Escolar Documentos
Profissional Documentos
Cultura Documentos
ATOMIC WEIGHTS OF THE ELEMENTS 2013
Enviado por
akvssakthivel0 notas0% acharam este documento útil (0 voto)
24 visualizações8 páginasAtomic Weights
Título original
2011 Atomic Weights
Direitos autorais
© © All Rights Reserved
Formatos disponíveis
PDF, TXT ou leia online no Scribd
Compartilhar este documento
Compartilhar ou incorporar documento
Você considera este documento útil?
Este conteúdo é inapropriado?
Denunciar este documentoAtomic Weights
Direitos autorais:
© All Rights Reserved
Formatos disponíveis
Baixe no formato PDF, TXT ou leia online no Scribd
0 notas0% acharam este documento útil (0 voto)
24 visualizações8 páginasATOMIC WEIGHTS OF THE ELEMENTS 2013
Enviado por
akvssakthivelAtomic Weights
Direitos autorais:
© All Rights Reserved
Formatos disponíveis
Baixe no formato PDF, TXT ou leia online no Scribd
Você está na página 1de 8
IUPAC Commission on Isotopic Abundances and Atomic Weights.
ATOMIC WEIGHTS OF THE ELEMENTS
2013
These tables are based on the 2011 table at Pure Appl. Chem., 2013, 85, 1047-1078 with changes from for
the values of aluminium, arsenic, beryllium, cadmium, caesium, cobalt, fluorine, gold, holmium, manganese,
molybdenum, niobium, phosphorus, praseodymium, scandium, selenium, thorium, thulium and yttrium [see ciaaw
or IUPAC website or Chem. Int. 2013, 35(6), 17-18].
http://www.chem.qmul.ac.uk/iupac/AtWt/
A PDF format copy (1647 KBt) of the 2011 table is available from the abstract
World Wide Web version of atomic weight data originally prepared by G. P. Moss, from a file provided by D.
R. Lide.
Previous values may be consulted from the 1993 table, the 1995 table, the 1997 table, the 1999 table, the 2001
table, the 2005 table, the 2007 table, the 2009 table or the 2011 table.
The standard atomic weights of twelve elements having two or more stable isotopes have variability of atomic-
weight values in natural terrestrial materials. These are given in table 1 below. In the other lists the values quoted
are those suggested for material where the origin of the sample is unknown. For radioactive elements the isotope
with the longest half-life is quoted in parenthesis. The original paper should be consulted for full details of the
variation in atomic weight and the half life of the radioisotopes quoted below.
A number in parentheses indicates the uncertainty in the last digit of the atomic weight.
See below for the elements listed in Atomic Number Order or Name order.
See also a copy of the periodic table with atomic weights to five significant figures.
Table 1. List of Elements with Range of Atomic Weights.
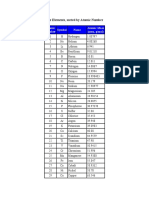
At No Symbol Name Minimum Atomic Wt Maximum Atomic Wt
1 H hydrogen 1.007 84 1.008 11
3 Li lithium 6.938 6.997
5 B boron 10.806 10.821
6 C carbon 12.0096 12.0116
7 N nitrogen 14.006 43 14.007 28
8 O oxygen 15.999 03 15.999 77
12 Mg magnesium 24.30 24.31
14 Si silicon 28.084 28.086
16 S sulfur 32.059 32.076
17 Cl chlorine 35.446 35.457
35 Br bromine 79.90 79.91
81 Tl thallium 204.382 204.385
See original paper for the range of these elements from different sources.
Table 2. List of Elements in Atomic Number Order.
