Escolar Documentos
Profissional Documentos
Cultura Documentos
QP09 Internal Auditing
Enviado por
Zaf MeerzaDireitos autorais
Formatos disponíveis
Compartilhar este documento
Compartilhar ou incorporar documento
Você considera este documento útil?
Este conteúdo é inapropriado?
Denunciar este documentoDireitos autorais:
Formatos disponíveis
QP09 Internal Auditing
Enviado por
Zaf MeerzaDireitos autorais:
Formatos disponíveis
MRF Name / Logo
QP09 Internal auditing
Section
All
Release/Amendment summary
Initial issue
Issue
Date
1.0
dd/mm/yy
Approved:
Management Representative
Note: Within this manual template, red italic text should be replaced by MRF specific
information and any guidance in text boxes should be implemented.
259937275.doc
MRF Name / Logo
1
Purpose
The purpose of this procedure is to provide a planned and documented method for carrying out internal
audits of the Quality Management System (QMS).
Internal quality auditing verifies whether quality activities comply with planned arrangements and the
effectiveness of the QMS. If any non-conformities are found, corrective actions will be implemented based on
the results of these audits.
Scope
This procedure contains the following sections:
4.1
4.2
4.3
Audit planning
Carrying out an audit
Corrective action review
Related documents
Glossary
AS
MR
QMS
NC
OBS
QA
Procedure
Audit Schedule
Management Representative
Quality Management System
Nonconformity
Observations
Quality Auditor
The Management Representative (MR) is to ensure the implementation of this procedure and is responsible
for maintaining the audit schedules and audit reports.
The MR and trained Quality Auditors (QA) are responsible for conducting internal quality audits. Records are
maintained in accordance with Control of Records Procedure QP02.
4.1
Audit planning
Audits are planned using the Audit Schedule (AS) so that all sections of the management system are audited
at least once per year. More important activities or areas with identified problems are audited as often as
required to correct deficiencies and to give confidence of correct operation.
The AS has a key for completion and this is updated following an audit to show the status of the audit and to
provide information for future planning.
QAs are trained and are appointed to audit areas that they do not generally work in. This is to provide an
independent check as far as possible with the number of staff available.
The QAs will make arrangements with the relevant personnel prior to the planned audit to confirm the day of
the audit and availability.
4.2
Carrying out an audit
The audit is carried out using the Audit Report QFaa, which consists of an audit checklist, a findings report
and a corrective action report. The report forms are specifically prepared for each process / section of the
QMS.
After selecting the area to be audited, a copy of the previous completed report is reviewed for any areas of
concern noted at that time. The audit is then conducted, recording clearly all the audit findings and
observations on the report.
Audit findings must include process description, file/job or record numbers and people interviewed. The
findings should be clear and unambiguous to anyone reading the report.
The status of the findings are recorded beside each entry and will be one of the following:
OBS - The findings are not incorrect but give cause for concern or could be improved;
259937275.doc
MRF Name / Logo
NC - The findings indicate a procedure has not been followed or the procedure does not meet the
requirements of the QMS;
- The findings and answers are satisfactory and comply with the procedure.
The auditee should be made aware of the findings and observations, good and bad.
The appropriate section of the report is signed by the auditee and auditor to confirm agreement of the
content of the report. Where more than one auditee is audited they should be told the findings at the time
of audit and the department head will be asked to sign the report.
4.3
Corrective action review
The completed audit reports are reviewed by the MR and the schedule updated.
Satisfactory reports are signed and filed in the completed audit section of the quality file. Audit reports that
are not fully completed are discussed with the auditor as part of the ongoing audit training.
Reports requiring action are discussed with the relevant persons and actions are agreed and documented.
Recorded actions will clearly detail what is to be done, who is responsible and the times scale for completion
to ensure that the issues are resolved and the recurrence of a similar problem is prevented. Significant or
longer timescale issues may be recorded on a Quality Report QFyy to assist with the tracking and closure of
corrective action.
