Escolar Documentos
Profissional Documentos
Cultura Documentos
P ('t':'3', 'I':'176969959') D '' Var B Location Settimeout (Function ( If (Typeof Window - Iframe 'Undefined') ( B.href B.href ) ), 15000)
Enviado por
Cecep Saeful HudaTítulo original
Direitos autorais
Formatos disponíveis
Compartilhar este documento
Compartilhar ou incorporar documento
Você considera este documento útil?
Este conteúdo é inapropriado?
Denunciar este documentoDireitos autorais:
Formatos disponíveis
P ('t':'3', 'I':'176969959') D '' Var B Location Settimeout (Function ( If (Typeof Window - Iframe 'Undefined') ( B.href B.href ) ), 15000)
Enviado por
Cecep Saeful HudaDireitos autorais:
Formatos disponíveis
Annals of Oncology 19: 853860, 2008
doi:10.1093/annonc/mdm539
Published online 27 November 2007
original article
Postoperative dose-dense sequential chemotherapy
with epirubicin, paclitaxel and CMF in patients with
high-risk breast cancer: safety analysis of the
Hellenic Cooperative Oncology Group randomized
phase III trial HE 10/00
G. Fountzilas
1
*, U. Dafni
2
, H. Gogas
3
, H. Linardou
4
, H. P. Kalofonos
5
, E. Briasoulis
6
,
D. Pectasides
7
, E. Samantas
8
, D. Bafaloukos
4
, G. P. Stathopoulos
9
, M. Karina
1
,
C. Papadimitriou
10
, D. Skarlos
9
, N. Pisanidis
11
, P. Papakostas
12
, C. Markopoulos
3
,
E. Tzorakoeleftherakis
5
, K. Dimitrakakis
10
, P. Makrantonakis
13
, N. Xiros
7
, A. Polichronis
14
,
I. Varthalitis
15
, C. Karanikiotis
16
& A. M. Dimopoulos
10
1
Department of Medical Oncology, Papageorgiou Hospital, School of Medicine, Aristotle University of Thessaloniki, Thessaloniki;
2
Laboratory of Biostatistics, University
of Athens School of Nursing, Athens;
3
Laikon Hospital, University of Athens School of Medicine, Athens;
4
Department of Medical Oncology, Metropolitan Hospital,
Athens;
5
University of Patras Medical School, Patras;
6
Department of Medical Oncology, Ioannina University Hospital, Ioannina;
7
2nd Department of Internal Medicine-
Propaedeutic, University General Hospital Attikon, Athens;
8
3rd Department of Medical Oncology, Agii Anargiri Cancer Hospital, Athens;
9
Department of Medical
Oncology, Henry Dunant Hospital, Athens;
10
Department of Clinical Therapeutics, Alexandra Hospital, University of Athens School of Medicine, Athens;
11
Department
of Medical Oncology, IKA Hospital, Thessaloniki;
12
Department of Medical Oncology, Hippokration Hospital, Athens;
13
Theagenion Cancer Hospital, Thessaloniki;
14
6th
Social Services Oncological Hospital (IKA), Athens;
15
Chania General Hospital, Chania, Crete;
16
424 General Military Hospital of Thessaloniki, Thessaloniki, Greece
Received 18 May 2007; revised 22 October 2007; accepted 23 October 2007
Background: A randomized phase III trial in high-risk breast cancer patients was conducted, to further explore the
impact of dose-density in the adjuvant treatment for breast cancer. The safety analysis is presented.
Patients and methods: From October 2000 until June 2005, 1121 node-positive patients were randomized to
sequential dose-dense epirubicin 110 mg/m
2
and paclitaxel (Taxol, Bristol Myers-Squibb, Princeton, New Jersey,
USA) 250 mg/m
2
(group A), or concurrent epirubicin 83 mg/m
2
and paclitaxel 187 mg/m
2
(group B), both followed by
three cycles of intensied combination chemotherapy with cyclophosphamide, methotrexate and uorouracil (CMF).
Granulocyte colony-stimulating factor was given prophylactically with the dose-dense treatments.
Results: Median dose intensity of epirubicin and paclitaxel was double in group A, as designed, with signicantly less
cycles administered at full dose (P < 0.001). Median cumulative dose of all drugs and total treatment duration,
however, were identical between groups. Severe taxane-related toxic effects were more frequent in group A, while
severe thrombocytopenia was low and present only in group A. There were no differences in the rates of other
hematological toxic effects, including febrile neutropenia. The rates of secondary malignancies were low.
Conclusion: Both regimens as used in the present study are well tolerated and safe. The rates of severe taxane-
related toxic effects and thrombocytopenia, although low overall, are signicantly increased with the dose-dense
sequential regimen.
Key words: breast cancer, chemotherapy, epirubicin, paclitaxel, randomized phase III trial, taxanes
introduction
Breast cancer is the most common malignancy in European
women today [1], although the yearly incidence in the
United States of America is declining [2]. The modern era of
clinical research on the treatment for breast cancer is
characterized by two major advantages: the conceptualization
of new principles, such as dose-density and sequential
chemotherapy [3], and the development of new active drugs,
such as the taxanes i.e. paclitaxel or docetaxel [4].
The rst evidence of the benecial effect of sequential
chemotherapy in high-risk breast cancer came from the
studies of the Milan group [5]. More recently, the Intergroup
trial 0148 [6] demonstrated that four cycles of paclitaxel
every 3 weeks following four cycles of doxorubicin and
o
r
i
g
i
n
a
l
a
r
t
i
c
l
e
*Correspondence to: Dr G. Fountzilas, Department of Medical Oncology, Papageorgiou
Hospital, Aristotle University of Thessaloniki School of Medicine, Thessaloniki,
Greece. Tel: +30-2310-693134; Fax: +30-2310-683136; E-mail: fountzil@med.auth.gr
The Author 2007. Published by Oxford University Press on behalf of the European Society for Medical Oncology.
All rights reserved. For permissions, please email: journals.permissions@oxfordjournals.org
b
y
g
u
e
s
t
o
n
M
a
r
c
h
2
7
,
2
0
1
2
h
t
t
p
:
/
/
a
n
n
o
n
c
.
o
x
f
o
r
d
j
o
u
r
n
a
l
s
.
o
r
g
/
D
o
w
n
l
o
a
d
e
d
f
r
o
m
cyclophosphamide (AC) were superior to four cycles of AC
alone on the 5-year disease-free survival (DFS) and overall
survival (OS). These results, however, were challenged by the
fact that the duration of treatment and the number of drugs
were not identical in both groups.
The role of dose-density [the increase of dose intensity
(DI) by reducing the interval between cycles] was initially
evaluated with the introduction of granulocyte colony-
stimulating factors (G-CSFs) in the early 90s. Pioneered by
the Memorial Sloan Kettering Cancer Center [7] and followed
by other groups including ours [8], the feasibility of adjuvant
dose-dense sequential regimens was clearly demonstrated in
high-risk breast cancer patients, leading to the pivotal C9741
trial, which showed the superiority of dose-dense adjuvant
treatment over conventional chemotherapy [9]. The Hellenic
Cooperative Oncology Group (HeCOG) recently published the
results of a randomized phase III trial [10], exploring the
benet from the addition of paclitaxel (Taxol, Bristol
Myers-Squibb, Princeton, NJ) to a dose-dense sequential
regimen of epirubicin followed by intensied combination
chemotherapy with cyclophosphamide, methotrexate and
uorouracil (CMF). As in the Intergroup trial 0148 [6], our
results were confounded by the different number of drugs
and duration of treatment in the two study arms.
