Escolar Documentos
Profissional Documentos
Cultura Documentos
pvb054 PDF
Enviado por
Mauricio TerrazasDescrição original:
Título original
Direitos autorais
Formatos disponíveis
Compartilhar este documento
Compartilhar ou incorporar documento
Você considera este documento útil?
Este conteúdo é inapropriado?
Denunciar este documentoDireitos autorais:
Formatos disponíveis
pvb054 PDF
Enviado por
Mauricio TerrazasDireitos autorais:
Formatos disponíveis
Thermal Conductance of Hydrophilic and Hydrophobic Interfaces
Zhenbin Ge, David G. Cahill, and Paul V. Braun
Department of Materials Science and Engineering, the Frederick Seitz Materials Research Laboratory,
and the Beckman Institute for Advanced Science and Technology, University of Illinois, Urbana, Illinois 61801, USA
(Received 9 January 2006; published 8 May 2006)
Using time-domain thermoreectance, we have measured the transport of thermally excited vibrational
energy across planar interfaces between water and solids that have been chemically functionalized with a
self-assembled monolayer (SAM). The Kapitza lengthi.e., the thermal conductivity of water divided by
the thermal conductance per unit area of the interfaceis analogous to the slip length for water
owing tangentially past a solid surface. We nd that the Kapitza length at hydrophobic interfaces (10
12 nm) is a factor of 23 larger than the Kapitza length at hydrophilic interfaces (36 nm). If a vapor
layer is present at the hydrophobic interface, and this vapor layer has a thermal conductivity that is
comparable to bulk water vapor, then our experimental results constrain the thickness of the vapor layer to
be less than 0.25 nm.
DOI: 10.1103/PhysRevLett.96.186101 PACS numbers: 68.08.p, 68.65.Ac
The structure and physical properties of interfaces be-
tween water and solid surfaces are critical inputs for under-
standing many microscopic processes in the biological and
physical sciences, and for the design of new nanoscale
systems [18]. At both hydrophilic and hydrophobic sur-
faces, the properties of water are modied in close prox-
imity to the interface. For example, near hydrophilic
surfaces, interfacial water has been found by computer
simulation to have a higher density than in the bulk [2],
while near hydrophobic surfaces, a thin layer of low-
density water is predicted by theory [911] and has been
observed in experiments [3,5,6,9,12]. Water conned be-
tween two hydrophobic surfaces is expected to spontane-
ously evaporate when the spacing between the surfaces is
sufciently small [13]. These differences in the structure of
interfacial water are also revealed by measurements of
rheological properties where strong violations of the no-
slip boundary condition have been observed for uid-ow
tangential to hydrophobic interfaces [8,14].
Here we describe a new approach for probing the prop-
erties of interfacial water using measurements of the trans-
port of thermal energy. Understanding this interface
thermal conductance results in fundamental insights into
the structure and properties of interfacial water and also
has more practical implications. For example, biological
systems contain both hydrophilic and hydrophobic sur-
faces [1,4]. Understanding the interfacial thermal conduc-
tance may enable a better understanding of thermal
transport in thermally based medical therapies [15] that
combine ultrafast lasers and metal nanoparticles as a
source of heat.
The thermal conductance per unit area of the interface G
is dened by 1 GT, where 1 is the heat ux normal to
the interface and T is the temperature drop at the inter-
face. The ratio of thermal conductivity divided by G can
then be used to dene the Kapitza length h G, a
characteristic length that gives the thickness of a layer
with thermal conductivity that has the same thermal
resistance as the interface. As the characteristic dimensions
of a system approach h, the interfacial thermal conduc-
tance plays an increasingly important role in thermal trans-
port [16].