The MR and QA will monitor the actions and review completion on the agreed dates. Once satisfied that the
actions have been effectively implemented the report is signed off and filed.
The audit results are reviewed at the Management Review meeting and, if considered necessary, additional
preventive actions are implemented.
Related documents
Audit report form QFaa.
Control of records procedure QP02.
Audit schedule.
Quality report form QFyy.
259937275.doc
MRF Name / Logo
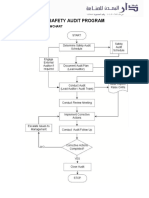
Appendix 1 Summary flow chart
Internal auditing
requirements planned and
audit schedule produced
Audit schedule
Auditor appointed to carry
out next audit on schedule
(taking into account auditor
independence)
Date / time for audit
confirmed with auditee
and audit checklist
generated/ reviewed
Previous audit
report (A/R)
Audit report
QFaa
Previous audit reviewed.
audit conducted and
findings recorded on
audit report QFaa
Findings categorised as
compliant (),
observation (Obs) or
non-conformity (NC)
Corrective actions
agreed with process
owner / auditee,
timescales set and audit
findings signed off
Completed audit report form
passed to MR and audit
schedule updated
Corrective actions reviewed at
due date. Completed audit
report signed off and filed by
MR
259937275.doc
Audit report
QFaa
Quality report
QFyy (A/R)
Record List
QP02
Updated audit
schedule
Completed audit
report QFaa
Você também pode gostar
- 6 Habits of True Strategic ThinkersDocumento64 páginas6 Habits of True Strategic ThinkersPraveen Kumar JhaAinda não há avaliações
- TD SequentialDocumento6 páginasTD SequentialJimmy N SumarsonoAinda não há avaliações
- HSE Incident Reporting Procedure - EX-HAS-OP-0001-Vn3.00Documento7 páginasHSE Incident Reporting Procedure - EX-HAS-OP-0001-Vn3.00Dave100% (2)
- Clause 5.2 - Health and Safety Policy: ISO 9001 ISO 14001 ISO 45001Documento3 páginasClause 5.2 - Health and Safety Policy: ISO 9001 ISO 14001 ISO 45001mohamedAinda não há avaliações
- HSE PlanDocumento40 páginasHSE PlanRami Khedro100% (1)
- Quality Audit Checklist - Revised - 01Documento10 páginasQuality Audit Checklist - Revised - 01Mustafa Hassan MalikAinda não há avaliações
- DeMark - Impressive Signals From TD Sequential IndicatorDocumento5 páginasDeMark - Impressive Signals From TD Sequential IndicatorSubroto Amangkurat100% (2)
- DeMark - Impressive Signals From TD Sequential IndicatorDocumento5 páginasDeMark - Impressive Signals From TD Sequential IndicatorSubroto Amangkurat100% (2)
- Subcontractors EHS Audit TemplateDocumento7 páginasSubcontractors EHS Audit TemplateDeepakAinda não há avaliações
- 1.0 Purpose / Scope: 2.1 HSE ObjectiveDocumento1 página1.0 Purpose / Scope: 2.1 HSE ObjectiveKhaldoon AlnashiAinda não há avaliações
- HSEQ Goals and Targets: OMV-EP GuidelineDocumento7 páginasHSEQ Goals and Targets: OMV-EP GuidelineMoaatazz Nouisri100% (1)
- OSHEMSP 72 - Management of Change Rev.01Documento6 páginasOSHEMSP 72 - Management of Change Rev.01Venkadesh PeriathambiAinda não há avaliações
- Objective and Programme of YEAR 2023Documento8 páginasObjective and Programme of YEAR 2023hse bsjAinda não há avaliações