At No Symbol Name Atomic Wt Notes
1 H Hydrogen 1.008 3, 6
2 He Helium 4.002602(2) 1, 2
3 Li Lithium 6.94 3, 6
4 Be Beryllium 9.0121831(5)
5 B Boron 10.81 3, 6
6 C Carbon 12.011 6
7 N Nitrogen 14.007 6
8 O Oxygen 15.999 6
9 F Fluorine 18.998403163(6)
10 Ne Neon 20.1797(6) 1, 3
11 Na Sodium 22.98976928(2)
12 Mg Magnesium 24.305 6
13 Al Aluminium 26.9815385(7)
14 Si Silicon 28.085 6
15 P Phosphorus 30.973761998(5)
16 S Sulfur 32.06 6
17 Cl Chlorine 35.45 3, 6
18 Ar Argon 39.948(1) 1, 2
19 K Potassium 39.0983(1)
20 Ca Calcium 40.078(4)
21 Sc Scandium 44.955908(5)
22 Ti Titanium 47.867(1)
23 V Vanadium 50.9415(1)
24 Cr Chromium 51.9961(6)
25 Mn Manganese 54.938044(3)
26 Fe Iron 55.845(2)
27 Co Cobalt 58.933194(4)
28 Ni Nickel 58.6934(4) 2
29 Cu Copper 63.546(3) 2
30 Zn Zinc 65.38(2) 2
31 Ga Gallium 69.723(1)
32 Ge Germanium 72.630(8)
33 As Arsenic 74.921595(6)
34 Se Selenium 78.971(8)
35 Br Bromine 79.904 6
36 Kr Krypton 83.798(2) 1, 3
37 Rb Rubidium 85.4678(3) 1
38 Sr Strontium 87.62(1) 1, 2
39 Y Yttrium 88.90584(2)
40 Zr Zirconium 91.224(2) 1
41 Nb Niobium 92.90637(2)
42 Mo Molybdenum 95.95(1) 1
43 Tc Technetium [97] 4
44 Ru Ruthenium 101.07(2) 1
45 Rh Rhodium 102.90550(2)
46 Pd Palladium 106.42(1) 1
47 Ag Silver 107.8682(2) 1
48 Cd Cadmium 112.414(4) 1
49 In Indium 114.818(1)
50 Sn Tin 118.710(7) 1
51 Sb Antimony 121.760(1) 1
52 Te Tellurium 127.60(3) 1
53 I Iodine 126.90447(3)
54 Xe Xenon 131.293(6) 1, 3
55 Cs Caesium 132.90545196(6)
56 Ba Barium 137.327(7)
57 La Lanthanum 138.90547(7) 1
58 Ce Cerium 140.116(1) 1
59 Pr Praseodymium 140.90766(2)
60 Nd Neodymium 144.242(3) 1
61 Pm Promethium [145] 5
62 Sm Samarium 150.36(2) 1
63 Eu Europium 151.964(1) 1
64 Gd Gadolinium 157.25(3) 1
65 Tb Terbium 158.92535(2)
66 Dy Dysprosium 162.500(1) 1
67 Ho Holmium 164.93033(2)
68 Er Erbium 167.259(3) 1
69 Tm Thulium 168.93422(2)
70 Yb Ytterbium 173.054(5) 1
71 Lu Lutetium 174.9668(1) 1
72 Hf Hafnium 178.49(2)
73 Ta Tantalum 180.94788(2)
74 W Tungsten 183.84(1)
75 Re Rhenium 186.207(1)
76 Os Osmium 190.23(3) 1
77 Ir Iridium 192.217(3)
78 Pt Platinum 195.084(9)
79 Au Gold 196.966569(5)
80 Hg Mercury 200.592(3)
81 Tl Thallium 204.38 6
82 Pb Lead 207.2(1) 1, 2
83 Bi Bismuth 208.98040(1)
84 Po Polonium [209] 4
85 At Astatine [210] 4
86 Rn Radon [222] 4
87 Fr Francium [223] 4
88 Ra Radium [226] 4
89 Ac Actinium [227] 4
90 Th Thorium 232.0377(4) 1, 4
91 Pa Protactinium 231.03588(2) 4
92 U Uranium 238.02891(3) 1, 3, 4
93 Np Neptunium [237] 4
94 Pu Plutonium [244] 4
95 Am Americium [243] 4
96 Cm Curium [247] 4
97 Bk Berkelium [247] 4
98 Cf Californium [251] 4
99 Es Einsteinium [252] 4
100 Fm Fermium [257] 4
101 Md Mendelevium [258] 4
102 No Nobelium [259] 4
103 Lr Lawrencium [262] 4
104 Rf Rutherfordium [267] 4
105 Db Dubnium [270] 4
106 Sg Seaborgium [271] 4
107 Bh Bohrium [270] 4
108 Hs Hassium [277] 4
109 Mt Meitnerium [276] 4
110 Ds Darmstadtium [281] 4
111 Rg Roentgenium [282] 4
112 Cn Copernicium [285] 4
113 Uut Ununtrium [285] 4, 5
114 Fl Flerovium [289] 4, 5
115 Uup Ununpentium [2889] 4, 5
116 Lv Livermorium [293] 4, 5
117 Uus Ununseptium [294] 4, 5
118 Uuo Ununoctium [294] 4, 5
1. Geological specimens are known in which the element has an isotopic composition outside the limits for
normal material. The difference between the atomic weight of the element in such specimens and that given
in the Table may exceed the stated uncertainty.
