Escolar Documentos
Profissional Documentos
Cultura Documentos
M. F. McCarty, J. C. Gustin - Pyruvate and Hydroxycitrate/carnitine May Synergize To Promote Reverse Electron Transport in Hepatocyte Mitochondria, Effectively Uncoupling' The Oxidation of Fatty Acids
Enviado por
Antonio Di DioTítulo original
Direitos autorais
Formatos disponíveis
Compartilhar este documento
Compartilhar ou incorporar documento
Você considera este documento útil?
Este conteúdo é inapropriado?
Denunciar este documentoDireitos autorais:
Formatos disponíveis
M. F. McCarty, J. C. Gustin - Pyruvate and Hydroxycitrate/carnitine May Synergize To Promote Reverse Electron Transport in Hepatocyte Mitochondria, Effectively Uncoupling' The Oxidation of Fatty Acids
Enviado por
Antonio Di DioDireitos autorais:
Formatos disponíveis
ROLE OF REVERSE ELECTRON TRANSPORT IN
KETOGENESIS
Recent anecdotial clinical experience strongly suggests
that pyruvate administration (4 g orally t.i.d.) interacts
synergistically with hydroxycitrate (HCA) and carnitine
to promote rapid fat loss and marked thermogenesis (see
Appendix).
HCA/carnitine has been recommended as a diet aid on
the basis of its presumed ability to promote the transport
of free fatty acids (FFAs) into hepatic mitochondria (13).
This transport step is believed to be rate-limiting for
hepatic ketogenesis (4,5). More specifically, the conver-
sion of cytoplasmic fatty acyl coA to fatty acyl carnitine
via the enzyme carnitine palmitoyl transferase I (CPT) is
pace-setting for fatty acid transport into mitochondria
and thus for ketogenesis (4,5). HCA the chief acid found
in fruits of the genus Garcinia disinhibits CPT by sup-
pressing synthesis of malonyl coA, a key allosteric inhi-
bitor of this enzyme (6); it achieves this by acting as a
potent competitive inhibitor of citrate lyase (79), whose
activity in hepatocytes is required for the cytoplasmic
generation of acetyl coA, the biosynthetic precursor of
malonyl coA. Carnitine is the essential cofactor for CPT
activity, and ordinary non-fasting hepatocyte levels of
this coenzyme appear to be subsaturating, such that
provision of extra carnitine accelerates hepatocyte keto-
genesis when CPT is activate (4). Thus, it has been
Medical Hypotheses (1999) 52(5), 407416
1999 Harcourt Brace & Co. Ltd
Article No. mehy.1997.0683
Pyruvate and hydroxycitrate/carnitine
may synergize to promote reverse
electron transport in hepatocyte
mitochondria, effectively uncoupling
the oxidation of fatty acids
M. F. McCarty, J. C. Gustin
NutriGuard Research, Encinitas, CA, USA
Summary In a recent pilot study, joint administration of pyruvate, hydroxycitrate (HCA), and carnitine to obese
subjects was associated with a remarkable rate of body-fat loss and thermogenesis, strongly suggestive of uncoupled
fatty-acid oxidation. Hepatocytes possess an uncoupling mechanism reverse electron transport that enables
fasting ketogenesis to proceed independent of respiratory control. Electrons entering the respiratory chain at the
coenzyme Q (CoQ) level via FAD-dependent acyl coA dehydrogenase, can be driven up the chain by the
electrochemical proton gradient to reduce NAD
+
; if these electrons are then shuttled to the cytoplasm, returning to the
respiratory chain at the CoQ level, the net result is heat generation at the expense of the proton gradient, enabling the
uncoupled flow of electrons to oxygen. Pyruvates bariatric utility may stem from its ability to catalyze the rapid
transport of high-energy electrons from mitochondria to the cytoplasm, thus stimulating electron shuttle mechanisms.
By enabling rapid mitochondrial uptake of fatty acids and thus disinhibiting hepatocyte ketogenesis, HCA/carnitine
should initiate reverse electron transport: concurrent amplification of electron shuttle mechanisms by pyruvate can be
expected to accelerate this reverse electron transport, thereby decreasing the electrochemical proton gradient. As a
result, hepatocytes may be able to convert fatty acids to CO
2
and heat with little net generation of ATP. These
considerations suggest that it may be feasible to render hepatocytes functionally equivalent to activated brown fat,
such that stored fat can be selectively oxidized in the absence of caloric restriction. Other measures which enhance
the efficiency of hepatocyte electron shuttle mechanisms may increase the efficacy of this strategy.
Received 2 September 1997
Accepted 15 October 1997
Correspondence to: Mark F. McCarty MD, NutriGuard Research, 1051
Hermes Avenue, Encinitas, CA 92024, USA
407
proposed that joint administration of HCA and carnitine
will be clinically useful as a strategy for disinhibiting and
activating CPT, thereby stimulating ketogenesis.
Although the process of ketogenesis reduces both
NAD
+
and FAD, the rate of ketogenesis appears to be
substantially independent of respiratory control (1013),
i.e. it can proceed at a high rate even when hepatocyte
metabolism is generating ADP at a low rate. The most
reasonable explanation for this phenomenon has been
offered by Berry and co-workers (11,13). They suggest
that, during ketogenesis, high-energy electrons enter
the respiratory chain at a greater rate than hepatocytes
can generate ADP, resulting in an electron glut and an
increased electrochemical proton gradient. Under these
circumstances, electrons entering the chain at the level of
coenzyme Q (via the FAD-dependent acyl coA dehydro-
genase reaction) can be pushed up the respiratory chain
to NAD dehydrogenase, which transfers them to NAD
+
.
These electrons can then be shipped to the cytosol via
the malate/aspartate shuttle and, after reducing NAD
+
or NAD(P) in the cytosol, can then re-enter the mito-
chondrial respiratory chain at the coenzyme (CoQ) level
via the glycerol-3-phosphate shuttle. The reverse elec-
tron transport step in this cycle is driven by the electro-
chemical proton gradient of the mitochondrial inner
membrane which diminishes as a result or, alterna-
tively, by conversion of ATP to ADP via the mitochondrial
ATP synthase. After two turns of this cycle, the electro-
chemical proton gradient will be sufficiently diminished
to enable the electrons to pass down the respiratory chain
from CoQ to oxygen, without any obligate coupling to
ATP synthesis. (Alternatively, the two ADPs generated by
two turns of the cycle will enable the coupled transport
of these electrons from CoQ to oxygen.) This mechanism
thus would effectively uncouple the transfer of electrons
from FADH
2
to oxygen during ketogenesis. This reverse
electron transport mechanism is well documented in
mitochondria and sub-mitochondrial particles in vitro
(1418); its role in vivo is still speculative, but it seems
to offer a very plausible explanation for the mysterious
uncoupling of hepatic ketogenesis.
PYRUVATE PROMOTES ELECTRON SHUTTLE
MECHANISMS
The well-documented bariatric benefits of pyruvate
(1924) remain unexplained. We propose that adminis-
tration of pyruvate enhances the efficiency of the shuttle
mechanisms required for reverse electron transport, by
serving as a biosynthetic precursor for key substrates
of these shuttles namely oxaloacetate and dihydroxy-
acetone phosphate; it is also conceivable that pyruvate
has an as-yet-uncharacterized allosteric effect on the acti-
vity or synthesis of enzymes which catalyze the shuttles.
When concurrent administration of HCA/carnitine disin-
hibits hepatic ketogenesis, flooding hepatic mitochondria
with reducing equivalents and initiating reverse electron
transport, the pyruvate-mediated activation of shuttle
mechanisms may substantially amplify the rate of reverse
electron transport, such that not only ketogenesis, but
also Krebs cycle activity is at least partially uncoupled,
enabling the complete oxidation of FFAs to CO
2
with
little net production of ATP and a substantial release of
heat. Thus, we propose that, under these circumstances,
electrons transferred to NAD
+
from the Krebs cycle can
be shuttled to enter the respiratory chain at the CoQ
level, and that a portion of these electrons will then
be subjected to reverse electron transport thereby
diminishing the electrochemical proton gradient and
enabling uncoupled respiration.
The efficient conversion of pyruvate to mitochondrial
oxaloacetate may be crucial to pyruvates utility. The
rapid production of acetyl coA during ketogenesis can
be expected to strongly activate pyruvate carboxylase,
whereas pyruvate dehydrogenase will be inhibited (l,2).
Furthermore, the generation of ketone bodies from excess
acetyl coA will promote pyruvate transport into mito-
chondria. Thus, pyruvate administered during ketogenesis
should be rapidly converted to mitochondrial oxalo-
acetate. Owing to the high redox potential in ketogenic
mitochondria, and the fact that reduction of oxaloacetate
is highly exergonic, much of this oxaloacetate will be
quickly reduced to malate, which readily exits mito-
chondria. In this way, pyruvate can be expected to catalyze
the transfer of high energy electrons from mitochondria
to the cytosol. The resulting decrease in mitochondrial
NADH should stimulate the Krebs cycle and promote
reverse electron transport. Provided that efficient en-
zymatic machinery is in place to transfer electrons from
cytoplasmic malate to dihydroxyacetone phosphate,
and to oxidize the resulting glycerol-3-phosphate at the
mitochondrial inner membrane, the exported electrons
can be quickly returned to CoQ in the respiratory chain,
enabling further reverse electron transport.