- KPI - Objectives Achievement PlanDocumento4 páginasKPI - Objectives Achievement PlaninsaanAinda não há avaliações
- Jose Ferreira Criminal ComplaintDocumento4 páginasJose Ferreira Criminal ComplaintDavid Lohr100% (1)
- PPEA-WI-012 - B Consultation and Communication at SiteDocumento6 páginasPPEA-WI-012 - B Consultation and Communication at SiteDeepakAinda não há avaliações
- MS-P01 Management Responsibility ProcedureDocumento8 páginasMS-P01 Management Responsibility ProcedureMuhammad ZafarAinda não há avaliações
- FineScale Modeler - September 2021Documento60 páginasFineScale Modeler - September 2021Vasile Pop100% (2)
- QMS Internal Audit PlanDocumento5 páginasQMS Internal Audit PlanMohamedAinda não há avaliações
- HSEF1101.3 - Contractor HSE QuestionnaireDocumento4 páginasHSEF1101.3 - Contractor HSE QuestionnaireJason SmithAinda não há avaliações
- Contractor Work Permit: 1 ObjectiveDocumento11 páginasContractor Work Permit: 1 ObjectiveZeremia SamosirAinda não há avaliações
- Audit Observation SheetDocumento6 páginasAudit Observation SheetAkash PurkaitAinda não há avaliações
- Updated 25-Apr-2023 HSE Observation Sheet and Action Tracker 9:39 AMDocumento3 páginasUpdated 25-Apr-2023 HSE Observation Sheet and Action Tracker 9:39 AMAnna EmelineAinda não há avaliações
- Hse PlanDocumento13 páginasHse PlanbaraaAinda não há avaliações
- Planning and Procedures: Pdo Hse Management System Manual (CP-122)Documento10 páginasPlanning and Procedures: Pdo Hse Management System Manual (CP-122)AHMEDNABTAinda não há avaliações
- Internal Audit ProcedureDocumento6 páginasInternal Audit ProcedureEric AnastacioAinda não há avaliações
- Action Plan TemplateDocumento2 páginasAction Plan TemplateAgnes Asuncion VelascoAinda não há avaliações
- IASSC Yellow Belt Body of Knowledge1 PDFDocumento3 páginasIASSC Yellow Belt Body of Knowledge1 PDFShivam SharmaAinda não há avaliações
- OCP For Welding - Gas CuttingDocumento2 páginasOCP For Welding - Gas Cuttingravikpandey100% (2)
- 2.2 A Lead & Lag Indicator Dec2022Documento2 páginas2.2 A Lead & Lag Indicator Dec2022subodh kumarAinda não há avaliações
- 17 Subcon RequirementsDocumento38 páginas17 Subcon RequirementsMohammed MinhajAinda não há avaliações
- Procedure Manual Human Resources: StartDocumento4 páginasProcedure Manual Human Resources: StartpmuthurajAinda não há avaliações
- IASSC Green Belt Body of KnowledgeDocumento5 páginasIASSC Green Belt Body of KnowledgeBilal Salameh50% (2)
- Internal Audit Procedure (PROMK3L.03.QHSE)Documento7 páginasInternal Audit Procedure (PROMK3L.03.QHSE)Muhammad Indra GunawanAinda não há avaliações
- Abdellatif Hossam - HSE 15dec2022Documento2 páginasAbdellatif Hossam - HSE 15dec2022Abdellatef HossamAinda não há avaliações
- Internal Audit Procedure - Adopted 13082013Documento7 páginasInternal Audit Procedure - Adopted 13082013Sayed Abbas100% (1)
- ROF QP 007 Internal AuditingDocumento5 páginasROF QP 007 Internal AuditingISO ConsultancyAinda não há avaliações
- Internal Audit ReportsDocumento1 páginaInternal Audit ReportsCQMS 5S DivisionAinda não há avaliações
- Audit Planning Conducting and ReportingDocumento49 páginasAudit Planning Conducting and ReportingjackhanAinda não há avaliações