2. Range in isotopic composition of normal terrestrial material prevents a more precise value being given; the
tabulated value should be applicable to any normal material.
3. Modified isotopic compositions may be found in commercially available material because it has been
subject to an undisclosed or inadvertant isotopic fractionation. Substantial deviations in atomic weight of
the element from that given in the Table can occur.
4. Element has no stable nuclides. The value enclosed in brackets, e.g. [209], indicates the mass number of
the longest-lived isotope of the element. However three such elements (Th, Pa, and U) do have a
characteristic terrestrial isotopic composition, and for these an atomic weight is tabulated.
5. The names and symbols for elements 113, 115, 117 and 118 are under review. The temporary system
recommended by J Chatt, Pure Appl. Chem., 1979, 51, 381-384 is used above.
6. See table 1 for details of range and original paper for the atomic weight of the element from different
sources.
Table 3. List of Elements in Name Order.
At No Symbol Name Atomic Wt Notes
89 Ac Actinium [227] 4
13 Al Aluminium 26.9815385(7)
95 Am Americium [243] 4
51 Sb Antimony 121.760(1) 1
18 Ar Argon 39.948(1) 1, 2
33 As Arsenic 74.921595(6)
85 At Astatine [210] 4
56 Ba Barium 137.327(7)
97 Bk Berkelium [247] 4
4 Be Beryllium 9.0121831(5)
83 Bi Bismuth 208.98040(1)
107 Bh Bohrium [270] 4
5 B Boron 10.81 3, 6
35 Br Bromine 79.904 6
48 Cd Cadmium 112.414(4) 1
55 Cs Caesium 132.90545196(6)
20 Ca Calcium 40.078(4) 1
98 Cf Californium [251] 4
6 C Carbon 12.011 6
58 Ce Cerium 140.116(1) 1
17 Cl Chlorine 35.45 3, 6
24 Cr Chromium 51.9961(6)
27 Co Cobalt 58.933194(4)
112 Cn Copernicium [285] 4
29 Cu Copper 63.546(3) 2
96 Cm Curium [247] 4
110 Ds Darmstadtium [281] 4
105 Db Dubnium [270] 4
66 Dy Dysprosium 162.500(1) 1
99 Es Einsteinium [252] 4
68 Er Erbium 167.259(3) 1
63 Eu Europium 151.964(1) 1
100 Fm Fermium [257] 4
114 Fl Flerovium [289] 4, 5
9 F Fluorine 18.998403163(6)
87 Fr Francium [223] 4
64 Gd Gadolinium 157.25(3) 1
31 Ga Gallium 69.723(1)
32 Ge Germanium 72.630(8)
79 Au Gold 196.966569(5)
72 Hf Hafnium 178.49(2)
108 Hs Hassium [277] 4
2 He Helium 4.002602(2) 1, 2
67 Ho Holmium 164.93033(2)
1 H Hydrogen 1.008 3, 6
49 In Indium 114.818(1)
53 I Iodine 126.90447(3)
77 Ir Iridium 192.217(3)
26 Fe Iron 55.845(2)
36 Kr Krypton 83.798(2) 1, 3
57 La Lanthanum 138.90547(7) 1
103 Lr Lawrencium [262] 4
82 Pb Lead 207.2(1) 1, 2
3 Li Lithium 6.94 3, 6
116 Lv Livermorium [293] 4, 5
71 Lu Lutetium 174.9668(1) 1
12 Mg Magnesium 24.305 6
25 Mn Manganese 54.938044(3)
109 Mt Meitnerium [276] 4
101 Md Mendelevium [258] 4
80 Hg Mercury 200.592(3)
42 Mo Molybdenum 95.95(1) 1
60 Nd Neodymium 144.242(3) 1
10 Ne Neon 20.1797(6) 1, 3
93 Np Neptunium [237] 4
28 Ni Nickel 58.6934(4)
41 Nb Niobium 92.90637(2)
7 N Nitrogen 14.007 6
102 No Nobelium [259] 4
76 Os Osmium 190.23(3) 1
8 O Oxygen 15.999 6
46 Pd Palladium 106.42(1) 1
15 P Phosphorus 30.973761998(5)
78 Pt Platinum 195.084(9)
94 Pu Plutonium [244] 4
84 Po Polonium [209] 4
19 K Potassium 39.0983(1)
59 Pr Praseodymium 140.90766(2)
61 Pm Promethium [145] 4
91 Pa Protactinium 231.03588(2) 4
88 Ra Radium [226] 4
86 Rn Radon [222] 4
75 Re Rhenium 186.207(1)
45 Rh Rhodium 102.90550(2)
111 Rg Roentgenium [282] 4
37 Rb Rubidium 85.4678(3) 1
44 Ru Ruthenium 101.07(2) 1
104 Rf Rutherfordium [267] 4
62 Sm Samarium 150.36(2) 1
21 Sc Scandium 44.955908(5)
106 Sg Seaborgium [271] 4
34 Se Selenium 78.971(8)
14 Si Silicon 28.085 6
47 Ag Silver 107.8682(2) 1
11 Na Sodium 22.98976928(2)
38 Sr Strontium 87.62(1) 1, 2
