Escolar Documentos
Profissional Documentos
Cultura Documentos
Task 1 Completed
Enviado por
Adawiyah Az-zahraTítulo original
Direitos autorais
Formatos disponíveis
Compartilhar este documento
Compartilhar ou incorporar documento
Você considera este documento útil?
Este conteúdo é inapropriado?
Denunciar este documentoDireitos autorais:
Formatos disponíveis
Task 1 Completed
Enviado por
Adawiyah Az-zahraDireitos autorais:
Formatos disponíveis
SCHOOL OF CHEMICAL ENGINEERING
EKC 451: PROCESS DESIGN AND ANALYSIS
TASK 1
Group 1:
Leong Sim Siong 104718
Kow Shu Wen 104713
Nor Hidahyah bt Ludin 104747
Nafilah bt Khalid 104743
Supervisor: Assoc. Prof Dr Zainal Ahmad
Date of Submission: 8 October 2012
TABLE OF CONTENTS
________________________________________________
CONTENTS PAGE
1.1 Problem Statement and Letter of Transmittal
1.1.1 Gantt Chart
1.1.2 Importance of Methanol Industry from Malaysian Chemical Industry Point of
View
1.1.3 Methanol Production Plant
1.1.4 Projected Demand and Supply
1
4
5
9
13
1.2 Process Alternatives
1.2.1 Process Scheme of the Three Alternatives
1.2.2 Discussion of the Three Alternatives
1.2.3 Choice of Process
19
20
30
38
1.3 Critical Assessments of the Process Chosen
1.3.1 Capacity of the Designed Plant
1.3.2 Complete Description of the Process Chosen
1.3.3 Process Chemistry, Reactions and Kinetics
1.3.4 Thermodynamics, Physical and Chemical Properties
1.3.5 Material Safety Data Sheet
1.3.6 Critical Assessments: Advantages and Disadvantages of the Process Chosen
1.3.7 Methanol Plants in Malaysia with Total Investments
45
45
47
54
57
59
82
84
1.4 Process Synthesis Structure and Analysis
1.4.1 Input-Output Structure with Feed and Product Specifications
1.4.2 Price of All Products, Byproducts and Raw Materials
1.4.3 Destination Codes and Component Classifications
1.4.4 Utilities
85
85
90
91
92
1.5 Process Flow Diagram
93
Appendix
97
References
104
LIST OF FIGURES PAGE
1.1-1 World consumption of methanol
1.1-2 Methanol demand by end use for 2011
1.1-3 Methanol demand by end use for 2016
1.1-4 Methanol supply and demand around the world
12
12
14
18
1.2-1 Block diagram of methanol production using catalytic oxidation of methane
1.2-2 Block diagram of methanol production using CO/CO
2
hydrogenation
1.2-3 Chemical route of enzymatic synthesis of methanol from CO
2
with in situ
regeneration of NADH
1.2-4 Block diagram of methanol production using enzymatic oxidation of CO
2
1.2-5 Change in world natural gas production by region (2008 2035)
22
26
27
29
43
1.3-1 Graph of increasing natural gas production capacity
1.3-2 Cross-sectional view of methanol reactor
1.3-3 The effect of adsorbate partial pressure on the adsorbate loading.
46
51
53
1.4-1 Block diagram for methanol production
86
1.5-1 Block diagram of methanol production using CO/CO
2
hydrogenation
1.5-2 Process synthesis flow of methanol production using CO/CO
2
hydrogenation
1.5-3 Process flow diagram of methanol production using CO/CO
2
hydrogenation
94
95
96
LIST OF TABLES PAGE
1.1-1 Malaysias methanol plant with capacities
1.1-2 Major global methanol manufacturing companies with respective
annual capacities and location
1.1-3 Global methanol supply from 2005 to 2012
1.1-4 Annual methanol capacity additions and timing 000 metric tons per
year
9
11
16
17
1.2-1 Discussion of the three process alternatives
1.2-2 The activity and selectivity of the catalyst for methanol synthesis
31
39
1.3-1 Thermodynamics data
1.3-2 Physical and chemical properties
1.3-3 Advantages and disadvantages of the process chosen
1.3-4 Methanol plants in Malaysia capacity, investment
57
58
82
84
1.4-1 Natural gas specifications
1.4-2 Methanol specifications
1.4-3 Prices of components in methanol production
1.4-4 Destination codes and component classification of related chemical
processes
87
88
90
91
Plant Design For The Production of AA Grade Methanol
[Prepared by: Leong Sim Siong, Kow Shu Wen, Nor Hidahyah Binti Ludin, Nafilah Binti Khalid] 1
1.1 PROBLEM STATEMENT AND LETTER OF
TRANSMITTAL
________________________________________________
MethaChem Sdn.Bhd
Universiti Sains Malaysia (Engineering Campus)
Seri Ampangan, 14300 Nibong Tebal, Pulau Pinang,
Fax: +604-5996401, Tel: +604-5941013
________________________________________________________________________
From:
Process Design Team,
Process Design and Analysis Department,
MethaChem Sdn.Bhd 8
th
October 2012
To:
Professor Madya Dr. Zainal Ahmad,
Plant Manager,
MethaChemSdn.Bhd.
Dear Sir,
Plant Design for the Production of AA grade Methanol
Enclosed is a copy of the primary stage of the plant design for the production of AA
grade methanol. The plants have been designed for the production of methanol. The
expected production capacities of methanol from the plants are 900,000 tons per year.
In Malaysia, the demand towards methanol has been gradually increased throughout the
past 10 years and the projected demand is even higher. Methanol is favorable especially
in the chemical and petrochemical industries. It is a basic building block for the
Plant Design For The Production of AA Grade Methanol
[Prepared by: Leong Sim Siong, Kow Shu Wen, Nor Hidahyah Binti Ludin, Nafilah Binti Khalid] 2
manufacture of petrochemicals and other derivatives such as Acetic Acid (solvents),
MTBE (octane enhancer), Formaldehyde (resins, adhesives) and others. Besides, the
Malaysia government also plans to enlarge the current methanol production capacity in
order to support the high demands. In view the potential profit from this industry, we
choose AA grade methanol production as our major plant in this design.
We choose natural gas as the raw material as it provides high conversion and selectivity
towards the production of AA grade methanol through catalytic industrial process
directly from carbon monoxide, carbon dioxide and hydrogen.
The scope of this design project involves developing the most suitable process not only
from the aspect of profits, but we also considered the safety and health requirement of the
society. We try to design the plants with less impact on the environment. The deliverables
of this project include the selection of the best path to produce AA grade methanol, the
cost, the process flow diagram, mass balance, energy balance, process and equipment
optimization, heat integration, reactor sizing, operating condition and also market
analysis.
This project is expected to be done by the end of December 2012. We will continue the
second stage and will deliver it by 9th November 2012, while the final complete design
report will be delivered by 21st December 2012 for approval.
Lastly, if you need further clarification and information regarding this project, please feel
free to contact us through the phone number or e-mail stated below. We will make further
correction if there is error inside our project. Thank you for your support and guidance
and we hope to hear from you soon.
Plant Design For The Production of AA Grade Methanol
[Prepared by: Leong Sim Siong, Kow Shu Wen, Nor Hidahyah Binti Ludin, Nafilah Binti Khalid] 3
Sincerely,
__________________________________
(LEONG SIM SIONG)
Leader of plant design (task 1)
h/p: 016-6338702
email: ssleong_1989@hotmail.com
________________________________
(KOW SHU WEN)
Member of plant design
h/p: 016-7374799
email: kagome0818@hotmail.com
__________________________________
(NOR HIDAHYAH BINTI LUDIN)
Member of plant design
h/p: 017-75877186
email: diya_hidaya@yahoo.com
_________________________________
(NAFILAH BINTI KHALID)
Member of plant design
h/p: 017-3959561
email: nafila_fila@yahoo.com
Plant Design For The Production of AA Grade Methanol
[Prepared by: Leong Sim Siong, Kow Shu Wen, Nor Hidahyah Binti Ludin, Nafilah Binti Khalid] 4
1.1.1 GANTT CHART
Project Name: Plant Design for the Production of AA grade Methanol
Company Name: MethaChem Sdn. Bhd.
Project Manager: Leong Sim Siong
Date of Report: 5
th
of October 2012
Task Start
Date
End date Days completed Days
remaining
Introduction 9/10/2012 9/19/2012 8 1
Process Alternatives 9/19/2012 9/21/2012 1 2
Critical Assessments 9/22/2012 9/25/2012 1 3
Process Synthesis Structure and Analysis 9/26/2012 9/30/2012 1 4
Process Flow Diagram 10/1/2012 10/5/2012 1 4
10/09/2012 15/09/2012 20/09/2012 25/09/2012 30/09/2012 05/10/2012
Introduction
Process Alternatives
Critical Assesments
Processs Synthesis Structure
and Analysis
Process Flow Diagram
Start Date
Days completed
Days remaining
Plant Design For The Production of AA Grade Methanol
[Prepared by: Leong Sim Siong, Kow Shu Wen, Nor Hidahyah Binti Ludin, Nafilah Binti Khalid] 5
1.1.2 IMPORTANCE OF METHANOL INDUSTRY FROM
MALAYSIAN CHEMICAL INDUSTRY POINT OF VIEW
AA grade methanol consists of 99.85% of methanol by mass, which is methanol with
very high purity.
1
In this modern era, methanol is playing an important role in humans
life. The importance of methanol industry from Malaysia chemical industrial point of
view is discussed in the following section.
Chemical Feedstock
2,3
Methanol is an important feedstock which is used to synthesis other useful chemical
compounds and materials. For example, methanol is the raw material for the
formaldehyde production, a common building block utilized to produce urea
formaldehyde resin and melamine resin. Hence, the high demand of methanol in
Malaysia is mainly due to the drastic development of formaldehyde industry.
Nevertheless, methyl tert-butyl ester (MTBE), which is a chemical compound
synthesized by the reaction of methanol and isobutylene, is playing a vital role as the
antiknock reagent in gasoline to protect and lengthen the lifetime of vehicle engine.
Besides, methanol is the basic and fundamental building block of many chemicals in our
daily lives. There are plenty of useful products which can be derived from methanol,
indicating that methanol is a critical reagent to improve humans standard of life. Some
types of materials that can be made from methanol including:
Plastics
Synthetic fibers
Paints
Resins
Solvents
Magnetic film
Adhesives
Insulation
Refrigerants
Plant Design For The Production of AA Grade Methanol
[Prepared by: Leong Sim Siong, Kow Shu Wen, Nor Hidahyah Binti Ludin, Nafilah Binti Khalid] 6
Transportation Fuel
3
Methanol is the simplest compound in alcohol homologous series, with only one carbon
atom per molecule; therefore, it is easy to burn and has a high octane rating. Such
properties enable methanol to behave as a superior transportation fuel compared to the
gasoline which is currently in used. Due to the high performance of methanol as burning
fuel for transportation engine, the lifetime of the engine can be lengthened as knocking is
greatly reduced. There are a lot of researches being carried out in order to lower the
production cost and promote the methanol-fueling program. Furthermore, methanol
burning will emit lesser particulate and smoke than reformulated gasoline. Therefore, the
utilization of methanol as burning fuel can reduce the environmental pollution and
improve the air quality in our nation.
4
In term of biodiesel production, methanol is a key component of the transesterification
process. Biodiesel is an alternative diesel fuel derived from vegetable oils or animal fats.
Due to the high viscosity and low volatility of vegetable oils and animal fats, they cannot
be used as combustible fuel as it may solidify and clog the engine chamber. Hence,
transesterification of the oils and fats with methanol is essential to produce fatty acid
methyl ester which is more suitable to be utilized as fuel. Fatty acid methyl ester, which
is commonly known as biodiesel, has smaller molecular weight compared to fatty acid
which contributes to its lower viscosity and higher volatility is more suitable to be used
as engine fuel. This is an important alternative energy source as the energy crisis of the
world is getting more serious in this modern era.
5
Plant Design For The Production of AA Grade Methanol
[Prepared by: Leong Sim Siong, Kow Shu Wen, Nor Hidahyah Binti Ludin, Nafilah Binti Khalid] 7
Fuel Cell Technology
6
Fuel cell is a newly developed electrochemical device to convert the chemical energy of a
reaction directly to electrical energy. Fuel cell is an alternative to replace conventional
energy converting device such as petroleum and natural gas which is getting depleted
currently. Methanol can used the fuel in direct-methanol fuel cell as it is easy to transport,
an energy-dense yet reasonably stable liquid at all environmental conditions. Although
the efficiency of the fuel cell is still quite low, more research is now being carried out to
improve the capability of the fuel cell to convert chemical energy of the methanol to
electrical energy. It is believed that fuel cell has a high potential to play an important role
as essential energy converting device in the near future.
Wastewater Denitrification
3
Methanol is also used in wastewater treatment system to remove nitrogen from the
effluent streams. The wastewater produced by some of the industries may contain high
level of ammonia, which will be converted into nitride through a bacterial degradation
process. If the water contains high concentration of nitride is being discharged into the
river, it can cause eutrophication as excessive algae will grow along the river due to the
high amount of nutrient. This will have a devastating effect on water ecosystems as
biological oxygen demand (BOD) of the river will increase. Furthermore, the algae will
block the sunlight from reaching the aquatic life and prohibiting photosynthesis.
Methanol can play an essential role in wastewater denitrification as it is an effective
biodegrade which can help to revitalize waterways tainted by the effects of nitrates cost-
effectively.
Availability of Raw Material
7
Malaysia, which is located beside South China Sea, has the potential to develop methanol
production industry as the raw material is easily available. There are several oilfields,
such as off-shore of the state of Terengganu, Sabah and Sarawak, which have abundant
natural gas reservoir. Since natural gas is one of the main components to produce
Plant Design For The Production of AA Grade Methanol
[Prepared by: Leong Sim Siong, Kow Shu Wen, Nor Hidahyah Binti Ludin, Nafilah Binti Khalid] 8
methanol, the availability of natural gas is attracting more investors to develop methanol
industry in Malaysia.
Economic Growth
The methanol production industry in Malaysia accounts for around 3% of the overall
production in the Asia-Pacific region. Its demand is expected to increase in the coming
years quite significantly due to a healthy demand from the formaldehyde-based resin and
automobile fuel sector. The growth in demand for methanol in Malaysia is expected to be
at the rate of 5% during the forecast period, and the majority of domestic demand would
be fulfilled by imports.
8
Therefore, it is profitable to construct methanol production plant
in Malaysia as the demand of methanol is higher than production. For example,
PETRONAS started methanol production at capacity of 660,000 ton per year in Labuan
on January of 2007. Nevertheless, the development and advancement of the methanol
production technology may boost the economic growth of our nation and hence improve
the standard of living in Malaysia.
Plant Design For The Production of AA Grade Methanol
[Prepared by: Leong Sim Siong, Kow Shu Wen, Nor Hidahyah Binti Ludin, Nafilah Binti Khalid] 9
1.1.3 METHANOL PRODUCING PLANT
As the production of methanol is important for the chemicals and petrochemicals industry,
a lot of methanol production plants have been operated throughout the world. Generally,
methanol is being produced as a raw material to make formaldehydes, acetic acid MTBE
(octane enhancer) and etc. With the rapid development of this industry, the world
methanol production capacity burst up causing the scale of ethylene production became
enormous; the size of a typical world-scale methanol unit now may ranges from 75 to 80
million metric ton per year.
Malaysia
Although currently a minor player on the Far East petrochemicals scene, Malaysia has a
strong and growing petrochemical industry with a nameplate methanol capacity
approaching 1.7 million tons per year
9
. In Malaysia, the methanol production plants use
natural gas as the feed. Table 1 below shows the Methanol plants available in Malaysia
with their production capacities. PML1 is shut down on 26th July 2012 after a fire
erupted at a chemical tanker at a nearby port early in the day. However, since the second
unit PML2 doesnt involve in the disaster, PML2 is still operating normally
10
.
Table 1.1-1: Malaysia's methanol plants with it capacities
Company Location Capacity (Tons/Year)
PETRONAS Methanol
(Labuan) SdnBhd
Labuan, Malaysia PML1 - 660,000
PML2 - 1.7 million
PETRONAS Chemical
Fertilizer (Kedah) SdnBhd
Gurun, Kedah 730,000
Plant Design For The Production of AA Grade Methanol
[Prepared by: Leong Sim Siong, Kow Shu Wen, Nor Hidahyah Binti Ludin, Nafilah Binti Khalid] 10
World
Methanol production plants can be found throughout the world. No less than 2.9 million
Metric Tons of new methanol capacities were announced for startup in North American
by 2015. Meanwhile from 2010 to 2015, North American domestic demand is expected
to grow by around 670,000 MT, meaning that the capacity additions far exceed demand
growth and a large volume of imports will not be needed due to this growth in capacity
11
.
With domestic capacity running at 85% on average, in effect we will see the region shift
from a situation in which imports represent around 95% of domestic demand to a market
in which imports represent around 58% of domestic demand. On a volume basis this
means that around 2 million MT less methanol will be sent to North America in 2015
when compared to 2010.
12
China will restructure its methanol industry in the next five years with improvements in
energy conversion rates, rationalization of uncompetitive assets, and controls on new
builds. In derivatives, China will encourage strategic new sectors in specialties, new
energy feedstock, and bio-pharma. The Methanol-to-Olefins (MTO) and Methanol-to-
Propylene (MTP) will receive emphasis with production in resource-rich regions.
MTO/MTP is fast emerging with yet unknown implications to the merchant methanol
market, growing from nothing in 2009 to 20.1 million MT of demand in 2016. With some
of the MTO/MTP plants sourcing from the merchant market and many plants sourcing
merchant material (with up to 2 million MT methanol demand rates) during extended
start-ups, this has a dramatic impact on merchant methanol.
13
There are no further capacity additions in the Middle East apart from Iran due to gas
availability issues. Iran has 14 methanol projects under consideration totaling 4 million
MT forecasted to come on-stream by the end of 2016. However, the November 2011
sanctions targeting the petrochemical industry are likely to delay these projects. There is
little demand growth in West Europe through to 2016 averaging just 2.5%. Africa is set
to become the new Middle East with ever increasing quantities of gas being discovered in
Ghana, Mozambique, Nigeria and Tanzania and the likelihood of further capacity being
Plant Design For The Production of AA Grade Methanol
[Prepared by: Leong Sim Siong, Kow Shu Wen, Nor Hidahyah Binti Ludin, Nafilah Binti Khalid] 11
added in Algeria and Egypt.
14
Projects totaling 9.6 million MT have been identified but
unlikely to be on-stream until after 2016.
Below are the methanol production plants and their location;
Table 1.1-2: Major Global Methanol Manufacturing Companies with Respective Annual
Capacities and Location
Company Location Production Capacity
(tons/year)
Atlantic Methanol Production Company
LLC (AMPCO)
Equatorial Guinea, West
Africa
1,000,000
Brunei Methanol Kg. Sungai Liang Daerah
Belakit KC11335, Brunei
850,000
Kaltim Methanol Industri PT, Indonesia 660,000
Yanzhou Group Yulin Branch Shangdong, China 2,300,000
Azerbaijian Methanol Company Baku, Azerbaijian 561,000
Chang Chun Petrochemical
Cooperation
Miao-Li, Taiwan 18,000
Cangzhou Fine Chemical Company Hebei, China 200,000
Caribbean Methanol (Methanol II) Caribbean methanol (II) 547,500
Methanex Chilean
Trinidad
New Zealand
Damietta, Egypt
3,800,000
2,100,000
530,000-2,400,000
1,300,000
Tianjin Soda Plant Tianjin City, China 500,000
New Oriental Energy & Chemical
Corp
Luoshan, Henan, China 200,000
Blue Chemical & CNOOC King Board
Chemical
Hainan Island, China 600,000
Anhui Huai Hua Group Anhui, China 100,000
Plant Design For The Production of AA Grade Methanol
[Prepared by: Leong Sim Siong, Kow Shu Wen, Nor Hidahyah Binti Ludin, Nafilah Binti Khalid] 12
Company Location Production Capacity
(tons/year)
Haicheng Xiyang Fireproofing
Company
Chongqing, China 300,000
Shandong Jiutai Chemical Technical
Company
Shandong, China 900,00
Panjin Zhongrun Chemical Plant Hebei, China 100,000
Plant Design For The Production of AA Grade Methanol
[Prepared by: Leong Sim Siong, Kow Shu Wen, Nor Hidahyah Binti Ludin, Nafilah Binti Khalid] 13
1.1.4 PROJECTED DEMAND/SUPPLY
1.1.4.1 Methanol Demand
The following pie chart shows world consumption of methanol:
Figure 1.1-1: World consumption of methanol
Over the last two decades, a major shift in regional methanol capacity and production has
occurred. Countries with large reserves of natural gas and often limited domestic
consumption have built world-scale methanol facilities to attempt to monetize their low-
cost natural gas. The largest producing region/country in 2008 was China and it will
continue to have the largest production capacity and be the largest producer in 2013. The
China blended nearly 1 billion gallons of methanol in gasoline followed by Western
Europe, United Stated as shown in Figure 1.1-1 above.