In our previous studies on G of solid-liquid interfaces,
we employed colloidal dispersions of hydrophilic Au
nanoparticles in aqueous solution [17,18]. The Au nano-
particles ranged in diameter from 3 to 24 nm and were
functionalized with a wide variety of hydrophilic mole-
cules; we determined G by modeling the cooling curves
measured by transient absorption in an optical pump-probe
experiment. In aqueous solution, despite the variety of
surface chemistries we investigated, G was surprisingly
constant, 150 < G <250 MWm
2
K
1
[17], indicating
that thermal coupling between hydrophilic surfaces and
water is relatively strong regardless of the detailed chem-
istry. Because it is impossible to reliably suspend hydro-
phobic metal nanoparticles in aqueous solutionhydro-
phobic particles will rapidly aggregatewe could not
answer an obvious next question: how do these values of
G for hydrophilic interfaces compare with G for hydro-
phobic interfaces? Recently, we developed a means to
measure G at planar interfaces by time-domain thermore-
ectance [19,20], thereby enabling the study of interfaces
between water and hydrophobic surfaces.
Two sample congurations were prepared as illustrated
in Fig. 1 for studying Al and Au based interfaces. First, a
130 nm thick lm of SiO
2
was deposited by e-beam
evaporation on the bottom of a sapphire substrate. This
layer serves as an antireection optical coating at 770 nm,
the laser wavelength used in the pump-probe measure-
ments. A polyimide precursor was spin coated on the top
of the sapphire substrate and cured at 250
C in air, result-
ing in a 2030 nm thick layer with low thermal conduc-
tivity; this layer ensures that most of the heat deposited in
the overlying metal lm ows into the liquid at short time
scales, maximizing the sensitivity of our experiment to the
thermal conductance of the solid-liquid interface. Different
PRL 96, 186101 (2006)
P HYS I CAL RE VI E W L E T T E RS
week ending
12 MAY 2006
0031-90070696(18)186101(4) 186101-1 2006 The American Physical Society
combinations of Ti, Al, and Au were then deposited on the
polyimide lms by sputtering. Ti layers, 25 nm thick,
serve as adhesion layers and the 2030 nm Al layer pro-
vides a sensitive thermometer in the experiment through its
relatively large thermoreectance at optical wavelengths
near 770 nm. This Al layer can be directly modied with
silane molecules [Fig. 1(a)], or can be coated by Ti, fol-
lowed by 1020 nm of Au, and subsequently modied
with well-known gold-thiol chemistry [Fig. 1(b)] [21].
We study both Al and Au based interfaces because Al
and Au offer complementary strengths and weaknesses for
our experiments. Molecular monolayers formed using thiol
chemistry on Au are better understood and probably more
homogeneous than layers formed by silane chemistry on
the native oxide of Al but the Au layer introduces an
additional thermal resistances between the Al thermometer
and the interfaces with water that we are most interested in
studying. Near an interface with a nonmetal, the relatively
weak electron-phonon coupling in Au results in a non-
negligible thermal resistance [22,23]. Therefore, the ther-
mal conductance of an interface with Au includes an addi-
tional conductance G
ep
in series: G
ep
g
p
, where g
describes the conductance per unit volume of the coupling
between electrons and phonons in Au; and is the lattice
thermal conductivity of Au. Using 2.7 Wm
1
K
1
[24]; g 3.1 10
16
Wm
3
K
1
[25], gives G
ep
290 MWm
2
K
1
. For Al, both g and are much larger
than for Au [g 4.9 10
17
Wm
3
K
1
[26];
10 Wm
1
K
1
[27] ], and G
ep
> 2 GWm
2
K
1
. Also,
the interface between the Au and Al layers introduces a
measurable thermal resistance, presumably because of
contamination of the Ti adhesion layer by residual gases
in the deposition chamber. The conductance of the
AlTiAu interfaces is measured separately by tting the
data at short delay times of less than 100 ps; this additional
interface conductance does not produce a signicant
change in the measurement of the water interfaces but
this effect is included in the thermal model for samples
containing Au.