16 S Sulfur 32.06 6
73 Ta Tantalum 180.94788(2)
43 Tc Technetium [97] 4
52 Te Tellurium 127.60(3) 1
65 Tb Terbium 158.92535(2)
81 Tl Thallium 204.38 6
90 Th Thorium 232.0377(4) 1, 4
69 Tm Thulium 168.93422(2)
50 Sn Tin 118.710(7) 1
22 Ti Titanium 47.867(1)
74 W Tungsten 183.84(1)
118 Uuo Ununoctium [294] 4, 5
115 Uup Ununpentium [289] 4, 5
117 Uus Ununseptium [294] 4, 5
113 Uut Ununtrium [285] 4, 5
92 U Uranium 238.02891(3) 1, 3, 4
23 V Vanadium 50.9415(1)
54 Xe Xenon 131.293(6) 1, 3
70 Yb Ytterbium 173.054(5) 1
39 Y Yttrium 88.90584(2)
30 Zn Zinc 65.38(2) 2
40 Zr Zirconium 91.224(2) 1
1. Geological specimens are known in which the element has an isotopic composition outside the limits for
normal material. The difference between the atomic weight of the element in such specimens and that given
in the Table may exceed the stated uncertainty.
2. Range in isotopic composition of normal terrestrial material prevents a more precise value being given; the
tabulated value should be applicable to any normal material.
3. Modified isotopic compositions may be found in commercially available material because it has been
subject to an undisclosed or inadvertant isotopic fractionation. Substantial deviations in atomic weight of
the element from that given in the Table can occur.
4. Element has no stable nuclides. The value enclosed in brackets, e.g. [209], indicates the mass number of
the longest-lived isotope of the element. However three such elements (Th, Pa, and U) do have a
characteristic terrestrial isotopic composition, and for these an atomic weight is tabulated.
5. The names and symbols for elements 113, 115, 117 and 118 are under review. The temporary system
recommended by J Chatt, Pure Appl. Chem., 1979, 51, 381-384 is used above.
6. See table 1 for details of range and original paper for the atomic weight of the element from different
sources.
Return to IUPAC Chemical Nomenclature home page
Você também pode gostar
- Atomic Weights of The Elements 2009Documento8 páginasAtomic Weights of The Elements 2009Balaram mondalAinda não há avaliações
- Webelements Table 5sf 2012-06-07Documento0 páginaWebelements Table 5sf 2012-06-07api-239300177Ainda não há avaliações
- Tabel Periodik Unsur MaspaDocumento2 páginasTabel Periodik Unsur MaspaAlphonse ElricAinda não há avaliações
- CULLITY, B. STORCK, S. Elements of X-Ray Diffraction. 3. Ed. - Ap. 13. Crystal Structure Data (P. 488-491) PDFDocumento4 páginasCULLITY, B. STORCK, S. Elements of X-Ray Diffraction. 3. Ed. - Ap. 13. Crystal Structure Data (P. 488-491) PDFFernando Freitas AlvesAinda não há avaliações
- 2019 Atomic WeightsDocumento7 páginas2019 Atomic WeightsMirella PopescuAinda não há avaliações
- Periodic Table ColorDocumento1 páginaPeriodic Table ColorHector I. Areizaga MartinezAinda não há avaliações
- Standard Atomic Weights (2001) : Name Symbol Atomic No. Atomic Weight FootnotesDocumento3 páginasStandard Atomic Weights (2001) : Name Symbol Atomic No. Atomic Weight FootnotesilijavujovicAinda não há avaliações
- Student Pocket HandbookDocumento64 páginasStudent Pocket Handbookadarsh_mrAinda não há avaliações
- Atomic WeightsDocumento8 páginasAtomic WeightsSeamus AlaricAinda não há avaliações
- The Periodic TableDocumento7 páginasThe Periodic TableJake Giuseppe PriceAinda não há avaliações
- Periodic Table: ChemistryDocumento1 páginaPeriodic Table: ChemistryRafael RamosAinda não há avaliações
- Elements CHEMISTRY 5Documento27 páginasElements CHEMISTRY 5Nick FullerAinda não há avaliações
- Table A.3: Table of Atomic MassesDocumento10 páginasTable A.3: Table of Atomic MassesPriadiAinda não há avaliações