15
Another significant factor in methanol demand is that the new mega-methanol plants
(1.02.0 million metric tons per year) are much larger than existing plants. Thus, they
will have reduced fixed costs, as well as greatly reduced natural gas costs because of their
strategically located feedstock, giving a significant cost advantage. This will drive down
the cost of methanol, and cause major shifts in trade patterns. This cost-competitive
position will also make the methanol-to-olefins technology more competitive with
Plant Design For The Production of AA Grade Methanol
[Prepared by: Leong Sim Siong, Kow Shu Wen, Nor Hidahyah Binti Ludin, Nafilah Binti Khalid] 14
existing olefins technologies. Locations for these large new methanol plants are Iran,
Saudi Arabia, Oman, and Trinidad and Tobago.
11
The pie chart below indicates the
Methanol Demand by End Use in the year of 2011 and 2016.
Figure 1.1-2: Methanol Demand by End Use for 2011
16
Figure 1.1-2: Methanol Demand by End Use for 2016
16
Plant Design For The Production of AA Grade Methanol
[Prepared by: Leong Sim Siong, Kow Shu Wen, Nor Hidahyah Binti Ludin, Nafilah Binti Khalid] 15
Worldwide, around 32% of methanol is consumed in the production of formaldehyde.
Demand is driven by the construction industry since formaldehyde is used primarily to
produce adhesives for the manufacture of various construction board products. This is
anticipated to fall to 25% by 2016 with Gasoline and Fuel applications becoming the
largest demand sector, totaling 31%.
17
While there is growing interest in methanol-to-olefins (MTO) technologies as a result of
the large new methanol capacities coming on line, the operational costs are what make
MTO attractive, since the capital costs are considerably larger than a traditional olefins
cracker. In the year 2011, there are 6% of methanol is used for MTO; however, it
increases to 22% in the year of 2016. The critical issue in evaluating production
economics and the feasibility of building such a complex is securing a long-term supply
agreement for inexpensive methanol, in order to offset the very large capital costs
associated with MTO plants. Another key factor making MTO economically attractive is
a secure source of isolated natural gas feeding the mega-methanol plant.
18
There is also much interest in developing methanol-to-propylene (MTP) technology
because of the interest in direct production of propylene (on-purpose) as opposed to
producing it as a coproduct of ethylene in steam cracking of various heavy feedstocks.
The pie chart above had shown the increment of methanol consumption to MTP in the
year 2016.
The following market for methanol is methyl tertiary-butyl ether (MTBE) with 10% of
world methanol demand in 2011. Methanol consumption for MTBE has been predicted to
be decline in the year 2016 and it may because of the consumption of MTBE have only
been for export markets or for the export-directed gasoline pool. In other regions of the
world, especially where lead compounds are currently used to maintain octane levels,
some growth for MTBE is still possible. Worldwide, methanol consumption for MTBE
will be declining since 2016; an average decline of 0.6% per year worldwide is likely
from 2011 to 2016, and very soon, MTBE will no longer be the a few largest world
markets for methanol. Obviously, the pie chart above had also shown that the methanol
Plant Design For The Production of AA Grade Methanol
[Prepared by: Leong Sim Siong, Kow Shu Wen, Nor Hidahyah Binti Ludin, Nafilah Binti Khalid] 16
demand increases from 55.4 million metric tons in the year 2011 to 92.3 million metric
tons in the year 2016.
1.1.4.2 Methanol Supply
Worldwide, over 90 methanol plants have a combined production capacity of about 75
million metric tons (almost 24 billion gallons or 90 billion liters), and each day more than
100,000 tons of methanol is used as a chemical feedstock or as a transportation fuel (33
million gallons or 125 million liters).
19
Methanol is also a truly global commodity, and
each day there is more than 80,000 metric tons of methanol shipped from one continent to
another. Key to increasing capacities is the availability of low cost raw materials and also
the world methanol demand is expected to cross 96 million metric tons by 2016 as per
Global Industry Analysts. The table below shows the global methanol supply in the year
2005 to 2010.
Table 1.1-3: Global Methanol Supply from 2005 to 2010
2005 2006 2007 2008 2009 2010
Supply
Total capacity 43,349 46,061 52,089 59,117 65,475 75,104
Macro Operation
Rate
82.5% 78.7% 74.9% 68.1% 64.3% 62.9%
Production 35,773 36,267 39,034 40,276 42,106 45,198
Imports 18,943 19,387 19,279 20,231 22,503 22,331
Total Supply 54,716 55,654 58,313 60,507 64,609 67,528
Approximately 10 million MT of new capacity was added in 2011, most of which was in
China (IHS Chemical, 2012):
- 1,000,000 Metric Tons per year
- 850,000 Metric Tons per year
470,000 Metric Tons per year (re-start of idled capacity)
Plant Design For The Production of AA Grade Methanol
[Prepared by: Leong Sim Siong, Kow Shu Wen, Nor Hidahyah Binti Ludin, Nafilah Binti Khalid] 17
7,770,000 Metric Tons per year
Methanex restarted their 470,000 Metric Tons per year methanol plant in Medicine Hat,
Alberta, Canada. The current lower natural gas price environment in North America has
made the Medicine Hat plant a competitive new supply to the region.
20
The rest
represents new methanol production. The table below shows the Annual Methanol
Capacity additions and timing ('000 Metric Tons per year).
12
Table 1.1-4: Annual Methanol Capacity additions and timing'000 Metric Tons per year
According to Global Industry Analysts, the global market for Methanol is forecast to
reach 100 million metric tons by the year 2017, stimulated by increase in production
capacity worldwide, infusion of substantial investment and entry of new players in the
market. In addition, burgeoning Methanol demand from developing markets of Asia,
Middle East, and Latin America will retain the rapid growth momentum. In the long-term,
Methanol will gain from its tremendous potential as alternate transport fuel and use in
environment friendly, bio-fuels and chemical feedstock.
21
Plant Design For The Production of AA Grade Methanol
[Prepared by: Leong Sim Siong, Kow Shu Wen, Nor Hidahyah Binti Ludin, Nafilah Binti Khalid] 18
Figure 1.1-3: Methanol Supply and Demand around the World
Please consider the chart above, which describes the global supply and demand outlook
from 2005 through current estimate for 2015.
22
Very obviously, the 6 million metric ton
per year jump in methanol demand in 2010 is the highlight of the chart. China with total
demand of around 20.5 million metric tons is driven by the relatively low calorific value
of methanol, as well as positive economic growth rate. Coupled with slight postponement
for some new capacities globally, aggregate operating rate has increased in 2010.
Capacity totals here include phantom capacity around the world. (i.e. Iran, Chile, China,
Malaysia, Russia, Europe produces having feedstock during the year).
22
Besides, global methanol markets are set to steadily improve, not only demand wise, but
in operational terms, reaching 61.4 million metric tons globally by 2015. In 2012, the
improvements noted in 2011 are expected to continue.
Plant Design For The Production of AA Grade Methanol
[Prepared by: Leong Sim Siong, Kow Shu Wen, Nor Hidahyah Binti Ludin, Nafilah Binti Khalid] 19
1.2 PROCESS ALTERNATIVES
________________________________________________
Methanol can be made from variety of feedstock, making it one of the most flexible
chemical commodities and energy sources available today. One of the most traditional
method to produce methanol through the production of synthesis gas as intermediate
product, which is composed of carbon monoxide and hydrogen gas as its main
components. There are a lot of ways to produce synthesis gas. Natural gas is the most
common raw material to produce synthesis gas due to the abundance of its resources in
Malaysia. Almost all methanol plants around the world are using natural gas as raw
material as natural gas can be converted into synthesis gas (syngas) which can then being
utilized to synthesis methanol. This process employs hydrogenation of carbon monoxide
and carbon dioxide to produce methanol, which is catalyzed by copper catalyst.
23
Apart from carbon oxides hydrogenation, direct oxidation of methane also can synthesis
methanol. The oxidation can be carried out by using catalysts such as palladium and
copper.
24
Bioprocess also can be utilized in methanol production industry. Enzymatic reduction of
carbon dioxide has been proven effective in methanol synthesis. Enzyme and cofactor
which immobilized on the surface of polystyrene particles is able to catalyze the carbon
dioxide reduction to produce methanol.
25
Plant Design For The Production of AA Grade Methanol
[Prepared by: Leong Sim Siong, Kow Shu Wen, Nor Hidahyah Binti Ludin, Nafilah Binti Khalid] 20
1.2.1 PROCESS SCHEME OF THREE ALTERNATIVES
1.2.1.1 Catalytic Oxidation of Methane
24
Direct oxidation of methane to methanol is one of the alternative routes to synthesis
methanol in a large scale. However, catalysts role is essential in this kind of reaction as
the selectivity and yield of methanol is highly dependent on the suitability of the catalyst
selected. Some of the substances have proven their capability to catalyze the methane
oxidation reaction and produce methanol. The catalysts which are going to discuss in the
following section are palladium and copper. The combination of palladium and copper as
catalyst can oxidize methane to produce methanol.
The reaction involves catalytic oxidation of methane by oxygen in a mixture of water and
trifluoroacetic acid also can synthesis methanol. A combination of metallic palladium and
copper chloride acts as the catalyst and either carbon monoxide or hydrogen is required
as a coreductant. Studies indicate that the overall transformation encompasses three
catalytic steps as the reaction mechanism:
1. Water gas shift reaction involving the oxidation of carbon monoxide to carbon
dioxide with the simultaneous formation of hydrogen. However, it is possible to
bypass this step by replacing carbon monoxide with hydrogen.
2. Combination of hydrogen with oxygen to yield hydrogen peroxide (or its
equivalent).
3. Metal catalyzed oxidation of the alkane by hydrogen peroxide (or its equivalent).
The activation parameters for the overall reaction, determined under the condition when
the rate is first-order in both methane and carbon monoxide are given by
Plant Design For The Production of AA Grade Methanol
[Prepared by: Leong Sim Siong, Kow Shu Wen, Nor Hidahyah Binti Ludin, Nafilah Binti Khalid] 21
The role of metallic palladium is two-fold. First, it generates hydrogen peroxide (or its
equivalent). Second, it causes non-specific over oxidation of the substrate using the
hydrogen peroxide thus generated. This latter reaction is suppressed when copper
chloride is added; instead, copper chloride appears to catalyze the selective hydroxylation
of the alkane by hydrogen peroxide.
The overall reaction is given by
Process Scheme
The raw material to produce methanol by using this route is methane. Natural gas is the
main source of methane. Methane is fed into a catalytic reactor containing catalyst, which
are palladium and copper. Besides methane, carbon monoxide and oxygen (from air) also
being fed into the reactor in a right proportional to react with methane such that methanol
can be produced. The temperature of the catalytic reactor is maintained at 600C so that
the reaction system is in the gaseous state.
The gaseous product from the reactor is then channeled into a condenser in order to lower
the temperature of the product. Hence aqueous methanol is condensed as liquid and
separated from non-condensable carbon dioxide, carbon monoxide, oxygen and nitrogen.
Plant Design For The Production of AA Grade Methanol
[Prepared by: Leong Sim Siong, Kow Shu Wen, Nor Hidahyah Binti Ludin, Nafilah Binti Khalid] 22
Some of the unreacted carbon monoxide is recycled back to the reactor while the
remaining is purged out to avoid the accumulation of nitrogen in the reaction system.
The liquid methanol is then purified in a distillation column to produce AA grade
methanol with purity of 99.85%. Water is separated from the methanol in distillation
column due to their boiling temperature difference.
Figure 1.2-1 Block diagram of methanol production using catalytic oxidation of methane
Desulphurizer
Natural gas
Catalytic Reactor
(Catalyst: Cu and
Pd)
Condenser/
Separator
Distillation
Column
Methane
Methanol + Unreacted gas
Crude Methanol
AA Grade Methanol
Purged
Stream
Recycled
Stream
Air + Carbon monoxide
Water
Plant Design For The Production of AA Grade Methanol
[Prepared by: Leong Sim Siong, Kow Shu Wen, Nor Hidahyah Binti Ludin, Nafilah Binti Khalid] 23
1.2.1.2 Hydrogenation of CO/CO
2
23
Formation of Syngas
Hydrogenation of CO/CO
2
to obtain methanol employs synthesis gas (syngas) as
intermediate which can be produced by coal and natural gas. Syngas is a mixture of
hydrogen and carbon monoxide (CO) as well as carbon dioxide (CO
2
). In order to
produce syngas from coal, partial oxidation and steam treatment is required.
(Partial oxidation)
(Steam Reforming)
However, most of the syngas today is produced by using natural gas as raw material.
Methane, which is the main component in natural gas, is the most important constituent
to produce syngas. This process is known as steam reforming, where methane reacts with
steam on a nickel catalyst at moderate pressure of 25 bar (or 25atm) and high temperature
(around 850C). The syngas is produced according to the following chemical equations:
Methanol Production
26
Carbon monoxide and hydrogen from the syngas can react to produce methanol as the
final product. The synthesis of methanol from syngas is typically conducted over a
heterogeneous catalytic system, the most popular catalyst being utilized in this reaction is
coprecipitated Cu/ZnO/Al
2
O
3
, which is a reduced form of CuO/ZnO/Al
2
O
3
. In this
catalyst, alumina is the support for the active catalyst Cu. Alumina can be replaced by
other similar supports such as ThO
2
. Under the pressure of 80 bar (80atm) and 230C,
this catalyst can catalyze the production of methanol from carbon monoxide and
hydrogen (syngas) with very high selectivity, which is higher than 99.8% according to
the following chemical equation:
Plant Design For The Production of AA Grade Methanol
[Prepared by: Leong Sim Siong, Kow Shu Wen, Nor Hidahyah Binti Ludin, Nafilah Binti Khalid] 24
Under this catalytic reaction, two moles of molecular hydrogen combine with one mole
of carbon monoxide to produce one mole of methanol. However, if methane is the raw
material of the syngas production, three moles of molecular hydrogen will be produced
for every one mole of carbon monoxide. Thus, there is hydrogen gas produced in excess,
which is considered a waste if it is released to atmosphere without further processing. In
order to maximize and optimize the usage of raw material, one way of dealing with the
excess hydrogen is suggested. Under such circumstances, carbon dioxide is injected into
the methanol synthesis reactor where it reacts with the excess hydrogen to form ethanol
according to the following equation:
Process Scheme
Natural gas is the raw material to produce methanol via this route. Natural gas is
hydrocarbon gas mixture consisting primarily of methane, with other impurities such as
ethane and hydrogen sulfide. Since the main material we wish to obtained from natural
gas is methane, the raw natural gas need to be desulphurised in order to remove
impurities which will alter the reaction and destroy the downstream units.
The desulphurised natural gas is then undergoing steam reforming by mixing with steam
and converted to synthesis gas in the reformer over nickel catalysts at 20 bars to 35 bar
pressure and at temperatures of 800C to 950C. The steam reformer is a top-fired
reformer with tubes made of centrifugally-cast high alloy steel and propriety cold outlet
manifold system to enhance reliability.
The reformed gas at the reformer outlet is a mixture of hydrogen, carbon monoxide,
carbon dioxide and residual methane. It is then cooled down and the heat from the
synthesis gas is recovered by steam generation and heating of the crude methanol
distillation section.
Plant Design For The Production of AA Grade Methanol
[Prepared by: Leong Sim Siong, Kow Shu Wen, Nor Hidahyah Binti Ludin, Nafilah Binti Khalid] 25
After the heat recovery, the synthesis gas is compressed to synthesis pressure, which
ranges from 40-110 bars (depending on plant capacity) before entering the methanol
reactor. In the reactor, crude methanol is produced.
Crude methanol from the reactor is then flashed and separated from the unreacted gas in
the separator and routed to the crude methanol distillation column. The unreacted gas is
recycled into the reactor in order to increase the net conversion of the reaction system.
Plant Design For The Production of AA Grade Methanol
[Prepared by: Leong Sim Siong, Kow Shu Wen, Nor Hidahyah Binti Ludin, Nafilah Binti Khalid] 26
Figure 1.2-2 Block diagram of methanol production using CO/CO
2
hydrogenation
Distillation
Column
AA Grade Methanol
Flashing Column
Purged
Stream
Methanol
Reactor
Recycled
Stream
Compressor
Desulphurizer
Natural gas
Steam Reformer
(Catalyst: Ni)
Heat Exchanger
Methane
Carbon Monoxide + Hydrogen
Steam
Water + Impurities
Plant Design For The Production of AA Grade Methanol
[Prepared by: Leong Sim Siong, Kow Shu Wen, Nor Hidahyah Binti Ludin, Nafilah Binti Khalid] 27
1.2.1.3 Enzymatic Reduction of CO2
This route involves the utilization of an immobilized enzyme-cofactor catalytic system to
produce methanol. Four enzymes including formate, formaldehyde, alcohol and
glutamate dehydrogenases were coimmobilized using the same particles as that used for
cofactor immobilization (enzymes and cofactor were immobilized separately). Cofactors
function as electron carries in these reactions. In particular, NAD (H) and NADP (H) are
widely needed cofactors for the biosynthesis of chiral alcohols, amino acids, aldehyde,
ketones and etc. In order to get methanol, reactions were performed by bubbling CO
2
in a
suspension solution of particle-attached enzymes and cofactor. It appeared that the
collision among the particles afforded sufficient interactions between the cofactor and
enzymes, and thus enabled the sequential transformation of CO
2
to methanol along with
cofactor regeneration. The most extensively used cofactor regeneration method for bio-
processing is substrate-driven enzyme catalyzed reactions.
25
The reaction for synthesis of methanol from CO2 is shown in the figure below.
Figure1.2-3: Chemical route of enzymatic synthesis of methanol from CO
2
with in situ
regeneration of NADH.
25
The overall reaction is given by the chemical equation below:
[]
Plant Design For The Production of AA Grade Methanol
[Prepared by: Leong Sim Siong, Kow Shu Wen, Nor Hidahyah Binti Ludin, Nafilah Binti Khalid] 28
Process Scheme
Carbon dioxide is the main component to produce methanol through this process. Carbon
dioxide is first entering a saturator such that the carbon dioxide stream is saturated with
water before entering the batch reactor.
In the reactor, there is a solution of glutamate in 0.1 M pH 7.0 phosphate buffer with
polystyrene particles carrying NADH and enzymes which is in suspension. Methanol can
be produced by bubbling the carbon dioxide saturated with water through the solution in
the reactor.
The aqueous solution from the reactor is easily separated from the polystyrene particles
by only simple filtration. Methanol is then separated from the solution by undergoing
distillation process. Methanol is separated from the catalytic solution due to the boiling
point difference of methanol and the remaining solution.
Plant Design For The Production of AA Grade Methanol
[Prepared by: Leong Sim Siong, Kow Shu Wen, Nor Hidahyah Binti Ludin, Nafilah Binti Khalid] 29
Figure 1.2-4 Block diagram of methanol production using enzymatic oxidation of CO
2
Saturator
Methanol
Reactor
Filter
Distillation
Column
Carbon Dioxide
Carbon Dioxide saturated with Water
Glutamate solution in
pH 7.0 phosphate buffer
Polystyrene particles
carrying NADH and
enzyme
Crude Methanol
AA Grade Methanol
Water + Impurities
Plant Design For The Production of AA Grade Methanol
[Prepared by: Leong Sim Siong, Kow Shu Wen, Nor Hidahyah Binti Ludin, Nafilah Binti Khalid] 30
1.2.2 DISCUSSION OF THE THREE ALTERNATIVES
The three alternatives of the process scheme to produce AA methanol is listed below:
1. Catalytic oxidation of methane
2. CO/CO
2
hydrogenation
3. Enzymatic reduction of carbon dioxide
All the alternatives will be discussed in terms of green chemistry and sustainability,
environmental impact, energy consumption, flexibility of operation, safety factors and
waste management. The description of each aspect is tabulated and summarized in the
table below. Each alternative has its advantages as well as disadvantages. Hence, a
thorough study and analysis on each alternative is required in order to select the best
process scheme to be utilized in the plant.
Plant Design For The Production of AA Grade Methanol
[Prepared by: Leong Sim Siong, Kow Shu Wen, Nor Hidahyah Binti Ludin, Nafilah Binti Khalid] 31
Table 1.2-1: Discussion of three process alternatives in terms of green chemistry and sustainability, environmental impact, energy
consumption, flexibility of operation, safety factors and waste management
ALTERNATIVES Catalytic Oxidation of
Methane
CO/CO
2
Hydrogenation Enzymatic Reduction of CO
2
Green Chemistry and Sustainability
Raw Material Natural gas Natural gas Carbon dioxide
Is the raw material
renewable or depleting?