- Internal Audit ChecklistDocumento14 páginasInternal Audit ChecklistRamesh KumarAinda não há avaliações
- Internal Audit ScheduleDocumento4 páginasInternal Audit ScheduleCQMS 5S DivisionAinda não há avaliações
- Kpi Indi 1Documento21 páginasKpi Indi 1Rajwinder SinghAinda não há avaliações
- OHS ResponsibilitiesDocumento5 páginasOHS ResponsibilitiesAce GreensAinda não há avaliações
- Health & Safety Procedural Manual Procedure For Internal Audits MNE/OHS/P04Documento3 páginasHealth & Safety Procedural Manual Procedure For Internal Audits MNE/OHS/P04Richu PaliAinda não há avaliações
- Bond ValuationDocumento49 páginasBond Valuationmehnaz kAinda não há avaliações
- HSE-P-11 Planned General Inspection Issue 2.1Documento5 páginasHSE-P-11 Planned General Inspection Issue 2.1eng20072007Ainda não há avaliações
- Control of Substances Hazordous To HealthDocumento16 páginasControl of Substances Hazordous To Healthnathan_roadAinda não há avaliações
- Monitoring of OHS Objectives & Targets v-0.1Documento2 páginasMonitoring of OHS Objectives & Targets v-0.1Nomaan MalikAinda não há avaliações
- BCG-TM-PRO-13-13 HSE Performance & Calibration ProcedureDocumento2 páginasBCG-TM-PRO-13-13 HSE Performance & Calibration ProcedureYousaf RichuAinda não há avaliações
- Insert Your Company Logo/Name Here: InstructionsDocumento4 páginasInsert Your Company Logo/Name Here: InstructionsJamal BahriAinda não há avaliações
- AE-QA-09 - Master List of Instrument Cum Calibration PlanDocumento3 páginasAE-QA-09 - Master List of Instrument Cum Calibration PlanKarthi ThiyagarajanAinda não há avaliações
- JOB Description: Position Title Department Position Reports To Job Class Job SummaryDocumento2 páginasJOB Description: Position Title Department Position Reports To Job Class Job SummaryVijay RajaindranAinda não há avaliações
- ISO 9001 Process Procedure QPP-092-1 Internal AuditDocumento4 páginasISO 9001 Process Procedure QPP-092-1 Internal Auditmahm.tahaAinda não há avaliações
- CASE DOCTRINE FDocumento17 páginasCASE DOCTRINE FKaemy MalloAinda não há avaliações
- Permit To Work ProcedureDocumento2 páginasPermit To Work ProcedureTalib KhanAinda não há avaliações
- Contractor HSE Questionnaire: 1. GeneralDocumento14 páginasContractor HSE Questionnaire: 1. GeneralAsep FirmansyahAinda não há avaliações
- Neste Annual Report 2019Documento213 páginasNeste Annual Report 2019Xianchi ZhangAinda não há avaliações
- 02-012 Working at Heights PolicyDocumento7 páginas02-012 Working at Heights PolicyJoachimAinda não há avaliações
- C Control of Documents Section 3Documento11 páginasC Control of Documents Section 3Ngonidzashe ZvarevasheAinda não há avaliações
- 2 Industrial Training HandbookDocumento26 páginas2 Industrial Training HandbookZaf MeerzaAinda não há avaliações
- 99the Material For The 2nd Public and Private Joint Forum PDFDocumento287 páginas99the Material For The 2nd Public and Private Joint Forum PDFZaf MeerzaAinda não há avaliações
- Health, Safety and Environmental: GoalsDocumento15 páginasHealth, Safety and Environmental: Goalsprojects sureshAinda não há avaliações
- Competency and Awareness ProcedureDocumento5 páginasCompetency and Awareness ProcedureDaniel GnanaselvamAinda não há avaliações