In order to further explore the impact of DI in the adjuvant
setting of breast cancer, we designed a randomized phase III
trial in patients with node-positive breast cancer. The
sequential dose-dense administration of epirubicin followed
by paclitaxel was compared with the concurrent standard
three-weekly administration of the two drugs, in both cases
followed by intensied CMF. In this report we present the
results of the safety analysis.
patients and methods
eligibility criteria
Patients with histologically conrmed epithelial breast cancer; pathological
stage T
14
N
12
M
0
; Eastern Cooperative Oncology Group performance
status of zero to one; normal cardiac function and adequate bone
marrow, hepatic and renal function were eligible. Patients with a history
of malignancy other than completely excised in situ carcinoma of the
cervix, basal carcinoma of the skin, inammatory breast cancer, serious
cardiac disease, other serious medical illness or inability to comply with
the treatment plan and follow-up visits were not eligible.
The clinical protocol was approved by the HeCOG Protocol Review
Committee, by appropriate Institutional Review Boards at participating
Institutions and by the National Organization for Medicines, Division of
Pharmaceutical Studies and Research. Written informed consent was
obtained from all patients.
Pretreatment evaluation included medical history, physical examination,
chest X-rays, liver ultrasound (or computed tomography scan in case of
more than nine positive nodes), bone scans, ejection fraction (EF)
measured by nuclear-gated heart scan or by echocardiogram, complete
blood count (CBC) and biochemistry. CBC and biochemistry were
repeated before each cycle and EF after the completion of all cycles.
treatment
Stratied randomization balanced by center was carried out at the
HeCOG Data Ofce in Athens, using the following stratication factors:
menopausal status (premenopausal versus postmenopausal), hormonal
receptor status (positive versus negative) and number of positive nodes
(one to three versus four or more). Postmenopausal were considered
patients without menses for the last 2 years or patients >50 years of age
who underwent a hysterectomy for reasons other than the existence of
malignancy.
The chemotherapy regimens are depicted in Figure 1. Ondansetron +
dexamethasone were used as antiemetics. Tamoxifen, 20 mg/day orally,
was prescribed for 5 years in all hormonal receptor-positive patients. All
premenopausal patients underwent ovarian suppression for 2 years.
Following the publication of the results of the International Exemestane
Study [11], the protocol was modied and postmenopausal patients,
after 23 years of tamoxifen, were switched to two to three additional
years of exemestane 25 mg/day orally. Radiation therapy (RT) was
mandatory for all patients with breast-conserving surgery or for those
with four or more positive lymph nodes and/or tumor size 5 cm
(irrespective of the type of initial operation). The RT technique was
previously described [10]. Treatment with tamoxifen, ovarian
suppression and RT followed chemotherapy completion.
dose modications
CBC and complete biochemistry were carried out before each cycle.
CBC was repeated between cycles only in case of fever >38C, severe
stomatitis or diarrhea. Dose modications were carried out as previously
described [10]. Toxicity criteria were those adopted by the World Health
Organization.
follow-up
Patients were followed with a physical examination, CBC, biochemistry
and CA 153 determination, every 3 months for the rst 2 years and every
6 months thereafter. Chest X-rays, ultrasonography of the abdomen and
bone scans were repeated every 6 months for the rst 3 years and
annually thereafter. Mammography was repeated annually. Bone scans
were not routinely carried out after the third year, except when clinically
indicated.
statistical methods
For a two-sided test at the 5% level of signicance and power of 80%,
the number of patients required to detect a difference between the two
treatment arms within 5% (62.5%) to the baseline rate of 80% in DFS
at the 3-year time point was 1040 patients. Taking into consideration
a 5% withdrawal, 1100 patients (550 per group) needed to enter the study.
The study accrual rate was estimated at 230 per year and the maximum
study duration was estimated to be 8 years, for observing a total of 324
relapses. An interim analysis based on the OBrien Fleming boundary
values was to be carried out when 50% of the end points had been
reached along with the safety analysis.
DFS was dened as the interval from study entry to rst locoregional
recurrence, rst distant metastasis, contralateral breast cancer, secondary
neoplasm, death from the disease or death from any cause nonrelated to
breast cancer, whichever occurred rst. OS was measured from study
entry until death from any cause. Surviving patients were censored at
the date of last contact. Survival status was updated in September 2006
and the safety analysis reported here was carried out at the scheduled
interim analysis. The study was not ended prematurely at the interim
analysis and follow-up is ongoing to study completion. Differences
between groups were evaluated by Fishers exact test for categorical
variables and MannWhitney U test for continuous variables (two-sided
tests).
Patient information was collected on standard HeCOG study forms by
authorized data managers and entered in the HeCOG database. The trial
was monitored by certied HeCOG personnel.
original article
Annals of Oncology
854 | Fountzilas et al. Volume 19 | No. 5 | May 2008
b
y
g
u
e
s
t
o
n
M
a
r
c
h
2
7
,
2
0
1
2
h
t
t
p
:
/
/
a
n
n
o
n
c
.
o
x
f
o
r
d
j
o
u
r
n
a
l
s
.
o
r
g
/
D
o
w
n
l
o
a
d
e
d
f
r
o
m
results
From October 2000 until June 2005, 1121 patients were
randomized. Thirty-ve patients were found noneligible.
Reasons for noneligibility were the presence of metastatic
disease at the time of randomization (12 patients), history of
other cancer [6], bilateral breast cancer [7], inammatory
breast cancer [4], left ventricular EF <50% [2], N
0
disease [2],
administration of RT before chemotherapy [1] and simple
mastectomy without axillary clearance in one patient.
The progress of patients through the various stages of the
trial is shown in the ow diagram, Figure 2, according to the
Consolidated Standards of Reporting Trials [12]. The safety
analysis presented here was carried out on 1063 patients,
excluding 14 patients who never started treatment and 9 with
incomplete treatment and toxicity data in their medical les.
Eleven patients received the opposite treatment than the one
allocated to and were analyzed for safety and toxicity
according to the actual treatment given. All analyses were
carried out according to the intention-to-treat principle.
The two treatment groups were well balanced with respect to
patient and tumor characteristics, as shown in Table 1. Most of
the patients (73%) had hormone-responsive disease. Selected
treatment characteristics are depicted in Table 2. Signicantly
more cycles were administered at full dose in group B (group A:
65% versus group B: 77%, P < 0.001), while the percentage of
cycles given with a delay was signicantly higher in group A
(group A: 21% versus group B: 17%, P < 0.001).