Depleting Depleting Renewable
Chemical Reaction Catalytic Oxidation:
Methane + oxygen + carbon
monoxide
Methanol + carbon
dioxide
(Catalyst: Pd + Ni)
Steam Reforming:
Methane + water
Carbon monoxide +
hydrogen
(Catalyst: Ni)
Methanol Production:
Carbon monoxide + hydrogen
Methanol
(Catalyst: Cu/Al
2
O
3
)
Enzymatic Reduction:
Carbon dioxide + Reductant
Methanol
(Catalyst: Enzyme and cofactor)
Catalyst Used Copper and metallic palladium Copper/ zinc oxide/ aluminum
oxide/ chromites oxide
Formate/ formaldehyde/
alcohol/ glutamate
dehydrogenase (enzymes);
NADH (cofactor)
Regeneration of Catalyst Yes Yes No
Conversion (%) 99 99.85 40.00-90.00
Operation Condition Temperature: 600
o
C
Pressure: 9.86-29.61atm
Temperature: 230
o
C
Pressure: 50-100 atm
Temperature: 40
o
C
Pressure: 1 atm
Byproduct(s) Carbon dioxide None None
Is the byproduct
hazardous?
No No No
Plant Design For The Production of AA Grade Methanol
[Prepared by: Leong Sim Siong, Kow Shu Wen, Nor Hidahyah Binti Ludin, Nafilah Binti Khalid] 32
Sustainability All reactants are non-
renewable but available
in abundance.
All reactants are non-
renewable but available
in abundance.
Carbon dioxide is
renewable source of
reactant.
Energy Consumption
Energy Consumption Great amount of energy
is required to sustain the
reactor at high
temperature (600C) in
order to activate the
methane C-H bond as it
is symmetrical
tetrahedron.
Large amount of energy
is required to promote
steam reforming at very
high temperature
(850C).
Autothermal reforming,
which is a secondary
reforming process, also
need a huge amount of
heat being supplied.
The energy consumption
is low as the reaction is
catalyzed by biological
enzyme and the reaction
temperature is low.
Environment Impact
Consequences The utilization of natural
gas as the source of
methane will cause the
natural gas reservoir
becomes depleted.
Since carbon dioxide is
the byproduct released,
it has the potential to
cause global warming.
The utilization of natural
gas as the source of
methane will cause the
natural gas reservoir
becomes depleted.
Since phosphate buffer
is employed in the
reactor, the wastewater
discharged from the
methanol production via
this route can cause
eutrophication to natural
water system.
Operation Flexibility
Response to the reactor
temperature change
Selectivity of methanol
from catalytic oxidation
may low due to the
further oxidation of
methanol to
The reaction condition is
highly flexible as
methane hydrogenation
can occur at a wider
temperature range.
The reaction condition
has to be carefully
controlled as enzyme is
temperature sensitive.
Enzyme will be
Plant Design For The Production of AA Grade Methanol
[Prepared by: Leong Sim Siong, Kow Shu Wen, Nor Hidahyah Binti Ludin, Nafilah Binti Khalid] 33
formaldehyde (IUPAC
name: methanal).
24
Reactor temperature
change may decrease
selectivity of methanol.
Require careful control
of reactor temperature.
Reactor temperature
change will not affect
the reaction much.
denatured under high
temperature.
Under low temperature,
the catalytic function of
enzyme is low so that it
unable to catalyze the
reaction.
Catalyst Stability Catalyst is stable over a
wide range of operating
condition.
However, the catalytic
function may be too
rigorous under extreme
condition.
Catalyst is stable over a
wide range of operating
condition.
Catalyst, which is
immobilized enzyme,
can only retain its
catalytic function over
very small range of
temperature.
Catalyst may be
denatured at high
temperature and its
performance is not
stable.
Purification Steps Gaseous methanol is
separated from other
non-condensable gases
by condensation.
Crude methanol is
purified by distillation.
Gaseous methanol is
separated from other
non-condensable gases
by flashing.
Crude methanol is
purified by distillation.
The polystyrene
particles with
immobilized enzyme are
separated from the liquid
stream by simple
filtration.
The liquid stream
containing methanol and
other substances can be
separated by distillation.
Plant Design For The Production of AA Grade Methanol
[Prepared by: Leong Sim Siong, Kow Shu Wen, Nor Hidahyah Binti Ludin, Nafilah Binti Khalid] 34
Safety Factors
Healthy concern The components that
will pose danger on
health are Cu, Pd and
methane.
Cu: Experience
gastrointestinal distress
(for short-term
exposure), experience
liver or kidney damage
(for long-term
exposure).
27
Pd: Cause skin, eyes or
respiratory tract
irritation and also skin
sensitization.
28
Methane: Acts as
asphyxiate which
displace oxygen in the
air and cause
asphyxiation (symptom
of oxygen
deprivation).
29
The components that
will pose danger on
health are carbon
monoxide, hydrogen and
methane.
Carbon monoxide:
Replaces oxygen in the
blood, thus killing off
cells and starving vital
organs of oxygen. Large
enough dose can kill
within minutes. It is
odorless, colorless and
tasteless, hence difficult
to be detected.
30
Hydrogen: Acts as
asphyxiate. It is also
odorless, colorless and
tasteless, hence difficult
to be detected.
31
Methane: Refer to
Catalytic Oxidation of
Methane column.
The component that will
pose danger on health is
polystyrene that is used
to hold immobilized
enzyme and cofactor.
Polystyrene: Contains
the toxic substances
such as styrene and
benzene, which are
suspected to be
carcinogenic and able to
cause cancer. It is also
suspected neurotoxins
that are hazardous to
human.
32
Plant Design For The Production of AA Grade Methanol
[Prepared by: Leong Sim Siong, Kow Shu Wen, Nor Hidahyah Binti Ludin, Nafilah Binti Khalid] 35
Potential of cause
accident
Operating temperature is
600C, which is quite
high to create a
dangerous working
environment to workers.
Methane is flammable
and may be explosive
when it is mixing with
air. It is also violently
reactive with oxidizers,
halogens and some
halogens-containing
compound and thus
releasing large amount
of unwanted energy.
Same as Catalytic
Oxidation of Methane.
However, this reaction
involves hydrogen as
intermediate which is
explosive when mixing
with air.
The operation is safe as
it is operating under low
temperature and
pressure.
There is no any
chemical which can
cause serious disaster
through the methanol
formation via this route.
Controllability The methanol selectivity
is difficult to be
controlled.
Further oxidation of
methanol can occur to
form formaldehyde,
which is a carcinogen
and able to cause cancer.
The reaction
controllability is
moderate, since the
operation condition is
flexible.
Runaway reaction
seldom occurs in this
reaction due to the slight
exothermic nature of the
reaction.
The reaction is
extremely difficult to be
controlled.
The reaction is batch
wise such that the
product can be different
from batch to batch.
The working
temperature range for
enzyme is very small,
thus the control of
temperature must be
very precise.
Plant Design For The Production of AA Grade Methanol
[Prepared by: Leong Sim Siong, Kow Shu Wen, Nor Hidahyah Binti Ludin, Nafilah Binti Khalid] 36
Waste Management
Waste Produced After a long time, the
catalyst used might be
deactivated. Deactivated
catalysts, Cu and Pd are
a waste released from
the process which
requires careful
regeneration.
Carbon monoxide in
the purged stream is a
hazardous waste which
requires special
treatment.
The nickel catalyst used
in steam reforming also
can be deactivated after
a period of time and
discharged back to
environment as a waste.
Unreacted carbon
monoxide and
hydrogen will be
purged out of the
reaction system.
Enzyme can be
denatured and lost its
catalytic function.
Hence, the polystyrene
where enzyme and
cofactor reside can be
considered as a waste
from this process.
Phosphate buffer is also
byproduct released from
this process scheme.
Waste Management Copper and palladium
can be reactivated as a
catalyst by certain
treatment process such
as calcination. The
substances that cover the
catalyst active site will
be removed.
Copper is 100%
recyclable. The copper
properties will not lose
after recycling; hence
can be used to produce
other useful products
such as electrical wire.
33
Palladium is catalyst
Nickel catalyst can be
recycled by two types of
processes: Pyroprocess
(high temperature
oxidation of a spent
catalyst) and
hydroprocess (selective
dissolution of
components in an
adequate leaching
solution). Catalytic
function of nickel can be
recovered in high
proportion.
36
The waste hydrogen can
be captured by new
Polystyrene is non-
biodegradable and hence
should be recycled. For
example, it can be
ground down and used
as a soil conditioner
which will improve
drainage and aeration.
Alternatively it can be
remoulded into new
polystyrene products, or
recycled to produce new
products entirely.
37
Phosphate acts as
nutrient in the natural
water system and it is
Plant Design For The Production of AA Grade Methanol
[Prepared by: Leong Sim Siong, Kow Shu Wen, Nor Hidahyah Binti Ludin, Nafilah Binti Khalid] 37
with very high recovery
value. Simple
regeneration process that
would allow the reuse of
the palladium compound
leads to various benefits
and substantial cost
savings connected to the
use of palladium
catalyst.
34
Since carbon monoxide
is extremely poisonous,
it must be removed from
the gas stream before
discharged. Carbon
monoxide can be
removed by adsorption
with -Al
2
O
3
and
TiO
2
.
35
invented technology so
that it can be used as a
source of energy for
vehicles and power
stations. Apart from that,
hydrogen can also be
recycled to the methanol
reactor as it is one of the
major reactants to
synthesis methanol.
The management of
waste carbon monoxide:
refer to column Catalytic
Oxidation of Methane.
able to cause excessive
growth of algae and
initiate eutrophication.
The removal of
phosphorous involves
the incorporation of
phosphate into TSS and
the subsequent removal
from these solids. The
most common method is
the addition of calcium
which will react with
phosphate and form
solid that can settle
down.
38
Plant Design For The Production of AA Grade Methanol
[Prepared by: Leong Sim Siong, Kow Shu Wen, Nor Hidahyah Binti Ludin, Nafilah Binti Khalid] 38
1.2.3 CHOICE OF PROCESS
In order to decide which of the 3 proposed methods is most suitable and feasible for our
plant design, all pros and cons of each individual process have been taken into account.
As shown in the Table 1.2-1, all three processes proposed have been discussed.
Method 1 - Catalytic Oxidation of Methane
Method 2 - CO/CO
2
Hydrogenation
Method 3 - Enzymatic Reduction of CO
2
From Table 1.2-1, it can be seen that the raw material involved in the production is
natural gas for the first two methods, except for the carbon dioxide used in Method 3. All
three methods required catalyst, copper and metallic palladium for Method 1, copper/
zinc oxide/ aluminum oxide for Method 2 and formate, formaldehyde, alcohol, glutamate
dehydrogenase and NADH for Method 3.
Among the process suggested as above, the CO/CO
2
hydrogenation is the most applicable
route path for methanol production in large scale. The reasons of choosing Method 2 as
the methanol synthesis process in the plant are discussed in term of methanol conversion
and selectivity, catalyst used, environmental issue, safety issue, economical indicators,
market analysis, flexibility and controllability.
Conversion and Selectivity
From Table 1.2-1, we can know that Method 1 and Method 2 have very high conversion
(99% for Method 1 and 99.85% for Method 2), which is far more superior compared to
enzymatic reduction (Method 3). However, for Method 1, the selectivity of methanol will
decrease when the operating condition is changing due to the further oxidation of
methanol to formaldehyde. In contrast, for Method 2, there is no any side reaction which
will produce other byproduct and hence lower the selectivity of methanol. Therefore, in
term of conversion and selectivity, Method 2 is the best choice compare to Method 1 and
Method 3.
Plant Design For The Production of AA Grade Methanol
[Prepared by: Leong Sim Siong, Kow Shu Wen, Nor Hidahyah Binti Ludin, Nafilah Binti Khalid] 39
Catalyst
Cu/ZnO/Al
2
O
3
has long been used in industrial methanol synthesis via Method 2 is
because of their high catalytic activity, long life time, high poison durability and
relatively low reaction temperature and pressure. In order to increase Cu/ZnO/Al
2
O
3
activity and stability, the basic catalyst was modified by different oxide additives such as
boron, silica, chromium, tungsten, palladium, manganese and Zirconium. Small amounts
of different oxide (Mn, Mg, Zr, Ce, Ba, Cr, and W) were added to CU/ZnO/Al
2
O
3
.
39
In
the table below, initial activity and the activity after 64 hrs, average carbon conversion
(mol %) and methanol selectivity are shown.
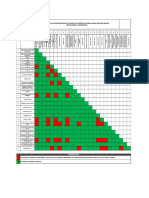
Table 1.2-2: The activity and selectivity of the catalyst for methanol synthesis
If Zr and Mn were used as promoters together, Cu/ZnO/Al
2
O
3
catalyst became more
stable and its activity after nearly 30 hrs was more than that of the catalyst without any
promoter. Furthermore, Zr could be a good support for Cu/ZnO catalyst because it makes
the catalyst more stable and also increase its activity.
39
Although the oxidation catalyst, copper and palladium, for Method 1 is also stable and
can be regenerated, its oxidation activity maybe too rigorous, thus causing methanol to be
further oxidized to formaldehyde, thus decreasing the selectivity of methanol.
Nevertheless, palladium is one of the rare metals on the Earth, which is very expensive
and non-economical.
Plant Design For The Production of AA Grade Methanol
[Prepared by: Leong Sim Siong, Kow Shu Wen, Nor Hidahyah Binti Ludin, Nafilah Binti Khalid] 40
For Method 3, the catalyst used is enzyme from biological microorganism. The catalytic
temperature range is quite narrow and the enzyme can be denatured easily.
On the other hand, the catalyst of Method 2 has been investigated thoroughly and there is
a lot of information available which can improve its catalytic performance. The stability
of catalyst has made method 2 being more superior compared to other method.
Environmental issue
From Table 1.1-2, it can be observed that carbon dioxide is one of the reactant to produce
methanol in methanol reactor via Method 2. The consumption of carbon dioxide to
produce methanol will reduce greenhouse effect and global warming. In contrast, carbon
dioxide is one of the byproduct released through the methane catalytic oxidation route
(Method 1). The production of carbon dioxide should be avoided as greenhouse effect is
majority contributed by carbon dioxide.
Nevertheless, in line with fulfillment of green technology, there is no any toxic and
hazardous byproduct produced from methanol synthesis via Method 2. The only
byproduct from Method 2 is water, which can be separated from methanol through
distillation process and it has no impact on the environment.
In term of catalyst recovery, Method 2 also has major advantage as nickel (for steam
reforming) and copper (for methanol synthesis) can be reactivated by certain processes
after a long use. Hence, there is no waste catalyst produced as long as the operation of the
plant. In contrast, for Method 3, the enzyme is not stable and cannot be regenerated once
it is denatured. Furthermore, the support of the enzyme polystyrene is not biodegradable
and able to produce polystyrene foam which is toxic to marine life and lethal to any sea
bird or creature which swallow it.
Hence, in term of environmental issue, Method 2 is the best option among the 3
alternative processes to be utilized in methanol synthesis.
Plant Design For The Production of AA Grade Methanol
[Prepared by: Leong Sim Siong, Kow Shu Wen, Nor Hidahyah Binti Ludin, Nafilah Binti Khalid] 41
Safety and Health Concern
In term of safety issue, health problem and potential to cause accident are considered. For
Method 1, hydrogen and hydrogen peroxide is the intermediate in this reaction. If there is
a rapture of the reactor, workers may be exposed to such dangerous chemicals. Hydrogen
can form explosive mixture with air while hydrogen peroxide is corrosive.
For Method 2, carbon monoxide and hydrogen are the hazardous chemicals employed
during the process. Carbon monoxide replaces oxygen in the blood, thus killing off cells
and starving vital organs of oxygen.
There is no any hazardous chemical being used or produced in Method 3. Hence, Method
3 is the best choice if safety issue is considered.
Flexibility
The raw material for Method 1 and Method 2 is natural gas, which is easily available in
Malaysia. Due to the abundance of natural gas, the price of raw material for Method 1
and Method 2 is quite low. Carbon dioxide, which is the raw material for Method 3, is
also easily available as the side product of the industrial synthesis of ammonia and
hydrogen.
However, the operation temperature range for Method 2 is far wider compared to Method
1 and Method 3. This is because the further oxidation of methanol to formaldehyde will
take place under temperature higher than set point. The catalytic activity of nickel maybe
too rigorous under extreme temperature and causes the methanol selectivity to decrease.
The enzyme utilized in Method 3 also not a stable catalyst and only can perform its
catalytic function under very limited temperature range. This is because enzyme from
microorganism can be denatured under slightly higher temperature. Furthermore, the
enzyme loss its catalytic activity when the temperature is low.
Hence, in term of flexibility, Method 2 should be the most suitable choice and more
superior compared to Method 1 and Method 3 as it can be operated under wider range of
temperature.
Plant Design For The Production of AA Grade Methanol
[Prepared by: Leong Sim Siong, Kow Shu Wen, Nor Hidahyah Binti Ludin, Nafilah Binti Khalid] 42
Controllability
In term of controllability, Method 2 is the best choice to synthesis methanol in the plant.
The reaction is not difficult to control with a properly installed controller.
On the other hand, Method 1 employs the catalytic oxidation of methane to methanol
where further oxidation of methanol to formaldehyde may take place. It is hard to control
the operating condition so that further oxidation of methanol can be avoided. The
formation of formaldehyde will pose serious health problem to the surrounding people,
increase the separation cost and decline the product quality.
Method 3 is using enzyme from microorganism as a catalyst. Therefore, the operating
temperature range is very narrow for this process and it is very difficult to control the
operation temperature to the set point desired.
Market Analysis and Economical Indicators
Consumption growth of natural gas was below average in all regions except North
America, where low prices drove robust growth. Outside North America, the largest
volumetric gains in consumption were in China (+21.5%), Saudi Arabia (+13.2%) and
Japan (+11.6%). These increases were partly offset by the largest decline on record in EU
gas consumption (-9.9%), driven by a weak economy, high gas prices, warm weather and
continued growth in renewable power generation.
Global natural gas production grew by 3.1%. The US (+7.7%) recorded the largest
volumetric increase despite lower gas prices, and remained the worlds largest producer.
Output also grew rapidly in Qatar (+25.8%), Russia (+3.1%) and Turkmenistan (+40.6%),
more than offsetting declines in Libya (-75.6%) and the UK (-20.8%)[16].
40
As was the
case for consumption, the EU recorded the largest decline in gas production on record (-
11.4%), due to a combination of mature fields, maintenance, and weak regional
consumption. The figure below shows the change in world natural gas production by
region, 2008-2035
Plant Design For The Production of AA Grade Methanol
[Prepared by: Leong Sim Siong, Kow Shu Wen, Nor Hidahyah Binti Ludin, Nafilah Binti Khalid] 43
Figure 1.2-5: Change in world natural gas production by region, 2008-2035(trillion
cubic feet)
41
In our plant, the natural gas will be our raw material that used to produce methanol.
Natural gas is found in oil fields, natural-gas fields and coal beds. Since natural gas isn't a
pure product, it needs to undergo processing before it can be used as consumer fuel. The
gas is extracted from a field using a decompression process called retrograde
condensation.
42
After the natural gas is extracted, it is stripped of the additional elements
such as acid and mercury to make it usable by consumers. Natural gas is definitely an
excellent feedstock for producing a good quality product.
Since natural gas is the raw material for Method 1 and Method 2, these two methods are
suitable to be employed in the methanol plant.
From the comparisons above, it is obvious that Method 2 is far more superior compared
to the other two method to synthesis methanol. This is because Method 2 has very high
conversion and selectivity, long catalyst life, high flexibility and controllability. In the
view of environmental issue, Method 2 utilizes carbon dioxide as reactant, which may
help to reduce greenhouse effect. Long catalyst lifetime is also an advantage for methanol
production via Method 2, which dispose no catalyst waste to the environment. Natural
gas also easily available and feed stock for methanol production via Method 2 is not a
great issue to be worried. Although Method 3 is safer compared to Method 2, Method 2 is
chosen as it is more superior to Method 3 in other aspects. Safety problem can be
Plant Design For The Production of AA Grade Methanol
[Prepared by: Leong Sim Siong, Kow Shu Wen, Nor Hidahyah Binti Ludin, Nafilah Binti Khalid] 44
improved by the implementation of inherent safety concept during the design stage of
the plant. Hence, Method 2 is chosen as the process being utilized in the plant.