Indeed, if one were to logically devise an optimal method
for expediting the transport of excess high-energy elec-
trons from mitochondria, one could probably do no better
than to administer an agent which, like pyruvate, promotes
the rapid intramitochondrial generation of oxaloacetate.
(A Trojan horse such as pyruvate is required to increase
mitochondrial oxaloacetate levels, since oxaloacetate
itself cannot cross the mitochondrial inner membrane.)
This may hold the key to pyruvates bariatric utility
even in the absence of reverse electron transport, pyruvate
should catalyze the shuttle-mediated discounting of
high-energy electrons, whereby electrons flow from
mitochondrial NADH through the cytoplasm to enter the
respiratory chain at CoQ.
408 McCarty and Gustin
Medical Hypotheses (1999) 52(5), 407416 1999 Harcourt Brace & Co. Ltd
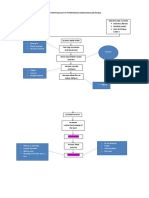
Figure 1 demonstrates how it would be theoretically
conceivable for reverse electron transport to completely
uncouple hepatocyte oxidative metabolism. If keto-
genesis and Krebs cycle activity transfer reducing equi-
valents to NAD
+
and FAD at rates x and y, respectively,
then a net reverse electron transport from CoQ to NAD
+
at a rate of 2(x+y) would collapse the electrochemical
proton gradient sufficiently (or generate sufficient ADP)
to enable respiration in the absence of net ATP produc-
tion, i.e. FFAs (and any other substrate oxidized by
hepatic mitochondria) could be converted to CO
2
and
heat at a rate independent of hepatocyte metabolic
activity. In practice, respiration will be partially coupled,
as hepatocytes have metabolic needs that generate ADP
thus, it cannot be expected that reverse electron transport
will ever achieve a rate as high as 2(x+y). Nonetheless,
a considerable degree of uncoupling can be anticipated
if reverse electron transport can be substantially boosted.
Electron flow during reverse electron transport may
well proceed in an oscillatory fashion. Reverse electron
flow will gradually collapse the electrochemical proton
gradient, slowing the reverse flow but accelerating
forward flow to oxygen. The increased forward flow,
to the degree that it is uncoupled, will recharge the
proton gradient, slowing forward flow but promoting
an increased rate of reverse flow. (And so, to quote the
King, et cetera, et cetera, et cetera.)
Pyruvate supplementation should also have thermo-
genic effects that drive the coupled oxidation of FFAs by
stimulating the futile cycle pyruvate oxaloacetate
phospho-enol-pyruvate pyruvate, utilizing one net
ATP (or if malic enzyme is induced, the cycle pyruvate
oxaloacetate malate pyruvate), and by acting as
substrate for gluconeogenesis, which requires 2ATP
equivalents plus an NADH per molecule of pyruvate
converted to glucose. The activation of pyruvate carbo-
xylase by HCA/camitine should promote these mecha-
nisms. The mitochondrial oxaloacetate generated from
pyruvate will also aid Krebs cycle activity, and the
pyruvate-stimulated production of malate will accelerate
mitochondrial uptake of anionic substrates such as
pyruvate and phosphate. An increase in hepatocyte malate
levels is typically seen in response to measures exercise
and gluconeogenic hormones that boost the respiratory
capacity of hepatocyte mitochondria (25).
It can be anticipated that, even with the concurrent use
of pyruvate, a proportion of the acetyl coA generated
from fatty acids will not be oxidized, but rather exported
from the liver as ketone bodies, committed to oxidation
in peripheral tissues. This peripheral oxidation of ketones
will presumably be coupled, but will in any case complete
the selective oxidation of fats, such that a low respiratory
quotient favorable to fat loss is achieved. Ketosis can
also suppress adipocyte lipolysis (26); while this may slow
the rate of fat loss, it should have a favorable impact on
cardiovascular risk factors. Some of the electrons evolved
during hepatocyte fatty acid oxidation will not flow to
oxygen, but rather will be used to convert acetoacetate
to beta-hydroxybutyrate.
SHUTTLE FUNCTION AS A KEY TO
THERMOGENESIS
It undoubtedly is not coincidental that the thermogenic
hormones thyroxine and (in rats) high-dose DHEA
potently induce the expression of two enzymes in hepato-
cytes the mitochondrial glycerol-3-phosphate dehydro-
genase and the cytoplasmic malic enzyme which can be
rate-limiting for electron shuttle mechanisms (27,28);
cafeteria-feeding, which is potently thermogenic, has an
analogous effect (13,30). Catecholamines also thermo-
genic for hepatocytes activate mitochondrial glycerol-
3-phosphate dehydro-genase by increasing hepatocyte
intracellular calcium levels (31). Even in the absence
of reverse electron transport, the transfer of electrons
from mitochondrial NADH to respiratory chain CoQ via
these shuttle mechanisms effectively lowers the P:O ratio
from 3 to 2 thus partially uncoupling hepatic oxidative
metabolism. Clearly, however, the greatest degree of un-
coupling can be anticipated when an up-regulation of
shuttle mechanisms is allied with a disinhibition of keto-
genesis that initiates reverse electron transport. We pro-
pose that this is precisely what happens when pyruvate
is administered in conjunction with HCA/carnitine.
Alternate means of up-regulating shuttle function might
be expected to synergize with these measures. Whereas
high-dose DHEA is not suitable for clinical use (owing to
its ability to give rise to excessive levels of sex hormones),
Lardy and colleagues have recently demonstrated that
7-hydroxy or 7-keto derivatives of DHEA which to some
degree are produced endogenously, and do not give rise
to sex-hormone activity are actually more potent than
Pyruvate, HCA and carnitine in uncoupling the oxidation of FFAs 409
1999 Harcourt Brace & Co. Ltd Medical Hypotheses (1999) 52(5), 407416
Fig. 1 Uncoupling of hepatocyte respiration via reverse electron
transport.
DHEA itself in inducing thermogenic shuttle enzymes
(as well as fatty acyl coA oxidase) in rat hepatocytes
(32,33). If these agents prove to be effective in this regard
in humans, without unacceptable side-effects or toxicities
(such as are seen with thyroid hormone), they may well
have a bright future in bariatric medicine, alone or in
conjunction with the techniques suggested here.
Folkers has demonstrated that many individuals are
functionally deficient in CoQ, in the sense that addition
of CoQ to their mitochondria can enhance the oxidation
of succinate by succinate dehydrogenase (34). Could
CoQ levels likewise sometimes be rate-limiting for the
glycerol-3-phosphate shuttle and reverse electron trans-
port? If so, supplemental CoQ might sometimes enhance
the efficacy of the measures recommended here. Some
years ago, an open study by Van Gaal provided evidence
that correction of documented CoQ deficiency (as assessed
by succinate dehydrogenase activity) might accelerate
weight loss in dieters (35).
If pyruvates bariatric utility is primarily reflective of
the fact that it is an efficient precursor for mitochondrial
oxaloacetate (as noted above, the possibility that it has
direct inductive effects cannot presently be discounted),
it is possible that aspartic acid will have comparable
activity. Aspartate is avidly transported into mitochondria
and de-aminated to yield oxaloacetate, as a key portion
of the malate-aspartate shuttle; as noted, oxaloacetate
cannot directly enter mitochondria, but can do so when
disguised as aspartate. If aspartate is in fact effective in
this regard, this may be of some practical importance as
aspartates are currently far less expensive than pyruvates.
(It may be noted that the process of de-amination and the
subsequent conversion of NH
4
+
to urea will expend an
additional 4 ATPs but also requires reduction of NAD
+
.)
Lactic acid, likewise inexpensive, is readily converted
to pyruvate and thus might be considered as an alter-
native to this compound. However, the oxidation of
lactate to pyruvate would be expected to increase cyto-
plasmic redox potential, which could slow the rate of
electron transport from mitochondria to the cytoplasm.
It should be noted that Stankos original testing of
pyruvate and dihydroxyacetone as remedies for ethanol-
induced fatty liver (36), was rooted in the fact that these
compounds can function as electron acceptors whereas
lactate is clearly an electron donor. However, there are
alternative pyruvate precursors the amino acids serine
and glycine that do not mediate reductions when con-
verted to pyruvate. Alanine, however, may be inappro-
priate for this purpose, as it is an inhibitor of pyruvate
kinase.