Octadecyltrichlorosilane (OTS) was used to function-
alize the native oxide of the Al surface making it hydro-
phobic. The advancing water contact angle is 0
adv
104
and the receding water contact angle is 0
rec
90
; the
thickness of the self-assembled monolayer (SAM) mea-
sured by ellipsometry, using an index of refraction n
1.45, is 2.4 0.3 nm. 2-[methoxy(polyethyleneoxy)-
propyl]-trichlorosilane (PEG-silane) was used to make
the Al surface hydrophilic [28]. The water contact angles
are 0
adv
16
and 0
rec
13
; the SAM thickness is
0.7 0.2 nm. The Au surface was functionalized with
1-octadecanethiol (C
18
) making it hydrophobic. The water
contact angles are 0
adv
103
and 0
rec
94
; the SAM
thickness is 2.3 0.3 nm. 11-mercapto-1-undecanol
(C
11
OH) was used to functionalize the Au surface making
it hydrophilic [21]. The water contact angles are 0
adv
18
and 0
rec
9
; the SAM thickness is 1.1 0.2 nm.
The thermal conductance of the interfaces with water
was measured using time-domain thermoreectance
[19,20]. Two thermal models were used to analyze the
raw data: unidirectional heat ow was used to analyze
the heat transfer when the metal surface was exposed to
air, and bidirectional heat ow was used to analyze the heat
transfer when the metal surface was immersed in water
[29]. Metal lm thicknesses are measured by Rutherford
backscattering spectrometry, as shown in Fig. 2; heat ca-
pacities are taken from literature values. The thermal con-
ductivity of the polyimide lm was separately measured to
be 0.17 Wm
1
K
1
using an AlpolyimideSi stack; the
thickness of the polyimide lm is adjusted in the unidirec-
tional heat ow model to obtain the best t between the
model and the data. The bidirectional thermal model then
has only one remaining free parameter, G, the thermal
conductance of metal-water interface, that we adjust to
obtain the best t. Because the thermal diffusivities of
water and polyimide are small, heat ow in these experi-
ments is primarily one dimensional; however, both of
thermal models take into account the full three-
dimensional heat ow in cylindrical coordinates [29,30].
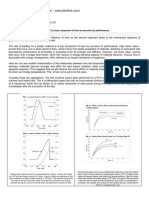
Examples of time-domain thermoreectance data and
ts are shown in Fig. 3 for hydrophobic and hydrophilic
Al-water and Au-water interfaces. Oscillations observed in
the short time region are the result of longitudinal acoustic
oscillations of the metal lms. G for the OTS modied
hydrophobic Al surface in water is determined to be 60
5 MWm
2
K
1
and G for C
18
modied hydrophobic Au
surface in water is 50 5 MWm
2
K
1
. The hydrophilic
surfaces resulted in signicant increase in G. G of the
PEG-Silane modied Al surface in water is 180
30 MWm
2
K
1
, and G of C
11
OH modied hydrophilic
Au surface in water is 100 20 MWm
2
K
1
.
(a) (b)
Polyimide~30nm
Sapphire
substrate 1mm
SiO
2
130nm
Ti ~ 5nm
Al ~ 20nm
Water
Polyimide~30nm
Sapphire
substrate 1mm
SiO
2
130nm
Ti ~ 5nm
Al ~ 20nm
Ti ~ 2nm
Au ~ 10nm
Water
FIG. 1 (color online). Schematic of sample structures for
studying (a) aluminum-water interface thermal conductance;
(b) gold-water interface thermal conductance.
PRL 96, 186101 (2006)
P HYS I CAL RE VI E W L E T T E RS
week ending
12 MAY 2006
186101-2
These results for planar hydrophilic functionalized Al
and Au surfaces are in good agreement with our previous
work using Au nanoparticles in aqueous solution [17,18].
Both sets of experiments give G ranging between 100 and
300 MWm
2
K
1
indicating that the thermal coupling be-
tween hydrophilic surfaces and water is strong regardless
of whether the surfaces are nanoparticles or planar lms.
Our experimental values for G at hydrophilic interfaces
are smaller by a factor of approximately 2 than the results
of molecular dynamics (MD) simulations of interfaces
between water and the hydrophilic head group (OH) of
a surfactant molecule; these simulations found G
300 MWm
2
K
1
[31]. The authors of Ref. [31] attributed
the large thermal conductance to the strong coupling of
water molecules with the hydroxyl head of the surfactant
molecules by hydrogen bonding. The smaller value for G
in our experiments on Au-C
11
OHwater interface com-
pared to Al-PEGwater may be due to the issues of
electron-phonon coupling in Au described above: since
we estimate G
ep
290 MWm
2
K
1
, the conductance
of the lattice vibrations at the interface may, in fact, be
close to G 150 MWm
2
K
1
, i.e., nearly identical to
our measured value for the Al-PEGwater interface.