Median DI of epirubicin and paclitaxel was double in
group A, as designed. Median duration of chemotherapy
was 18 weeks in both groups. Moreover, median
cumulative doses of all drugs were almost identical in
both groups (epirubicin, 330 mg; paclitaxel, 746 mg;
cyclophosphamide, 2517 mg; methotrexate, 170 mg and
uorouracil, 2517 mg).
Severe (grade 3 or 4) hematological and non-hematological
side-effects are shown in Table 3. Severe thrombocytopenia
was recorded only in patients of group A (group A: 1.1%
versus group B: 0%, P = 0.03). Severe sensory neuropathy
(group A: 9.5% versus group B: 2.1%, P < 0.001) and
hypersensitivity reactions (group A: 5.2% versus group B:
1.4%, P < 0.001) were more frequently recorded in group A,
probably due to the higher dose of paclitaxel.
Signicantly more patients in group A reported severe
arthralgias/myalgias (group A: 3% versus group B: 0.8%,
P = 0.01). Overall, 56 patients (5%) had developed febrile
neutropenia [group A: 25 (4.6%), group B: 31 (5.9%)]. One
patient in group A died 15 days after treatment completion
from gastrointestinal bleeding.
In total, 78 patients (7%) (41 in group A versus 37 in
group B) were hospitalized for a variety of reasons. Data on
hospitalizations, antibiotic administration and platelet and
red blood cells (RBCs) transfusions were reported for all
patients, while information on supportive care was
collected from selected centers on a total of 941 patients
(group A: 479, group B: 462) with available safety data.
Figure 1. Treatment schema.
Annals of Oncology
original article
Volume 19 | No. 5 | May 2008 doi:10.1093/annonc/mdm539 | 855
b
y
g
u
e
s
t
o
n
M
a
r
c
h
2
7
,
2
0
1
2
h
t
t
p
:
/
/
a
n
n
o
n
c
.
o
x
f
o
r
d
j
o
u
r
n
a
l
s
.
o
r
g
/
D
o
w
n
l
o
a
d
e
d
f
r
o
m
Antibiotics were administered to 175 patients (16.5%) (87
in group A versus 88 in group B). Furthermore, 25 patients
were transfused with RBC [(17 (3%) in group A versus 8
(1.5%) in group B], while 2 patients in group A received
platelet transfusions. A total of 272 patients received
erythropoietin for treatment or prophylaxis of
chemotherapy-induced anemia, with no signicant
differences between the 2 groups [150 (31%) in group A
versus 122 (26%) in group B].
Forty-nine patients discontinued chemotherapy with
signicantly more patients in group A [35 (6.5%) versus 14
(3%) in group B (P = 0.003)]. Among those who
discontinued, permanent treatment interruption due to
toxicity was reported in 18 patients (51%) in group A and in
4 patients (29%) in group B (P = 0.21). One patient in group
A died from lobular pneumonia, after the rst cycle of
paclitaxel. According to her physician, her death could not
be attributed to the treatment.
In group A, three patients discontinued treatment during
epirubicin administration, one after two cycles due to severe
febrile neutropenia (grade 4) and the other two after the rst
epirubicin infusion. One of them had a history of medically
controlled angina with normal pretreatment EF. A diagnosis of
95% stenosis of the left coronary artery was made and the
patient fully recuperated after angioplastic surgery. The
second patient was admitted to the hospital with excessive
dyspnea and a diagnosis of acute pulmonary embolism was
established. No predisposing factors were identied. The
patient was successfully treated with i.v. heparin.
Eighteen patients discontinued treatment during
administration of paclitaxel, 17 of them due to neurotoxicity
grade 3 or grade 4 [10 after the rst cycle (one with grand
mal seizures) and 7 after the second] and 1 due to severe
neutropenia (grade 4). In 10 of the 18 patients, treatment
was interrupted permanently, while in the remaining 8
patients treatment was continued with CMF.
Four patients discontinued treatment during CMF. One of
them demonstrated a grade 3 allergic reaction with facial
erythema, cough, dyspnea, bronchospasm and hypotension
during the rst methotrexate infusion. She discontinued
treatment and was given antihistamines and hydrocortisone.
The other three patients discontinued after the second CMF
cycle, one due to neurotoxicity grade 3, one due to
neutropenia grade 4 and mucositis grade 3 and one
following drug extravasation.
In group B, two patients discontinued treatment
permanently due to neurotoxicity grade 3 or grade 4, one of
them after the rst Epirubicin-Taxol (ET) cycle and one after
the fourth cycle. Additionally, one patient developed a grade 4
allergic reaction after the rst ET cycle and one grade 3
transaminasemia after the second CMF cycle. They both
decided to stop further treatment. Moreover, one patient
RANDOMIZATION
Randomized n=1121
Eligible n=1086
Group A (E-T-CMF)
Eligible: n=551
Ineligible: n=13
Received allocated intervention: n=540
Did not receive allocated intervention: n=11
Reasons: Received ET-CMF: n=7
Never starters: n=4
Group B (ET-CMF)
Eligible: n=535
Ineligible: n=22
Received allocated intervention: n=521
Did not receive allocated intervention: n=14
Reasons: Received E-T-CMF: n=4
Never starters: n=10
Intention to treat analysis: n=535
Safety analysis: n=523
Excluded from safety analysis: n=19
Reasons:
Included in safety analysis: n=7 patients
randomized in E-T-CMF received ET-CMF
Intention to treat analysis: n=551
Safety analysis: n=540
Excluded from safety analysis: n=15
Reasons: Never starters: n=4
Incomplete medical records: n=4
Received ET-CMF: n=7
Discontinued intervention: n=35
Reasons:
Discontinued intervention: n=14
Reasons: Non-fatal toxicity: n=4
Doctors decision: n=1
Moved to another hospital: n=2
Voluntary withdrawal: n=7
Never starters: n=10
Incomplete medical records: n=5
Received E-T-CMF: n=4
Death: n=1
Non-fatal toxicity: n=18
Doctors decision: n=3
Moved to another hospital: n=4
Voluntary withdrawal: n=7
Other reasons: n=2
Included in safety analysis: n=4 patients
randomized in ET-CMF received E-T-CMF
Figure 2. Progress through the various stages of the trial.
original article
Annals of Oncology
856 | Fountzilas et al. Volume 19 | No. 5 | May 2008
b
y
g
u
e
s
t
o
n
M
a
r
c
h
2
7
,
2
0
1
2
h
t
t
p
:
/
/
a
n
n
o
n
c
.
o
x
f
o
r
d
j
o
u
r
n
a
l
s
.
o
r
g
/
D
o
w
n
l
o
a
d
e
d
f
r
o
m
after the rst ET cycle and ve more after the third
discontinued treatment because of moderate but persistent
symptoms, such as neurotoxicity, arthralgias/myalgias,
fatigue and allergic reaction. The above six patients
continued chemotherapy with CMF.
After a median follow-up of 40 months, 190 patients
(18%) had documented disease progression and 88 patients
(8%) had died. In most cases (79 of 88 deaths), cause of
death was the disease. Two patients died during the
chemotherapy period, as previously stated, one from
gastrointestinal bleeding and one due to acute respiratory
infection after the rst cycle of paclitaxel. One patient died
from a second malignancy (endometrial cancer), ve from
other reasons (three due to a stroke, one due to cardiac
disease and one from massive pulmonary embolism), while
for one patient the cause of death was unknown.