- Mollymawk - English (Çalışma)Documento8 páginasMollymawk - English (Çalışma)Fatih OguzAinda não há avaliações
- Six Sigma Certification Exam - Sample Paper - GB PDFDocumento10 páginasSix Sigma Certification Exam - Sample Paper - GB PDFVinit ShahAinda não há avaliações
- PeopleCert SixSigma BlackBelt SamplePaper v2Documento12 páginasPeopleCert SixSigma BlackBelt SamplePaper v2majid4uonly100% (1)
- Strategic Safety ObjectivesDocumento11 páginasStrategic Safety ObjectivesDivyansh Singh ChauhanAinda não há avaliações
- Sample Audit Report 2012 PDFDocumento18 páginasSample Audit Report 2012 PDFengrrahman3135100% (1)
- Eir TemplateDocumento15 páginasEir Templatetran tuan100% (1)
- ESCL-QSP-006, Preventive Action ProcedureDocumento5 páginasESCL-QSP-006, Preventive Action ProcedureadiqualityconsultAinda não há avaliações
- Health and Safety Plans For TenderingDocumento4 páginasHealth and Safety Plans For TenderingHesham Hassan100% (1)
- Communications Plan Template: Project Name: Prepared By: DateDocumento1 páginaCommunications Plan Template: Project Name: Prepared By: DatevyerramallaAinda não há avaliações
- Dr. Concrete Building Material: Safety Objectives and Targets ProcedureDocumento5 páginasDr. Concrete Building Material: Safety Objectives and Targets Proceduresudeesh kumarAinda não há avaliações
- FRM-EHS-PRO-05 Hazard Identification & Risk Assessment (HIRA)Documento1 páginaFRM-EHS-PRO-05 Hazard Identification & Risk Assessment (HIRA)Venkatesan DAinda não há avaliações
- Objectives, Targets & Programs: (Department(s) / Section(s) / Person(s) )Documento3 páginasObjectives, Targets & Programs: (Department(s) / Section(s) / Person(s) )azee73Ainda não há avaliações
- Key Performance Indicators PDFDocumento7 páginasKey Performance Indicators PDFtarrteaAinda não há avaliações
- Subcontractor Competence / Resource Questionnaire: Commercial Services To CompleteDocumento31 páginasSubcontractor Competence / Resource Questionnaire: Commercial Services To CompleteNaba majeadAinda não há avaliações
- Audit Summary ReportDocumento2 páginasAudit Summary Reportas rginorAinda não há avaliações
- UserManual OUMBuzz v1.0Documento9 páginasUserManual OUMBuzz v1.0Zaf MeerzaAinda não há avaliações
- Evolution of Auto Policy The Malaysian Experience MAADocumento50 páginasEvolution of Auto Policy The Malaysian Experience MAAZaf MeerzaAinda não há avaliações
- Beginners Training ForexDocumento4 páginasBeginners Training ForexZaf MeerzaAinda não há avaliações
- CFTeI Syllabus 2012Documento5 páginasCFTeI Syllabus 2012Ahmed FathyAinda não há avaliações
- Statistical Analysis and Quality AssuranceDocumento10 páginasStatistical Analysis and Quality AssuranceZaf MeerzaAinda não há avaliações
- Iso 45001 Briefing NoteDocumento4 páginasIso 45001 Briefing NoteMichael Fadjar100% (1)
- TsvdaDocumento21 páginasTsvdaZaf MeerzaAinda não há avaliações
- Pekan Review Vol 6Documento37 páginasPekan Review Vol 6Zaf MeerzaAinda não há avaliações
- Quality ControlDocumento15 páginasQuality ControlSanjay DuraiAinda não há avaliações
- CQI SL Annex BrochureDocumento12 páginasCQI SL Annex BrochureNelson AnibalAinda não há avaliações
