 PHB Azohydromonas LatamtlopezAinda não há avaliações
- Energies: Second Generation Ethanol Production From Brewers' Spent GrainDocumento12 páginasEnergies: Second Generation Ethanol Production From Brewers' Spent GrainmulerAinda não há avaliações
- Jarzebski 1989 PDFDocumento6 páginasJarzebski 1989 PDFblooom_00Ainda não há avaliações
- Economics of Bioethanol From Rice StrawDocumento8 páginasEconomics of Bioethanol From Rice StrawJosé Alberto CastilloAinda não há avaliações
- 2.4 Bioreaction StoichiometryDocumento43 páginas2.4 Bioreaction StoichiometryAstra Beckett100% (1)
- Sustainable synthesis of ciclopentene derivatives through multicomponent reactions in continuous flow regimeNo EverandSustainable synthesis of ciclopentene derivatives through multicomponent reactions in continuous flow regimeAinda não há avaliações
- Bioresource Technology: Sheng-Yi Chiu, Chien-Ya Kao, Ming-Ta Tsai, Seow-Chin Ong, Chiun-Hsun Chen, Chih-Sheng LinDocumento6 páginasBioresource Technology: Sheng-Yi Chiu, Chien-Ya Kao, Ming-Ta Tsai, Seow-Chin Ong, Chiun-Hsun Chen, Chih-Sheng LinsamagamaAinda não há avaliações
- Functional Fibers Via Biomimesis: NTC Project: M05-CD01 1Documento10 páginasFunctional Fibers Via Biomimesis: NTC Project: M05-CD01 1Danyboy LopezAinda não há avaliações
- Bioengineering 05 00001Documento15 páginasBioengineering 05 00001Davi OliveiraAinda não há avaliações
- Development and Validation of A Kinetic Model For Enzymatic Saccharification of Lignocellulosic BiomassDocumento8 páginasDevelopment and Validation of A Kinetic Model For Enzymatic Saccharification of Lignocellulosic BiomassJoséAinda não há avaliações
- Bioautomation, 2007, 7, 34 - 45Documento12 páginasBioautomation, 2007, 7, 34 - 45olympia1047Ainda não há avaliações
- Pengembangan Model Matematika Proses Simultan Antara Sakarifikasi Dan Fermentasi Pada ProduksiDocumento6 páginasPengembangan Model Matematika Proses Simultan Antara Sakarifikasi Dan Fermentasi Pada ProduksiAnggitsb NainggolanAinda não há avaliações
- Journal of Food Engineering: A B A C C A C ADocumento10 páginasJournal of Food Engineering: A B A C C A C ALaura Daniela RodriguezAinda não há avaliações
- Tobajas Et Al. - 2009 - Unstructured Kinetic Model For Reuterin and 1,3-..Documento7 páginasTobajas Et Al. - 2009 - Unstructured Kinetic Model For Reuterin and 1,3-..Bryan Alexis CastrillonAinda não há avaliações
- GCCDocumento265 páginasGCCzhenguoliAinda não há avaliações
- NDP-25 Data SheetDocumento4 páginasNDP-25 Data SheetsetyaAinda não há avaliações
- CMP Tutorial PDFDocumento83 páginasCMP Tutorial PDFMax HaroutunianAinda não há avaliações
- Digital Booklet - Oh My My (Deluxe) PDFDocumento8 páginasDigital Booklet - Oh My My (Deluxe) PDFMehmet Akif DelibaşAinda não há avaliações
- Chapter 8 - 1935 Rife Ray #4 Rife MachineDocumento2 páginasChapter 8 - 1935 Rife Ray #4 Rife MachineKhalid IbrahimAinda não há avaliações
- On The Job Winter 2013Documento3 páginasOn The Job Winter 2013alanhynesAinda não há avaliações
- Masonry - Block Joint Mortar 15bDocumento1 páginaMasonry - Block Joint Mortar 15bmanish260320Ainda não há avaliações
- Geothermal Project TimelinesDocumento10 páginasGeothermal Project TimelinesAldwin EncarnacionAinda não há avaliações
- Click and Learn How To Get Free TikTok FansDocumento4 páginasClick and Learn How To Get Free TikTok FansFreedmanMcFadden9Ainda não há avaliações
- RX-78GP03S Gundam - Dendrobium Stamen - Gundam WikiDocumento5 páginasRX-78GP03S Gundam - Dendrobium Stamen - Gundam WikiMark AbAinda não há avaliações
- Fan Motor Basic PartsDocumento7 páginasFan Motor Basic PartsMARIO BULANADIAinda não há avaliações
- Chopra Scm5 Ch13Documento58 páginasChopra Scm5 Ch13Faried Putra SandiantoAinda não há avaliações
- BE Spec Flash EconomizerDocumento4 páginasBE Spec Flash Economizeronkarrathee100% (1)
- Zf6 6r60 Zip BookletDocumento8 páginasZf6 6r60 Zip BookletPablo Farfan Alvarez100% (1)
- Swaroop (1) ResumeDocumento4 páginasSwaroop (1) ResumeKrishna SwarupAinda não há avaliações
- CE ThesisDocumento210 páginasCE ThesisKristin ArgosinoAinda não há avaliações
- Pump House SopDocumento5 páginasPump House SopCode NameAinda não há avaliações
- First Page PDFDocumento1 páginaFirst Page PDFNebojsa RedzicAinda não há avaliações
- Bangkok-Singapore CDM JournalDocumento20 páginasBangkok-Singapore CDM JournalvasidhartaAinda não há avaliações
- Sneha Foundation PlusDocumento17 páginasSneha Foundation PlusBikash KumarAinda não há avaliações
- Feasibility Study Notes Revised PDFDocumento10 páginasFeasibility Study Notes Revised PDFGilbert BettAinda não há avaliações
- Scan 1111111111Documento1 páginaScan 1111111111angela1178Ainda não há avaliações
- Sanghvi: Protein Self TestDocumento11 páginasSanghvi: Protein Self TestNewborn2013Ainda não há avaliações
- 23 - Battery Sizing DischargeDocumento19 páginas23 - Battery Sizing Dischargechanchai T100% (4)
- Bomba Submersa FE - Petro STPDocumento6 páginasBomba Submersa FE - Petro STProbsonlagambaAinda não há avaliações
- 2.0 Intro To Small Basic GraphicsDocumento18 páginas2.0 Intro To Small Basic GraphicspatoturboAinda não há avaliações
- Rajasthan BrochureDocumento31 páginasRajasthan BrochureMayank SainiAinda não há avaliações
- Mass Flow SensorDocumento0 páginaMass Flow Sensorwong_arifAinda não há avaliações
- SAP MM Module OverviewDocumento15 páginasSAP MM Module OverviewAmit Kumar100% (1)
- SMA Inverter Catalogue PDFDocumento290 páginasSMA Inverter Catalogue PDFxodewaAinda não há avaliações