We are not aware of any MD simulations of the thermal
conductance across an interface between water and a solid
hydrophobic surface. The closest case is a water-octane
interface, in which G 65 MWm
2
K
1
[31]. This is
similar to our results on hydrophobic-water interfaces,
but additional simulations that include the long-range van
der Waals attractions [32] will be helpful in identifying the
microscopic mechanisms that control thermal transport
across hydrophobic interfaces.
Even if we neglect the issues of electron-phonon cou-
pling, i.e., the nite value of G
ep
, the value of the interface
conductance that we measure includes 4 processes: (i) ow
of heat from metal lms to functional groups (i.e., S or Si
group), (ii) ow of vibrational energy along the length of
the molecular chain, (iii) transport of vibrational energy
10 100 1000
1
2
3
4
5
6
1
2
3
4
5
6
1
2
3
4
5
6
1
2
3
4
5
6
7
t (ps)
-
V
i
n
/
V
o
u
t
-
V
i
n
/
V
o
u
t
-
V
i
n
/
V
o
u
t
-
V
i
n
/
V
o
u
t
G = 60 5 MW m
-2
K
-1
, h = 10 nm
G = 50 5 MW m
-2
K
-1
, h = 12 nm
G = 180 30 MW m
-2
K
-1
, h=3 nm
G = 10020 MW m
-2
K
-1
, h = 6 nm
(a)
(b)
(c)
(d)
Au
S S S
Au
S
O H
S
O H
S
O H
S i
O
O
O
Al
S i
O
O
O
Si
O
O
O
O
O
6-9
Al
Si
O
O
O
O
O
6-9
FIG. 3. Time-domain thermoreectance data for thermal trans-
port across hydrophobic (a), (b) and hydrophilic (c), (d) metal-
water interfaces. The ratio of the in-phase to out-of-phase signals
of the lock-in amplier is plotted as a function of the delay time t
between pump and probe. Solid circles, dry state; open circles, in
water. Dashed lines are ts based on the unidirectional model
with the polyimide thickness as the one free parameter (heat
dissipated through air is negligible). Solid lines are ts based on
the bidirectional model (heat is dissipated through both water
and the polyimide layer) with the solid-liquid interface thermal
conductance, G, as the one free parameter. (a) OTS modied Al
surface, hydrophobic, G 60 5 MWm
2
K
1
, Kapitza
length h 10 nm; (b) C
18
modied Au surface, hydrophobic,
G 50 5 MWm
2
K
1
, h 12 nm; (c) PEG-silane modi-
ed Al surface, hydrophilic, G 180 30 MWm
2
K
1
, h
3 nm; (d) C
11
OH modied Au surface, hydrophilic, G 100
20 MW m
2
K
1
, h 6 nm.
0 200 400 600 800
0
1000
2000
3000
4000
400 800 1200 1600
0
1000
2000
3000
4000
Channel
Energy (keV)
C
o
u
n
t
s
Ti
Al
Polymer
FIG. 2 (color online). Rutherford backscattering spectrometry
data for determining metal lm thicknesses of the samples. Open
circles are measured intensities. Solid line is a t using SIMNRA
5.02 with polymer and metal thicknesses as the free parameters.
For this particular sample, the Ti thickness is 5.3 nm and Al
thickness is 34.0 nm. The depth of the valley between the Al in
the thin lm and the Al in the sapphire substrate is determined by
the thickness of polymer.
PRL 96, 186101 (2006)
P HYS I CAL RE VI E W L E T T E RS
week ending
12 MAY 2006
186101-3
from the terminal group to the contacting, possibly low
density, surrounding water, and (iv) ow of heat across the
possibly low-density water gap to the bulk water phase.
Because well-aligned polyethylene has a high thermal
conductivity, 30 times higher than water [33], the tempera-
ture drop along the molecular chains, i.e., process (ii), is
probably not signicant. The large difference between G
for hydrophilic and hydrophobic interfaces suggests that
the third and/or fourth mechanism(s) play a critical role in
thermal transport across hydrophobic interfaces.