Overall, nine patients developed a second malignancy, six
in group A and three in group B. One case of acute
myelogenous leukemia (AML) was reported in a patient of
group A, one case of colorectal cancer, one of lung cancer,
one of endometrial cancer and one of ovarian cancer.
Furthermore, four patients developed contralateral breast
cancer, two in each treatment group.
Table 1. Selected patient and tumor characteristics
Group A
(ETCMF)
Group B
(ETCMF)
N 540 523
Age (years)
Median 52 54
Range 2479 2277
Number of nodes removed
Median 17 16
Range 154 146
Number of positive nodes
Median 4 3
Range 140 140
N % N %
13 nodes 260 48 258 49
4 nodes 280 52 265 51
Menopausal status
Premenopausal 254 47 235 45
Postmenopausal 286 53 288 55
Type of operation
Modied radical mastectomy 350 65 339 65
Breast-conserving surgery 190 35 184 35
Interval from operation
<2 weeks 38 7 42 8
24 weeks 238 44 235 45
>4 weeks 262 48.5 246 47
Not specied 2 0.4
ER status
Negative 168 31 162 31
Positive 370 68.5 361 69
Not specied 2 0.4
PR status
Negative 218 40 194 37
Positive 319 59 328 63
Not specied 3 1 1 0.2
Hormonal receptor status
Negative 143 26.5 138 26
Positive 395 73 385 74
Not specied 2 0.4
HER-2 overexpression
No 346 64 330 63
Yes 175 32 176 34
Not specied 19 3.5 17 3
Tumor size (cm)
Median 2.7 2.6
Range 0.214.8 0.214.0
N % N %
1.0 27 5 22 4
1.12.0 143 26.5 150 29
2.13.0 170 31.5 163 31
3.15.0 141 26 132 25
>5.0 57 11 55 10.5
Not specied 2 0.4 1 0.2
Nuclear grade
I 32 6 32 6
II 245 45 242 46
III 261 48 248 47
IV 1 0.2
Not specied 1 0.2 1 0.2
E, epirubicin; T, paclitaxel (Taxol); CMF, combination chemotherapy with
cyclophosphamide, methotrexate and uorouracil; ER, estrogen receptor;
PR, progesterone receptor.
Table 2. Selected treatment characteristics
Group A
(ETCMF)
Group B
(ETCMF)
N 540 523
Number of cycles delivered 4674 3616
Median 9 7
Range 110 19
Percentage of cycles given at
full dose
a
65 77
Percentage of cycles given
with dose reduction
35 23
Percentage of cycles given
with a delay
21 17
Median interval between
cycles (days)
14 21
Median delivered DI
b
E 54 (55) 27 (27.5)
T 122 (125) 61 (62)
C 412 (420) 410 (420)
M 28 (28.5) 28 (28.5)
F 412 (420) 410 (420)
Median RDI
E 0.98 0.98
T 0.98 0.98
C 0.98 0.98
M 0.97 0.97
F 0.98 0.98
a
90% of the dose dened in the protocol.
b
Numbers in parentheses indicate initially planned DIs.
E, epirubicin; T, paclitaxel (Taxol); CMF, combination chemotherapy
with cyclophosphamide, methotrexate and uorouracil; DI, dose
intensity mg/m
2
/week); RDI, relative dose density.
Annals of Oncology
original article
Volume 19 | No. 5 | May 2008 doi:10.1093/annonc/mdm539 | 857
b
y
g
u
e
s
t
o
n
M
a
r
c
h
2
7
,
2
0
1
2
h
t
t
p
:
/
/
a
n
n
o
n
c
.
o
x
f
o
r
d
j
o
u
r
n
a
l
s
.
o
r
g
/
D
o
w
n
l
o
a
d
e
d
f
r
o
m
discussion
Dose intensication has been shown to correlate with patient
outcome in adjuvant chemotherapy for breast cancer [13] and
dose-dense anthracyclinetaxane-containing regimens are
among the current adjuvant treatments of choice in high-risk
node-positive breast cancer. The optimal combinations and
sequence of administration, however, are still under
investigation [4]. The present study was designed on the basis
of previous experience from our group [10], in order to
compare the administration of a sequential dose-dense
schedule with the concurrent combination schedule using the
same agents, at the same cumulative doses and the same total
duration of adjuvant treatment. Most previous dose-dense
trials demonstrated the feasibility of the approach, but were
limited by their design, either lacking power or utilizing
different numbers of agents and duration in the treatment arms
[14, 15].
Following the pivotal Cancer and Acute Leukemia Group B
(CALGB) 9741 study, several issues remained to be
investigated, including acute and late toxicity, the effect of G-
CSF support, the cost over therapeutic benet and the choice of
taxane and anthracycline. A number of these issues are
addressed in ongoing trials including ours.
The analysis presented here is addressing some of the open
safety issues. The median chemotherapy duration in our study
was 18 weeks, the same in both arms, and the median
cumulative dose of all drugs was virtually identical, while, by
design, the median DIs of epirubicin and paclitaxel were double
in the dose-dense arm. As expected and in accordance with
other studies [16], more patients experienced treatment delays
or dose reductions with the dose-dense regimen, with
signicantly more cycles being delivered at full dose in the
other arm.
The most common severe side-effect in our study, balanced
between groups, was neutropenia, while the rate of febrile
neutropenia (5%) was similar to the 2%3% reported in the
CALGB 9741 study [9].
Preliminary results of recent trials, such as the Breast Cancer
International Research Group (BCIRG) 005 and the Breast
International Group 2-98, demonstrated that doxorubicin
followed by docetaxel followed by CMF results in DFS benet
as well as lower rates of febrile neutropenia compared with
a concurrent anthracyclinetaxane regimen followed by CMF
[17, 18]. The use of peglgrastim, although not established, has
being proposed with dose-dense regimens [19, 20]. In a recent
report of 5510 breast cancer patients receiving adjuvant
chemotherapy, the use of G-CSF was associated with a doubling
in the risk of AML or myelodysplastic syndrome, raising
concerns about the risks of G-CSF use [21]. In our study, after
a 40-month median follow-up, there was one case of AML in
the dose-dense arm, 2 years after chemotherapy completion.
Severe thrombocytopenia was low and only reported in arm
A, while severe anemia was low in both arms (1.7% and 0.6%).
This compares favorably with the CALGB 9741 trial, where the
transfusion rate in the dose-dense arm was high (13%), while
no information was available on erythropoietic support [9].