- SSHK1PERDocumento0 páginaSSHK1PERitsnotUnUnSUlae24Ainda não há avaliações
- List of Periodic Table Elements Sorted by Atomic NumberDocumento3 páginasList of Periodic Table Elements Sorted by Atomic NumberKrishnaMehta100% (2)
- Hydraulic Sulfur Concrete (HSC)Documento57 páginasHydraulic Sulfur Concrete (HSC)gcontechAinda não há avaliações
- ElementsDocumento9 páginasElementsgopuvenkatAinda não há avaliações
- Chemistry Data Booklet Standard Grade and Intermediate 2: © Scottish Qualifications Authority 2007Documento12 páginasChemistry Data Booklet Standard Grade and Intermediate 2: © Scottish Qualifications Authority 2007anilkumarappapurapuAinda não há avaliações
- Periodic Table of the Elements GroupDocumento1 páginaPeriodic Table of the Elements GroupSepehr Masoumi-AlamoutiAinda não há avaliações
- AiCHe Student Pocket Handbook 85Documento63 páginasAiCHe Student Pocket Handbook 85DigitalMastersTXAinda não há avaliações
- Chemistry Formulas - List of Chemistry FormulasDocumento34 páginasChemistry Formulas - List of Chemistry FormulasGirdhar TiwariAinda não há avaliações
- Atomic MassesDocumento5 páginasAtomic MassesJesús CastilloAinda não há avaliações
- 1-Radiation and RadioactivityDocumento20 páginas1-Radiation and Radioactivityعلاء محمدAinda não há avaliações
- Atomic Weights 2013Documento8 páginasAtomic Weights 2013LuisCastilloAinda não há avaliações
- Periodic Table of ElementsDocumento1 páginaPeriodic Table of ElementsCH'NG KIA CHUANAinda não há avaliações
- Elements Numbered in Terms of Atomic NumberDocumento9 páginasElements Numbered in Terms of Atomic NumbergopuvenkatAinda não há avaliações
- IUPAC Periodic Table of The Elements: Ti CRDocumento2 páginasIUPAC Periodic Table of The Elements: Ti CRMarcus LimaAinda não há avaliações
- Elements Arranged in Terms of Atomic NumberDocumento10 páginasElements Arranged in Terms of Atomic NumbergopuvenkatAinda não há avaliações
- Sample Dispersion & RI Guide PDFDocumento54 páginasSample Dispersion & RI Guide PDFcbs78Ainda não há avaliações
- Periodic table elements in Chinese charactersDocumento3 páginasPeriodic table elements in Chinese charactersTheodore HaralabisAinda não há avaliações
- The Elements, Sorted by Atomic Number: Atomic Number Symbol Name Atomic Mass (Amu, G/mol)Documento4 páginasThe Elements, Sorted by Atomic Number: Atomic Number Symbol Name Atomic Mass (Amu, G/mol)Kurt Navales NacarioAinda não há avaliações
- Atomic Number Symbol Name Atomic Weight (Amu, G/mol)Documento5 páginasAtomic Number Symbol Name Atomic Weight (Amu, G/mol)Strata GamingAinda não há avaliações
- Atomic Mass and the Mole ConceptDocumento4 páginasAtomic Mass and the Mole ConceptS.packialakshmiAinda não há avaliações
- CBC Databook 1Documento36 páginasCBC Databook 1anees19oct50% (2)
- Atomic Properties of The Elements TableDocumento1 páginaAtomic Properties of The Elements TableMaahiAinda não há avaliações
- Elements by Atomic MassDocumento4 páginasElements by Atomic MassHaider AliAinda não há avaliações
- VOGEL Química Analítica Qualitativa 7ed PDFDocumento356 páginasVOGEL Química Analítica Qualitativa 7ed PDFMiguel Machado Manhães100% (1)
- IUPAC Periodic Table 19feb2009Documento2 páginasIUPAC Periodic Table 19feb2009blarghghghAinda não há avaliações
- Aqa Chm6x W Ins Jun11Documento2 páginasAqa Chm6x W Ins Jun11Illharm SherrifAinda não há avaliações
- Atomic Name, Atomic Number, Atomic Mass With SymbolDocumento4 páginasAtomic Name, Atomic Number, Atomic Mass With SymbolabhishekAinda não há avaliações