With regard to the demonstrated utility of dihydroxy-
acetone as an aid to thermogenesis in animals (19), it
should be noted that phosphorylation of this compound
yields the oxidized component of the glycerol-3-
phosphate shuttle. Thus, dihydroxyacetone appears to be
a preferable (though much more expensive) alternative
to glycerol. The latter compound appears to have a tem-
porary anorexic activity in rat (37), although controlled
clinical evaluations of its impact as an adjuvant to low-
calorie diets have failed to confirm efficacy in this regard
(38,39). Hepatocyte levels of dihydroxyacetone phosphate
can be increased most conveniently with fructose; the
liver-specific fructokinase produces fructose-1-phosphate,
which is then cleaved by aldolase to yield glyceraldehyde
and dihydroxyacetone phosphate. While fructose alone
is clearly not a diet aid, it would be interesting to deter-
mine whether catalytic amounts might stimulate reverse
electron transport in the context of the measures re-
commended here. Dihydroxyacetone phosphate levels
should also be increased somewhat by gluconeogenic
prescursors including pyruvate and aspartate.
ACTIVATION OF PYRUVATE KINASE WITH
METFORMIN AND BIOTIN
The drug metformin may also have utility as an adjuvant
in this weight-loss strategy (3). By activating flux through
pyruvate kinase (40,41), metformin may enhance endo-
genous generation of pyruvate, decrease the loss of
administered pyruvate to gluconeogenesis, and con-
currently enhance the thermogenic impact of the futile
cycles centered on pyruvate. The tendency of metformin
to promote weight loss and decreased appetite in dia-
betics is well known (4245). Its use in conjunction with
ketogenic techniques, in diabetics or borderline diabetics,
may in any case be desirable to prevent excessive stimu-
lation of gluconeogenesis by accelerated hepatic FFA
oxidation (40,44,4651).
An alternative (or adjunctive) approach to activating
pyruvate kinase may be offered by high-dose biotin.
In rodent studies, pharmacological doses of biotin can
produce significant increases in the expression of gluco-
kinase in both hepatocytes and pancreatic cells (5256);
activation of guanylate cyclase by supraphysiological
concentrations of biotin may mediate this effect (57,58).
Increased activity of glucokinase in hepatocytes can be
expected to increase pyruvate kinase activity both by
enhancing the level of its allosteric activator, fructose-
1,6,-dipkosphate, and by stimulating increased synthesis
of the enzyme (5962). An elevation of glucokinase should
aid electron shuttle function by increasing the concentra-
tion of dihydroxyacetone phosphate, and can be expected
to promote thermogenic futile cycles involving glycolytic
intermediates (61). A further benefit of biotin could be
that, by increasing glucokinase activity in cells, it may
act to offset a potential adverse effect of high-dose HCA
on glucose-stimulated insulin secretion (63). A recent
report that biotin lessens weight gain (presumably, fat
410 McCarty and Gustin
Medical Hypotheses (1999) 52(5), 407416 1999 Harcourt Brace & Co. Ltd
gain) in a congenitally obese strain of rat despite slightly
increasing food intake suggests that biotin does indeed
have thermogenic potential (64). Since 3 mg t.i.d. has
been shown to aid glycemic control in human diabetics
(65), this dose schedule is likely to be adequate for
increasing hepatocyte glucokinase activity clinically.
A THERMOGENIC ROLE FOR GLUCAGON
In the pilot study described in the Appendix, patients
were advised to perform fasting aerobic exercise and to
eat substantial amounts of protein. Each of these measures
can be expected to increase glucagon activity. Glucagon
promotes intramitochondrial fatty acid transport and
ketogenesis, both by decreasing the synthesis of malonyl-
coA (via suppression of acetyl-coA carboxylase activity)
and by decreasing the potency of malonyl-coA as an
allosteric inhibitor of carnitine palmitoyl transferase
(66,67); thus, measures which enhance glucagon activity
have been recommended as adjuvants to HCA/carnitine
(2). Glucagons ability to stimulate pyruvate carboxylase
and thus promote gluconeogenesis may simply reflect
allosteric activation by the increased levels of acetyl-coA
stemming from accelerated fatty acid oxidation (68);
glucagon also promotes mitochondrial uptake of pyruvate
(25,69). Mitochondria from glucagon-treated animals
have greater respiratory activity ex vivo (70); this is due
in part to increased efficiency of electron flow from
NADH through NADH dehydrogenase to CoQ (71). It
seems not unlikely that, conversely, glucagon could
increase the efficiency of reverse flow from CoQ to NADH
during ketogenesis, though experimental demonstration
of this appears to be lacking. Homeostatically, it would
make good sense for glucagon to increase the efficiency
of reverse electron transport, thereby promoting more
rapid ketogenesis. Glucagon acts as a signal of carbo-
hydrate deficiency, inducing the conversion of the bodys
chief stored substrates, protein and fat, to glucose and
ketones (respectively), thus helping to fuel the central
nervous system during starvation. Efficient ketogenesis
is evidently crucial in this regard, as it enables sparing of
tissue protein.
When insulin levels are low, glucagon infusion has a
marked thermogenic impact in humans (72); stimulation
of gluconeogenesis and ureagenesis undoubtedly contri-
butes to this effect. As these processes are driven by ATP,
they can be expected to promote the coupled oxidation
of hepatic lipid. A high protein intake will activate these
processes, both by stimulating glucagon output, and
by providing substrate for them. In the context of the
measures recommended here, the chief benefit of these
endergonic processes may be that they stimulate electron
flow from the Krebs cycle, for which ADP is an allosteric
activator.
OVERVIEW
There may be a certain logical inevitability to the ideas
proposed here. The clinical results described in the
Appendix can only credibly be attributed to a substantial
uncoupling of fatty acid oxidation. Since HCA/carnitine
can be expected to have its most profound impact on the
liver, this is likely to be the chief site of this uncoupling.
The other major thermogenic organ in man is skeletal
muscle; since pyruvate has been shown to enhance
exercise endurance (73,74), it seems most unlikely that
pyruvate promotes uncoupling in muscle. The liver is
known to have a mechanism for uncoupled oxidative
metabolism reverse electron transport which should
be stimulated by HCA/carnitine. It is therefore logical to
suspect that an amplification of this mechanism is respon-
sible for the uncoupling observed during joint adminis-
tration of pyruvate and HCA/carnitine. Pyruvates role
as an efficient precursor for mitochondrial oxaloacetate
suggests that pyruvate administration will indeed aid
reverse electron transport.
It is very clear to us that the results reported in the
Appendix demonstrate a marked synergism between
pyruvate and HCA/carnitine. When used in conjunction
with low-calorie diets or an overfeeding regimen, pyruvate
(at 1530 g daily) diminished body fat by about 1 kg over
21 days relative to placebo (2224); in the context of a
low-fat weight-maintaining regimen in hyperlipidemic
subjects, about half of whom were obese, the incremental
fat loss associated with pyruvate (2244 g daily) was
0.4 kg over six weeks (75). In a double-blind study in
which overweight volunteers received HCA/carnitine/
chromium picolinate while asked to avoid fatty foods, eat
more fiber, and get more physical activity, the additional
fat loss attributable to the supplement was 0.6 kg over
four weeks (76). Used alone, either of these techniques
appears to have some utility, but they should rightly be
construed as adjuvants to traditional weight-management
techniques. The results reported here with the combined
use of these measures are clearly far greater than the sum
of their individual efficacies and, if confirmable, suggest
a new and definitive approach to obesity control.
If indeed it is possible to at least partially uncouple FFA
oxidation in the liver, this organ might reasonably be
compared to a vast slab of activated brown fat. Under
these circumstances, unprecedented rates of selective fat
loss can be anticipated.
The liver evidently can only degrade FFAs that reach
it. Thus, these methods should have their most rapid
impact on fat stores such as visceral adipocytes that
are relatively lipolytically active. Indeed, diets and exer-
cise regimens that successfully achieve fat loss tend
to have a disproportionate impact on visceral fat stores
(7780). A reduction in lipolytically active fat stores can
Pyruvate, HCA and carnitine in uncoupling the oxidation of FFAs 411
1999 Harcourt Brace & Co. Ltd Medical Hypotheses (1999) 52(5), 407416
be expected to be of practical benefit with respect to the
insulin resistance syndrome (syndrome X) and its fre-
quent sequel, type II diabetes (8185). Conversely, the
subcutaneous fat stores which would be more gradually
responsive to the measures suggested here presumably
the gynoid depots are precisely those which have
minimal impact on health (and, if desired, could be
targeted for liposuction).