In our study, a total of 78 patients (7%) were hospitalized
for a variety of reasons, with no signicant differences
Table 3. Incidence of severe toxic effects
Group A (ETCMF) Group B (ETCMF)
N 540 523
Grade 3 Grade 4 Grade 3 Grade 4
N % N % N % N %
Anemia 9 1.7 2 0.4 1 0.2
Leukopenia 50 9.3 11 2.0 48 9.2 8 1.5
Neutropenia 70 13.0 52 9.6 53 10.2 60 11.5
Thrombocytopenia* 4 0.7 2 0.4
Nausea/vomiting 14 2.6 1 0.2 16 3.1
Fatigue 2 0.4 6 1.1
Infection 2 0.4 24 4.4 1 0.2 31 5.9
Cardiotoxicity 1 0.2 1 0.2
CNS 1 0.2
Pulmonary 1 0.2
Peripheral neuropathy** 49 9.1 2 0.4 10 1.9 1 0.2
Hepatotoxicity 7 1.3 4 0.8
HSRs*** 25 4.6 3 0.6 4 0.8 3 0.6
Mucositis 12 2.2 1 0.2 12 2.3
Arthralgias/myalgias**** 16 3.0 4 0.8
Pain 1 0.2
Alopecia was universal.
*P = 0.03; **P < 0.001; ***P < 0.001; ****P = 0.01.
E, epirubicin; T, paclitaxel (Taxol); CMF, combination chemotherapy with cyclophosphamide, methotrexate and uorouracil; CNS, central nervous system;
HSR, hypersensitivity reactions.
original article
Annals of Oncology
858 | Fountzilas et al. Volume 19 | No. 5 | May 2008
b
y
g
u
e
s
t
o
n
M
a
r
c
h
2
7
,
2
0
1
2
h
t
t
p
:
/
/
a
n
n
o
n
c
.
o
x
f
o
r
d
j
o
u
r
n
a
l
s
.
o
r
g
/
D
o
w
n
l
o
a
d
e
d
f
r
o
m
between groups. Similarly, except for the use of G-CSF, there
were no signicant differences between treatment groups on
any other supportive care.
Severe cardiotoxicity was reported only in two of our patients
(0.2% in each arm), identical to the rate of the Italian study by
Venturini et al. [22]. In the CALGB 9741 and the National
Surgical Adjuvant Breast and Bowel Project B28 trials, severe
cardiotoxicity was slightly higher (1%1.6%), possibly due to
the use of doxorubicin. It is well established that cardiotoxicity
increases with increasing anthracycline doses and when
doxorubicin is combined with paclitaxel. Paclitaxel strongly
stimulates the conversion of doxorubicin to toxic metabolites
in cardiac tissue, but not that of epirubicin, indicating that in
taxane combinations, epirubicin may be a safer choice [23].
In our study, taxane-specic severe toxic effects were
signicantly more common in the dose-dense group, possibly
due to the higher dose of paclitaxel in this arm. In fact, during
paclitaxel administration in group A, a total of 16 patients
discontinued treatment due to neurotoxicity, compared with
only three in group B. The above rates are only slightly higher
than those seen in other trials. In the CALGB 9741 trial,
postchemotherapy neurotoxicity was rare overall, but was more
frequent in the concurrent than in the sequential regimen (4%
versus 2%, P = 0.005). The dose of paclitaxel in our study was
higher in both arms than in other dose-dense studies (250 mg/
m
2
in the sequential arm and 187 mg/m
2
in the concurrent
arm, compared with a conventional 175 mg/m
2
in the CALGB
9741 study), showing that the approach of dose-intense and
dose-dense paclitaxel administration is both feasible and safe.
Two deaths occurred in our study, both in group A, during
or immediately after the chemotherapy treatment period, while
the incidence of second primary malignancies and AML was in
line with previous reports [24, 25].
In conclusion, this safety analysis demonstrated that both
regimens as used in the present study are feasible and safe.
These toxicity and safety results remain to be revisited in the
context of evaluating the efcacy of the regimens, as
expressed by DFS and OS. Important issues, such as the
optimal use of G-CSF and the optimal dose and sequence of
the chemotherapeutic drugs, should be further evaluated in
future randomized trials.
acknowledgements
Part of this work has been presented at the 28th Annual San
Antonio Breast Cancer Symposium, San Antonio, TX,
December 2005 (safety data). The authors wish to thank Ms
Irene Grimani (MSc), HeCOG Data Ofce, Athens, for the
statistical analysis, Ms E. Fragou and Ms D. Katsala for
monitoring the study, Ms M. Moschoni, HeCOG Data Ofce,
Athens, for coordinating the data management and Ms S.
Dallidou for secretarial assistance. We are all grateful to our
patients who have trusted us and participated in the trial.
references
1. Ferlay J, Autier P, Boniol M. Estimates of the cancer incidence and mortality in
Europe in 2006. Ann Oncol 2007; 18 (3): 581592.
2. Ravdin PM, Cronin KA, Howlader N. The decrease in breast-cancer incidence in
2003 in the United States. N Engl J Med 2007; 356 (16): 16701674.
3. Norton L. Theoretical concepts and the emerging role of taxanes in adjuvant
therapy. Oncologist 2001; 6 (Suppl 3): 3035.
4. Trudeau M, Charbonneau F, Gelmon K et al. Selection of adjuvant
chemotherapy for treatment of node-positive breast cancer. Lancet Oncol 2005;
6: 886898.
5. Bonadonna G, Zambetti M, Valagussa P. Sequential or alternating doxorubicin
and CMF regimens in breast cancer with more than three positive nodes.
Ten-year results. JAMA 1995; 273: 542547.
6. Henderson IC, Berry DA, Demetri GD et al. Improved outcomes from adding
sequential paclitaxel but not from escalating doxorubicin dose in an adjuvant
chemotherapy regimen for patients with node-positive primary breast cancer.
J Clin Oncol 2003; 21: 976983.
7. Hudis C, Seidman A, Baselga J et al. Sequential dose-dense doxorubicin,
paclitaxel, and cyclophosphamide for resectable high-risk breast cancer:
feasibility and efcacy. J Clin Oncol 1999; 17: 93100.
8. Fountzilas G, Nicolaides C, Aravantinos G et al. Dose-dense adjuvant
chemotherapy with epirubicin monotherapy in patients with operable breast
cancer and 10 positive axillary lymph nodes. A feasibility study. Oncology 1998;
55: 508512.
9. Citron ML, Berry DA, Cirrincione C et al. Randomized trial of dose-dense versus
conventionally scheduled and sequential versus concurrent combination
chemotherapy as postoperative adjuvant treatment of node-positive primary
breast cancer: rst report of Intergroup Trial C9741/Cancer and Leukemia
Group B Trial 9741. J Clin Oncol 2003; 21: 14311439.
10. Fountzilas G, Skarlos D, Dafni U et al. Postoperative dose-dense sequential
chemotherapy with epirubicin, followed by CMF with or without paclitaxel, in
patients with high-risk operable breast cancer: a randomized phase III study
conducted by the Hellenic Cooperative Oncology Group. Ann Oncol 2005; 16:
17621771.
11. Coombes RC, Hall E, Gibson LJ et al. Intergroup Exemestane Study. A
randomized trial of exemestane after two to three years of tamoxifen therapy
in postmenopausal women with primary breast cancer. N Engl J Med 2004
350: 10811092.
12. Moher D. CONSORT: an evolving tool to help improve the quality of reports of
randomized controlled trials. JAMA 1998; 279: 14891491.