- A Brief Introduction To Polymeric MaterialsDocumento39 páginasA Brief Introduction To Polymeric MaterialsDeepa T PESU CIVILAinda não há avaliações
- IB Chemistry Data Book 2009Documento48 páginasIB Chemistry Data Book 2009phantomdancerAinda não há avaliações
- 1 Appendix A. Properties of The Elements: HHHHHHHHHHHHHHHHHHHHHHHHHHHHHHHHHHHHDocumento4 páginas1 Appendix A. Properties of The Elements: HHHHHHHHHHHHHHHHHHHHHHHHHHHHHHHHHHHHtarek moahmoud khalifaAinda não há avaliações
- Irodov - Problems in Atomic and Nuclear PhysicsDocumento132 páginasIrodov - Problems in Atomic and Nuclear Physicsyomanshivi100% (2)
- Periodic Table of The Elements: Atomic Number SymbolDocumento3 páginasPeriodic Table of The Elements: Atomic Number SymbolmurugangdAinda não há avaliações
- Periodic Table ColorDocumento2 páginasPeriodic Table ColorAnjana LadkaAinda não há avaliações
- Metallabenzenes: An Expert ViewNo EverandMetallabenzenes: An Expert ViewL. James WrightAinda não há avaliações
- Experimental and Theoretical Approaches to Actinide ChemistryNo EverandExperimental and Theoretical Approaches to Actinide ChemistryJohn K. GibsonAinda não há avaliações
- Heterogeneous Catalysis at Nanoscale for Energy ApplicationsNo EverandHeterogeneous Catalysis at Nanoscale for Energy ApplicationsAinda não há avaliações
- Materials Data for Cyclic Loading: Low-Alloy SteelsNo EverandMaterials Data for Cyclic Loading: Low-Alloy SteelsNota: 5 de 5 estrelas5/5 (2)
- Materials Data for Cyclic Loading: Aluminium and Titanium AlloysNo EverandMaterials Data for Cyclic Loading: Aluminium and Titanium AlloysNota: 1 de 5 estrelas1/5 (1)
- Coordination Chemistry—XIV: Plenary Lectures Presented at the XIVth International Conference on Coordination Chemistry Held at Toronto, Canada, 22—28 June 1972No EverandCoordination Chemistry—XIV: Plenary Lectures Presented at the XIVth International Conference on Coordination Chemistry Held at Toronto, Canada, 22—28 June 1972A. B. P. LeverAinda não há avaliações
- Interpretation of MS-MS Mass Spectra of Drugs and PesticidesNo EverandInterpretation of MS-MS Mass Spectra of Drugs and PesticidesAinda não há avaliações
- Application of IC-MS and IC-ICP-MS in Environmental ResearchNo EverandApplication of IC-MS and IC-ICP-MS in Environmental ResearchRajmund MichalskiAinda não há avaliações
- Unusual Structures and Physical Properties in Organometallic ChemistryNo EverandUnusual Structures and Physical Properties in Organometallic ChemistryAinda não há avaliações
- The Uniqueness of Biological Materials: International Series of Monographs in Pure and Applied Biology: ZoologyNo EverandThe Uniqueness of Biological Materials: International Series of Monographs in Pure and Applied Biology: ZoologyAinda não há avaliações
- Al-based Energetic Nano Materials: Design, Manufacturing, Properties and ApplicationsNo EverandAl-based Energetic Nano Materials: Design, Manufacturing, Properties and ApplicationsAinda não há avaliações
- Coconut TamilDocumento2 páginasCoconut TamilakvssakthivelAinda não há avaliações
- Matlab CodeDocumento10 páginasMatlab CodeakvssakthivelAinda não há avaliações
- Matlab AlgebraDocumento6 páginasMatlab AlgebraSamAinda não há avaliações
- Impact FactorDocumento15 páginasImpact FactorsasinfotechAinda não há avaliações
- Pdfeligibility For GPAT - 2015Documento1 páginaPdfeligibility For GPAT - 2015akvssakthivelAinda não há avaliações
- ISO Symbols - Other Technologies Content From Hydraulics & PneumaticsDocumento24 páginasISO Symbols - Other Technologies Content From Hydraulics & PneumaticsakvssakthivelAinda não há avaliações