The ability of hepatocytes to achieve reverse electron
transport, and thus decouple ketogenesis from the meta-
bolic energy needs of hepatocytes, is by no means an
evolutionary fluke. Clearly, ketogenesis is crucial to
meeting the energy needs of the central nervous system
while enabling protein sparing during prolonged fasts;
it would be most counterproductive if sluggish hepato-
cyte metabolism in fasted animals were to impede the
efficiency of ketone generation. The central thesis here
is that it may prove possible perhaps via pyruvate,
HCA, and carnitine to amplify reverse electron transport
in hepatocytes, thus substantially uncoupling hepatocyte
oxidative metabolism and promoting the efficient con-
version of portal FFAs to CO
2
and heat. In vitro studies, in
which hepatocytes are exposed concurrently to pyruvate,
HCA, carnitine, and FFAs, should readily determine
whether these proposals have validity. In the meantime,
clinical efforts along the lines described in the Appendix
should continue, and attempt to verify not only the
efficacy, but also the safety of these measures.
In regard to safety considerations, it may be noted that
the strategy suggested here utilizes only nutrients, a
natural metabolite, and a food compound (HCA) tradi-
tionally consumed in Indian cuisine none of which has
shown any evident toxicity in previous clinical experience.
Also, with respect to the increased gallstone formation
commonly observed with rapid-weight-loss diets (8689),
this appears to be largely attributable to the gallbladder
stasis associated with semi-starvation (8991); since the
methods proposed here do not require caloric restriction,
there is little reason to suspect that they will induce
gallstones.
APPENDIX
Summary of an informal pilot trial
A short pilot study was conducted to test the impact of a
supplement regimen, in conjunction with dietary and
exercise advice, on fat loss and physique modification
in overweight volunteers. The daily regimen provided the
following, to be consumed in three equal doses: calcium
pyruvate, 12 g; HCA 1.5 g; L-carnitine 250 mg; chromium
(as chromium picolinate) 600 mcg. The supplements
were to be consumed in the morning (prior to exercise
or eating), with lunch, and at bedtime. Participants were
asked to walk at least 20 min each morning, on an empty
stomach; they were asked to walk at a brisk pace at which
they could converse normally. Dietary advice consisted
of recommendations to eat a high-protein, low-fat diet
(30% and 10% of calories, respectively) in an amount that
provided 0.751 g protein per pound of lean mass daily
(or 100 g daily for women with lean mass under 100 lb).
These recommendations corresponded to total daily
caloric intakes ranging from 13003100 kcal daily, de-
pendent on body size; the average recommended intake
was around 2000 kcal daily. This was to be consumed
in numerous small meals daily, and no calories were to
be ingested in the 3 h prior to bedtime. An ample intake
of water was also suggested, and fiber-rich foods were
recommended.
Body composition was evaluated at baseline and at
weekly intervals thereafter using Futrex 5000, a new infra-
red technique for quantifying the depth of underlying
subcutaneous fat (92,93). All measurements were made
by the junior author (JCG) on the dominant arm, using a
contact point on the anterior aspect of the exterior
midline of the biceps, halfway between the antecubital
fossa and acromion (as recommended by the Futrex
manual.) On each occasion (except the last), measurements
were taken in triplicate and averaged. (Unfortunately,
time constraints prevented triplicate measurements during
the final evaluation. This probably had little impact on
the final results, as JGs replicate measurements typically
vary by less than 2%). Heights and weights were also
determined, thus enabling calculation of body-mass
indexes (BMIs).
A total of 23 volunteers the majority Samoan-American
appeared for enrollment. They were admitted in three
groups on 6/28/97, 7/2/97, and 7/5/97; final evaluation
for all subjects was on 7/26/97. Three subjects did not
return after baseline evaluation, and one subject did
not return for final evaluation. Two subjects refused to
be weighed at baseline (insisting on reporting their own
weights) and therefore could not be properly assessed.
One subject developed a rash after three days, and
pyruvate was discontinued. There were thus 16 parti-
cipants who were evaluable. The initial body weights of
these subjects ranged from 69 to 231 kg, with a mean
weight of 117 kg. Percentage body fat ranged, from 26%
to 54%, with a mean value of 41%. The average BMI
was 39.3 (range 26.263.7).
Most subjects tolerated the regimen quite well. As
noted, one subject developed a rash and pyruvate dis-
continued. The largest enrolled subject (initial weight
231 kg), who also lost the most weight and fat, had a
prior history of gout, and experienced an attack of gout
during the final week which prevented him from walking.
One subject noted borborygmus and gas.
Most subjects reported feeling warm during the study.
412 McCarty and Gustin
Medical Hypotheses (1999) 52(5), 407416 1999 Harcourt Brace & Co. Ltd
In the first week, three subjects reported sweating and/or
subjective feelings of heat (including a woman who at
baseline had complained that she was always cold). Since
this was thought to be a possible sign of increased
thermogenesis, the other subjects were queried as to
whether they were experiencing sensations of warmth,
and the subjects were virtually unanimous in affirming
this. One subject (again, the largest one) noted profuse
sweating and indicated that he needed to turn a fan on
himself at bedtime to enable himself to get to sleep.
The other virtually unanimous subjective response was
of considerably increased physical energy.
Self-reported compliance with the dietary and exercise
recommendations was excellent in many subjects, but
others confessed to occasional junk-food binges, a failure
to achieve the suggested intake of protein, or sporadic
adherence to morning walking exercise. Self-reported
compliance with the supplement regimen in general
was quite good, although a few subjects noted that they
had missed several doses. Participants were free-living
and, with the exception of a whey protein supplement
provided to several subjects, their food was self-chosen.
Exercise was not monitored. Thus, the conditions of the
study were closer to real world application than is the
case in most carefully supervised clinical studies. Further-
more, no subjects were excluded from the final analysis
owing to sporadic (or in a few instances, non-existent)
compliance with either dietary, exercise, or supplemen-
tation recommendations.
Since subjects were enrolled for varying periods of
time (34 weeks), results are reported as average weight
loss and average fat loss per week. In the entire group of
16 subjects, average weekly weight loss was 1.5 kg; average
weekly fat loss was 2.3 kg. This evidently implies a weekly
gain of lean mass averaging 0.8 kg.
The largest subject achieved, within 24 days, a weight
loss of 11.8 kg and, remarkably, Futrex analysis indicated
a fat loss of 22.7 kg. If this subject is excluded from
the analysis as atypical, the average weekly weight and
fat losses in the remaining 15 subjects were 1.4 kg and
2.0 kg, respectively.
Since grossly obese subjects have often been noted
to lose significant weight initially when their diet is regu-
lated (preventing binges on favorite. foods), a separate
analysis was made of the five subjects with initial weight
under 200 lb (91 kg). In these subjects, average weekly
weight loss and fat loss averaged 1.3 kg and 1.8 kg re-
spectively not greatly different from the group as a
whole. The subject with the lowest body weight, as well
as the subject with the lowest initial percentage body
fat, achieved rates of fat loss of 2.0 and 2.7 kg per
week, respectively. Thus, benefit does not appear to be
contingent on severe obesity.
To ascertain the regularity of response, it is appropriate
to note the poorest response in the 16 subjects. A 74-kg
woman lost 3.2 kg of weight and 4.5 kg of fat over four
weeks. During the final week, she had been traveling
to Australia, and had been completely noncompliant with
the diet and exercise recommendations.
It is notable that, in every subject, lean mass increased
during the study. Thus, response was quite different from
that seen during very-low-calorie dieting. The subjective
increase in energy (which helped some subjects comply
with the recommended exercise) and in body warmth, is
also hardly typical of response during calorie deprivation.
Perhaps the most telling indication of the success of
the regimen was the mob scene which greeted the in-
vestigators at the final evaluation dozens of friends and
relatives of the volunteers had shown up, demanding to
be included in the study!
The authors are acutely aware that a study of longer
duration would have been more meaningful particularly
since it is important to confirm durability of response to
this regimen. Unfortunately, the businessman sponsoring
the study (not affiliated with Nutrition 21) refused to
provide pyruvate capsules beyond the four-week point,
when he became apprised of the fact that pyruvate had
already been patented for use as a diet aid.
If one assumes that 1 lb of fat corresponds to about
3500 kcal, whereas 1 lb of lean averages 700 kcal, the
average daily calorie deficit was approximately 2370 kcal
(2100 kcal if the heaviest subject is excluded form
analysis). Given the fact that the subjects were not sub-
jected to severe caloric restriction (indeed, a few com-
plained that they were being asked to eat too much!),
and were asked to do only walking exercise of modest
duration, these responses are likely to be unprecedented,
and are strongly suggestive of a dramatic increase in
thermogenesis a view consistent with the many reports
of heat and sweating.
Even if skeptics were to disregard the Futrex analyses
as unreliable, the average monthly weight loss approxi-
mately 6.5 kg is exceptional in the context of a regimen
that does not include severe caloric restriction, draconian
exercise, or drugs.