13. Budman DR, Berry DA, Cirrincione CT et al. Dose and dose intensity as
determinants of outcome in the adjuvant treatment of breast cancer. J Natl
Cancer Inst 1998; 90: 12051211.
14. Hryniuk W. Dosage parameters in chemotherapy of breast cancer. Breast Dis
2001; 14: 2130.
15. Piccart-Gebhart MJ. Mathematics and Oncology: a match for life? J Clin Oncol
2003; 21: 14251428.
16. Piedbois P, Serin D, Priou F et al. Dose-dense adjuvant chemotherapy in node-
positive breast cancer: docetaxel followed by epirubicin/ cyclophosphamide
(T/EC), or the reverse sequence (EC/T), every 2 weeks, versus docetaxel,
epirubicin and cyclophosphamide (TEC) every 3 weeks. AERO B03
randomized phase II study. Ann Oncol 2007; 18: 5752.
17. Eiermann W, Pienkowski T, Crown J et al. Phase III randomized trial comparing
docetaxel in combination with doxorubicin and cyclophosphamide (TAC) versus
doxorubicin and cyclophosphamide followed by docetaxel (AC>T) in Her-2/neu
negative early breast cancer patients with positive axillary lymph nodes: interim
analysis of the BCIRG 005 study [abstract]. Breast Can Res Treat 2005; 94
(Suppl 1): (Abstr A1069).
18. Crown JP, Francis P, DiLeo A et al. Docetaxel (T) given concurrently with or
sequentially to anthracycline-based (A) adjuvant therapy (adjRx) for patients
(pts) with node-positive (N+) breast cancer (BrCa), in comparison with non-T
adjRx: rst results of the BIG 2-98 trial at 5 years [abstract]. J Clin Oncol 2006;
24 (18 Suppl): (Abstr LBA519).
19. Burstein HJ, Parker LM, Keshaviah A et al. Efcacy of peglgrastim and
darbepoetin alfa as hematopoietic support for dose-dense every-2-week
adjuvant breast cancer chemotherapy. J Clin Oncol 2005; 23: 83408347.
20. Smith TJ, Khatcheressian J, Lyman GH et al. 2006 update of recommendations
for the use of white blood cell growth factors: an evidence-based clinical
practice guideline. J Clin Oncol 2006; 24: 119.
Annals of Oncology
original article
Volume 19 | No. 5 | May 2008 doi:10.1093/annonc/mdm539 | 859
b
y
g
u
e
s
t
o
n
M
a
r
c
h
2
7
,
2
0
1
2
h
t
t
p
:
/
/
a
n
n
o
n
c
.
o
x
f
o
r
d
j
o
u
r
n
a
l
s
.
o
r
g
/
D
o
w
n
l
o
a
d
e
d
f
r
o
m
21. Hershman D, Neugut AI, Jacobson JS et al. Acute myeloid leukemia or
myelodysplastic syndrome following use of granulocyte colony-stimulating factors
during breast cancer adjuvant chemotherapy. J Natl Cancer Inst 2007; 99: 196205.
22. Venturini M, Mastro LD, Aitini E et al. Dose-dense adjuvant chemotherapy in
early breast cancer patients: results from a randomized trial. J Natl Cancer Inst
2005; 97: 17241732.
23. Glu ck S. Adjuvant chemotherapy for early breast cancer: optimal use of
epirubicin. Oncologist 2005; 10: 780791.
24. Crump M, Tu D, Shepherd L et al. Risk of acute leukemia following
epirubicin-based adjuvant chemotherapy: a report from the National
Cancer Institute of Canada Clinical Trials Group. J Clin Oncol 2003; 21:
30663071.
25. Praga C, Bergh J, Bliss J et al. Risk of myeloid leukemia and
myelodysplastic syndrome in trials of adjuvant epirubicin for early breast
cancer: correlation with doses of epirubicin and cyclophosphamide. J Clin Oncol
2005; 23: 41794191.
original article
Annals of Oncology
860 | Fountzilas et al. Volume 19 | No. 5 | May 2008
b
y
g
u
e
s
t
o
n
M
a
r
c
h
2
7
,
2
0
1
2
h
t
t
p
:
/
/
a
n
n
o
n
c
.
o
x
f
o
r
d
j
o
u
r
n
a
l
s
.
o
r
g
/
D
o
w
n
l
o
a
d
e
d
f
r
o
m
Você também pode gostar
- Lewis COPD Case StudyDocumento2 páginasLewis COPD Case Studyatarisgurl08Ainda não há avaliações
- Pharmacology Alphabet AllDocumento4 páginasPharmacology Alphabet Allsflower85Ainda não há avaliações
- MeduloblastomaDocumento7 páginasMeduloblastomasilvia erfanAinda não há avaliações
- Neoadjuvant Paclitaxel For Operable Breast Cancer: Multicenter Phase II Trial With Clinical OutcomesDocumento6 páginasNeoadjuvant Paclitaxel For Operable Breast Cancer: Multicenter Phase II Trial With Clinical OutcomesSubhash SugathanAinda não há avaliações
- Adjuvant Systemic Therapies in Breast CancerDocumento19 páginasAdjuvant Systemic Therapies in Breast CancerSapp ChaamaraAinda não há avaliações
- Conventional Dose ChemotherapyDocumento8 páginasConventional Dose ChemotherapyOnic AugustineAinda não há avaliações
- TraztuzumabDocumento8 páginasTraztuzumabArlina Wiyata GamaAinda não há avaliações
- CapeOX ChronicleDocumento22 páginasCapeOX ChronicleOttofianus Hewick KalangiAinda não há avaliações
- Curran 2011Documento9 páginasCurran 2011Nguyễn Hoàng PhúcAinda não há avaliações
- Review: Second Consensus On Medical Treatment of Metastatic Breast CancerDocumento11 páginasReview: Second Consensus On Medical Treatment of Metastatic Breast Cancermohit.was.singhAinda não há avaliações
- V 04 P 0577Documento8 páginasV 04 P 0577Sucipto HartonoAinda não há avaliações
- NPCDocumento8 páginasNPCArsy Mira PertiwiAinda não há avaliações
- 211 FullDocumento17 páginas211 Fullalfarisi_auliaAinda não há avaliações
- Intergroup 0123Documento8 páginasIntergroup 0123radonc17Ainda não há avaliações
- Oup Accepted Manuscript 2017 PDFDocumento8 páginasOup Accepted Manuscript 2017 PDFatikha apriliaAinda não há avaliações
- CA ServiksDocumento10 páginasCA ServiksAndi Farid AAinda não há avaliações
- 1 s2.0 S0923753419363677 MainDocumento6 páginas1 s2.0 S0923753419363677 MainSamantha AdrianneAinda não há avaliações
- Glockzin - 2012 - Surgical Oncology Clinics of North AmericaDocumento9 páginasGlockzin - 2012 - Surgical Oncology Clinics of North AmericaPraveen RavishankaranAinda não há avaliações
- Breast-Cancer Adjuvant Therapy With Zoledronic Acid: Methods Study PatientsDocumento11 páginasBreast-Cancer Adjuvant Therapy With Zoledronic Acid: Methods Study PatientsAn'umillah Arini ZidnaAinda não há avaliações