Plant Design For The Production of AA Grade Methanol
[Prepared by: Leong Sim Siong, Kow Shu Wen, Nor Hidahyah Binti Ludin, Nafilah Binti Khalid] 45
1.3 CRITICAL ASSESSMENTS OF THE PROCESS
CHOSEN
________________________________________________
1.3.1 CAPACITY OF THE DESIGNED PLANT
The production capacities for our designed plant are 900,000 tons of methanols per year.
It is about 3% from the overall Asia-Pacific region. The capacities are decided based on
the following factors:
1) The demand for the production formaldehyde, methyl tert-butyl ether (MTBE)
and acetic acid.
2) The availability of the feed stocks (natural gas)
3) The size of the plant as well as the equipment
In order to meet the goal, the availability of the feed stock in Malaysia is our main
consideration. Currently, Petronas Gas Bhd is the main supplier of natural gas with
production capacity of about382 mmscfd (million standard cubic feet per day) that is
equivalent to 1.0817x10
7
m
3
per day. From The Star Online dated 4
th
September, Gas
Malaysia Bhd had recently signed an agreement with Petronas Gas Bhd to increase the
natural gas supply by 110 mmscfd to 492 mmscfd from its current 382 mmscfd capacity
on a step-up basis, with 40 mmscfd for 2013, 30 mmscfd for 2014 and 40 mmscfd for
2015. However, after 2015, they still cannot secure the supply of the natural gas.
Since the supply of natural gas in Malaysia is keep on increasing, hence, Petronas has the
ability to supply the natural gas for the methanol production. It is approximated that for
0.032 billion tons of methanol is produced from 100,000 mmcf of natural gas. So, for
900,000 ton of methanol, the natural gas needed is around 281.25 mmcf per year which is
less than 0.3 % of the total natural gas production. Based on the availability of the natural
gas in Malaysia, it is sufficient enough to get one million tons per year of methanol.
Plant Design For The Production of AA Grade Methanol
[Prepared by: Leong Sim Siong, Kow Shu Wen, Nor Hidahyah Binti Ludin, Nafilah Binti Khalid] 46
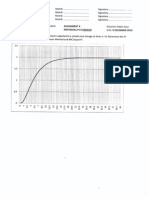
Figure 1.3-1: Graph of Increasing Natural Gas Production Capacity
43
Even though, there is much natural gas available in Malaysia, we decided not to increase
the production capacity of the designed plant greater than 900,000 ton per year. It is
because natural gas also used for other industries. In addition, most of the natural gas is
used in energy sector such as generation of electricity. We also want to apply the
inherently safety design by reduce the storage and production of methanol. Also, the
natural gas availability can be depleted soon due to high demand of it for other industries.
0
100
200
300
400
500
2012 2013 2014 2015
P
r
o
d
u
c
t
i
o
n
C
a
p
a
c
i
t
y
Year
Increasing of Natural Gas Production Capacity
Natural Gas Capacity
(mmscfd)
Plant Design For The Production of AA Grade Methanol
[Prepared by: Leong Sim Siong, Kow Shu Wen, Nor Hidahyah Binti Ludin, Nafilah Binti Khalid] 47
1.3.2 COMPLETE DESCRIPTION OF THE PROCESS CHOSEN
44
In a conventional CO/CO
2
hydrogenation process, the feedstock that used here is natural
gas. Most probably, the natural has is converted into synthesis gas first before reacts in
second section. In our production line, natural gas (96% methane) is received from
National Gas Company (NGC) to provide a carbon and hydrogen components.
The plant designed will utilize a conventional steam reforming to produce carbon
monoxide and hydrogen, which will in turn being converted into methanol in the reactor.
Generally, the process consists of five steps:
1. Mercury removal of natural gas
2. Desulphurization of natural gas
3. Synthesis gas generation
4. Methanol synthesis
5. Methanol purification
The five processes will be described in details in the following sections.
Mercury removal of natural gas
There is trace amount of mercury exists in the natural gas. Since the mercury is a
powerful poison for the catalyst along the process line, it must be removed before the
natural gas entering the production plant. Mercury can accumulates as a liquid in heat
exchangers and destroy them. Hence, a mercury removal unit is the first operation
encountered by natural gas in the plant.
The raw natural gas with mercury is first fed into an adsorbed. Sulfur impregnated
activated carbon is used as the adsorbent of mercury in this removal process as sulphur
will react with mercury and fix it in the pore of activated carbon. The mercury removal
process is carried out under temperature of 50C and pressure of 20 bars.
Plant Design For The Production of AA Grade Methanol
[Prepared by: Leong Sim Siong, Kow Shu Wen, Nor Hidahyah Binti Ludin, Nafilah Binti Khalid] 48
Desulphurization of natural gas
Sculpture is the main catalyst poison in methanol production process. The existence of
hydrogen sulphide in the methanol synthesis loop will greatly reduce the catalytic activity
of nickel and copper. Hence, it must be removed prior to the steam reformer in order to
protect catalysts in the steam reformer and reactor.
The desulphurization reactor is located at the upstream of steam reforming, which is
operating at a temperature of 350C. The feed is channel through zinc oxide beds where
hydrogen sulphide is adsorbed according to the following equation:
Synthesis gas generation
Synthesis gas (known as syngas) is generated using two steps of catalytic reforming
process, which consist of steam reforming and auto-thermal reforming.
Steam Reforming
Under steam reforming, syngas, which is mainly composed of carbon monoxide and
hydrogen, is synthesized by the steam reforming of methane under nickel catalyst on an
Al
2
O
3
support structure according to the following equation:
The reaction is highly endothermic such that heat should be provided to raise the reaction
temperature. According to Le Chateliers principle, the high temperature will favor the
formation of carbon monoxide and hydrogen. Hence, in order to keep the methane
concentration in the reformed gas to a minimum level, the temperature at the reformer
should be as high as possible.
Apart from that, the carbon monoxide produced may also react with the steam to produce
carbon dioxide and hydrogen in the steam reformer, according to the following equation:
1.3-1
1.3-2
Plant Design For The Production of AA Grade Methanol
[Prepared by: Leong Sim Siong, Kow Shu Wen, Nor Hidahyah Binti Ludin, Nafilah Binti Khalid] 49
The steam reforming reactions take place in a temperature range from approximately
600C at the inlet to the catalyst bed to approximately 900C at the outlet. The pressure in
the steam reformer is around 25 bar. The steam reformer requires high alloy, nickel-based,
centrifugally cast and inside machined tubes to withstand the combination of high
pressure and temperatures, oxidizing atmosphere on outside and reducing atmosphere on
the inside.
The reformed gas, which consists of gas mixture of hydrogen, carbon monoxide, inert,
unreacted methane and un-decomposed steam leaves the steam reformer tubes and routed
to oxygen blown auto-thermal reformer.
Auto-thermal Reforming
After steam reforming, the effluent will be directed into a secondary reformer, which is
known as auto-thermal reformer. The catalyst used in this reformer is the same of the
stream reformer; however, the operation is carried out under higher temperature (1000 C)
and higher pressure (50 bar). Oxygen also being supplied to the reactor such that it can
react with hydrogen in order to lower the H
2
to CO ratio:
Apart from that, oxygen also partially oxidizes methane and ethane to produce carbon
monoxide and hydrogen gas.
After undergoing auto-thermal reforming, all the remaining methane and heavier
hydrocarbon such as ethane will be converted into syngas. The reformed gas will then
enter a water adsorption column to remove the unreacted steam before methanol reactor.
1.3-3
1.3-4
1.3-5
1.3-6
Plant Design For The Production of AA Grade Methanol
[Prepared by: Leong Sim Siong, Kow Shu Wen, Nor Hidahyah Binti Ludin, Nafilah Binti Khalid] 50
Methanol synthesis
The reformed gas is then routed into the methanol reactor such that hydrogen and carbon
monoxide can react to synthesis methanol in the presence of a highly selective copper
based catalyst. The chemical equation which represents the synthesis of methanol is
shown below:
Carbon dioxide also can react with hydrogen under such condition to form methanol
according to the equation:
The reactions are highly exothermic and a lot of heat will be generated, which must be
promptly removed from its source. The reactions occur in a methanol reactor which is
designed to induce the methanol synthesis reaction.
The reformed as will be compressed to 80 bar before entering at the top of the reactor and
routed through the tubes where the synthesis reactions take place. The heat of reaction is
removed from the catalyst by partial evaporation of boiler feed water circulating between
the reactor shell and steam drum, mount on the top of the reactor in order to keep the
temperature of reactor at approximately 230C. The product mixture is then discharged at
the bottom of the reactor. The cross sectional of methanol reactor is shown in the figure
below.
1.3-7
1.3-8
Plant Design For The Production of AA Grade Methanol
[Prepared by: Leong Sim Siong, Kow Shu Wen, Nor Hidahyah Binti Ludin, Nafilah Binti Khalid] 51
Figure 1.3-2: Cross-sectional view of methanol reactor
44
Methanol Purification
The crude methanol produced in methanol reactor contains water, dissolved gases and a
quantity of undesired but unavoidable by-products which have either lower or higher
boiling points than methanol. In order to produce AA grade methanol with 99.85% of
methanol by mass, purification of crude methanol is essential. Purification of methanol
takes place in distillation column where impurities are removed.
Before entering distillation column, the reactor effluent is cooled to 10C and flashed at
50 bar to separate the residual syngas (carbon monoxide, carbon dioxide and hydrogen)
from the methanol-water solution. The vapor leaving is then recycled to increase the
overall methanol conversion.
Plant Design For The Production of AA Grade Methanol
[Prepared by: Leong Sim Siong, Kow Shu Wen, Nor Hidahyah Binti Ludin, Nafilah Binti Khalid] 52
The methanol-water stream is then heated to 95C and sent to distillation column to
separate the water from the methanol. In this tower, a methanol-rich stream, containing
approximately 99.86% of methanol is taken as top product and sent to a central methanol
storage vessel. The water comes out as bottom product contains a lot of impurities and
need to be sent to wastewater treatment plant for further treatment.
Pressure Swing Adsorption
Pressure swing adsorption is an additional and optional step to recover unreacted
reactants from methanol reactor. A pressure swing adsorption column is located at the
downstream of the methanol reactor. The adsorption column is packed with zeolite which
is playing a vital role as carbon dioxide adsorbent in the plant. The separation is based on
the concept that adsorption capacity of zeolite is different under different pressure.
Pressure swing adsorption is particular useful for the separation of carbon dioxide.
45
The objective of the adsorption column is to recover hydrogen and carbon monoxide back
into the methanol reactor whereas carbon dioxide is adsorbed by adsorbent (zeolite)
under pressure of 3 bar. After the adsorbent becomes saturated with carbon dioxide, the
adsorbent will be depressurized to atmospheric in order to release the carbon dioxide
trapped, and hence regenerate the adsorbent. The carbon dioxide will be purged out of the
reaction system to be flared. By this way, hydrogen and carbon monoxide will be
recovered and recycled in the reaction loop without being discharged into the atmosphere.
This will help to prevent air pollution (hydrogen and carbon monoxide are air pollutants)
and also reduce the amount of raw material to be fed as well.
Plant Design For The Production of AA Grade Methanol
[Prepared by: Leong Sim Siong, Kow Shu Wen, Nor Hidahyah Binti Ludin, Nafilah Binti Khalid] 53
Figure 1.3-3: The effect of adsorbate partial pressure on the adsorbate loading. This
concept is utilized in pressure swing adsorption.
Plant Design For The Production of AA Grade Methanol
[Prepared by: Leong Sim Siong, Kow Shu Wen, Nor Hidahyah Binti Ludin, Nafilah Binti Khalid] 54
1.3.3 PROCESS CHEMISTRY, REACTION AND KINETICS
46
Steam Reformer
In steam reformer, methane from natural gas reacts with steam to produce carbon
monoxide and hydrogen gas (known as syngas). The reactions that take place in this
reactor are given by Equations 1 and 2, which is catalyzed by Ni/MgO on Al2O3 support
structure.
Equation 1.3-9 is known as reforming reaction and it is highly endothermic. The reaction
produces more number of moles of products than reactants. Therefore, the forward
reaction is favoured by high temperature and low pressure.
On the other hand, Equation 1.3-10 is known as water-gas shift reaction. This reaction is
exothermic and producing same number of moles of product with reactants. Hence, this
reaction is favoured by low temperature and independent of pressure.
The rate laws for Equations 1.3-9 and 1.3-10 are shown below.
where
1.3-9
1.3-10
Plant Design For The Production of AA Grade Methanol
[Prepared by: Leong Sim Siong, Kow Shu Wen, Nor Hidahyah Binti Ludin, Nafilah Binti Khalid] 55
where
In these equations, the reaction rate is in kmol/m cat/s, the pressure is in bar and the
temperature is in Kelvin. The activation energy units are J/kmol.
Methanol Reactor
The production of methanol from syngas from gas reforming is the reaction that occurs in
methanol reactor. The reactions that take place in methanol reactor are shown in the
following equations.
Equation 1.3-11 is the desired reaction which produce methanol. This reaction is highly
exothermic and produces fewer moles than it reacts. Hence, this forward reaction is
favoured by low temperature and high pressure. Equation 1.3-12 is the reverse of
Equation 1.3-10, which is also the water-gas shift reaction. Both reactions are equilibrium
limited and are controlled by reaction kinetics shown in the equations below. The rate
constants shown below are taken from the study of the reaction conducted at a
temperature of 225C.
1.3-11
1.3-12
Plant Design For The Production of AA Grade Methanol
[Prepared by: Leong Sim Siong, Kow Shu Wen, Nor Hidahyah Binti Ludin, Nafilah Binti Khalid] 56
where
In this equation, the reaction rate is in mol/g cat/h and the pressure is in atm.
Plant Design For The Production of AA Grade Methanol
[Prepared by: Leong Sim Siong, Kow Shu Wen, Nor Hidahyah Binti Ludin, Nafilah Binti Khalid] 57
1.3.4 THERMODYNAMICS, PHYSICAL AND CHEMICAL PROPERTIES
Table 1.3-1: Thermodynamics Data
47,48,49,50,51,52
Compound
Standard
enthalpy of
formation,
H
(kJ/mol)
Gibbs
energies of
formation,
G
(kJ/mol)
Heat of
fusion at
melting
point and
1 atm H
(kJ/mol)
Heat of
vaporization at
boiling point and
1 atm H
(kJ/mol)
T
c
(K)
P
c
(atm)
Heat of
formation at
25C and 1
atm H
(kJ/mol)
Heat of
combustion at
25C and 1 atm
H (kJ/mol)
Specific heat
capacity, C
p
At
25C (kJ/kg. K)
Carbon
monoxide
-110.5 (g) -137.2 (g) 0.837 6.042 133 34.5 -110.52 (g) -282.99 (g)
-
Carbon
dioxide
-393.5 (g)
-413.8 (aq)
-394.4 (g)
-386.0 (aq)
8.33 - 304.2 72.9
-412.9 (l)
-393.5 (g)
-
0.839 (g)
Water
-285.8 (l)
-241.8 (g)
-273.2 (l)
-228.6 (g)
6.0095 40.656 647.4 218.3
-285.84(l)
-241.83 (g)
-
4.1813 (l)
2.080 (g)
Hydrogen 0 (g) 0 (g) 0.12 0.904 33.3 12.8 0 (g) -285.84 (g)
14.30 (g)
Methanol -238.66 (l) -166.35 (l) 3.167 35.27 513.2 78.5
-238.6 (l)
-201.2 (g)
726.6 (l)
-764.0 (g)
2.14 (l)
Methane -74.85 (g) -50.8 (g) 0.94 8.179 190.7 45.8 -74.85 (g) -890.36 (g)
2.191 (g)
Hydrogen
sulfide
-20.6 (g)
-39.7 (aq)
-33.6 (g)
-27.9 (aq)
2.38 18.67 373.6 88.9 -19.96 (g) -562.59 (g)
1.015 (g)
Oxygen 0 (g) 0 (g) 0.444 6.82 154.4 49.7 0 (g) -
0.918 (g)
Plant Design For The Production of AA Grade Methanol
[Prepared by: Leong Sim Siong, Kow Shu Wen, Nor Hidahyah Binti Ludin, Nafilah Binti Khalid] 58
Table 1.3-2: Physical and Chemical Properties
Name Formula
Molecular
weight
(g/mol)
Color &
phase
Density
(g/L) at
0C
Melting
point,
C
Boiling
point,
C
Vapour
pressure, kPa
Molecular structure
Carbon
monoxide
CO 28.01
Colorless
gas
1.25
198.9
[2]
-191.5
101.325 at -
191C
.C:
Carbon
dioxide
CO
2
44.01
Colorless
gas
1.53 - -78.5
5826.189 at
20C
Water H
2
O 18.0153
Clear
liquid
1 0 100
101.325 at 100
C
Hydrogen H
2
2.00
Colorless
gas
0.08987 - -253
101.325 at -253
C
H-H
Methanol CH
3
OH 32.04
Colorless
liquid
0.792
(20C)
-98 64 12.821 at 20C
Methane CH
4
16.05
Colorless
gas
0.6784 -182.6 -161.6 -
Hydrogen
sulfide
H
2
S 34.08
Colorless
gas
0.089 -82.8 -60 1838.804
Oxygen O
2
32.00
Colorless
gas
1.429 54.38 90.20 1 at 21.2C :O=O:
Plant Design For The Production of AA Grade Methanol
[Prepared by: Leong Sim Siong, Kow Shu Wen, Nor Hidahyah Binti Ludin, Nafilah Binti Khalid] 59
1.3.5 MATERIAL SAFETY DATA SHEET
1.3.5.1 Carbon monoxide
46,53
Hazard identification: Potential health effects
Route of exposure Potential Health Effects
Inhalation Short term exposure: changes in body temperature, changes in blood
pressure, nausea, vomiting, chest pain, difficulty breathing, irregular heartbeat,
headache, drowsiness, fatigue, dizziness, disorientation, hallucinations, pain in
extremities, tremors, loss of coordination, hearing loss, visual disturbances,
eye damage, bluish skin colour, suffocation, blood disorders, convulsions,
coma
Long term exposure: nausea, vomiting, loss of appetite, headache, dizziness,
visual disturbances, blood disorders, heart disorders, heart damage, nerve
damage, reproductive effects, birth defects, brain damage
Eye contact Short term exposure: frostbite, blurred vision
Long term exposure: no information is available
Skin contact Short term exposure: blisters, frostbit
Long term exposure: no information is available
Ingestion Short term exposure: ingestion of a gas is unlikely
Long term exposure: ingestion of a gas is unlikely
First Aid Measures
Inhalation Remove to uncontaminated area. Give artificial respiration if not breathing. If
breathing is difficult, oxygen should be administered by qualified personnel.
Get immediate medical attention.
Eye contact Immediately flush eyes with plenty of water for at least 15 minutes. Then get
immediate medical attention.
Skin contact Immediately flush with plenty of lukewarm water. If warm water is not
available, gently wrap affected parts in blankets. Get immediate medical
attention.
Plant Design For The Production of AA Grade Methanol
[Prepared by: Leong Sim Siong, Kow Shu Wen, Nor Hidahyah Binti Ludin, Nafilah Binti Khalid] 60
Ingestion If a large amount is swallowed, get medical attention.
Fire-Fighting Measures
Suitable extinguishing
media
Carbon dioxide, regular dry chemical
Large fires: Use regular foam or flood with fine water spray.
Fire and explosion
hazard
Vapour/air mixtures are explosive. Containers may rupture or explode if
exposed to heat.
Fire fighting Move container from fire area if it can be done without risk. Cool containers
with water spray until well after the fire is out. Stay away from the ends of
tanks.
Accidental Release Measures
Water release Keep out of water supplies and sewers
Occupational
spill/release
Avoid heat, flames, sparks and other sources of ignition. Stop leak if possible
without personal risk. Reduce vapours with water spray. Keep unnecessary
people away, isolate hazard area and deny entry. Remove sources of ignition
Handling and Storage
Handling -
Storage Store in accordance with all current regulations and standards. Store in a cool,
dry place. Store in a well-ventilated area. Avoid direct sunlight. Avoid heat,
flames, sparks and other sources of ignition. Keep separated from incompatible
substances.
Exposure Control / Personal Protection
Occupational exposure
limits (10-hours
reference period)
Exposure limits : 35 ppm (40 mg/m3)
Engineering measures /
Ventilation
Ventilation equipment should be explosion-resistant if explosive
concentrations of material are present. Provide local exhaust or process
enclosure ventilation system. Ensure compliance with applicable exposure
Plant Design For The Production of AA Grade Methanol
[Prepared by: Leong Sim Siong, Kow Shu Wen, Nor Hidahyah Binti Ludin, Nafilah Binti Khalid] 61
limits.