Although not discussed in the body of this paper, the
nutrient chromium picolinate was included in the supple-
ment regimen. In the two largest controlled studies to
date evaluating the impact of this nutrient on body com-
position in overweight subjects (400800 mcg chromium
daily), a significant incremental fat loss (relative to placebo)
averaging 0.4 kg per month was observed (94,95). Pre-
sumably, the supplemental chromium may have had an
additive impact on the fat loss achieved during the
present study. However, whether it might also have had
a synergistic interaction with the other compounds
administered (for example, by down-regulating insulin
secretion, allowing glucagon to work more effectively),
Pyruvate, HCA and carnitine in uncoupling the oxidation of FFAs 413
1999 Harcourt Brace & Co. Ltd Medical Hypotheses (1999) 52(5), 407416
obviously cannot be determined from this study, but
requires consideration in future studies.
As a scientific study, the pilot trial described above
has many overt deficiencies. Clearly, confirmatory studies
are urgently needed using double-blind design, a more
accurate technique for body composition assessment
(i.e. immersion densitometry or DEXA), more extended
duration, assessment of blood parameters to monitor
safety, and respiratory analysis or the double-labeled
water technique to quantify metabolic rates and respira-
tory quotients. However, despite the fact that the current
study was virtually an exercise in guerilla nutrition
conducted not for science per se but to aid in product
development the results are so striking that they seem
very likely to point the way to a new and important
approach to modification of body composition. Whether
or not the theoretical mechanism suggested in the body
of this paper proves to have validity, the authors are
convinced that the joint administration of pyruvate, HCA,
L-carnitine, and chromium picolinate, in adequate and
appropriate doses, has very considerable potential as a
novel strategy for physique modification. We urge experi-
enced well-trained bariatric scientists (which the authors
cannot claim to be) to attempt replication of methods
described here; frankly, we do not expect properly skep-
tical scientists to believe the remarkable and counter-
intuitive results reported above until they have seen it
with their own eyes. That being said, we nonetheless
affirm that we have attempted to be scrupulously honest
in collecting, analyzing, and reporting the data from this
informal study.
With regard to safety considerations, it is conceivable
that the rash experienced by one subject reflected a trace
contaminant in the 12 g of pyruvate consumed daily.
An allergic reaction to the Garcinia extract used (50%
HCA as the calcium salt) is also a possibility, but seems
unlikely since the rash cleared while Garcinia intake
continued. The flare-up of gout experienced by one
subject might be attributable to ketosis, which reduces
renal clearance of uric acid (96,97); the high protein
intake might also have been a contributory factor. No
subjects complained of abdominal distress suggestive of
gallstones.
REFERENCES
1. McCarty M. F. Promotion of hepatic lipid oxidation and
gluconeogenesis as a strategy for appetite control. Med
Hypotheses 1994; 42: 215225.
2. McCarty M. F. Inhibition of citrate lyase may aid aerobic
endurance. Med Hypotheses 1995; 45: 247254.
3. McCarty M. F. Utility of metformin as an adjunct to
hydroxycitrate/carnitine for reducing body fat in diabetics. Med
Hypotheses 1998; 51: 399403.
4. McGarry J. D., Robles-Valdes C., Foster D. W. Role of carnitine in
hepatic ketogenesis. Proc Natl Acad Sci 1975; 72: 43854388.
5. McGarry J. D., Foster D. W. Regulation of hepatic fatty acid
oxidation and ketone body production. Ann Rev Biochem 1980;
49: 395420.
6. McGarry J. D., Foster D. W. In support of the roles of malonyl-
coA and carnitine acyltransferase I in the regulation of hepatic
fatty acid oxidation and ketogenesis. J Biol Chem 1979;
254: 81638168 .
7. Watson J. A., Fang M., Lowenstein J. M. Tricarballylate and
hydroxycitrate: substrate and inhibitor of ATP: citrate
oxaloacetate lyase. Arch Biochem Biophys 1969; 135: 209217.
8. Lowenstein N. J. Effect of (-)hydroxycitrate on fatty acid
synthesis by rat liver in vivo. J Biol Chem 1971; 246: 629632.
9. Sullivan A. C., Hamilton J. C., Triscari J. Metabolic inhibitors of
lipid biosynthesis as anti-obesity agents. In: Curtis-Prior P. B.,
ed. Biochemical Pharmacology of Obesity. Amsterdam: Elsevier
Science Publishers, 1983: 311325.
10. Debeer L. J. Mannaerts G., De Schepper P. J. Effects of octanoate
and oleate on energy metabolism in the perfused rat liver. Eur J
Biochem 1974; 47: 591600.
11. Berry M. N., Clark D. G., Grivell A. R., Wallace P. G. The
calorigenic nature of hepatic ketogenesis: an explanation for the
stimulation of respiration induced by fatty acid substrates. Eur J
Biochem 1983; 131: 205214.
12. Scholz R., Schwabe U., Soboll S. Influence of fatty acids on
energy metabolism. 1. Stimulation of oxygen consumption,
ketogenesis and CO
2
production following addition of
octanoate and oleate in perfused rat liver. Eur J Biochem 1984;
141: 223230.
13. Berry M. N., Clark D. G., Grivell A. R., Wallace P. G. The
contribution of hepatic metabolism to diet-induced
thermogenesis. Metabolism 1985; 34: 141147.
14. Chance B., Hollunger G. Energy-linked reduction of
mitochondrial pyridine nucleotide. Nature 1960; 185: 666672.
15. Klingenberg M., Schollmeyer P. On the reversibility oxidative
phosphorylation. III. Effect of adenosine triphosphate on the
respiratory chain in respiratory inhibited mitochondria. Biochem
Z 1961; 335: 243262.
16. Packer L. Metabolic and structural states of mitochondria.
III. Reversal of electron transport and mitochondrial swelling.
J Biol Chem 1962; 237: 1327l331.
17. Snoswell A. M. The reduction of diphosphopyridine nucleotide
of rabbit-heart sarcosomes by succinate. Biochim Biophys Acta
1962; 60: 143157.
18. Scholes T. A., Hinkle P. C. Energetics of ATP-driven reverse
electron transfer from cytochrome c to fumarate and from
succinate to NAD in submitochondrial particles. Biochemistry
1984; 23: 33413345.
19. Stanko R. T., Adibi S. A. Inhibition of lipid accumulation and
enhancement of energy expenditure by the addition of
pyruvate and dihydroxyacetone to a rat diet. Metabolism 1986;
35: 182186.
20. Stanko R. T., Ferguson T. L., Newman C. W., Newman R. K.
Reduction of carcass fat in swine with dietary addition of
dihydroxyacetone and pyruvate. J Animal Sci 1989;
67: 12721278.
21. Cortez M. Y., Torgan C. E., Brozinick J. T. Jr et al. Effects of
pyruvate and dihydroxyacetone consumption on the growth
and metabolic state of obese Zucker rats. Am J Clin Nutr 1991;
53: 847853.
22. Stanko R. T., Tietze D. T., Arch J. E. Body composition, energy
utilization, and nitrogen metabolism with a severely restricted
diet supplemented with dihydroxyacetone and pyruvate. Am J
Clin Nutr 1992; 55: 771776.
23. Stanko R. T., Tietze D. L., Arch J. E. Body composition, energy
utilization, and nitrogen metabolism with a 4.25-MJ/d low-
414 McCarty and Gustin
Medical Hypotheses (1999) 52(5), 407416 1999 Harcourt Brace & Co. Ltd
energy diet supplemented with pyruvate. Am J Clin Nutr 1992;
56: 630635.
24. Stanko R. T., Arch J. E. Inhibition of regain in body weight and
fat with addition of 3-carbon compounds to the diet with
hyperenergetic refeeding after weight reduction. Int J Obesity
1996; 20: 925930.
25. Bobyleva-Guarriero V., Wehbie R. S., Lardy H. A. The role of
malate in hormone-induced enhancement of mitochondrial
respiration. Arch Biochem Biophys 1986; 245: 477482.
26. Larsen T. S., Nilsson N. , Blix A. S. Effects of volatile fatty acids
and ketone bodies on lipolysis in isolated adipocytes from
Norwegian reindeer (Rangifer tarandus). Acta Phvsiol Scand
1983; 117: 451455.
27. Tyzbir R. S., Kunin A. S., Sims N. M., Danforth E. Jr. Influence of
diet composition on serum triiodothyronine (T
3
) concentration,
hepatic mitochondrial metabolism and shuttle system activity
in rats. J Nutr 1981; 111: 252259.
28. Lardy H., Shrago E. Biochemical aspects of obesity. Ann Rev
Biochem 1990; 59: 689710.
29. Bobyleva V., Kneer N., Bellei M. et al. Concerning the
mechanism of increased thermogenesls in rats treated with
dehydroepiandrosterone. J Bioenerg Biomemb 1993; 25: 313321.
30. Stirling J. L., Stock M. J. Metabolic origins of thermogenesis
induced by diet. Nature 1968; 220: 801802.
31. Wernette M. E., Ochs R. S., Lardy H. A. Ca
2+
stimulation of rat
liver mitochondrial glycerophosphate dehydrogenase. J Biol
Chem 1981; 256: 1276712771.