The difference in the Kapitza lengths of hydrophobic
and hydrophilic interfaces places constraints on the thick-
ness of a possible vapor layer at hydrophobic interfaces.
The existence of a nm-scale water-vapor layer at hydro-
phobic interfaces, or, in other words, the degree of dry-
ing of a hydrophobic interface, has been controversial for
many years [1,32]. If we can assume that the thermal
conductivity of the vapor layer has the same thermal con-
ductivity as bulk water-vapor, approximately 30 times
smaller than water, then the thickness of this vapor layer
must be less than h30 0.25 nm, where h is the
difference in the Kapitza lengths for hydrophilic and hy-
drophobic interfaces. Recently, neutron diffraction experi-
ments on the structure of interfacial water near hydro-
phobic interfaces were modeled by an error function of
width c and a midpoint shifted by a distance u from the
hydrocarbon-water interface; for naturally aerated heavy
water, c 0.35 nm and u 1.0 nm and the offset was as
small as u 0.2 nm in heavy-water bubbled by Ar to
remove dissolved gases [3]. We, however, did not observe
signicant changes in the thermal conductance when we
used Ar-bubbled deionized water instead of the deionized
water directly from a mili-Q dispenser, or when we used
water degassed in vacuum overnight.
The slip length in uid ow past a hydrophobic surface
has also been controversial and no consensus has emerged
on the value of the slip length in the limit of small shear
rates or how the slip length varies with shear rate [8,14,34].
Surface morphology is thought to play an important role in
the rheology experiments because uid ow tangential to a
surface can be strongly modied by a low density of
protrusions [14]. In this regard, measurements of the inter-
face thermal conductance may be much less sensitive to the
perfection of the experimental system, and therefore, more
easily interpreted in terms of intrinsic mechanisms.
Through this work, we establish the interface thermal
conductance G for hydrophilic and hydrophobic surfaces
(Fig. 3). The smallest conductance for hydrophobic-water
interfaces measured to date is 50 MWm
2
K
1
, with a
Kapitza length, h G 12 nm. This large thermal
resistance might be a signicant factor modulating heat
ow in biological systems as well as nanoscale systems
that contain high densities of hydrophobic interfaces [31].
This work was supported by US DOE Grant
No. DEFG02-01ER45938 and the WaterCAMPWS under
NSF Agreement No. CTS-0120978. Rutherford backscat-
tering spectrometry was carried out in the Center for
Microanalysis of Materials, University of Illinois, which
is partially supported by the US Department of Energy
under Grant No. DEFG02-91-ER45439.
[1] P. Ball, Nature (London) 423, 25 (2003).
[2] G. Cicero et al., J. Am. Chem. Soc. 127, 6830 (2005).
[3] D. A. Doshi et al., Proc. Natl. Acad. Sci. U.S.A. 102, 9458
(2005).
[4] J. Israelachvili and H. Wennerstrom, Nature (London)
379, 219 (1996).
[5] D. Schwendel et al., Langmuir 19, 2284 (2003).
[6] R. Steitz et al., Langmuir 19, 2409 (2003).
[7] X. Y. Zhang, Y. X. Zhu, and S. Granick, Science 295, 663
(2002).
[8] Y. X. Zhu and S. Granick, Phys. Rev. Lett. 87, 096104
(2001).
[9] K. Lum, D. Chandler, and J. D. Weeks, J. Phys. Chem. B
103, 4570 (1999).
[10] F. H. Stillinger, J. Solution Chem. 2, 141 (1973).
[11] J. D. Weeks, Annu. Rev. Phys. Chem. 53, 533 (2002).
[12] U. K. Sur and V. Lakshminarayanan, J. Colloid Interface
Sci. 254, 410 (2002).
[13] A. Luzar, J. Phys. Chem. B 108, 19 859 (2004).
[14] C. Cottin-Bizonne et al., Phys. Rev. Lett. 94, 056102(2005).