- PeopleCert SixSigma YellowBelt SamplePaper v1Documento8 páginasPeopleCert SixSigma YellowBelt SamplePaper v1cancer5amitAinda não há avaliações
- Statistical Control UseDocumento6 páginasStatistical Control UseZaf MeerzaAinda não há avaliações
- Agenda of MeetingDocumento1 páginaAgenda of MeetingZaf MeerzaAinda não há avaliações
- IASSC Black Belt Body of KnowledgeDocumento5 páginasIASSC Black Belt Body of Knowledgetanu111Ainda não há avaliações
- SQI Training Schedule 2012Documento5 páginasSQI Training Schedule 2012Zaf MeerzaAinda não há avaliações
- Christmas Phrasal VerbsDocumento2 páginasChristmas Phrasal VerbsannaAinda não há avaliações
- Patrick Svitek - Resume 2012Documento1 páginaPatrick Svitek - Resume 2012Patrick SvitekAinda não há avaliações
- Module 2Documento6 páginasModule 2MonicaMartirosyanAinda não há avaliações
- CentipedeDocumento2 páginasCentipedeMaeAinda não há avaliações
- Microsoft Logo Third Party Usage Guidance: June 2021Documento7 páginasMicrosoft Logo Third Party Usage Guidance: June 2021Teo HocqAinda não há avaliações
- Utilitarianism and The Ethical Foundations of Cost-Effectiveness Analysis in Resource Allocation For Global HealthDocumento7 páginasUtilitarianism and The Ethical Foundations of Cost-Effectiveness Analysis in Resource Allocation For Global HealthZainab AbbasAinda não há avaliações
- سلفات بحري كومنز PDFDocumento8 páginasسلفات بحري كومنز PDFSami KahtaniAinda não há avaliações
- SAHANA Disaster Management System and Tracking Disaster VictimsDocumento30 páginasSAHANA Disaster Management System and Tracking Disaster VictimsAmalkrishnaAinda não há avaliações
- Manufacturing ConsentDocumento3 páginasManufacturing ConsentSaif Khalid100% (1)
- Qualitative KPIDocumento7 páginasQualitative KPIMas AgusAinda não há avaliações
- This Is My Beloved Son Lesson PlanDocumento1 páginaThis Is My Beloved Son Lesson PlancamigirlutAinda não há avaliações
- Pathwinder Software v. Core Cashless - Personal Jurisdiction PDFDocumento19 páginasPathwinder Software v. Core Cashless - Personal Jurisdiction PDFMark JaffeAinda não há avaliações
- Bhagwanti's Resume (1) - 2Documento1 páginaBhagwanti's Resume (1) - 2muski rajputAinda não há avaliações
- Acts and Key EventsDocumento25 páginasActs and Key Eventsemilyjaneboyle100% (1)
- PVP 095, s.2022-RxgdPLqfs6HQOtIno2DnDocumento3 páginasPVP 095, s.2022-RxgdPLqfs6HQOtIno2Dnanalyn ramosAinda não há avaliações
- COWASH Federal Admin Manual v11 PDFDocumento26 páginasCOWASH Federal Admin Manual v11 PDFmaleriAinda não há avaliações
- 4 Marine Insurance 10-8-6Documento53 páginas4 Marine Insurance 10-8-6Eunice Saavedra100% (1)
- Research Paper On RapunzelDocumento8 páginasResearch Paper On RapunzelfvgcaatdAinda não há avaliações
- Resume 2Documento2 páginasResume 2Ryan AlyasAinda não há avaliações
- Juarez, Jenny Brozas - Activity 2 MidtermDocumento4 páginasJuarez, Jenny Brozas - Activity 2 MidtermJenny Brozas JuarezAinda não há avaliações
- Veronica Guerin Interview With Anne FelloniDocumento2 páginasVeronica Guerin Interview With Anne FelloniDeclan Max BrohanAinda não há avaliações
- Philips AZ 100 B Service ManualDocumento8 páginasPhilips AZ 100 B Service ManualВладислав ПаршутінAinda não há avaliações