Personal protective equipment
Respiratory protection Any supplied-air respirator and self-contained breathing apparatus with a full
face piece.
Hand protection Wear insulated gloves.
Eye protection For the gas: Eye protection not required, but recommended.
For the liquid: Wear splash resistant safety goggles. Contact lenses should
not be worn. Provide an emergency eye wash fountain and quick drench
shower in the immediate work area.
Skin and body
protection (clothing)
For the gas: Protective clothing is not required.
For the liquid: Wear appropriate protective, cold insulating clothing.
Physical and chemical properties
Form Gas
Color Colorless
Odor Odorless
Taste Tasteless
Molecular weight 28.01
Vapor pressure 760 mmHg @-191C
Vapor density 0.968 (air=1)
Specific gravity Not applicable
Boiling point -191.5C
Freezing point -205C
Water solubility 2.3% @ 20C
Solvent solubility Soluble: alcohol, benzene, acetic acid, ethyl acetate, chloroform, cuprous
chloride solutions
Flash point (C) Not available
Plant Design For The Production of AA Grade Methanol
[Prepared by: Leong Sim Siong, Kow Shu Wen, Nor Hidahyah Binti Ludin, Nafilah Binti Khalid] 62
Ignition temperature
Explosion limits
Auto ignition
temperature
1292F (700C)
Flammability limit
(lower %)
12.5 % by volume
Flammability limit
(upper %)
74 % by volume
Stability and reactivity
Reactivity Stable at normal temperatures and pressure.
Condition to avoid Avoid heat, flames, sparks and other sources of ignition. Minimize contact
with material. Avoid inhalation of material or combustion by-products. Keep
out of water supplies and sewers.
Incompatibilities Oxidizing materials, halogens, metal oxides, metals, combustible materials,
lithium
Hazardous
decomposition products
Thermal decomposition products: oxides of carbon
1.3.5.2 Carbon dioxide
47
Hazard identification: Potential Health Effects
Route of exposure Potential Health Effects
Inhalation Short term exposure: changes in blood pressure, ringing in the ears, nausea,
difficulty breathing, irregular heartbeat, headache, drowsiness, dizziness,
tingling sensation, tremors, weakness, visual disturbances, suffocation,
convulsions, unconsciousness, coma
Long term exposure: no information on significant adverse effect
Eye contact Short term exposure: frostbite, blurred vision
Long term exposure: no information on significant adverse effect
Plant Design For The Production of AA Grade Methanol
[Prepared by: Leong Sim Siong, Kow Shu Wen, Nor Hidahyah Binti Ludin, Nafilah Binti Khalid] 63
Skin contact Short term exposure: blisters, frostbit
Long term exposure: no information on significant adverse effect
Ingestion Short term exposure: ingestion of a gas is unlikely
Long term exposure: ingestion of a gas is unlikely
First Aid Measures
Route of exposure First Aid measures
Inhalation Remove to uncontaminated area. Give artificial respiration if not breathing. If
breathing is difficult, oxygen should be administered by qualified personnel.
Get immediate medical attention.
Eye contact Immediately flush eyes with plenty of water for at least 15 minutes. Then get
immediate medical attention.
Skin contact Immediately flush with plenty of lukewarm water. If warm water is not
available, gently wrap affected parts in blankets. Get immediate medical
attention.
Ingestion If a large amount is swallowed, get medical attention.
Fire-Fighting Measures
Suitable extinguishing
media
Use extinguishing agents appropriate for surrounding fire.
Fire and explosion
hazard
Negligible fire hazard. Containers may rupture or explode if exposed to heat.
Fire fighting Move container from fire area if it can be done without risk. Cool containers
with water spray until well after the fire is out. Stay away from the ends of
tanks.
Accidental Release Measures
Water release -
Occupational
spill/release
Do not touch spilled material. Stop leak if possible without personal risk. Keep
unnecessary people away, isolate hazard area and deny entry. Ventilate closed
spaces before entering.
Handling and Storage
Plant Design For The Production of AA Grade Methanol
[Prepared by: Leong Sim Siong, Kow Shu Wen, Nor Hidahyah Binti Ludin, Nafilah Binti Khalid] 64
Handling -
Storage Store and handle in accordance with all current regulations and standards.
Protect from physical damage. Store in a well-ventilated area. Keep separated
from incompatible substances.
Exposure Control / Personal Protection
Occupational exposure
limits (10-hours
reference period)
Exposure limits: 5000 ppm (9000 mg/m3)
Engineering measures /
Ventilation
Provide local exhaust ventilation system. Ensure compliance with applicable
exposure limits
Personal protective equipment
Respiratory
protection
Any self-contained breathing apparatus and supplied-air respirator with a full
face piece.
Hand protection Wear insulated gloves.
Eye protection For the gas: Eye protection not required, but recommended.
For the liquid: Wear splash resistant safety goggles. Contact lenses should
not be worn. Provide an emergency eye wash fountain and quick drench
shower in the immediate work area.
Skin and body
protection (clothing)
For the gas: Protective clothing is not required.
For the liquid: Wear appropriate protective, cold insulating clothing.
Physical and chemical properties
Form Gas
Color Colorless
Odor Odorless
Taste Acid taste
Molecular weight 44.01
Vapor pressure 43700 mmHg @ 21C
Vapor density 1.5 (air=1)
Specific gravity 1.527 @ 21C (water=1)
Plant Design For The Production of AA Grade Methanol
[Prepared by: Leong Sim Siong, Kow Shu Wen, Nor Hidahyah Binti Ludin, Nafilah Binti Khalid] 65
Boiling point -78.50 to -61.7 C (liquid)
Freezing point -71 F (-57C) @ 4000 mmHg
Water solubility 2.3% @ 20C
Solvent solubility Soluble: alcohol, acetone, hydrocarbons, organic solvent
Flash point (C) None
Ignition temperature
Explosion limits
Auto ignition
temperature
Flammability limit
(lower %)
Flammability limit
(upper %)
Stability and reactivity
Reactivity Stable at normal temperatures and pressure.
Condition to avoid Protect from physical damage and heat. Containers may rupture or explode if
exposed to heat. Avoid contact with water or moisture.
Incompatibilities combustible materials, oxidizing materials, metal salts, reducing agents, metal
carbide, metals, bases, potassium, sodium, ethylene, amine combustible
materials, lithium
Hazardous
decomposition products
Thermal decomposition products: oxides of carbon
Plant Design For The Production of AA Grade Methanol
[Prepared by: Leong Sim Siong, Kow Shu Wen, Nor Hidahyah Binti Ludin, Nafilah Binti Khalid] 66
1.3.5.3 Water
48
Hazard identification: Potential Health Effects
Route of exposure Potential Health Effects
Inhalation Acute over exposure: Inhalation can result in asphyxiation and is often fatal.
Chronic overexposure: Chronic inhalation overexposure not encountered.
Eye contact Acute overexposure: No effects noted.
Chronic overexposure: No effects noted.
Skin contact Acute overexposure: Prolonged but constant contact with liquid may cause a
mild dermatitis.
Chronic overexposure: Mild to severe dermatitis.
Ingestion Acute overexposure: Excessive ingestion of liquid form can cause gastric
distress and mild diarrhoea.
Chronic overexposure: No effects noted.
First Aid Measures
Route of exposure First Aid measures
Inhalation Remove to fresh air; Provide artificial respiration; Provide oxygen.
Eye contact None
Skin contact None
Ingestion None
Fire-Fighting Measures
Suitable extinguishing
media
Not applicable
Fire and explosion
hazard
Rapid temperature rise of liquid can result in explosive vaporization,
particularly if in a sealed container.
Fire fighting -
Handling and Storage
Handling and Storage A high pressure containment vessel should be used for the vapor at high
Plant Design For The Production of AA Grade Methanol
[Prepared by: Leong Sim Siong, Kow Shu Wen, Nor Hidahyah Binti Ludin, Nafilah Binti Khalid] 67
temperatures.
Do not allow filled, closed containers to solidify as compound expands upon
freezing.
Exposure Control
Occupational exposure
limits (10-hours
reference period)
Exposure limits : 35 ppm (40 mg/m
3
)
Engineering measures /
Ventilation:
Remove hot vapor from environment using local exhaust systems.
Personal protective equipment
Respiratory protection None required.
Hand protection Use insulating gloves when extensive exposure to solid state or high
temperature liquid state is contemplated.
Eye protection Goggles or full face splash shield when dealing with hot liquid.
Skin and body
protection (clothing)
Use heat protective garment when exposed to large quantities of heated
vapour.
Physical and chemical properties
Form Liquid
Color Clear
Odor No odor
Taste -
Molecular weight 18
Vapor pressure 760 mm Hg @ 100C
Vapor density 1 (water=1)
Specific gravity Not applicable
Boiling point 100C
Freezing point 0C
Water solubility 100%
Plant Design For The Production of AA Grade Methanol
[Prepared by: Leong Sim Siong, Kow Shu Wen, Nor Hidahyah Binti Ludin, Nafilah Binti Khalid] 68
Solvent solubility
Flash point (C) Not applicable
Ignition temperature
Explosion limits
Auto ignition
temperature
Not applicable
Flammability limit in air
(% by vol.)
Not applicable
Stability and reactivity
Reactivity Stable at normal temperatures and pressure.
Condition to avoid Avoid heat, flames, sparks and other sources of ignition. Minimize contact with
material. Avoid inhalation of material or combustion by-products. Keep out of
water supplies and sewers.
Incompatibilities Oxidizing materials, halogens, metal oxides, metals, combustible materials,
lithium
Hazardous
decomposition products
Thermal decomposition products: oxides of carbon
1.3.5.4Hydrogen
49
Hazard identification: Potential Health Effects
Route of exposure Potential Health Effects
Inhalation Short term exposure: nausea, vomiting, difficulty breathing, irregular
heartbeat, headache, fatigue, dizziness, disorientation, mood swings, tingling
sensation, loss of coordination, suffocation, convulsions,
unconsciousness, coma
Long term exposure: no information is available
Eye contact Short term exposure: no information on significant adverse effects
Long term exposure: no information is available
Plant Design For The Production of AA Grade Methanol
[Prepared by: Leong Sim Siong, Kow Shu Wen, Nor Hidahyah Binti Ludin, Nafilah Binti Khalid] 69
Skin contact Short term exposure: no information on significant adverse effect
Long term exposure: no information is available
Ingestion Short term exposure: ingestion of a gas is unlikely
Long term exposure: ingestion of harmful amounts is unlikely
First Aid Measures
Route of exposure First Aid measures
Inhalation Remove to uncontaminated area. Give artificial respiration if not breathing. If
breathing is difficult, oxygen should be administered by qualified personnel.
Get immediate medical attention.
Eye contact Flush eyes with plenty of water.
Skin contact Wash exposed skin with soap and water.
Ingestion If a large amount is swallowed, get medical attention.
Fire-Fighting Measures
Suitable extinguishing
media
Carbon dioxide, regular dry chemical
Large fires: Flood with fine water spray.
Fire and explosion
hazard
Severe fire hazard. Severe explosion hazard. Vapour/air mixtures are explosive.
Pressurized containers may rupture or explode if exposed to sufficient heat.
Electrostatic discharges may be generated by flow or agitation resulting in
ignition or explosion.
Fire fighting Move container from fire area if it can be done without risk. Cool containers
with water spray until well after the fire is out. Stay away from the ends of
tanks.
Accidental Release Measures
Water release -
Occupational
spill/release
Avoid heat, flames, sparks and other sources of ignition. Do not touch spilled
material. Stop leak if possible without personal risk. Reduce vapours with water
spray. Keep unnecessary people away, isolate hazard area and deny entry.
Remove sources of ignition. Ventilate closed spaces before entering
Handling and Storage
Plant Design For The Production of AA Grade Methanol
[Prepared by: Leong Sim Siong, Kow Shu Wen, Nor Hidahyah Binti Ludin, Nafilah Binti Khalid] 70
Handling -
Storage Store and handle in accordance with all current regulations and standards.
Grounding and bonding required.
Engineering measures /
Ventilation
Ventilation equipment should be explosion-resistant if explosive concentrations
of material are present. Provide local exhaust ventilation system. Ensure
compliance with applicable exposure limits
Personal protective equipments
Respiratory
protection
Under conditions of frequent use or heavy exposure, respiratory protection
may be needed.
Respiratory protection is ranked in order from minimum to maximum.
Consider warning properties before use.
Hand protection Wear appropriate chemical resistant gloves.
Eye protection Eye protection not required, but recommended.
Skin and body
protection (clothing)
Protective clothing is not required.
Physical and chemical properties
Form Gas
Color Colorless
Odor Odorless
Taste Tasteless
Molecular weight 2.0
Vapor pressure 760 mmHg @ -253C
Vapor density 0.07 (air=1)
Specific gravity Not applicable
Boiling point -253C
Freezing point -259C
Water solubility 1.82% @ 20C
Solvent solubility Slightly Soluble: alcohol, ether
Plant Design For The Production of AA Grade Methanol
[Prepared by: Leong Sim Siong, Kow Shu Wen, Nor Hidahyah Binti Ludin, Nafilah Binti Khalid] 71
Flash point (C) -
Ignition temperature -
Explosion limits -
Auto ignition
temperature
932F (500C)
Flammability limit
(lower %)
4.0 %
Flammability limit
(upper %)
75 %
Stability and reactivity
Reactivity Stable at normal temperatures and pressure.
Condition to avoid Avoid heat, flames, sparks and other sources of ignition. Minimize contact with
material. Containers may rupture or explode if exposed to heat.
Incompatibilities Metals, oxidizing materials, metal oxides, combustible materials, halogens,
metal salts, halo carbons
Hazardous
decomposition products
Thermal decomposition products: miscellaneous decomposition product
1.3.5.5Methanol
50
Hazard identification: Potential Health Effects
Route of exposure Potential Health Effects
Inhalation Acute Exposure: headache, weakness, drowsiness, light-headedness, nausea,
difficult breathing, drunkenness, eye irritation, blurred vision, blindness, loss of
consciousness, vertigo, fatigue, convulsions, and possibly death, depending on
exposure.
Eye contact Acute Contact: Upon direct contact with liquid, it can progress to permanent
blindness.
Skin contact Acute Contact: drying, cracking, and inflammation of the skin due to the
Plant Design For The Production of AA Grade Methanol
[Prepared by: Leong Sim Siong, Kow Shu Wen, Nor Hidahyah Binti Ludin, Nafilah Binti Khalid] 72
defatting action of the product.
Ingestion Ingestion may cause serious poisoning with effects similar to those of inhalation
and absorption through the skin.
First Aid Measures
Route of exposure First Aid measures
Inhalation Move patient to fresh air and keep warm and at rest. Monitor for respiratory
distress
Eye contact Immediately flush eyes with copious amounts of tepid water for at least
15minutes.
Skin contact Immediately flush eyes with copious amounts of tepid water for at least 15
minutes while removing contaminated clothing and shoes, followed by washing
area thoroughly with soap and water.
Ingestion If patient is conscious, immediately give two glasses of water and induce
vomiting.
Fire-Fighting Measures
Suitable extinguishing
media
Water may be ineffective but may be used to dilute spills to non-flammable
mixtures.
Small Fire: Dry Chemical, CO2, Water spray or alcoholresistant foam
Large Fire: Water Spray, fog or alcoholresistant foam
Fire and explosion
hazard
-
Fire fighting Move container from fire area if you can do it without risk. Apply cooling water
to sides of containers that are exposed to flames until well after fire is out. Stay
away from ends of tanks due to exploding potential when tanks are involved in
a fire.
Accidental Release Measures
Water release -
Occupational Isolate spill or leak area immediately for at least 330 to 660 feet (100 to 200
Plant Design For The Production of AA Grade Methanol
[Prepared by: Leong Sim Siong, Kow Shu Wen, Nor Hidahyah Binti Ludin, Nafilah Binti Khalid] 73
spill/release meters) in all directions. Stay upwind, out of low areas, and ventilate closed
spaces before entering. Eliminate all ignition sources.
Handling and Storage
Handling Use proper personal protective equipment when working with or around
methanol. No smoking or open flame in storage, use, or handling areas. Use
explosionproof electrical equipment. Ensure proper electrical grounding
procedures are in place.
Storage Store in totally enclosed equipment, designed to avoid ignition and human
contact. Tanks must be grounded, vented, and should have vapour emission
controls. Handlers must eliminate ignition sources or purge the tank with an
inert gas such as nitrogen.
Exposure Control / personal Protection
Occupational exposure
limits (10-hours
reference period)
Exposure limits : 35 ppm (40 mg/m
3
)
Engineering measures /
Ventilation:
Ventilation equipment should be explosion-resistant if explosive concentrations
of material are present. Provide local exhaust or process enclosure ventilation
system. Ensure compliance with applicable exposure limits.
Personal protective equipment
Respiratory protection Less than 200 ppm: No protection required if TWA is not exceeded.
200 to 250 ppm: Protection required if the daily TWA is exceeded, fresh
air supplied system must be used if protection is needed.
Greater than 200 ppm: A fresh air supply system must be used if protection
is needed
Hand protection -
Eye protection Use chemical (indirectly vented) goggles when there is a potential for contact
with product including vapour. A fullface shield may be worn over goggles
for additional protection, but not as substitute for goggles.
Plant Design For The Production of AA Grade Methanol
[Prepared by: Leong Sim Siong, Kow Shu Wen, Nor Hidahyah Binti Ludin, Nafilah Binti Khalid] 74
Skin and body
protection
Equipment should prevent repeated or prolonged skin contact with the
product. This may include rubber boots, resistant gloves, and other
impervious and resistant clothing. Compatible materials may include butyl
rubber, natural rubber, neoprene, nitrile rubber, viton and others.
Physical and chemical properties
Form Liquid
Color Colorless
Odor Faintly sweet pungent odor like ethyl alcohol
Taste -
Molecular weight 32.04
Vapor pressure 12.82 mmHg @ 20C
Vapor density 1.11 @ 20 C
Specific gravity 0.792 @ 20 C
Boiling point 64C at atmospheric pressure
Melting point -98C
Solubility 100 %
Solvent solubility
Flash point (C) 11C, closed cup
Ignition temperature
Explosion limits
Auto ignition
temperature
725F (385C)
Flammability limit
(lower %)
6.0% Volume in Air
Flammability limit
(upper %)
36.5% Volume in Air
Stability and reactivity
Incompatibilities Incompatible with beryllium dihydride, metals (potassium, magnesium, etc.),
oxidants (barium, perchlorate, bromine, chlorine, etc.),
Plant Design For The Production of AA Grade Methanol
[Prepared by: Leong Sim Siong, Kow Shu Wen, Nor Hidahyah Binti Ludin, Nafilah Binti Khalid] 75
Dangerous because can react vigorously with oxidizing materials
Hazardous
decomposition products
Excessive heating and/or incomplete combustion will generate carbon
monoxide, formaldehyde, and possible unburned methanol.
1.3.5.6 Methane
51
Hazard identification: Potential Health Effects
Route of exposure Potential Health Effects
Inhalation Acts as a simple asphyxiate.
Eye contact Contact with rapidly expanding gas may cause burns or frostbite. Contact with
cryogenic liquid can cause frostbite and cryogenic burns.
Skin contact Contact with rapidly expanding gas may cause burns or frostbite. Contact with
cryogenic liquid can cause frostbite and cryogenic burns.
Ingestion Ingestion is not a normal route of exposure for gases. Contact with cryogenic
liquid can cause frostbite and cryogenic burns.
First Aid Measures
Route of exposure First Aid measures
Inhalation Move exposed person to fresh air. Loosen tight clothing such as a collar, tie,
belt or waistband. Get medical attention immediately.
Eye contact Immediately flush eyes with plenty of water for at least 15 minutes,
occasionally lifting the upper and lower eyelids. Get medical attention
immediately
Skin contact Immediately flush skin with plenty of water for at least 15 minutes.
Ingestion Refer to the inhalation section.
Fire-Fighting Measures
Fire fighting media Use water spray (fog), foam or dry chemical.
Fire and explosion
hazard
Extremely flammable in the presence of the open flames, sparks and static
discharge and oxidizing materials.
Plant Design For The Production of AA Grade Methanol
[Prepared by: Leong Sim Siong, Kow Shu Wen, Nor Hidahyah Binti Ludin, Nafilah Binti Khalid] 76
Accidental Release Measures
Water release Keep out of water supplies and sewers
Occupational
spill/release
Avoid heat, flames, sparks and other sources of ignition. Stop leak if possible
without personal risk. Reduce vapours with water spray. Keep unnecessary
people away, isolate hazard area and deny entry. Remove sources of ignition
Handling and Storage
Handling Use only with adequate ventilation and explosion-proof electrical equipment.