[15] L. R. Hirsch et al., Proc. Natl. Acad. Sci. U.S.A. 100,
13 549 (2003).
[16] D. G. Cahill et al., J. Appl. Phys. 93, 793 (2003).
[17] Z. B. Ge, D. G. Cahill, and P. V. Braun, J. Phys. Chem. B
108, 18 870 (2004).
[18] O. M. Wilson et al., Phys. Rev. B 66, 224301 (2002).
[19] R. M. Costescu, M. A. Wall, and D. G. Cahill, Phys. Rev. B
67, 054302 (2003).
[20] C. A. Paddock and G. L. Eesley, J. Appl. Phys. 60, 285
(1986).
[21] C. D. Bain et al., J. Am. Chem. Soc. 111, 321 (1989).
[22] A. Majumdar and P. Reddy, Appl. Phys. Lett. 84, 4768
(2004).
[23] Y. S. Ju, J. Heat Transfer 127, 1400 (2005).
[24] G. K. White, S. B. Woods, and M. T. Elford, Philos. Mag.
4, 688 (1959).
[25] J. H. Hodak, A. Henglein, and G. V. Hartland, J. Chem.
Phys. 112, 5942 (2000).
[26] G. Tas and H. J. Maris, Phys. Rev. B 49, 15 046 (1994).
[27] R. J. Gripshover, J. B. Vanzytveld, and J. Bass, Phys. Rev.
163, 598 (1967).
[28] N. Tillman et al., J. Am. Chem. Soc. 110, 6136 (1988).
[29] D. G. Cahill, Rev. Sci. Instrum. 75, 5119 (2004).
[30] A. Feldman, High Temp. High Press. 31, 293 (1999).
[31] H. A. Patel, S. Garde, and P. Keblinski, Nano Lett. 5, 2225
(2005).
[32] D. Chandler, Nature (London) 437, 640 (2005).
[33] D. B. Mergenthaler et al., Macromolecules 25, 3500
(1992).
[34] C. H. Choi, K. J. A. Westin, and K. S. Breuer, Phys. Fluids
15, 2897 (2003).
PRL 96, 186101 (2006)
P HYS I CAL RE VI E W L E T T E RS
week ending
12 MAY 2006
186101-4
Você também pode gostar
- The Subtle Art of Not Giving a F*ck: A Counterintuitive Approach to Living a Good LifeNo EverandThe Subtle Art of Not Giving a F*ck: A Counterintuitive Approach to Living a Good LifeNota: 4 de 5 estrelas4/5 (5794)
- The Gifts of Imperfection: Let Go of Who You Think You're Supposed to Be and Embrace Who You AreNo EverandThe Gifts of Imperfection: Let Go of Who You Think You're Supposed to Be and Embrace Who You AreNota: 4 de 5 estrelas4/5 (1090)
- Never Split the Difference: Negotiating As If Your Life Depended On ItNo EverandNever Split the Difference: Negotiating As If Your Life Depended On ItNota: 4.5 de 5 estrelas4.5/5 (838)
- Hidden Figures: The American Dream and the Untold Story of the Black Women Mathematicians Who Helped Win the Space RaceNo EverandHidden Figures: The American Dream and the Untold Story of the Black Women Mathematicians Who Helped Win the Space RaceNota: 4 de 5 estrelas4/5 (894)
- Elon Musk: Tesla, SpaceX, and the Quest for a Fantastic FutureNo EverandElon Musk: Tesla, SpaceX, and the Quest for a Fantastic FutureNota: 4.5 de 5 estrelas4.5/5 (474)
- The Hard Thing About Hard Things: Building a Business When There Are No Easy AnswersNo EverandThe Hard Thing About Hard Things: Building a Business When There Are No Easy AnswersNota: 4.5 de 5 estrelas4.5/5 (344)
- The Sympathizer: A Novel (Pulitzer Prize for Fiction)No EverandThe Sympathizer: A Novel (Pulitzer Prize for Fiction)Nota: 4.5 de 5 estrelas4.5/5 (119)
- The Emperor of All Maladies: A Biography of CancerNo EverandThe Emperor of All Maladies: A Biography of CancerNota: 4.5 de 5 estrelas4.5/5 (271)
- The Little Book of Hygge: Danish Secrets to Happy LivingNo EverandThe Little Book of Hygge: Danish Secrets to Happy LivingNota: 3.5 de 5 estrelas3.5/5 (399)