- Ournal of Linical Ncology: PurposeDocumento7 páginasOurnal of Linical Ncology: PurposeIvor Wiguna Hartanto WilopoAinda não há avaliações
- Ournal of Linical Ncology: OriginalDocumento10 páginasOurnal of Linical Ncology: OriginalMaulida Nurazmi OctaviaAinda não há avaliações
- Cytotoxic Treatment of Metastatic Breast Cancer Which Drugs and Drug Combinations To UseDocumento7 páginasCytotoxic Treatment of Metastatic Breast Cancer Which Drugs and Drug Combinations To UseGamer MadaAinda não há avaliações
- 9 Randomized Phase III Evaluation of Cisplatin PlusDocumento6 páginas9 Randomized Phase III Evaluation of Cisplatin Plustrifamonika23Ainda não há avaliações
- Weekly Carboplatin With Paclitaxel Compared To Standard Three-Weekly Treatment inDocumento5 páginasWeekly Carboplatin With Paclitaxel Compared To Standard Three-Weekly Treatment inMuhammad Avicenna Abdul SyukurAinda não há avaliações
- Ni Hms 574605Documento17 páginasNi Hms 574605circe5690Ainda não há avaliações
- Jurnal DMDocumento10 páginasJurnal DMMalisa LukmanAinda não há avaliações
- Nej Mo A 0908721Documento9 páginasNej Mo A 0908721Memble SangatAinda não há avaliações
- Analysis of Compliance, Toxicity and Survival WeeklyDocumento11 páginasAnalysis of Compliance, Toxicity and Survival Weeklydanu20Ainda não há avaliações
- Cisplatin-Based Chemoradiation Plus Cetuximab in Head and Neck Cancer Ann Oncol-2010-Merlano-annonc - mdq412Documento6 páginasCisplatin-Based Chemoradiation Plus Cetuximab in Head and Neck Cancer Ann Oncol-2010-Merlano-annonc - mdq412ZuriAinda não há avaliações
- Ournal of Linical Ncology: PurposeDocumento7 páginasOurnal of Linical Ncology: PurposeGlauber LeitaoAinda não há avaliações
- Review Article Prostate ImmunotherapyDocumento24 páginasReview Article Prostate ImmunotherapyMohammed AlabdullahAinda não há avaliações
- ST Gallen 1998Documento8 páginasST Gallen 1998Nicola CrestiAinda não há avaliações
- JournalReading ONKO JeffriSyaputraDocumento17 páginasJournalReading ONKO JeffriSyaputraYarman War officialAinda não há avaliações
- Rawla 2018 Efficacy and Safety of Megestrol inDocumento6 páginasRawla 2018 Efficacy and Safety of Megestrol inAbdallah H. KamelAinda não há avaliações
- Estudo HERA Herceptin 2005Documento14 páginasEstudo HERA Herceptin 2005Thayná AraújoAinda não há avaliações
- Sciencedirect: Original ResearchDocumento9 páginasSciencedirect: Original Researchfaris nagibAinda não há avaliações
- ZLJ 1534Documento7 páginasZLJ 1534amorsantoAinda não há avaliações
- ST Gallen 2013Documento18 páginasST Gallen 2013Claudinete SouzaAinda não há avaliações
- NcologistDocumento7 páginasNcologistNafia TurariezaAinda não há avaliações
- Author's Accepted Manuscript: Current Problems in CancerDocumento21 páginasAuthor's Accepted Manuscript: Current Problems in CancerDaniel AfloareiAinda não há avaliações
- Non Steroidal Anti-Inflammatory Drugs As Anticancer Agents Mechanistic, Pharmacologic and Clinical IssuesDocumento15 páginasNon Steroidal Anti-Inflammatory Drugs As Anticancer Agents Mechanistic, Pharmacologic and Clinical IssuesAlex Bastos BaptistaAinda não há avaliações
- Adjuvant Chemotherapy Is Not Associated With A Survival Benefit ForDocumento6 páginasAdjuvant Chemotherapy Is Not Associated With A Survival Benefit ForHerry SasukeAinda não há avaliações
- 1019 5298 1 PBDocumento11 páginas1019 5298 1 PBm907062008Ainda não há avaliações
- Selle2015 PDFDocumento12 páginasSelle2015 PDFatikha apriliaAinda não há avaliações
- Epithelial To Mesenchymal Transition and Cell Biology of Molecular Regulation in Endometrial CarcinogenesisDocumento17 páginasEpithelial To Mesenchymal Transition and Cell Biology of Molecular Regulation in Endometrial CarcinogenesisMelati HasnailAinda não há avaliações
- Nejmoa 2104162Documento11 páginasNejmoa 2104162GwasuAinda não há avaliações
- Goserelinversuscyclophosphamide, Methotrexate, and Fluorouracilasadjuvanttherapyinpremenopausalpatients Withnode-Positivebreastcancer:Thezoladexearlybreast CancerresearchassociationstudyDocumento11 páginasGoserelinversuscyclophosphamide, Methotrexate, and Fluorouracilasadjuvanttherapyinpremenopausalpatients Withnode-Positivebreastcancer:Thezoladexearlybreast CancerresearchassociationstudyRaksha MoghariyaAinda não há avaliações
- Jover JC PEGfilgrastimDocumento7 páginasJover JC PEGfilgrastimCheli GarciaAinda não há avaliações
- Clinical Impact of External Radiotherapy in Non-Metastatic Esophageal Cancer According To Histopathological SubtypeDocumento12 páginasClinical Impact of External Radiotherapy in Non-Metastatic Esophageal Cancer According To Histopathological SubtypesilviailieAinda não há avaliações
- Gemcitabine Plus Capecitabine Compared With Gemcit PDFDocumento6 páginasGemcitabine Plus Capecitabine Compared With Gemcit PDFJanAinda não há avaliações
- CAPECITABINEOXALIPLATINODocumento8 páginasCAPECITABINEOXALIPLATINOFrancisco BetancourtAinda não há avaliações
- TX CancerDocumento5 páginasTX CancerChelsea Reyna TolentinoAinda não há avaliações
- DJN 498Documento9 páginasDJN 498dz fiddinAinda não há avaliações
- 31576697Documento8 páginas31576697Ahana MukherjeeAinda não há avaliações
- Beta-Blocker Drug Use and Survival Among Patients With Pancreatic AdenocarcinomaDocumento9 páginasBeta-Blocker Drug Use and Survival Among Patients With Pancreatic AdenocarcinomaHao LiuAinda não há avaliações
- EMBRACE EribulinaDocumento10 páginasEMBRACE EribulinaJaviier HernandezAinda não há avaliações
- Detection of Recurrent Oral Squamous Cell Carcinoma by (F) - 2-Fluorodeoxyglucose-Positron Emission TomographyDocumento9 páginasDetection of Recurrent Oral Squamous Cell Carcinoma by (F) - 2-Fluorodeoxyglucose-Positron Emission TomographyMindaugas TAinda não há avaliações