Storage Keep container in a cool, well-ventilated area, tightly closed and sealed until
ready for use. Avoid all possible sources of ignition. Cylinders should be
stored upright, with valve protection cap in place, and firmly secured to prevent
falling or being knocked over. Cylinder temperatures should not exceed 52 C
Exposure Control
Occupational exposure
limits (8-hours reference
period)
Exposure limits: 1000 ppm
Engineering measures /
Ventilation
Use only with adequate ventilation and explosion-proof ventilation equipment
Personal protective equipment
Respiratory
protection
Use a properly fitted, air-purifying or air-fed respirator complying with an
approved standard
Hand protection Insulated gloves suitable for low temperatures
Eye protection Safety eyewear complying with an approved standard to avoid exposure to
liquid splashes, mists or dusts.
Skin and body
protection
Personal protective equipment
Physical and chemical properties
Plant Design For The Production of AA Grade Methanol
[Prepared by: Leong Sim Siong, Kow Shu Wen, Nor Hidahyah Binti Ludin, Nafilah Binti Khalid] 77
Form Gas
Color Colorless
Odor
Taste Tasteless
Molecular weight 16.05
Vapor pressure Not applicable
Vapor density 0.55 (air=1)
Specific gravity
Boiling point -161.6C
Freezing point -182.2C
Water solubility Very slight
Solvent solubility
Flash point (C) Closed cup: -188.15C
Ignition temperature
Explosion limits
Auto ignition
temperature
539.85C
Flammability limit
(lower %)
5 %
Flammability limit
(upper %)
16 %
Stability and reactivity
Reactivity Stable
Condition to avoid -
Incompatibilities Oxidizing materials
Hazardous
decomposition products
Under normal conditions of storage and use, hazardous decomposition products
should not be produced.
Plant Design For The Production of AA Grade Methanol
[Prepared by: Leong Sim Siong, Kow Shu Wen, Nor Hidahyah Binti Ludin, Nafilah Binti Khalid] 78
1.3.2.7 Oxygen
54
Hazard identification: Potential Health Effects
Route of exposure Potential Health Effects
Inhalation Breathing 80% or more oxygen at atmospheric pressure for more
than a few hours may cause nasal stuffiness, cough, sore throat, chest pain
and breathing difficulty
Eye contact No adverse effect.
Skin contact No adverse effect.
Ingestion -
First Aid Measures
Route of exposure First Aid measures
Inhalation Move victim to fresh air or if in elevated pressures reduce oxygen pressures
to one atmosphere
Eye contact Not applicable
Skin contact Not applicable
Ingestion -
Fire-Fighting Measures
Extinguishing media Oxygen is non flammable but will support combustion. Use extinguishing
media appropriate for surrounding fire.
Fire and explosion
hazard
Oxygen may form explosive compounds when exposed to combustible
materials or oil, grease, and other hydrocarbon materials.
Accidental Release Measures
Environmental
precautions
Avoid dispersal of spilled material and runoff and contact with soil,
waterways, drains and sewer
Occupational
spill/release
Stop leak if without risk. Use spark-proof tools and explosion-proof
equipment.
Handling and Storage
Handling Use equipment rated for cylinder pressure and suitable hand truck for
cylinder movement. Avoid contact with combustible materials.
Storage Keep container in a cool, well-ventilated area, tightly closed and sealed until
ready for use. Avoid all possible sources of ignition. Cylinders should be
stored upright, with valve protection cap in place, and firmly secured to
prevent falling or being knocked over. Cylinder temperatures should not
exceed 52 C
Exposure Control
Engineering measures /
Ventilation
Provide ventilation and/or local exhaust to prevent accumulation of high
concentrations of gas (greater than 23%).
Plant Design For The Production of AA Grade Methanol
[Prepared by: Leong Sim Siong, Kow Shu Wen, Nor Hidahyah Binti Ludin, Nafilah Binti Khalid] 79
Personal protective equipment
Respiratory protection None required
Hand protection None required
Eye protection None required
Skin and body
protection
None required
Physical and chemical properties
Form Gas
Color Colorless
Odor Odorless
Taste -
Molecular weight 32
Vapor pressure Not applicable at 70C
Vapor density 1.105 (air=1)
Specific gravity 1.1 (Air =1)
Boiling point -183C
Freezing point -218.8C
Water solubility 0.049
Solvent solubility
Flash point (C) Not applicable
Ignition temperature
Explosion limits
Auto ignition
temperature
Flammability limit
(lower %)
Flammability limit
(upper %)
Stability and reactivity
Reactivity Stable
Condition to avoid None
Incompatibilities Oils, grease, hydrocarbons and flammable materials.
Hazardous
decomposition products
-
Plant Design For The Production of AA Grade Methanol
[Prepared by: Leong Sim Siong, Kow Shu Wen, Nor Hidahyah Binti Ludin, Nafilah Binti Khalid] 80
1.3.2.8Steam
55
Hazard identification: Potential health effects
Route of exposure Potential Health Effects
Inhalation Potentially life-threatening burns to the nose, throat and lungs. Following
exposure, pulmonary edema is very likely to occur and may be fatal.
Eye contact Severe burns will occur and blindness may result.
Skin contact Severe burns to the skin. Burns may be life-threatening if a significant portion
of the skin is involved. Permanent scarring is likely to result
Ingestion Ingestion not anticipated being a likely route of occupational exposure.
First Aid Measures
Inhalation Use artificial respiration to support vital functions. Seek immediate medical
attention and transport victim to hospital or other medical facility.
Eye contact Cover eye(s) with cool compresses.
Skin contact Cover burns with loose, damp compresses. Burns to more than 20% of the
body should be treated as life-threatening; victim should be transported to
hospital immediately.
Ingestion -
Fire-Fighting Measures
Suitable extinguishing
media
Use extinguishing media appropriate for surrounding fire
Fire and explosion hazard Piping carrying this product may rupture in the heat of a fire, creating an
extreme contact hazard to fire-fighters if a sudden release occurs.
Fire fighting Structural fire-fighters must wear Self-Contained Breathing Apparatus and full
protective equipment.
Accidental Release Measures
Water release -
Occupational spill/release Evacuate immediate area. Once the valves have been closed and the release is
stopped, the remaining water, once cooled, should be cleaned-up according to
site response plan.
Handling and Storage
Storage and handling Open valves associated with piping system slowly. Pipes and process lines
must be properly labelled. This liquid should be kept away from
incompatible materials. Valves should be tightly closed when system is not in
use. All process lines, valves, and related equipment should be routinely
inspected, undergo periodic maintenance, and checked to ensure equipment is
properly labeled and not damaged.
Exposure Control / Personal Protection
Occupational exposure
limits
There are no specific exposure limits for Steam (Water).
Engineering measures /
Ventilation
No special ventilation or engineering controls are associated with routine use
of this liquid. Engineering controls should be developed to avoid sudden
Plant Design For The Production of AA Grade Methanol
[Prepared by: Leong Sim Siong, Kow Shu Wen, Nor Hidahyah Binti Ludin, Nafilah Binti Khalid] 81
release of this product
Personal protective equipment
Respiratory protection None normally required for routine use.
Hand protection Wear glove protection appropriate to the specific tasks. Leather gloves to
protect against mechanical injury or gloves to protect against thermal burns if
contact is possible.
Eye protection Safety glasses. If potential for release exists, a full-face shield must be worn.
Skin and body
protection (clothing)
Use body protection appropriate for task. Safety shoes are recommended.
Physical and chemical properties
Form Liquid
Color Colorless
Odor Odorless liquid which is pressurized and heated to either 175 psi and 420F or
820 psi and 750F.
Taste -
Molecular weight 18.02
Vapor pressure Approximately 18 mm Hg
Vapor density -
Specific gravity 1 (water = 1)
Boiling point 100C
Freezing point 0C
Water solubility Soluble at 0C
Solvent solubility -
Flash point (C) Not available
Ignition temperature -
Explosion limits -
Auto ignition temperature Not applicable
Flammability limit
(lower %)
Not applicable
Flammability limit
(upper %)
Not applicable
Stability and reactivity
Stability Stable
Condition to avoid Avoid exposure to incompatible (water-reactive) materials and accidental
release.
Incompatibilities Materials that is water-reactive.
Hazardous decomposition
products
None
Plant Design For The Production of AA Grade Methanol
[Prepared by: Leong Sim Siong, Kow Shu Wen, Nor Hidahyah Binti Ludin, Nafilah Binti Khalid] 82
1.3.6 CRITICAL ASSESSMENT: ADVANTAGES AND
DISADVANTAGES OF THE PROCESS CHOSEN
During designing the production plant, we had considered many criteria before we
decided to choose the suitable plant. This is important in order to get the best features for
our plant. Table 1.3-3 below shows the criteria that we had consider before choosing the
designed plant.
Table 1.3-3: Advantages and Disadvantages of Process Chosen
Criteria Advantages Disadvantages
Raw Materials Used
(Natural Gas)
Available in
abundance
Non-renewable
source
Need to be purified
first
Reactor
(Fixed-Bed Tubular Reactor)
Favours temperature
profile along the
reactor
Enhances activity
and lifetime of the
catalyst
Able to overcome
limitation of
thermodynamic
equilibrium
Enhances kinetic
limited reaction
Control
stoichimetrical feed
Reaction Condition
(Low temperature of methanol
synthesis 300-500
o
C)
High heat efficiency
High conversion per
run
Excellent
adaptability to CO
2
Low operation cost
Separation and Purification High purity ~99.85%
Plant Design For The Production of AA Grade Methanol
[Prepared by: Leong Sim Siong, Kow Shu Wen, Nor Hidahyah Binti Ludin, Nafilah Binti Khalid] 83
Catalyst Used Catalyst can be
regenerated
Deactivation of
catalyst in short life
time
Environmental concerns CO
2
can be recycled
back to the process
No harmful
byproduct
Greenhouse effect
Not emission-free
Safety and Health Concerns The reaction
conditions are not
too extreme
CO, H
2
and methane
are harmful to human
Methane is explosive
if mixed with air
Waste management Nickel catalyst can
be recycled
Catalytic function of
nickel can be
recovered in high
proportion.
The waste hydrogen
can be captured by
new invented
technology such as
membrane or
Pressure Swing
Absorption (PSA).
Carbon monoxide
can be removed by
adsorption with -
Al
2
O
3
and TiO
2
.
Plant Design For The Production of AA Grade Methanol
[Prepared by: Leong Sim Siong, Kow Shu Wen, Nor Hidahyah Binti Ludin, Nafilah Binti Khalid] 84
1.3.7 METHANOL PLANTS IN MALAYSIA
Table 1.3-4: Methanol Plants in Malaysia - capacity, investment
Plant Technology Used Production Capacity Total Investment
Petronas Methanol
(Labuan) SdnBhd
Lurgis Low
Pressure Combined
Technology
56
1,700,000 tons per
year
RM 1,140 million
Petronas Chemical
Fertilizer Kedah
SdnBhd
High Pressure Co-
production
730,000 tons per year Unknown
Plant Design For The Production of AA Grade Methanol
[Prepared by: Leong Sim Siong, Kow Shu Wen, Nor Hidahyah Binti Ludin, Nafilah Binti Khalid] 85
1.4 PROCESS SYNTHESIS STRUCTURE AND
ANALYSIS
________________________________________________
1.4.1 INPUT-OUTPUT STRUCTURE WITH FEED AND PRODUCT
SPECIFICATIONS
Figure 1.4-1 shows all the processes of the methanol production plant. There are only
three input stream into the synthesis line of the methanol plant:
1. Natural gas (contains mainly methane, small quantity of ethane and trace amount
of hydrogen sulphide and mercury) as raw material.
2. Steam for reforming processes it will react with methane to form syngas.
3. Oxygen for oxygen blown autothermal reforming.
Several output streams of the plant also shown in the figure below:
1. AA grade methanol as the product.
2. Water from water adsorption column and final distillation column.
3. Carbon dioxide from pressure swing adsorption column.
4. Mercury (trace amount) from mercury removal unit.
5. Hydrogen sulphide from desulphurizer.
Plant Design For The Production of AA Grade Methanol
[Prepared by: Leong Sim Siong, Kow Shu Wen, Nor Hidahyah Binti Ludin, Nafilah Binti Khalid] 86
Mercury Removal
Desulphurization
Steam Reforming
Autothermal Reforming
Water Adsorption
CO/CO2 Hydrogenation
Flash Separation
Pressure Swing Adsorption
Distillation
Natural Gas
Methane
Ethane
Mercury
Hydrogen Sulphide
Mercury
(Trace amount)
Methane
Ethane
Hydrogen Sulphide
Methane
Ethane
Hydrogen Sulphide
Methane
Ethane
Carbon Monoxide
Carbon Dioxide
Hydrogen
Steam
Carbon Monoxide
Carbon Dioxide
Hydrogen
Steam
Carbon Monoxide
Carbon Dioxide
Hydrogen
Carbon Monoxide
Carbon Dioxide
Hydrogen
Methanol
Water
Carbon Monoxide
Carbon Dioxide
Hydrogen
Steam
Oxygen
Water
AA Methanol
Water
Methanol
Water
Carbon Monoxide
Hydrogen
Carbon Dioxide
Inputs Operations Outputs
Figure 1.4-1: Block diagram for Methanol Production
Plant Design For The Production of AA Grade Methanol
[Prepared by: Leong Sim Siong, Kow Shu Wen, Nor Hidahyah Binti Ludin, Nafilah Binti Khalid] 87
Feed and Product Specifications
Table 1.4-1
57
and 1.4-2
58
show the properties or specification for natural gas (carbon
monoxide (CO) and carbon dioxide (CO
2
)) which are raw materials for the production of
AA grade methanol. From the table, we can conclude that pre-treatment is not needed due
to the purity of both raw materials are 88% and above. The major impurity for both
materials is hydrogen which is both reacts to produce methanol.
Table 1.4-1: Natural Gas Specifications
Property Limitations ASTM Test Method
Hydrocarbon Methane, Ethane,
Propane, C4 and higher
mole percent: 88% ,
Minimum: 6%
max.1.7% max.0.3%
D-1945
Other Gaseous Species
Hydrogen, Carbon dioxide +
Nitrogen + Oxygen, Oxygen,
Carbon Monoxide, Other Species
Methanol ,Sulphur, Total
|| mole percent: 0.1%
max.5.0% max.0.5%
max.0.1% max.0%
mass max.1.0 grains / 100
SCF, max. (32 ppm mass,
max.)
D 2650D1945D-
1945D 2650No
Test Method ASTM D
5504
Performance Related
Properties
Motor Octane Number, Wobbe
Number
115 Minimum
1290-1380 BTU/SCF
*D 2623D 3588
Contaminates
Pressure Water Dew, Point
Temperature, max.
D 1142
Pressure Hydrocarbon Dew Point,
Temperature, max.
Below which will form 1%
condensate
D 1142
Odorant
Notes:
Plant Design For The Production of AA Grade Methanol
[Prepared by: Leong Sim Siong, Kow Shu Wen, Nor Hidahyah Binti Ludin, Nafilah Binti Khalid] 88
* Test Method D 2623 was obsolete by ASTM in 1991. Wobbe Index (WI), also known
as Wobbe Number, is a measure of fuel energy flow rate through a fixed orifice under
given inlet conditions. Numerically, WI= (dry, higher heating value)/ (Specific gravity).
The compressed natural gas shall not contain dust, sand, dirt, gums, oils, or other
substances in an amount sufficient to be injurious to the fuel station equipment or the
vehicle being fuelled.
The water and hydrocarbon dew point at fuel pressure shall be at least 10 F below the
99.0% winter design temperature listed, Climatic Conditions for the United States, in
American Society of Heating, Refrigerating and Air Conditioning Engineers (ASHRAE)
Handbook, 1989 fundamentals volume. Testing for water and hydrocarbon vapour shall
be in accordance with ASTM D 1142, utilizing the Bureau of Mines apparatus.
The natural gas at ambient conditions must have a distinctive odour potent enough for
its presence to be detected down to a concentration in air of 1% by volume.
|| For Genset applications, when using fuel containing sulphur in excess of 16 ppm, the
oil change interval must be reduced.
Table 1.4-2: Methanol Specifications
Property Limitations ASTM Test Method
Purity, wt%, min. 99.85 IMPCA 001
Specific Gravity, 20C/20C 0.791-0.793 ASTM D891
Distillation Range, C 1.0
ASTM D1078 at 760 mm Hg
1.0 (incl. 64.60.1) ASTM D1078
Color, Pt-Co, max. 5 ASTM D1209
Odor characteristic no residual ASTM D1296
Carbonizable impurities 30 ASTM E346
Appearance Clear, no sediment Visual
Nonvolatile content mg/kg, 10 ASTM D1353
Plant Design For The Production of AA Grade Methanol
[Prepared by: Leong Sim Siong, Kow Shu Wen, Nor Hidahyah Binti Ludin, Nafilah Binti Khalid] 89
max.
Potassium Permanganate
minutes, min.
60 ASTM D1363
Acetone + Aldehydes wt%,
max.
0.003 ASTM E346
Acetones, wt. %, max. 0.002 ASTM E346 modified
Ethanol, wt.%, max 0.001 ASTM E346
Chloride, Cl', wt%, max 0.00005 IMPCA 002
Sulfur, wt%, max 0.00005 ASTM D3961 or
D5453
Total iron, wt%, max. 0.00001 ASTM D394
Hydrocarbons Pass Test ASTM D1722
Acidity, wt%, maxes. 0.003 ASTM D1613
Water, wt%, max. 0.1 ASTM E1064 or
D346
Water miscibility No turbidity
Plant Design For The Production of AA Grade Methanol
[Prepared by: Leong Sim Siong, Kow Shu Wen, Nor Hidahyah Binti Ludin, Nafilah Binti Khalid] 90
1.4.2 PRICE OF ALL PRODUCTS, BYPRODUCTS AND RAW
MATERIALS
Table 1.4-3 shows the price of product, by-product and raw materials involved in
Methanol production.
Table 1.4-3: Prices of Components in Methanol Production
Component Price (USD/MT) Price (RM/MT)
Natural gas USD 81.39 USD 529.06 RM 248.32- RM 1614.162
Steam USD 13.36 RM 40.74
Hydrogen USD 1248.769 RM 3810.00
Methanol Europe USD 439
North America USD 439
Asia Pacific USD 425
RM 1338.95
RM 1338.95
RM 1296.25
Oxygen USD 210 RM 640.00
Plant Design For The Production of AA Grade Methanol
[Prepared by: Leong Sim Siong, Kow Shu Wen, Nor Hidahyah Binti Ludin, Nafilah Binti Khalid] 91
1.4.3 DESTINATION CODES AND COMPONENT
CLASSIFICATION
Table 1.4-4 shows the destination code and component classification of related chemicals
in Methanol production.
Table 1.4-4: Destination Code and Component Classification of Related Chemicals in
Process
Component Component Classification Destination Code
Methane Reactant Autothermal Reformer
Steam Reactant Adsorption Column
Carbon Monoxide Intermediate reactant Reactor/Recycle Stream
Carbon Dioxide Intermediate reactant Reactor/Recycle Stream/Flare
tower
Hydrogen Intermediate reactant Reactor/Recycle Stream
Ethane Trace element Autothermal Reformer
Mercury Trace element Elimination
Hydrogen Sulphide Trace element Elimination
Copper/Zinc
oxide/Aluminum oxide
Catalyst Regeneration
Water By-product Waste
Methanol Primary Product Primary Product
Plant Design For The Production of AA Grade Methanol
[Prepared by: Leong Sim Siong, Kow Shu Wen, Nor Hidahyah Binti Ludin, Nafilah Binti Khalid] 92
1.4.4 UTILITIES
Three important utilities in Methanol plant are water, steam and electricity. The plant will
be shut down if any one of them absence.
i) Water
Water is used in heat exchanger as coolant due to its good cooling properties, cheap and
abundance. It can also be recycled from other operating equipment in the plant. It is non-
hazardous, thus making it able to be disposed without any further treatment.
ii) Steam
Steam is act as a heating medium and will be transferred to the necessary part of the plant
for heating purpose. The heat is transferred to the reactor for the reaction of natural gas.
iii) Electricity
Electricity is important in order to operate the whole process, below are some of the
process which required electricity:
a. Pump for circulation of raw materials, product and recycle stream.
b. Control system of the plant.
c. Heater, reboiler
d. Compressor
The commonly used motor will be electric motor as they are more efficient and are very
reliable in a wide range of wattage.
Plant Design For The Production of AA Grade Methanol
[Prepared by: Leong Sim Siong, Kow Shu Wen, Nor Hidahyah Binti Ludin, Nafilah Binti Khalid] 93
1.5 PROCESS FLOW DIAGRAM
________________________________________________
Block diagram, process synthesis flow diagram and process flow diagram for methanol
synthesis via CO/CO
2
hydrogenation route are shown in Figure 1.5-1, Figure 1.5-2 and
Figure 1.5-3 respectively.