32. Lardy H., Partridge B., Kneer N., Wei Y. Ergosteroids: induction
of thermogenic enzymes in liver of rats treated with steroids
derived from dehydroepiandrosterone. Proc Natl Acad Sci 1995;
92: 66176619.
33. Bobyleva V., Bellei M., Kneer N., Lardy H. The effects of the
ergosteroid 7-oxo-dehydroepiandrosterone on mitochondrial
membrane potential: possible relationship to thermogenesis.
Biochem Biophys Acta 1997; 341: 122128.
34. Folkers K., Watanabe T. Bioenergetics in clinical medicine XIV.
Studies on an apparent deficiency of coenzyme Q-10 in patients
with cardiovascular and related diseases. J Med 1978; 9: 6779.
35. Van Gaal L., De Leeuw I., Vadhanavikit S., Folkers K. Exploratory
study of coenzyme Q10 in obesity. In: Biomedical and Clinical
Aspects of Coenzyme Q, Vol. 4. Amsterdam: Elsevier,
1984: 369374.
36. Stanko R. T., Mendelow H., Shinozuka H., Adibi S. A. Prevention
of alcohol-induced fatty liver by natural metabolites and
riboflavin. J Lab Clin Med 1978; 91: 228235.
37. Wirtshafter D., Davis J. D. Body weight: reduction by long-term
glycerol treatment. Science 1977; 198: 12711274.
38. Leibel R. L., Drewnowski A., Hirsch J. Effect of glycerol on
weight loss and hunger in obese patients. Metabolism 1980;
29: 12341236.
39. Bjrvell H., Hylander B., Rssner S. Effects of glycerol addition to
diet in weight-reducing clubs. Int J Obesity 1984; 8: 129133.
40. Argaud D., Roth H., Weirnsperger N., Leverve X. M. Metformin
decreases gluconeogenesis by enhancing the pyruvate kinase
flux in isolated rat hepatocytes . Eur J Biochem 1993;
213: 13411348 .
41. McCarty M. F. A proposal for the locus of metformins clinical
action potentiation of the activation of pyruvate kinase by
fructose-1,6-diphosphate. Med Hypotheses 1997; 52: 8993.
42. Clarke B. F., Duncan L. J. P. Comparison of chlorpropamide and
metformin treatment on weight and blood-glucose response of
uncontrolled obese diabetics. Lancet 1968; i: 123126.
43. Clarke B. F., Campbell I. W. Comparison of metformin and
chlorpropamide in non-obese, maturity-onset diabetics
uncontrolled by diet. Br Med J 1977; 2: 15671578.
44. Stumvoll M., Nurjhan N., Perriello G. et al. Metabolic effects of
metformin in non-insulin-dependent diabetes mellitus. N Engl J
Med 1995; 333: 550554.
45. Lee A., Bray G. A. Metformin decreases food consumption in
obese non-insulin-dependent (NIDDM) diabetics. Diabetes 1996;
45(Suppl 2): 170A.
46. Williamson J. R., Browning E. T., Olson M. S. Interrelations
between fatty acid oxidation and the control of gluconeogenesis
in perfused rat liver. Adv Enzyme Reg 1968; 6: 67100.
47. Ferrannini E., Barret E. J., Bevilacqua S., DeFronzo R. A. Effect of
fatty acids on glucose production and utilization in man. J Clin
Invest 1983; 72: 17371747.
48. Rebrin K., Steil G. M., Mittelman S. D., Bergman R. N. Causal
linkage between insulin suppression of lipolysis and
suppression of liver glucose output in dogs. J Clin Invest 1996;
98: 741749.
49. Jackson R. A., Hawa M. I., Haspan J. B. et al. Mechanism of
metformin action in non-insulin-dependent diabetes. Diabetes
1987; 36: 632640
50. Shepherd M., Kushwaha R. Effect of metformin on basal and
postprandial lipid and carbohydrate metabolism in NIDDM
subjects. Diabetes 1994; 43 (Suppl 1): 74A.
51. Cusi K., Consoli A., DeFronzo R. A. Metabolic effects of
metformin on glucose and lactate metabolism in non-insulin-
dependent diabetes mellitus. J Clin Endocrinol Metab 1996;
81: 40594067.
52. Dakshinamurti K., Cheah-Tan C. Biotin-mediated synthesis of
hepatic glucokinase in the rat. Arch Biochem Biophys 1968;
127: 1721.
53. Editorial. Biotin and glucokinase in the diabetic rat. Nutr Rev
1970; 28: 242244.
54. Chauhan J., Dakshinamurti K. Transcriptional regulation of the
glucokinase gene by biotin in starved rats. J Biol Chem 1991;
266: 1003510038.
55. Borboni P., Magnaterra R., Rabini R. A. et al. Effect of biotin on
glucokinase activity, mRNA expression and insulin release in
cultured beta-cells. Acta Diabetol 1996; 33: 154158.
56. Zhang H., Osada K., Furukawa Y. Biotin administration
improves the impaired glucose tolerance of streptozotocin-
induced diabetic Wistar rats. 16th International Congress of
Nutrition, Montreal, 1997. Abstract book, p. 264.
57. Vesely D. L. Biotin enhances guanylate cyclase activity. Science
1982; 216: 13291330.
58. Spence J. T., Koudelka A. P. Effects of biotin upon the
intracellular level of cGMP and the activity of glucokinase in
cultured rat hepatocytes. J Biol Chem 1984; 259: 63636396.
59. Felu J. E., Hue L., Hers H.-G. Hormonal control of pyruvate
kinase activity and of gluconeogenesis in isolated hepatocytes.
Proc Natl Acad Sci 1976; 73: 27622766.
60. Ljungstrm O., Berglund L., Engstrm L. Studies on the kinetic
effects of adenosine-3:5-monophosphate-dependent
phosphorylation of purified pig-liver pyruvate kinase type L.
Eur J Biochem 1976; 68: 497506.
61. Hue L. The role of futile cycles in the regulation of carbohydrate
metabolism in the liver. Adv Enzymol 1981; 52: 247331.
62. Vaulont S., Kahn A. Transcriptional control of metabolic
regulation genes by carbohydrates. FASEB J 1994; 8: 2835.
63. Matschinsky F. M. A lesson in metabolic regulation inspired
by the glucokinase glucose sensor paradigm. Diabetes 1996;
45: 223241.
64. Zhang H., Osada K., Maebashi M. et al. A high biotin diet
improves the impaired glucose tolerance of long-term
spontaneously hyperglycemic rats with non-insulin-dependent
diabetes mellitus. J Nutr Sci Vitaminol 1996; 42: 517526.
65. Maebashi M., Makino Y., Furukawa Y. et al. Therapeutic
Pyruvate, HCA and carnitine in uncoupling the oxidation of FFAs 415
1999 Harcourt Brace & Co. Ltd Medical Hypotheses (1999) 52(5), 407416
evaluation of the effect of biotin on hyperglycemia in patients
with non-insulin dependent diabetes mellitus. J Clin Biochem
Nutr 1993; 14: 211218.
66. Mabrouk G. M., Helmy I. M., Thampy K. G., Wakil S. J. Acute
hormonal control of acetyl-coA carboxylase. J Biol Chem 1990;
265: 63306338.
67. Pgorier J.-P., Garcia-Garcia M.-V., Prip-Buus C. et al. Induction
of ketogenesis and fatty acid oxidation by glucagon and cyclic
AMP in cultured hepatocytes from rabbit fetuses. Biochem J
1989; 264: 93100.
68. Agius L., Alberti G. M. M. Regulation of flux through pyruvate
dehydrogenase and pyruvate carboxylase in rat hepatocytes.
Eur J Biochem 1985; 152: 699707.
69. Papa S., Paradies G. On the mechanism of translocation of
pyruvate and other monocarboxylic acids in rat-liver
mitochondria. Eur J Biochem 1974; 49: 265274.
70. Yamazaki R. K. Glucagon stimulation of mitochondrial
respiration. J Biol Chem 1975; 250: 79247930.
71. Halestrap A. P. Glucagon treatment of rats activates the
respiratory chain of liver mitochondria at more than one site.
Biochim Biophys Acta 1987; 927: 280290.
72. Nair K. S. Hyperglucagonemia increases resting metabolic rate
in man during insulin deficiency. J Clin Endocrinol 1987;
64: 896901.
73. Stanko R. T., Robertson R. J., Spina R. J. et al. Enhancement of
arm exercise endurance capacity with dihydroxyacetone and
pyruvate. J Appl Physiol 1990; 68: 119124.
74. Stanko R. T., Robertson R. J., Galbreath R. W. et al. Enhanced leg
exercise endurance with a high-carbohydrate diet and
dihydroxyacetone and pyruvate. J Appl Physiol 1990;
69: 16511656.
75. Stanko R. T., Reynolds H. R., Hoyson R. et al. Pyruvate
supplementation of a low-fat diet: effects on plasma lipid
concentrations and body composition in hyperlipidemic
patients. Am J Clin Nutr 1994; 59: 423427.