- The World Is Flat 3.0: A Brief History of the Twenty-first CenturyNo EverandThe World Is Flat 3.0: A Brief History of the Twenty-first CenturyNota: 3.5 de 5 estrelas3.5/5 (2219)
- The Yellow House: A Memoir (2019 National Book Award Winner)No EverandThe Yellow House: A Memoir (2019 National Book Award Winner)Nota: 4 de 5 estrelas4/5 (98)
- Devil in the Grove: Thurgood Marshall, the Groveland Boys, and the Dawn of a New AmericaNo EverandDevil in the Grove: Thurgood Marshall, the Groveland Boys, and the Dawn of a New AmericaNota: 4.5 de 5 estrelas4.5/5 (265)
- A Heartbreaking Work Of Staggering Genius: A Memoir Based on a True StoryNo EverandA Heartbreaking Work Of Staggering Genius: A Memoir Based on a True StoryNota: 3.5 de 5 estrelas3.5/5 (231)
- Team of Rivals: The Political Genius of Abraham LincolnNo EverandTeam of Rivals: The Political Genius of Abraham LincolnNota: 4.5 de 5 estrelas4.5/5 (234)
- The Unwinding: An Inner History of the New AmericaNo EverandThe Unwinding: An Inner History of the New AmericaNota: 4 de 5 estrelas4/5 (45)
- Lattice Vibrations and Phonon Dispersion RelationsDocumento12 páginasLattice Vibrations and Phonon Dispersion RelationsKartikay5Ainda não há avaliações
- Moon River (Acoustic)Documento6 páginasMoon River (Acoustic)Mauricio TerrazasAinda não há avaliações
- Evaluation of Ultrasonic Attenuation in Oxide Thin Films Using Wavelength DependenceDocumento2 páginasEvaluation of Ultrasonic Attenuation in Oxide Thin Films Using Wavelength DependenceMauricio TerrazasAinda não há avaliações
- Bte PDFDocumento11 páginasBte PDFMauricio TerrazasAinda não há avaliações
- Kovacs PDFDocumento18 páginasKovacs PDFMauricio TerrazasAinda não há avaliações
- Air On The G String: B B B BDocumento2 páginasAir On The G String: B B B BMauricio TerrazasAinda não há avaliações
- Transp3 PDFDocumento16 páginasTransp3 PDFMauricio TerrazasAinda não há avaliações
- The Fourier Transform and Its ApplicationsDocumento428 páginasThe Fourier Transform and Its Applicationsmachinelearner100% (3)
- PCA preamp data sheetDocumento3 páginasPCA preamp data sheetMauricio TerrazasAinda não há avaliações
- psdOFPSK PDFDocumento16 páginaspsdOFPSK PDFMauricio TerrazasAinda não há avaliações
- psdOFPSK PDFDocumento16 páginaspsdOFPSK PDFMauricio TerrazasAinda não há avaliações
- Tolgahan Çogulu PDFDocumento9 páginasTolgahan Çogulu PDFMauricio TerrazasAinda não há avaliações
- Non Ferrous AlloysDocumento18 páginasNon Ferrous Alloysamele25Ainda não há avaliações
- Hs diagramHDstaaendeDocumento5 páginasHs diagramHDstaaendeDjalma SouzaAinda não há avaliações
- Lec 1 Stresses in Solid BodyDocumento36 páginasLec 1 Stresses in Solid Bodyumair100% (1)
- Carnot Vapor Compression SystemsDocumento1 páginaCarnot Vapor Compression SystemsSUMITAinda não há avaliações
- Rivets ConnectionsDocumento30 páginasRivets Connectionsfaizankhan23Ainda não há avaliações
- Example of Application of AISI 360 10 and Parallel With EC3Documento5 páginasExample of Application of AISI 360 10 and Parallel With EC3Aakash KhatriAinda não há avaliações
- Vehicle Crash Can FEA Model Simulation and Validation With Experiment Data NLENG.2014.0030.R1Documento19 páginasVehicle Crash Can FEA Model Simulation and Validation With Experiment Data NLENG.2014.0030.R1rrmerlin_2Ainda não há avaliações