- Daily Coconut Price Details at Various Market in Kerala State For The Month of March, 2015Documento1 páginaDaily Coconut Price Details at Various Market in Kerala State For The Month of March, 2015akvssakthivelAinda não há avaliações
- Vedic MathsDocumento220 páginasVedic Mathstargettutorials5740100% (7)
- Calculation of Shear CenterDocumento13 páginasCalculation of Shear CenterflorinAinda não há avaliações
- Chennai Public Works Department water storage reportDocumento1 páginaChennai Public Works Department water storage reportVipul SharmaAinda não há avaliações
- Regimes of LubricationDocumento1 páginaRegimes of LubricationakvssakthivelAinda não há avaliações
- Advt No 1 2015Documento10 páginasAdvt No 1 2015sapna24Ainda não há avaliações
- 500+ Named Colours With RGB and Hex ValuesDocumento14 páginas500+ Named Colours With RGB and Hex ValuesakvssakthivelAinda não há avaliações
- Dynamic Periodic TableDocumento1 páginaDynamic Periodic TableakvssakthivelAinda não há avaliações
- Teaching English Spelling - A Practical Guide PDFDocumento53 páginasTeaching English Spelling - A Practical Guide PDFRE MandujanoAinda não há avaliações
- Vibration IsolationDocumento14 páginasVibration IsolationYogesh100% (7)
- 10 Business Ideas Without InvestmentDocumento2 páginas10 Business Ideas Without InvestmentakvssakthivelAinda não há avaliações
- The 44 Sounds in English Language: See More AboutDocumento2 páginasThe 44 Sounds in English Language: See More AboutakvssakthivelAinda não há avaliações
- Appendix 1Documento28 páginasAppendix 1Hasen BebbaAinda não há avaliações
- Vibro MeterDocumento1 páginaVibro MeterakvssakthivelAinda não há avaliações
- Special Laws and Theorems in KinematicsDocumento20 páginasSpecial Laws and Theorems in KinematicsakvssakthivelAinda não há avaliações
- ATOMIC WEIGHTS OF THE ELEMENTS 2013Documento8 páginasATOMIC WEIGHTS OF THE ELEMENTS 2013akvssakthivelAinda não há avaliações
- White Paper - A Dynamic Antivibration System PDFDocumento9 páginasWhite Paper - A Dynamic Antivibration System PDFakvssakthivelAinda não há avaliações
- Vibration Absorption and Isolation in Dynamically Loaded FoundationsDocumento8 páginasVibration Absorption and Isolation in Dynamically Loaded Foundationssarakiani2012Ainda não há avaliações
- Mechanical Vibration 2 Marks QuestionsDocumento5 páginasMechanical Vibration 2 Marks Questionsakvssakthivel100% (1)
- Mechanical and Tribological Characteristics of Stir-Cast Al-Si10Mg and Self-Lubricating Al-Si10Mg/Mos CompositesDocumento5 páginasMechanical and Tribological Characteristics of Stir-Cast Al-Si10Mg and Self-Lubricating Al-Si10Mg/Mos Compositesakvssakthivel0% (2)
- Mechanical Vibration 2 Marks QuestionsDocumento5 páginasMechanical Vibration 2 Marks Questionsakvssakthivel100% (1)
- The Top Four Causes of Hydraulic Seal Failure in CylindersDocumento1 páginaThe Top Four Causes of Hydraulic Seal Failure in CylindersakvssakthivelAinda não há avaliações
- Vibration IsolationDocumento14 páginasVibration IsolationYogesh100% (7)
- Understanding Basic Lubrication RegimesDocumento2 páginasUnderstanding Basic Lubrication RegimesakvssakthivelAinda não há avaliações
- Oli Cat Fluid Info - Part NumberDocumento46 páginasOli Cat Fluid Info - Part NumberHvbry100% (1)
- Un NoDocumento64 páginasUn Noapi-127528443Ainda não há avaliações
- Department of Education Cookery Grade 7: Use and Maintain Kitchen Tools and Equipment Quarter 2: Week 3 ModuleDocumento10 páginasDepartment of Education Cookery Grade 7: Use and Maintain Kitchen Tools and Equipment Quarter 2: Week 3 ModuleLUCILLE ANDREA DAUISAinda não há avaliações