76. Kaats G. R., Pullin D., Parker L. K. et al. Reductions of body fat as
a function of taking a dietary supplement containing garcinia
cambogia extract, chromium picolinate & l-carnitine: a double-
blind placebo controlled study. Oral presentation, 3rd
International Conference on Anti-Aging Medicine and
Biomedical Technology, Las Vegas, 1995.
77. Fujioka S., Matsuzawa Y., Tokunaga K. et al. Improvement of
glucose and lipid metabolism associated with selective
reduction of intra-abdominal visceral fat in premnopausal
women with visceral fat obesity. Int J Obesity 1991; 15: 853859.
78. Zamboni M., Armellini F., Turcato E. et al. Effect of weight loss
on regional body fat distribution in premenopausal women. Am
J Clin Nutr 1993; 58: 2934.
79. Ross R., Rissanen J. Mobilization of visceral and subcutaneous
adipose tissue in response to energy restriction and exercise.
Am J Clin Nutr 1994; 60: 695703.
80. Buemann B., Tremblay A. Effects of exercise training on
abdominal obesity and related metabolic complication. Sports
Med 1996; 21: 191212.
81. Bjrntorp P. Portal adipose tissue as a generator of risk factors
for cardiovascular disease and diabetes. Arteriosclerosis 1990;
10: 493496.
82. Bjrntorp P. Metabolic implications of body fat distribution.
Diab Care 1991; 14: 11321143.
83. McCarty M. F. Reduction of free fatty acids may ameliorate risk
factors associated with abdominal obesity. Med Hypotheses
1995; 44: 278286.
84. Paolisso G., Tataranni P. A., Foley J. E. et al. A high concentration
of fasting plasma non-esterified fatty acids is a risk factor for the
development of NIDDM. Diabetologia 1995; 38: 12131217.
85. Boden G. Role of fatty acids in the pathogenesis of insulin
resistance and NIDDM. Diabetes 1996; 45: 310.
86. Broomfield P. H., Chopra R., Sheinbaum R. C. et al. Effects of
ursodeoxycholic acid and aspirin on the formation of lithogenic
bile and gallstones during loss of weight. N Engl J Med 1988;
319: 15671572.
87. Liddle R. A., Goldstein R. B., Saxton J. Gallstone formation
during weight-reduction dieting. Arch Intern Med 1989;
149: 17501753.
88. Yang H., Petersen G. M., Roth M.-P. et al. Risk factors for
gallstone formation during rapid loss of weight. Dig Dis Sci
1992; 37: 912918.
89. Hoy M. K., Heshka S., Allison D. B. et al. Reduced risk of liver-
function-test abnormalities and new gallstone formation with
weight loss on 3350-kJ (800-kcal) formula diets. Am J Clin Nutr
1994; 60: 249254.
90. Wattchow D. A., Hall J. C., Whiting M. J. et al. Prevalence and
treatment of gallstones after gastric bypass surgery for morbid
obesity. Br Med J 1983; 286: 763.
91. Messing B., Bories C., Kunstlinger F., Bernier J.-J. Does total
parenteral nutrition induce gallbladder sludge formation and
lithiasis? Gastroenterology 1983; 84: 10121019.
92. Conway J. M., Norris K. H., Bodwell C. E. A new approach for the
estimation of body composition: infrared interactance. Am J Clin
Nutr 1984; 40: 11231130.
93. Elia M., Parkinson S. A., Diaz E. Evaluation of near infra-red
interactance as a method for predicting body composition. Eur J
Clin Nutr 1990; 44: 113121.
94. Kaats G. R., Blum K., Fisher J. A., Adelman J. A. Effects of
chromium picolinate supplementation on body composition: a
randomized, double-masked, placebo-controlled study. Curr
Ther Res 1996; 57: 747756.
95. Kaats G. R. Manuscript in submission, 1997.
96. Cheifetz P. N. Uric acid excretion and ketosis in fasting.
Metabolism 1965; 14: 12671272.
97. Runcie J., Thomson T. J. Total fasting, hyperuricaemia and gout.
Postgrad Med J 1969; 45: 251253.
416 McCarty and Gustin
Medical Hypotheses (1999) 52(5), 407416 1999 Harcourt Brace & Co. Ltd
Você também pode gostar
- Allowed Food ListDocumento14 páginasAllowed Food Listfreed1100% (4)
- Biochem CH 27 Integration of MetabolismDocumento6 páginasBiochem CH 27 Integration of MetabolismSchat ZiAinda não há avaliações
- Biblioteca Sagrado FemininoDocumento4 páginasBiblioteca Sagrado FemininoIsabel Angelica0% (1)
- HW-NUR8103 Lecture - Carbohydrate Metabolism IDocumento3 páginasHW-NUR8103 Lecture - Carbohydrate Metabolism IyanAinda não há avaliações
- GlycolysisDocumento7 páginasGlycolysisDoyen DanielAinda não há avaliações
- Medical Biochemistry (Week-15)Documento5 páginasMedical Biochemistry (Week-15)wasimsafdarAinda não há avaliações
- Biological ShuttlesDocumento4 páginasBiological ShuttlesJoseph YayenAinda não há avaliações
- Kitar KrebsDocumento5 páginasKitar KrebsAlfonso RobertAinda não há avaliações
- Ynah (Genbio)Documento3 páginasYnah (Genbio)nathaniel alcantaraAinda não há avaliações
- Presentation Carbohydrate MetabolismDocumento33 páginasPresentation Carbohydrate MetabolismMắt BétAinda não há avaliações
- Werner 2016Documento17 páginasWerner 2016SILVA SINTIAAinda não há avaliações
- 1. Chuyển hóa carbohydrate (metabolism of carbohydrates) : GlycolysisDocumento20 páginas1. Chuyển hóa carbohydrate (metabolism of carbohydrates) : GlycolysisAnh Tuyet NguyenAinda não há avaliações
- The Krebs Cycle - Harnessing Chemical Energy For Cellular RespirationDocumento4 páginasThe Krebs Cycle - Harnessing Chemical Energy For Cellular RespirationTami AbordoAinda não há avaliações
- LO5Documento2 páginasLO5Yo TuAinda não há avaliações
- Principles QuestionsDocumento20 páginasPrinciples QuestionsGlupiaSprawaAinda não há avaliações
- Bsa-Cs 1A: Pineda, Rhoniel DDocumento4 páginasBsa-Cs 1A: Pineda, Rhoniel DRhoniel PinedaAinda não há avaliações
- BioChem Metabolism2Documento4 páginasBioChem Metabolism2Shecana Rose CincoAinda não há avaliações
- Step 1: Generating A Proton Motive ForceDocumento22 páginasStep 1: Generating A Proton Motive Forcemuhammad sami ullah khanAinda não há avaliações
- GlycolysisDocumento7 páginasGlycolysiscutegal88Ainda não há avaliações
- Veech Therapeutic Implications of Ketone BodiesDocumento11 páginasVeech Therapeutic Implications of Ketone BodiesmreadesAinda não há avaliações
- Only A Small Amount of Energy Available in Glucose Is Captured in Glycolysis Cellular RespirationDocumento9 páginasOnly A Small Amount of Energy Available in Glucose Is Captured in Glycolysis Cellular RespirationYousif KashatAinda não há avaliações
- The Krebs CycleDocumento5 páginasThe Krebs CycleCarlo CondeAinda não há avaliações
- Fermentation: Steps in The Citric Acid CycleDocumento5 páginasFermentation: Steps in The Citric Acid CycleDenise TuazonAinda não há avaliações
- Assignment Number: 1Documento10 páginasAssignment Number: 1Palesa NtsekalleAinda não há avaliações
- Aerobic and Anaerobic RespirationDocumento7 páginasAerobic and Anaerobic RespirationFatmata Haja KamaraAinda não há avaliações
- Biochem GlycolysisDocumento7 páginasBiochem Glycolysisnabilahuda92Ainda não há avaliações
- Carbohydrate MetabolismDocumento15 páginasCarbohydrate MetabolismPRIYANSHU KAUSHALAinda não há avaliações
- CHAPTER 19 Tricarboxylic Acid CycleDocumento11 páginasCHAPTER 19 Tricarboxylic Acid Cycle楊畯凱Ainda não há avaliações
- Echevarria Jonille S. BSP 2Documento4 páginasEchevarria Jonille S. BSP 2Jonille EchevarriaAinda não há avaliações
- Sumalpong - Special AssignmentDocumento10 páginasSumalpong - Special AssignmentJEROME JAY SUMALPONGAinda não há avaliações
- Ketogenic Diet - A New Light Shining On Old But Gold ChemistryDocumento22 páginasKetogenic Diet - A New Light Shining On Old But Gold ChemistryMateo PeychauxAinda não há avaliações