Plant Design For The Production of AA Grade Methanol
[Prepared by: Leong Sim Siong, Kow Shu Wen, Nor Hidahyah Binti Ludin, Nafilah Binti Khalid] 94
Mercury
Removal Unit
R-101
Desulphurizer
R-102
Steam Reformer
R-103
Water Adsorption
Column
C-101
Methanol Reactor
R-104
Separator
V-101
Distillation Column
C-102
Natural Gas
Methane
Ethane
Carbon Dioxide
Hydrogen Sulphide
Trace element (Mercury)
Methane
Ethane
Carbon Dioxide
Hydrogen Sulphide
Methane
Ethane
Carbon Dioxide
Steam
Methane
Ethane
Carbon Monoxide
Carbon Dioxide
Hydrogen
Unreacted Steam
Carbon Monoxide
Carbon Dioxide
Hydrogen
Unreacted Steam
Carbon Monoxide
Carbon Dioxide
Hydrogen
Carbon Monoxide
Carbon Dioxide
Hydrogen
Autothermal Reformer
R-104
Oxygen
Methanol
Water
Carbon Monoxide
Carbon Dioxide
Hydrogen
Crude Methanol
Methanol
Water
AA Methanol
Adsorption Column
T-103
Hydrogen
Carbon Monoxide
Carbon Dioxide
Water
Figure 1.5-1 Block diagram of methanol production using CO/CO
2
hydrogenation
Plant Design For The Production of AA Grade Methanol
[Prepared by: Leong Sim Siong, Kow Shu Wen, Nor Hidahyah Binti Ludin, Nafilah Binti Khalid] 95
Natural Gas
Methane
Ethane
Carbon Dioxide
Hydrogen Sulphide
Trace element (Mercury)
Mercury Removal
Temperature: 50C
Pressure: 20 bar
Desulphurization
Temperature: 350C
Pressure: 20 bar
Steam Reforming
Temperature: 850C
Pressure: 25 bar
Autothermal Reforming
Temperature: 1000C
Pressure: 50 bar
Water Adsorption
Temperature: 80C
Pressure: 50 bar
CO/CO2 Hydrogenation
Temperature: 230C
Pressure: 80 bar
Flash Separation
Temperature: 10C
Pressure: 50 bar
Distillation
Temperature: 95C
Pressure: 1 bar
Methane
Ethane
Carbon Monoxide
Carbon Dioxide
Hydrogen
Steam
Carbon Monoxide
Carbon Dioxide
Hydrogen
Steam
Methane
Ethane
Carbon Dioxide
Hydrogen Sulphide
Methane
Ethane
Carbon Dioxide
Carbon Monoxide
Carbon Dioxide
Hydrogen
Methanol
Water
Carbon Monoxide
Carbon Dioxide
Hydrogen
Crude Methanol
Methanol
Water
Carbon Monoxide
Carbon Dioxide
Hydrogen
AA Methanol
Water
Carbon Dioxide
Steam
Oxygen
Pressure Swing Adsorption
Temperature: 10C
Pressure: 3 bar
Hydrogen
Carbon Monoxide
Figure 1.5-2 Process synthesis flow of methanol production using CO/CO
2
hydrogenation
Plant Design For The Production of AA Grade Methanol
[Prepared by: Leong Sim Siong, Kow Shu Wen, Nor Hidahyah Binti Ludin, Nafilah Binti Khalid] 96
R-104
E-101
E-102
E-103
E-104
R-105
V-101
T-102
E-105
E-106
E-107
C-105
E-110
E-109
V-103
R-102
3
Steam
7
11
12
19
20
21
22
23
24
25
26
32
27
44
Natural Gas
R-101
1
5
C-103 C-104
17
T-101
13
28
36 Purge to flare
Wastewater
AA Methanol
Cooling Water
Cooling Water
14
16
Cooling Water
Steam
E-108
33
34
35
Steam
Cooling Water
P-101
29
30 31
43
R-102
Desulphurizer
E-101
Steam Reformer
Preheater
C-103/104
Staged Syngas
Compressor
R-103
Steam Reformer
E-102
Syngas Cooler
E-104
Methanol Reactor
Preheater
R-101
Mercury Remover
R-104
Autothermal reformer
T-101
Water Adsorption Column
E-103
Interstage Cooler
2
E-110
Recycle Stream
Heater
R-105
Methanol Reactor
E-105
Reactor Effluent
Cooler
C-103
Recycle Stream
Compressor
V-101
Flash Separator
E-106/107
Distillation Column
Preheater
E-109
Condenser
T-102
Distillation Column
V-103
Methanol Reflux
Drum
P-101
Distillate Pump
E-108
Reboiler
C-101
C-102
C-101
Steam Reformer
Feed Compressor
C-102
Steam Reformer
Effluent
Compressor
Oxygen
8
10
9 6
15
18
T-103
37
38 39 42
4
T-103
Adsorption
Column
40
41
R-103
Steam
Steam
Flue gas
Flue gas
Figure 1.5.3 Process Flow Diagram (PFD) of methanol production using CO/CO2 hydrogenation
Plant Design For The Production of AA Grade Methanol
[Prepared by: Leong Sim Siong, Kow Shu Wen, Nor Hidahyah Binti Ludin, Nafilah Binti Khalid] 97
Appendix
a) Minutes of meetings
MINUTES OF MEETING WITH SUPERVISOR 1
Date : 17
th
September 2012 (Monday)
Time : 3.00 pm 4.00 pm
Venue : Office of Chemical Engineering School
Attendance:
1. Dr. Leo Cheo Peng (Supervisor)
2. Kow Shu Wen
3. Leong Sim Siong
4. Nor Hidahyah binti Ludin
5. Nafilah Binti Khalid
Agenda:
1. Supervisor has discussed with us about our project title and gives some related
information to us.
2. She asked us to start the task 1 by searching for the related plants and process of
our title and gather all related information.
3. She has given us some motivation so that we can complete this design project in a
good team work.
4. We finish the meeting at 4.00pm.
Prepared by,
-----------------------------
(Kow Shu Wen)
Plant Design For The Production of AA Grade Methanol
[Prepared by: Leong Sim Siong, Kow Shu Wen, Nor Hidahyah Binti Ludin, Nafilah Binti Khalid] 98
MINUTES OF MEETING WITH SUPERVISOR 2
Date : 24
th
September 2012 (Monday)
Time : 2.15 pm 3.30 pm
Venue : Office of Chemical Engineering School
Attendance:
1. Dr. Leo Cheo Peng (Supervisor)
2. Kow Shu Wen
3. Leong Sim Siong
4. Nor Hidahyah binti Ludin
5. Nafilah Binti Khalid
Agenda:
1. We have asked the questions that we are confused with the requirement.
2. Supervisor has explained it to us and also gives some information for us.
3. She has asked us to search our information using US pattern.
4. She also told us to put in references besides the sentences and the page number of
our report.
5. We finish the meeting at 5.00pm.
Prepared by,
-----------------------------
(Kow Shu Wen)
Plant Design For The Production of AA Grade Methanol
[Prepared by: Leong Sim Siong, Kow Shu Wen, Nor Hidahyah Binti Ludin, Nafilah Binti Khalid] 99
MINUTES OF MEETING WITH SUPERVISOR 3
Date : 2
nd
October 2012 (Tuesday)
Time : 3.30 pm 4.30 pm
Venue : Office of Chemical Engineering School
Attendance:
1. Assoc. Prof. Dr. Zainal Ahmad (Supervisor)
2. Kow Shu Wen
3. Leong Sim Siong
4. Nor Hidahyah binti Ludin
5. Nafilah Binti Khalid
Agenda:
1. During this meeting, we show our supervisor the process flow diagram (PFD) and
block diagram that we prepared last week.
2. Dr. Zainal comments on our flow diagram and give his opinion on the cooler,
boiler and distillation part. He asked us to find more information about the
operating conditions for each equipment inside the PFD.
3. We also take the opportunity to ask the supervisor how to do the controllability of
question 2 for the Choice of Process.
Prepared by,
-----------------------------
(Leong Sim Siong)
Plant Design For The Production of AA Grade Methanol
[Prepared by: Leong Sim Siong, Kow Shu Wen, Nor Hidahyah Binti Ludin, Nafilah Binti Khalid] 100
MINUTES OF MEETING WITH SUPERVISOR 4
Date : 4
th
October 2012 (Thursday)
Time : 3.00 pm 4.00 pm
Venue : Office of Chemical Engineering School
Attendance:
1. Assoc. Prof. Dr. Zainal Ahmad (Supervisor)
2. Kow Shu Wen
3. Leong Sim Siong
4. Nor Hidahyah binti Ludin
5. Nafilah Binti Khalid
Agenda:
1. During this meeting, we show our supervisor the edited process flow diagram
(PFD) and block diagram that we prepared last week.
2. Dr. Zainal comments on our flow diagram again and give his opinion on the
reactor part. He asked us to find more information about conversion of methane in
the steam reformer and PSA in the PFD.
3. We also take the opportunity to ask the supervisor to check our Task 1 report.
Prepared by,
-----------------------------
(Nafilah Binti Khalid)
Plant Design For The Production of AA Grade Methanol
[Prepared by: Leong Sim Siong, Kow Shu Wen, Nor Hidahyah Binti Ludin, Nafilah Binti Khalid] 101
MINUTES OF MEETING 1
Date : 14
th
September 2012 (Friday)
Time : 2.00 pm 3.00 pm
Venue : Caf Lembaran
Attendance:
1. Kow Shu Wen
2. Leong Sim Siong
3. Nor Hidahyah binti Ludin
4. Nafilah Binti Khalid
Agenda:
1. Before we start our meeting, we have search the fundamental knowledge on our
title to understand the process in order to let our meeting more effective.
2. We have an overview of task 1 from problem 1 to problem 5.
3. Then, we have discussed the problem 1 and problem 2 thoroughly and try to
understand the requirement of it before start our project.
4. The purpose for this meeting is to discuss and understand the requirement of
problem 1 and problem 2.
5. After that, both of the leaders of problem 1 and problem 2 divided the tasks to
each member respectively.
6. We finish our meeting at 3.00pm.
Prepared by,
-----------------------------
(Nor Hidahyah binti Ludin)
Plant Design For The Production of AA Grade Methanol
[Prepared by: Leong Sim Siong, Kow Shu Wen, Nor Hidahyah Binti Ludin, Nafilah Binti Khalid] 102
MINUTES OF MEETING 2
Date : 17
th
September 2012 (Monday)
Time : 9.00 pm 10.00 pm
Venue : Cafe Lembaran
Attendance:
1. Kow Shu Wen
2. Leong SimSiong
3. Nor Hidahyah binti Ludin
4. Nafilah Binti Khalid
Agenda:
1. The purposes for this meeting are:
i. Check and combine the tasks of problem 1 and problem 2 that we have
done.
ii. Divided the jobs by a leader to each member after discussed and
understood the requirement of problem 3, 4 and 5 together.
2. The leader of Task 1 checked the works done by the members among us.
3. The leader has pointed out her/his opinion and discussed among us if there has
any part does not meet the goals.
4. After that, the leader combined all the works of problem 1 together and it is the
same goes to the problem 2.
5. An alternative of the process is chosen from the problem 2 in order to proceed
with the following problem.
6. The sub-questions of problem 3, 4 and 5 are discussed one by one and then
distributed to each member equally after we have understood the question.
Prepared by,
---------------------------
(Nafilah Binti Khalid)
Plant Design For The Production of AA Grade Methanol
[Prepared by: Leong Sim Siong, Kow Shu Wen, Nor Hidahyah Binti Ludin, Nafilah Binti Khalid] 103
MINUTES OF MEETING 3
Date : 4
h
October 2012 (Thursday)
Time : 8.00pm 10.00 pm
Venue : Caf Lembaran
Attendance:
1. Kow Shu Wen
2. Leong Sim Siong
3. Nor Hidahyah binti Ludin
4. Nafilah Binti Khalid
Agenda:
1. The purposes for this meeting are:
iii. Check and combine the works of problem 3, 4 and 5 that we have done
within the days given by the leader.
iv. Finalize the works of all the problem of each member by leader.
2. The works of problem 3, 4 and 5 are checked by the leader and combined it
together after he/she has ensured all the works are meet the required statement of
this problem.
3. One of the members having problems on a sub-question of problem 3, hence, the
leader told that he will help to find it after back to the room.
4. Check the input-output structure; recycle structure, general structure of the
separation system, and also the destination codes and component classification,
and utilities so that the information would not contradict with the information that
mentioned before or after.
5. Combine all the problems, edit all the minor mistake that we had did and put in
our references of the report.
Prepared by,
-----------------------------
(Nor Hidahyah Binti Ludin)
Plant Design For The Production of AA Grade Methanol
[Prepared by: Leong Sim Siong, Kow Shu Wen, Nor Hidahyah Binti Ludin, Nafilah Binti Khalid] 104
REFERENCES
1
Federal Specification: Methanol (Methyl Alcohol). [Online], Available from:
http://www.gbhenterprises.com/federal%20grade%20aa%20methanol%20standard.pdf
[Accessed on: 16
th
September 2012]
2
Methanol Wikipedia, the free encyclopedia. [Online], Available from:
http://en.wikipedia.org/wiki/Methanol [Accessed on: 16
th
September 2012]
3
Methanol Applications Methanol Institute. [Online], Available from:
http://www.methanol.org/Methanol-Basics/Methanol-Applications.aspx [Accessed on:
16
th
September 2012]
4
Methanol as an alternative transportation fuel in the US: Options for sustainable and/or
energy-secure transportation. [Online], Available from:
http://www.afdc.energy.gov/pdfs/mit_methanol_white_paper.pdf [Accessed on: 16
th
September 2012]
5
Hideki Fukuda, Akihido Kondo and Hiedo Noda, Biodiesel Fuel Production by
Transesterification of Oils
6
Direct Methanol Fuel Cell Wkikipedia, the free encyclopedia. [Online], Available
from: http://en.wikipedia.org/wiki/Direct_methanol_fuel_cell [Accessed on: 16
th
September 2012]
7
Gas Malaysia Sdn Bhd Total Energy Solutions for 21
st
Century. [Online], Available
from: http://www.gasmalaysia.com/about_gas/natural_gas_in_malaysia.htm [Accessed
on: 17
th
September 2012]
8
Methanol Industry in Malaysia by Taiyou Research in Malaysia. [Online], Available
from: www.marketresearch.com/Taiyou.../Methanol-Malaysia-7053992/ [Accessed on:
17
th
September 2012]
9
PETRONAS Petrochemicals Quick Facts. Available from:
http://www.petronas.com.my/our-business/downstream/petro-chemicals/Pages/petronas-
petrochemicals-quick-facts.aspx. [Accessed on: 18
th
September 2012]
10
PETRONAS shuts Malaysia methanol No.1 plant after ship blast. July 2012; Available
from: http://www.icis.com/Articles/2012/07/26/9581129/petronas-shuts-malaysia-
methanol-no-1-plant-after-ship-blast.html. [Accessed on: 18
th
September 2012]
11
(MEEA), T.M.o.E.E.A., Downstream Gas Industry.
Plant Design For The Production of AA Grade Methanol
[Prepared by: Leong Sim Siong, Kow Shu Wen, Nor Hidahyah Binti Ludin, Nafilah Binti Khalid] 105
12
(MEEB), M.o.E.E.A. Downstream Gas Indutry. 2011; Available from:
http://www.google.com.my/url?sa=t&rct=j&q=Meanwhile+from+2010+to+2015%2C+N
orth+American+domestic+demand+is+expected+to+grow+by+around+670%2C000+MT
%2C&source=web&cd=1&cad=rja&ved=0CCIQFjAA&url=http%3A%2F%2Fwww.ene
rgy.gov.tt%2Fcontent%2FDownstream_Gas_Industry_Annual_Report.pdf&ei=SrNqUPy
6O5HMrQfUloHACA&usg=AFQjCNHxmuKe458HGg3sLVwGCV4Q4xKceA.
[Accessed on: 20
th
September 2012]
13
Downstream Gas Indutries- Annual Report 2011.
14
San Jose, C. Surge in Production Capacity Drives the Global Methanol Market. May
2012; Available from:
http://www.prweb.com/releases/methanol_market/formaldehyde_market/prweb9490126.
htm [Accessed on: 20
th
September 2012]
15
Saade, G.A. Methanol. Available from:
http://chemical.ihs.com/nl/Public/2009/0907/0907.html [Accessed on: 20
th
September
2012]
16
Johnson, D., Global Methanol Market. 2012.
17
wg_chem0203, Chemical Industry Plan.
18
Johnson, D. Global Methanol Market. June 2012; Available from:
http://www.google.com.my/url?sa=t&rct=j&q=2011+methanol+demand+by+end+use~~
+55.4+million+metric+tons&source=web&cd=1&cad=rja&ved=0CCIQFjAA&url=http
%3A%2F%2Fwww.ptq.pemex.com%2Fproductosyservicios%2Feventosdescargas%2FD
ocuments%2FForo%2520PEMEX%2520Petroqu%25C3%25ADmica%2F2012%2FPEM
EX_DJohnson.pdf&ei=8BVrUNGkFoforQfQ7ICICA&usg=AFQjCNFpw21RipZOXAlK
OrFE6W2zsZMzVQ [Accessed on: 21
th
September 2012]
19
Institute, M. The Methanol Indsutry. Available from:
http://www.methanol.org/Methanol-Basics/The-Methanol-Industry.aspx?lang=ar-SA
[Accessed on: 21
th
September 2012]
20
Corporation, M., METHANEX REPORTS FIRST QUARTER RESULTS AND
INCREASES DIVIDED 9%. 2012.
21
Surge In Production Capacity. 9 May 2012.
22
Methanol Industry in Focus, 2011. p. 12.
23
How Methanol is Made?. [Online], Available from:
http://www.methanol.org/Methanol-Basics/Overview/How-is-Methanol-Made-.aspx
[Accessed on: 24
th
September 2012]
Plant Design For The Production of AA Grade Methanol
[Prepared by: Leong Sim Siong, Kow Shu Wen, Nor Hidahyah Binti Ludin, Nafilah Binti Khalid] 106
24
Ayusman Sen and Minren Lin, Catalytic Partial Oxidation of Methane to Methanol
and Formaldehyde
25
Bilal El-Zahab, Dustin Donnelly, Ping Wang, Particle-Tethered NADH for Production
of Methanol from CO
2
Catalyzed by Coimmobilized Enzymes
26
Sunggyu Lee, Chapter 9: Methanol Synthesis from Syngas
27
Basic Information about Copper in Drinking Water. [Online], Available from:
http://water.epa.gov/drink/contaminants/basicinformation/copper.cfm [Accessed on: 24
th
September 2012]
28
Palladium (Pd) Chemical Properties, Health and Environmental Effects. [Online],
Available from: http://www.lenntech.com/periodic/elements/pd.htm [Accessed on: 24
th
September 2012]
29
2-Health Effects of Methane: OSH Answers. [Online], Available from:
http://www.ccohs.ca/oshanswers/chemicals/chem_profiles/methane/health_met.html
[Accessed on: 24
th
September 2012]
30
The Dangers of Carbon Monoxide: Silent Shadow. [Online], Available from:
http://www.silentshadow.org/the-dangers-of-carbon-monoxide.html [Accessed on: 24
th
September 2012]
31
Hydrogen Safety. [Online], Available from:
http://www.fchea.org/core/import/PDFs/factsheets/Hydrogen%20Safety_NEW.pdf
[Accessed on: 24
th
September 2012]
32
The Danger of Polystyrene Business Barbados. [Online], Available from:
http://businessbarbados.com/green-business/the-dangers-of-polystyrene/ [Accessed on:
24
th
September 2012]
33
Copper Recycling and Sustainability Introduction. [Online], Available from:
http://resources.schoolscience.co.uk/cda/16plus/sustainability/index.html [Accessed on:
25
th
September 2012]
34
DSM Licensing Palladium Catalyst Recovery Technology. [Online], Available
from: http://www.dsm.com/en_US/html/dlc/palladium_recycling.htm [Accessed on: 25
th
September 2012]
35
Carbon monoxide adsorption onto the metal oxides of -Al
2
O
3
and TiO
2
. [Online],
Available from: https://researchspace.auckland.ac.nz/handle/2292/9552 [Accessed on:
25
th
September 2012]
Plant Design For The Production of AA Grade Methanol
[Prepared by: Leong Sim Siong, Kow Shu Wen, Nor Hidahyah Binti Ludin, Nafilah Binti Khalid] 107
36
Matkovi V., Markovi B., Soki M., Vukovi N, Recycling of Spent Nickel based
Catalysts.