- Lecture 1. Shaft DesignDocumento18 páginasLecture 1. Shaft DesignhasanAinda não há avaliações
- Slab On GradeDocumento60 páginasSlab On GradeMAAinda não há avaliações
- Correlation of Yield Strength and Tensile Strength With Hardness For Steels - SpringerDocumento7 páginasCorrelation of Yield Strength and Tensile Strength With Hardness For Steels - SpringerJigar M. UpadhyayAinda não há avaliações
- 1 s2.0 S0734743X08000778 MainDocumento11 páginas1 s2.0 S0734743X08000778 Mainfa.jamshidiAinda não há avaliações
- Strain Rate EffectDocumento2 páginasStrain Rate EffectAnil YildizAinda não há avaliações
- Terfenol D DatasheetDocumento2 páginasTerfenol D DatasheetFirstAid2Ainda não há avaliações
- Aluminium Extrusions Technical Design GuideDocumento174 páginasAluminium Extrusions Technical Design GuideUmesh Chamara100% (1)
- Journal of Alloys and Compounds: A. Yarmou Shamsabadi, R. Bakhtiari, G. Eisaabadi BDocumento10 páginasJournal of Alloys and Compounds: A. Yarmou Shamsabadi, R. Bakhtiari, G. Eisaabadi BJustin DixonAinda não há avaliações
- Elemen MesinDocumento23 páginasElemen MesinMonika NikaAinda não há avaliações
- 2 ULS - Bending With or Without Axial Force (2014) PDFDocumento15 páginas2 ULS - Bending With or Without Axial Force (2014) PDFLuke LdhAinda não há avaliações
- Tanaka1981Documento28 páginasTanaka1981Madhwan ChandrawanshiAinda não há avaliações
- Test Certificate: Reference No.Documento3 páginasTest Certificate: Reference No.Maninder ChaudharyAinda não há avaliações
- Leespring EngguideDocumento27 páginasLeespring EngguideAnonymous h6qnMVb8eAinda não há avaliações
- Biot (1962) Mechanics of Deformation and Acoustic Propagation in Porous MediaDocumento18 páginasBiot (1962) Mechanics of Deformation and Acoustic Propagation in Porous MedianovaoneAinda não há avaliações
- Ghulam Ishaq Khan Institute of Engineering Sciences and TechnologyDocumento10 páginasGhulam Ishaq Khan Institute of Engineering Sciences and TechnologyWajid AliAinda não há avaliações
- Experiment 6. Adsorption Official Power PointDocumento40 páginasExperiment 6. Adsorption Official Power PointElaine Tan100% (4)
- Tiang Pancang JhsDocumento4 páginasTiang Pancang JhsFodki 2019Ainda não há avaliações
- 269-Design and Tensiometric Analysis of The C-Clamp For Railroad TracksDocumento4 páginas269-Design and Tensiometric Analysis of The C-Clamp For Railroad TracksIvancevic Ilija100% (1)
- IS 800-2007 Example 002w321321weDocumento6 páginasIS 800-2007 Example 002w321321weputra wiraAinda não há avaliações
- R7410303-Finite Element MethodsDocumento4 páginasR7410303-Finite Element MethodssivabharathamurthyAinda não há avaliações
- Reinforced Concrete DesignDocumento217 páginasReinforced Concrete Designmortada JasimAinda não há avaliações
- LLDPE Datasheet 2017Documento1 páginaLLDPE Datasheet 2017Endayenew MollaAinda não há avaliações
- Course Title: Material Science and Strength of Materials Course Code: 4053 Course Category: B Periods/Week: 6 Periods/Semester: 84 Credits: 6Documento4 páginasCourse Title: Material Science and Strength of Materials Course Code: 4053 Course Category: B Periods/Week: 6 Periods/Semester: 84 Credits: 6VaisakVenugopalAinda não há avaliações