- Bio Lec 2Documento14 páginasBio Lec 2Yousef SabaAinda não há avaliações
- The Krebs CycleDocumento10 páginasThe Krebs CycleHenry MafuaAinda não há avaliações
- Scan Doc0001Documento23 páginasScan Doc0001Nichola TappinAinda não há avaliações
- Some Carbon CycleDocumento35 páginasSome Carbon CycleJay CalAinda não há avaliações
- ATP Synthesis and Storage: Original ArticleDocumento15 páginasATP Synthesis and Storage: Original ArticleRiley RilanAinda não há avaliações
- Beta OxidationDocumento16 páginasBeta Oxidationharshitjajoo0215Ainda não há avaliações
- 2023 BCH313 Gluconeogenesis and Synthesis of Complex Carbohydrates NotesDocumento9 páginas2023 BCH313 Gluconeogenesis and Synthesis of Complex Carbohydrates NotesNOLUBABALOAinda não há avaliações
- Chem 1223Documento5 páginasChem 1223Ramil LucasAinda não há avaliações
- Cell RespirationDocumento29 páginasCell RespirationJohn OsborneAinda não há avaliações
- BiochemDocumento10 páginasBiochemHoàng LâmAinda não há avaliações
- RESPIRATION PROCESS and ShuttleDocumento10 páginasRESPIRATION PROCESS and ShuttleMichael NyaongoAinda não há avaliações
- Handout Week 3 Cell Respiration For StudDocumento4 páginasHandout Week 3 Cell Respiration For StudJhamilla AdajarAinda não há avaliações
- Is The Process by Which Chemical Energy in OrganicDocumento6 páginasIs The Process by Which Chemical Energy in Organicaiqi2712Ainda não há avaliações
- Glycolysis and TCA CycleDocumento38 páginasGlycolysis and TCA CycleAboubakar Moalim Mahad moh'dAinda não há avaliações
- Practical Session of Biokimia (2020)Documento6 páginasPractical Session of Biokimia (2020)tekatekikompreAinda não há avaliações
- მიტოქონდრიის ფუნქცია და კიბოDocumento1 páginaმიტოქონდრიის ფუნქცია და კიბოEMD GROUPAinda não há avaliações
- Chapter 8 Biochemistry and Clinical Pathology Complete Notes by Noteskarts Acc To ER20Documento41 páginasChapter 8 Biochemistry and Clinical Pathology Complete Notes by Noteskarts Acc To ER20prat.medbooksAinda não há avaliações
- CHAPTER 23 Fatty Acid CatabolismDocumento9 páginasCHAPTER 23 Fatty Acid Catabolism楊畯凱Ainda não há avaliações
- Hexose Monophosphate Pathway PathwayDocumento3 páginasHexose Monophosphate Pathway PathwayShiva100% (6)
- Cell RespirationDocumento29 páginasCell RespirationJohn OsborneAinda não há avaliações
- Gluconeogenesis - IntroductionDocumento29 páginasGluconeogenesis - IntroductionSaswat MohapatraAinda não há avaliações
- Generation of ATP From Glucose GlycolysisDocumento84 páginasGeneration of ATP From Glucose GlycolysisMohamed MaestroAinda não há avaliações
- The Metabolic Advantage of Tumor CellsDocumento12 páginasThe Metabolic Advantage of Tumor CellsmrsilvAinda não há avaliações
- Beta Oxidación de Ácidos GrasosDocumento10 páginasBeta Oxidación de Ácidos GrasosLeonardo RestrepoAinda não há avaliações
- Cellular Respiration HandoutsDocumento5 páginasCellular Respiration HandoutsivyAinda não há avaliações
- Module 3C - Cellular RespirationDocumento9 páginasModule 3C - Cellular RespirationDiane Balaba OsingAinda não há avaliações
- The Ri Arboxylic Cid (TCA) Cycle (Citric Acid Cycle) (Krebs Cycle)Documento22 páginasThe Ri Arboxylic Cid (TCA) Cycle (Citric Acid Cycle) (Krebs Cycle)Kiya AlemuAinda não há avaliações
- MetabolismeDocumento31 páginasMetabolismeLalu Aldi PratamaAinda não há avaliações
- Different Types of Transporters in MitochondriaDocumento15 páginasDifferent Types of Transporters in MitochondriaAnkush YadavAinda não há avaliações
- Citric Acid Cycle-With Quiz BiochemistryDocumento24 páginasCitric Acid Cycle-With Quiz BiochemistrySteph VeeAinda não há avaliações
- Mechanisms and Regulation of Carbohydrate Transport in BacteriaNo EverandMechanisms and Regulation of Carbohydrate Transport in BacteriaAinda não há avaliações
- COPD Case Study 1Documento15 páginasCOPD Case Study 1ifrah tariqAinda não há avaliações
- Aims and Objectives of Cooking FoodDocumento6 páginasAims and Objectives of Cooking FoodShovan HazraAinda não há avaliações
- Chemistry Project On Analysis of Vegetable and Fruit JuicesDocumento13 páginasChemistry Project On Analysis of Vegetable and Fruit JuicesAnuj Chaudhary100% (1)
- 8ac - Balanced Diet 2Documento17 páginas8ac - Balanced Diet 2trisAinda não há avaliações
- Report Card On Access To Obesity Treatment For Adults in CanadaDocumento38 páginasReport Card On Access To Obesity Treatment For Adults in CanadaCityNewsToronto0% (1)
- Selection and Formulation of Balance DietDocumento10 páginasSelection and Formulation of Balance DietNrs Sani Sule MashiAinda não há avaliações
- Food & Vitamin Related Health Relaterd Articles - Vol 1Documento517 páginasFood & Vitamin Related Health Relaterd Articles - Vol 1FW040100% (1)
- Effect of Yogic Practice On Selected Biochemical Variables Among Obese Middle Age School BoysDocumento4 páginasEffect of Yogic Practice On Selected Biochemical Variables Among Obese Middle Age School Boysgovindasamy100% (1)
- Pol Scie PPT FinalDocumento33 páginasPol Scie PPT FinalVenz LacreAinda não há avaliações
- BMI CalculatorDocumento76 páginasBMI CalculatorNhil Cabillon QuietaAinda não há avaliações
- BCH301Documento19 páginasBCH301yabdallahAinda não há avaliações
- Healthy Mass 4000Documento99 páginasHealthy Mass 4000Alex Hladun50% (6)
- Conditioning 2Documento8 páginasConditioning 2Rejanath Suarial100% (2)
- Anatomy Practice Test 1Documento25 páginasAnatomy Practice Test 1Estevan MartinezAinda não há avaliações
- Feed Your Flora: How To Promote Healthy Gut Bacteria: Steven Lalevich, RDDocumento16 páginasFeed Your Flora: How To Promote Healthy Gut Bacteria: Steven Lalevich, RDDraganMilenkovićAinda não há avaliações
- AirWaterLifeBottledWaterORP PH ComparisonChartDocumento1 páginaAirWaterLifeBottledWaterORP PH ComparisonChartDumitru ScortanuAinda não há avaliações
- Bài tập ôn thi giữa học kỳ 1 - Tiếng Anh 7Documento3 páginasBài tập ôn thi giữa học kỳ 1 - Tiếng Anh 7Hằng Nguyễn ThịAinda não há avaliações
- Reading Part A AppendicitisDocumento6 páginasReading Part A Appendicitisfernanda1rondelli0% (2)
- UNILEVER Green ManufacturingDocumento20 páginasUNILEVER Green ManufacturingLavina MehtaAinda não há avaliações
- Acidosis Alkalosis BiochemistryDocumento28 páginasAcidosis Alkalosis BiochemistryDocSam048Ainda não há avaliações
- Cinderella Solution ReviewDocumento2 páginasCinderella Solution Reviewkumar sk50% (2)
- Olive Oil As A Functional FoodDocumento19 páginasOlive Oil As A Functional FoodRadwan Ajo100% (1)
- Vera Simovska, MD., PHD, SummaryDocumento4 páginasVera Simovska, MD., PHD, SummaryAss. Prof. Vera Simovska, MD., PhD.Ainda não há avaliações
- Post Exercise Ingestion of Carbohydrate Protein and WaterDocumento30 páginasPost Exercise Ingestion of Carbohydrate Protein and WaterCarolina López ValenciaAinda não há avaliações
- Dietary Reference IntakeDocumento7 páginasDietary Reference IntakePolene AfableAinda não há avaliações
- Pathophysiology of Hypertensive Cardiovascular Disease: Chest Pain Muscle Weakness Shortness of BreathDocumento2 páginasPathophysiology of Hypertensive Cardiovascular Disease: Chest Pain Muscle Weakness Shortness of BreathCyril Jane Caanyagan AcutAinda não há avaliações
- Reverse DietingDocumento3 páginasReverse DietingAndrea CsatlósAinda não há avaliações
- Project Task: Context and Conditions of AssessmentDocumento15 páginasProject Task: Context and Conditions of AssessmentSUKH100% (1)