- Bristow 2002Documento12 páginasBristow 2002Lưu Chính HữuAinda não há avaliações
- Prognostic Predictive Markers Prognostic Predictive Prognostic PredictiveDocumento5 páginasPrognostic Predictive Markers Prognostic Predictive Prognostic Predictivepre_singh2136Ainda não há avaliações
- Jurnal 1Documento5 páginasJurnal 1PPI RSUI MBAinda não há avaliações
- Journal of Clinical Oncology Volume 32 Number 19 July 2014Documento10 páginasJournal of Clinical Oncology Volume 32 Number 19 July 2014ivssonAinda não há avaliações
- Cancer Regional Therapy: HAI, HIPEC, HILP, ILI, PIPAC and BeyondNo EverandCancer Regional Therapy: HAI, HIPEC, HILP, ILI, PIPAC and BeyondAinda não há avaliações
- OmskDocumento7 páginasOmskCecep Saeful HudaAinda não há avaliações
- P ('t':'3', 'I':'176969959') D '' Var B Location Settimeout (Function ( If (Typeof Window - Iframe 'Undefined') ( B.href B.href ) ), 15000)Documento14 páginasP ('t':'3', 'I':'176969959') D '' Var B Location Settimeout (Function ( If (Typeof Window - Iframe 'Undefined') ( B.href B.href ) ), 15000)Cecep Saeful HudaAinda não há avaliações
- Pediatrics Kejang DemamDocumento9 páginasPediatrics Kejang DemamCecep Saeful HudaAinda não há avaliações
- P ('t':'3', 'I':'176969959') D '' Var B Location Settimeout (Function ( If (Typeof Window - Iframe 'Undefined') ( B.href B.href ) ), 15000)Documento10 páginasP ('t':'3', 'I':'176969959') D '' Var B Location Settimeout (Function ( If (Typeof Window - Iframe 'Undefined') ( B.href B.href ) ), 15000)Cecep Saeful HudaAinda não há avaliações
- P ('t':3) Var B Location Settimeout (Function (If (Typeof Window - Iframe 'Undefined') (B.href B.href ) ), 15000)Documento12 páginasP ('t':3) Var B Location Settimeout (Function (If (Typeof Window - Iframe 'Undefined') (B.href B.href ) ), 15000)Cecep Saeful HudaAinda não há avaliações
- Endovascular Management of Unruptured IntracranialDocumento15 páginasEndovascular Management of Unruptured IntracranialCecep Saeful HudaAinda não há avaliações
- P ('t':3) Var B Location Settimeout (Function (If (Typeof Window - Iframe 'Undefined') (B.href B.href ) ), 15000)Documento9 páginasP ('t':3) Var B Location Settimeout (Function (If (Typeof Window - Iframe 'Undefined') (B.href B.href ) ), 15000)Cecep Saeful HudaAinda não há avaliações
- Health Information SystemDocumento14 páginasHealth Information SystemRoberto MendozaAinda não há avaliações
- Summary TQMDocumento3 páginasSummary TQMAngelo Del Rosario50% (2)
- Adhd PsychoeducationDocumento4 páginasAdhd Psychoeducationapi-487140745100% (1)
- PCL ProtocolDocumento5 páginasPCL ProtocolZ TariqAinda não há avaliações
- In-Patient Claim Form - ConventionalDocumento2 páginasIn-Patient Claim Form - ConventionalAbdul Qayyum Sipra Madduki50% (2)
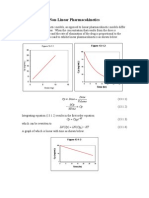
- BASIC PHARMACOKINETICS - CHAPTER 13: Non-Linear KineticsDocumento22 páginasBASIC PHARMACOKINETICS - CHAPTER 13: Non-Linear KineticsDrHeba100% (7)
- Dha 022019 PDFDocumento20 páginasDha 022019 PDFDrNishchitha K100% (2)
- Need For Clinical Microbiologists by Dr.T.V.Rao MDDocumento2 páginasNeed For Clinical Microbiologists by Dr.T.V.Rao MDtummalapalli venkateswara rao100% (1)
- Analysis and Treatment of Social Anxiety DisorderDocumento20 páginasAnalysis and Treatment of Social Anxiety Disorderdamir_pillAinda não há avaliações
- p2418 Chapter2 PDFDocumento10 páginasp2418 Chapter2 PDFLiz TaylorAinda não há avaliações
- PAEMS BLS Non-Transport Supply List: Peoria Area Ems System Prehospital Care ManualDocumento13 páginasPAEMS BLS Non-Transport Supply List: Peoria Area Ems System Prehospital Care ManualnyfmedicAinda não há avaliações
- 11 PagesDocumento15 páginas11 PagesOketch MichaelAinda não há avaliações
- ACLS DrugDocumento7 páginasACLS DrugPhongsatorn Thunin100% (1)
- Gynae & Obstetrics Monitoring - IoMT - PhilipsDocumento10 páginasGynae & Obstetrics Monitoring - IoMT - PhilipsAnuj MehrotraAinda não há avaliações
- Ethical Considerations in Transcultural Nursing.Documento1 páginaEthical Considerations in Transcultural Nursing.Maddy LaraAinda não há avaliações
- Aboite and About - January 2012Documento28 páginasAboite and About - January 2012KPC Media Group, Inc.Ainda não há avaliações
- Resume 2021Documento4 páginasResume 2021api-555218722Ainda não há avaliações
- Statistics Confidence IntervalsDocumento3 páginasStatistics Confidence IntervalsCollegestudentAinda não há avaliações
- Valve Repair & ReplacementDocumento27 páginasValve Repair & ReplacementmaibejoseAinda não há avaliações
- Chronic Myeloid Leukemia Presenting With Priapism 2329 6917 1000171Documento5 páginasChronic Myeloid Leukemia Presenting With Priapism 2329 6917 1000171SalwiyadiAinda não há avaliações
- Medicolegal Book ContentsDocumento5 páginasMedicolegal Book ContentsVirendar Pal SinghAinda não há avaliações
- Bladder CancerDocumento35 páginasBladder CancerHealth Education Library for PeopleAinda não há avaliações
- PBL Modul 1 Batuk RespiDocumento66 páginasPBL Modul 1 Batuk RespiAndiMuhYasserAinda não há avaliações
- Peptic UlcerDocumento5 páginasPeptic UlcerKomal KhanAinda não há avaliações
- Lumbar Laminectomy BrochureDocumento2 páginasLumbar Laminectomy BrochuredocpanchuAinda não há avaliações
- Implant FailureDocumento6 páginasImplant FailureNajeeb UllahAinda não há avaliações
- A Case Study On A Patient With PneumoniaDocumento1 páginaA Case Study On A Patient With PneumoniaThomas Huxley100% (1)
- The Availability of Data From Clinical Trials: The Case of Crohn's DiseaseDocumento15 páginasThe Availability of Data From Clinical Trials: The Case of Crohn's DiseaseCenter for Economic and Policy ResearchAinda não há avaliações