37
Polystyrene. [Online], Available from:
http://www.greenusarecycling.com/polystyrene.html [Accessed on: 25
th
September 2012]
38
Phosphorus Removal from Wastewater. [Online], Available from:
http://www.lenntech.com/phosphorous-removal.htm [Accessed on: 25
th
September 2012]
39
F.Meshkini, a.M.T.M., a M.Bahmanib, Effect of metal oxide additives on the properties
of Cu/ZnO/Al2O3 catalysts in methanol synthesis from syngas. 2007.
40
Natural Gas.
41
International Energy Outlook 2011. 2011; Available from:
http://www.eia.gov/forecasts/ieo/nat_gas.cfm [Accessed on: 28
th
September 2012]
42
Howell, N. How is Natural gas Produced. Available from:
http://www.ehow.com/how_4686639_natural-gas-produced.html [Accessed on: 28
th
September 2012]
43
HAN, C.E., Gas Malaysia: 80% of gas taken up, additional volume to be delivered via
extended pipeline. Tuesday September 4, 2012.
44
Integrated Low Pressure Methanol Process: Synthesis Gas Production by Conventional
Steam Reforming of Natural Gas or Oil Associated Gas.
45
Pressure Swing Adsorption Wikipedia, the free encyclopedia. [Online], Available
from: http://en.wikipedia.org/wiki/Pressure_swing_adsorption [Accessed on: 29
th
September 2012]
46
Methane-Syngas-Methanol Microprocessing.
47
MATERIAL SAFETY DATA SHEET, 1989, MATHESON TRI-GAS, INC. p. 8.
48
MATERIAL SAFETY DATA SHEET, 1989, MATHESON TRI-GAS, INC. p. 7.
49
Material Safety Data Sheet - Water 1991, MOTHER NATURE, Inc.
50
MATERIAL SAFETY DATA SHEET, 1990, MATHESON TRI-GAS, INC. p. 6.
51
Material Safety Data Sheet, 2012, Atlantic Methanol Production Company LLC. p. 9.
52
Material Safety Data Sheet, 2010, AIRGAS INC. p. 7.
53
Sequoia Ecosystem and Recreation Preserve Act of 1999, 1999.
Plant Design For The Production of AA Grade Methanol
[Prepared by: Leong Sim Siong, Kow Shu Wen, Nor Hidahyah Binti Ludin, Nafilah Binti Khalid] 108
54
MATERIAL SAFETY DATA SHEET, 1995, Air Products and Chemicals, Inc. p. 7.
55
MATERIAL SAFETY DATA SHEET, 2005, Air liquide p. 7.
56
2012; Available from: http://www.petronas.com.my/our-business/downstream/petro-
chemicals/Pages/other-petro-chemical-plants.aspx [Accessed on: 29
th
September 2012]
57
Corporation, D.D., Specifications for Natural Gas 2001.
58
LLC, A.M.P.C., Methanol Sales Speficications.
Você também pode gostar
- Career Change From Real Estate to Oil and Gas ProjectsNo EverandCareer Change From Real Estate to Oil and Gas ProjectsNota: 5 de 5 estrelas5/5 (1)
- Chemical Engineering Process SimulationNo EverandChemical Engineering Process SimulationDominic FooAinda não há avaliações
- Qatar University: Department of Chemical EngineeringDocumento13 páginasQatar University: Department of Chemical EngineeringMuataman KhAinda não há avaliações
- ReportttttttttttttttttttttttttttttttttttttttttttttttttttttttttttttttttttttttttttttttttttttttttttttttttttttttttttDocumento10 páginasReportttttttttttttttttttttttttttttttttttttttttttttttttttttttttttttttttttttttttttttttttttttttttttttttttttttttttttM Asrar SidonAinda não há avaliações
- Final Year Project Proposal Presentation Group #13Documento14 páginasFinal Year Project Proposal Presentation Group #13Usama JahangirAinda não há avaliações
- Project ManagementDocumento29 páginasProject ManagementIzzah Hafizah YahayaAinda não há avaliações
- Baoliang SunDocumento45 páginasBaoliang SunCagla AcikbasAinda não há avaliações
- A Systematic Approach For Conceptual and Sustainable Process Design: Production of Methylamines From Methanol and AmmoniaDocumento141 páginasA Systematic Approach For Conceptual and Sustainable Process Design: Production of Methylamines From Methanol and Ammonialucia corradini deaneAinda não há avaliações
- A Project Report-Wps OfficeDocumento28 páginasA Project Report-Wps OfficeDivyamAinda não há avaliações
- CPE520 - Mini Project Management ReportDocumento49 páginasCPE520 - Mini Project Management Reporthakim amirAinda não há avaliações
- b07 Production of 150,000 Mta of Monopropylene Glycol From PropyleneDocumento471 páginasb07 Production of 150,000 Mta of Monopropylene Glycol From PropyleneHarshini SivanganamAinda não há avaliações
- Maintenance Management Performance of Malaysian Palm Oil MillsDocumento294 páginasMaintenance Management Performance of Malaysian Palm Oil Millsnazimbaluch100% (14)
- PlantDesign ReportDocumento166 páginasPlantDesign ReportMela SariAinda não há avaliações
- Design and Fabrication of Plastic ShredderDocumento56 páginasDesign and Fabrication of Plastic ShredderVinayaga Projectinstitute100% (1)
- CHE451 - Chemical Process Design and EconomicsDocumento99 páginasCHE451 - Chemical Process Design and EconomicsDaniel Grabovsky0% (1)
- 7th Methanol Markets & Tech - About Event - About ConferenceDocumento3 páginas7th Methanol Markets & Tech - About Event - About ConferenceidownloadbooksforstuAinda não há avaliações
- Project 1 Technical and Economic Feasibility Study 2023 1Documento12 páginasProject 1 Technical and Economic Feasibility Study 2023 1Anele HadebeAinda não há avaliações
- IP ProjectDocumento4 páginasIP ProjecthoikliangAinda não há avaliações
- Industrial Training ReportDocumento32 páginasIndustrial Training ReportsyamimiAinda não há avaliações
- Design of Styrene Monomer PlantDocumento242 páginasDesign of Styrene Monomer PlantshaliniAinda não há avaliações
- Project Thesis Refining of Used Motor Oil Using Solvent ExtractionDocumento77 páginasProject Thesis Refining of Used Motor Oil Using Solvent ExtractionSyed Waqas Haider50% (2)
- Group 2 FInal YEar PrersentationDocumento15 páginasGroup 2 FInal YEar PrersentationMuhammad Zohaib SafdarAinda não há avaliações
- Group 4 - EH2206M - A2Documento50 páginasGroup 4 - EH2206M - A2NNAinda não há avaliações
- Oil and Gas Treatment Technology After Exploitation: Xu Lei Lei PengjuDocumento3 páginasOil and Gas Treatment Technology After Exploitation: Xu Lei Lei PengjuIOSRJEN : hard copy, certificates, Call for Papers 2013, publishing of journalAinda não há avaliações
- TCC LTDDocumento17 páginasTCC LTDjithukpAinda não há avaliações
- Eia Report of A CEMENT INDUSTRYDocumento19 páginasEia Report of A CEMENT INDUSTRYabdullah al mahinAinda não há avaliações
- Cpe520 - Mini Project Report - Eh2204g PDFDocumento40 páginasCpe520 - Mini Project Report - Eh2204g PDFAwiBurhanuddinAinda não há avaliações
- Technology Assessment of Liquid Waste in Rubber Factory Using Analytical Hierarchy Process and Promethee MethodsDocumento9 páginasTechnology Assessment of Liquid Waste in Rubber Factory Using Analytical Hierarchy Process and Promethee MethodsMateo bolañosAinda não há avaliações
- Unitoperations and ProcessesDocumento19 páginasUnitoperations and ProcessesnirbhaykumarAinda não há avaliações
- Auto Product CycleDocumento10 páginasAuto Product CycleRajendranath VigrahalaAinda não há avaliações
- Presentation Group2 Adeel, Waqas, ShafqatDocumento15 páginasPresentation Group2 Adeel, Waqas, ShafqatMuhammad Zohaib SafdarAinda não há avaliações
- Master Plan For Automotive IndustryDocumento109 páginasMaster Plan For Automotive IndustryAvilash100% (1)
- Design & Manufacture and Material Performance: Li Jingui, Yuan Xunhua and Zhang QifuDocumento7 páginasDesign & Manufacture and Material Performance: Li Jingui, Yuan Xunhua and Zhang QifuJudith LugoAinda não há avaliações
- IChEC 2016 Problem Solving GuidebookDocumento22 páginasIChEC 2016 Problem Solving GuidebookRizkyanto NugrohoAinda não há avaliações
- Government Polytechnic Nashik Certificate for Vehicle Recycling SeminarDocumento7 páginasGovernment Polytechnic Nashik Certificate for Vehicle Recycling SeminarawesomeyogeshwarAinda não há avaliações
- 2017CHE003 FinalPaper Draft 8 1Documento90 páginas2017CHE003 FinalPaper Draft 8 1Evan DulayAinda não há avaliações
- Integrated Design Project: CDB 2013 Separation Process I CDB 2043 Reaction EngineeringDocumento34 páginasIntegrated Design Project: CDB 2013 Separation Process I CDB 2043 Reaction EngineeringMuhammad AnwarAinda não há avaliações
- 111Documento62 páginas111441103042Ainda não há avaliações
- Supply Chain Management For Biofuels in Malaysia A Feasibility Study of Logistics OptimizationDocumento8 páginasSupply Chain Management For Biofuels in Malaysia A Feasibility Study of Logistics OptimizationnikrahiniAinda não há avaliações
- Internship Report SampleDocumento21 páginasInternship Report SampleMuhammad Khairul Haziq50% (2)
- Conventional and Advanced Treatment Technologies For Palm Oil Mill Effluents: A Systematic Literature ReviewDocumento20 páginasConventional and Advanced Treatment Technologies For Palm Oil Mill Effluents: A Systematic Literature ReviewradAinda não há avaliações
- Methanol To Gasoline: PERP 2011S7Documento7 páginasMethanol To Gasoline: PERP 2011S7hhvgAinda não há avaliações
- Chemical Process Design & EconomicsDocumento46 páginasChemical Process Design & Economicskatamani temple100% (2)
- Power TrainDocumento8 páginasPower TrainpaparezaAinda não há avaliações
- Properties of Formaldehyde PDFDocumento120 páginasProperties of Formaldehyde PDFKolliparaDeepakAinda não há avaliações
- CHM415Documento136 páginasCHM415palmer okiemuteAinda não há avaliações
- Micro Project: Title of The ProjectDocumento12 páginasMicro Project: Title of The Projectomkar digamabar sononeAinda não há avaliações
- GP1 Final Report Template Ss09.10Documento17 páginasGP1 Final Report Template Ss09.10Al- DhaheriAinda não há avaliações
- Application of Process Simulation Software METSIMDocumento7 páginasApplication of Process Simulation Software METSIMBart FrienderAinda não há avaliações
- LcaDocumento13 páginasLcanor_tahir_6Ainda não há avaliações
- Hariom ReportDocumento60 páginasHariom Reportहरिओम हरी100% (2)
- Simulating Methanol Recovery in Biodiesel ProductionDocumento6 páginasSimulating Methanol Recovery in Biodiesel ProductionSteven Putra HalimAinda não há avaliações
- A New Approach On Implementing TPM in A Mine - A Case Study: SciencedirectDocumento12 páginasA New Approach On Implementing TPM in A Mine - A Case Study: SciencedirectMaysa MarthaAinda não há avaliações
- 4 Properties of FormaldehydeDocumento120 páginas4 Properties of FormaldehydeJonathan ByamunguAinda não há avaliações
- JCPR 2015 7 1 897 901Documento5 páginasJCPR 2015 7 1 897 901Ahmed AliAinda não há avaliações
- Career Episode 1Documento8 páginasCareer Episode 1avinashpatil2408100% (1)
- Productivity Improvement Using Industrial EngineerDocumento8 páginasProductivity Improvement Using Industrial EngineerjasmineAinda não há avaliações
- EKB3013 Chemical Process Industries ModifiedDocumento8 páginasEKB3013 Chemical Process Industries ModifiedmarkAinda não há avaliações
- MEB Project Sem I 20232024Documento3 páginasMEB Project Sem I 20232024Harshini BaskaranAinda não há avaliações
- Assignment 2 My PortionDocumento15 páginasAssignment 2 My PortionYongama Jordan TshoziAinda não há avaliações
- MKMPLA17Documento12 páginasMKMPLA17Adawiyah Az-zahraAinda não há avaliações
- Assignment PetroleumDocumento1 páginaAssignment PetroleumAdawiyah Az-zahraAinda não há avaliações
- TGV Mines Booking Number: 633671 Avengers: Infinity WarDocumento1 páginaTGV Mines Booking Number: 633671 Avengers: Infinity WarAdawiyah Az-zahraAinda não há avaliações
- TIB vs Competitors: Takful Ikhas, AIA, Prudential, Great EasternDocumento5 páginasTIB vs Competitors: Takful Ikhas, AIA, Prudential, Great EasternAdawiyah Az-zahraAinda não há avaliações
- Pages From Aspen Plus 2013 Assignment IIDocumento2 páginasPages From Aspen Plus 2013 Assignment IIAdawiyah Az-zahraAinda não há avaliações
- Sample LettersDocumento5 páginasSample LettersFalak KhanAinda não há avaliações
- Safety FactorDocumento4 páginasSafety FactorAdawiyah Az-zahraAinda não há avaliações
- English Year 5 Paper 1 Answers SchemeDocumento3 páginasEnglish Year 5 Paper 1 Answers SchemesitiAinda não há avaliações
- Soalan Exam Interaktif - PPTMDocumento40 páginasSoalan Exam Interaktif - PPTMAdawiyah Az-zahraAinda não há avaliações
- Soalan1 AssignmentDocumento2 páginasSoalan1 AssignmentAdawiyah Az-zahraAinda não há avaliações
- Parental Lock/Controls: Content FiltersDocumento3 páginasParental Lock/Controls: Content FiltersAdawiyah Az-zahraAinda não há avaliações
- Assignment 4 (Group Work)Documento1 páginaAssignment 4 (Group Work)Adawiyah Az-zahraAinda não há avaliações
- HR Job Application Process EngineerDocumento1 páginaHR Job Application Process EngineerAdawiyah Az-zahraAinda não há avaliações
- Do AaaaaDocumento1 páginaDo AaaaaAdawiyah Az-zahraAinda não há avaliações
- Lecture ScheduleDocumento1 páginaLecture ScheduleAdawiyah Az-zahraAinda não há avaliações
- LSP 404: Technical & Engineering English (MB1) EKC 361: Process Dynamics & Control (KCK2)Documento1 páginaLSP 404: Technical & Engineering English (MB1) EKC 361: Process Dynamics & Control (KCK2)Adawiyah Az-zahraAinda não há avaliações
- Plant Safety (Chemical ProcessesDocumento10 páginasPlant Safety (Chemical ProcessesAdawiyah Az-zahraAinda não há avaliações
- Experiment 2 - Study of Packed Column DistillationDocumento7 páginasExperiment 2 - Study of Packed Column DistillationAdawiyah Az-zahra100% (1)
- Steam Tables Sige An Kop L IsDocumento2 páginasSteam Tables Sige An Kop L IsAdawiyah Az-zahraAinda não há avaliações
- Transport Phenomena L4Documento21 páginasTransport Phenomena L4Adawiyah Az-zahraAinda não há avaliações
- Application of Packed Bed Column Distillation in IndustryDocumento3 páginasApplication of Packed Bed Column Distillation in IndustryAdawiyah Az-zahra0% (1)
- Shortlist Company (Tapah-Penang)Documento2 páginasShortlist Company (Tapah-Penang)Adawiyah Az-zahraAinda não há avaliações
- Intership ProgrammeDocumento1 páginaIntership ProgrammeAdawiyah Az-zahraAinda não há avaliações
- Renewable Energy Systems DesignDocumento5 páginasRenewable Energy Systems DesignAdawiyah Az-zahraAinda não há avaliações
- ApplicationNote PH Measurement JamJellies EN 30538204Documento4 páginasApplicationNote PH Measurement JamJellies EN 30538204MarcinAinda não há avaliações
- Determination of Seismic Bearing Capacity of Shallow Strip Footings On SlopesDocumento10 páginasDetermination of Seismic Bearing Capacity of Shallow Strip Footings On SlopesboyzesAinda não há avaliações
- Standby battery product overviewDocumento2 páginasStandby battery product overviewHillary McgowanAinda não há avaliações
- Masterfiber: Monofilament Polypropylene Fibres For Cementitious SystemDocumento2 páginasMasterfiber: Monofilament Polypropylene Fibres For Cementitious SystemHsaam Hsaam100% (1)
- Matrices Incompatibilidad BogDocumento11 páginasMatrices Incompatibilidad BogIngenieria ScadiaAinda não há avaliações
- Activity 1 Pxy, Txy DiagramsDocumento3 páginasActivity 1 Pxy, Txy DiagramsPatricia ManaogAinda não há avaliações
- Optional Strain Rate Forms For The Johnson CookDocumento17 páginasOptional Strain Rate Forms For The Johnson CookTien KimAinda não há avaliações
- Carbohydrates OutlineDocumento3 páginasCarbohydrates OutlineKalka BoroAinda não há avaliações
- Biochemistry of Bone & Muscle: Dr. Syahrijuita, M.Kes, SP - THT-KLDocumento69 páginasBiochemistry of Bone & Muscle: Dr. Syahrijuita, M.Kes, SP - THT-KLyantiAinda não há avaliações
- 2019 - 1.16. Tvrdo I Meko LemljenjeDocumento24 páginas2019 - 1.16. Tvrdo I Meko LemljenjeticmaAinda não há avaliações
- ISO 4406 Fluid Cleanliness GuideDocumento1 páginaISO 4406 Fluid Cleanliness GuideJai BhandariAinda não há avaliações
- Photosynthesis Lab: Leaf Disk Floating RatesDocumento9 páginasPhotosynthesis Lab: Leaf Disk Floating RatesNakuru SandwichAinda não há avaliações
- Electrophoresis Buffers And Solutions GuideDocumento4 páginasElectrophoresis Buffers And Solutions GuidepersefoniAinda não há avaliações
- Evapco Evaporative Condenser Engineering ManualDocumento32 páginasEvapco Evaporative Condenser Engineering Manualrodolfocv923590% (1)
- Chapter 2 Fluid StaticsDocumento13 páginasChapter 2 Fluid StaticsguhanAinda não há avaliações
- Weberdry 130 PR Grey-WhiteDocumento2 páginasWeberdry 130 PR Grey-WhiteabbAinda não há avaliações
- Macromolecules: Question Paper 1Documento8 páginasMacromolecules: Question Paper 1Bridgette LombardAinda não há avaliações
- Solvent RecoveryDocumento22 páginasSolvent RecoveryNikul Rathod100% (1)
- Boscoseal Torch OnDocumento3 páginasBoscoseal Torch OnjbonvierAinda não há avaliações
- Formation of Delta Ferrite in 9 WT.% CR Steel Investigated by In-Situ X-Ray Diffraction Using Synchrotron RadiationDocumento9 páginasFormation of Delta Ferrite in 9 WT.% CR Steel Investigated by In-Situ X-Ray Diffraction Using Synchrotron Radiationsmallik3Ainda não há avaliações
- Practical Proteins and Amino Acids Identification PDFDocumento23 páginasPractical Proteins and Amino Acids Identification PDFFarahDeebaAinda não há avaliações
- Analysis of Hard WaterDocumento3 páginasAnalysis of Hard WaterPankaj Patel100% (4)
- ACT Reading Practice TestsDocumento81 páginasACT Reading Practice TestsYann VautrinAinda não há avaliações
- Coursera BioinfoMethods-II Lab03Documento10 páginasCoursera BioinfoMethods-II Lab03Harly CNAinda não há avaliações
- How The Concept of The Element Evolved From Ancient Greek To The PresentDocumento23 páginasHow The Concept of The Element Evolved From Ancient Greek To The PresentPineda, Sean AlfredAinda não há avaliações
- Gating ManualDocumento84 páginasGating Manualmr.nguyenk100% (4)
- Growing shrimp and tilapia together in indoor aquacultureDocumento13 páginasGrowing shrimp and tilapia together in indoor aquaculturetushar patelAinda não há avaliações
- What Is Langmuir Adsorption IsothermDocumento5 páginasWhat Is Langmuir Adsorption Isothermalimisaghian62Ainda não há avaliações
- Algebra:: N N N N N N 1B 2B ZoneDocumento6 páginasAlgebra:: N N N N N N 1B 2B Zonevenedick sapongayAinda não há avaliações