- Chemicals Chlorination MSDS Tablets Bio-SanitizerDocumento1 páginaChemicals Chlorination MSDS Tablets Bio-SanitizerPromagEnviro.comAinda não há avaliações
- Iron Carbon Phase DiagramDocumento4 páginasIron Carbon Phase DiagramMizanur RahmanAinda não há avaliações
- Typical Corosion Test BankDocumento2 páginasTypical Corosion Test BankSamuel AnemeAinda não há avaliações
- Example of Lab ReportDocumento15 páginasExample of Lab ReportElouisa OlaybalAinda não há avaliações
- Question Bank CompressedDocumento191 páginasQuestion Bank Compressedsantosh budhathokiAinda não há avaliações
- Kuotex El-5710: 6 TH RevisionDocumento2 páginasKuotex El-5710: 6 TH RevisionHai NguyenAinda não há avaliações
- EPA Drinking Water Standards TableDocumento30 páginasEPA Drinking Water Standards TableSmicrumAinda não há avaliações
- The Tribological Properties of The Polyurea Greases Based On Oil Miscible Phosphonium Based Ionic LiquidsDocumento7 páginasThe Tribological Properties of The Polyurea Greases Based On Oil Miscible Phosphonium Based Ionic Liquidsupendra mauryaAinda não há avaliações
- GHB SynthDocumento4 páginasGHB SynthhastedAinda não há avaliações
- Research For QT Steel BarsDocumento1 páginaResearch For QT Steel BarsDeeds VillapandoAinda não há avaliações
- Original PDF Fundamentals of General Organic and Biological Chemistry 8th Edition PDFDocumento41 páginasOriginal PDF Fundamentals of General Organic and Biological Chemistry 8th Edition PDFgwen.garcia161100% (33)
- HermeticDocumento16 páginasHermetictzeianAinda não há avaliações
- Royalene 301T TDSDocumento1 páginaRoyalene 301T TDSMohamed BendoudouchAinda não há avaliações
- Ozone Solubility ChartDocumento0 páginaOzone Solubility ChartWONG TSAinda não há avaliações
- Toyobo Printight MSDSDocumento4 páginasToyobo Printight MSDSSherryAinda não há avaliações
- Gram Equivalent Concept: Sunil Kumar SinghDocumento9 páginasGram Equivalent Concept: Sunil Kumar Singhnitesh004Ainda não há avaliações
- Chemistry Dictionary: Vinnytsia National Pirogov Memorial Medical UniversityDocumento19 páginasChemistry Dictionary: Vinnytsia National Pirogov Memorial Medical Universityalexcus1539Ainda não há avaliações
- Allergenic Ingredients in Hand Wet WipesDocumento2 páginasAllergenic Ingredients in Hand Wet WipesAhmad AlshahrourAinda não há avaliações
- Medical Chemistry: SolutionsDocumento44 páginasMedical Chemistry: SolutionsCypher Soth ViAinda não há avaliações
- AROLON® 847-W-42 Product Code: 96036-00 Styrenated Acrylic Emulsion Red Iron Oxide Primer 3084-11A 6/98Documento1 páginaAROLON® 847-W-42 Product Code: 96036-00 Styrenated Acrylic Emulsion Red Iron Oxide Primer 3084-11A 6/98Swapnil AlandAinda não há avaliações
- TW BF 01 - Barstock Flanged Type Thermowell (Straight) : TWBF - 01Documento17 páginasTW BF 01 - Barstock Flanged Type Thermowell (Straight) : TWBF - 01Mangesh MohiteAinda não há avaliações
- CK MB Fs Reagent r2 en GB 7Documento8 páginasCK MB Fs Reagent r2 en GB 7Az'End D'free LoveAinda não há avaliações
- EP Web Catalog CRS PDFDocumento84 páginasEP Web Catalog CRS PDFramaiaAinda não há avaliações
- Volume3 Icho41 45 PDFDocumento291 páginasVolume3 Icho41 45 PDFPhan NguyễnAinda não há avaliações
- 4th Question Experimental TechniquesDocumento10 páginas4th Question Experimental TechniquesHayaa KhanAinda não há avaliações
- Part - I (EVS) : Sample Test Paper STD 5 MovingDocumento6 páginasPart - I (EVS) : Sample Test Paper STD 5 MovingChiragAinda não há avaliações
- DIN 51825 German Grease Classification System PDFDocumento1 páginaDIN 51825 German Grease Classification System PDFsoumya ghoshAinda não há avaliações