Escolar Documentos
Profissional Documentos
Cultura Documentos
Entrapping Enzyme in A Functionalized Nanoporous Support
Enviado por
chuotchu012024Descrição original:
Título original
Direitos autorais
Formatos disponíveis
Compartilhar este documento
Compartilhar ou incorporar documento
Você considera este documento útil?
Este conteúdo é inapropriado?
Denunciar este documentoDireitos autorais:
Formatos disponíveis
Entrapping Enzyme in A Functionalized Nanoporous Support
Enviado por
chuotchu012024Direitos autorais:
Formatos disponíveis
Published on Web 08/28/2002
Entrapping Enzyme in a Functionalized Nanoporous Support
Chenghong Lei, Yongsoon Shin, Jun Liu,*, and Eric J. Ackerman*
Pacific Northwest National Laboratory, Richland, Washington 99352
Received May 9, 2002
There is a long history for enzyme (protein) immobilization using
solid supports via adsorption, encapsulation, and covalently linking.1
One of the most widely used methods for immobilizing enzymes
is encapsulation inside sol-gel silica.2 However, due to small pore
size and non-open-pore structure, most studies showed lower
specific activity than that of the free enzymes in solution.3
Unlike sol-gel silica, mesoporous silica provides a rigid, uniform
open-pore structure. Functionalized mesoporous silica (FMS) has
exhibited very high affinity for binding heavy metal ions with
mercapto functional groups.4 FMS could have great potential for
high enzyme loading, provided that (1) the pore size is sufficiently
large for the enzyme to be comfortably hosted and also for its
substrate and product to access and diffuse easily through open
pore channel and (2) appropriate functional groups provide high
affinity for protein molecules. Recently, mesoporous silica has
begun to attract attention for enzyme immobilization.5,6 The best
results showed that 84% of the initial specific activity remained
after immobilization albeit with very low protein loading (0.2%,
w/w), presumably due to the small pore size (<6 nm). To our
knowledge, the great potential advantage of FMS for enzyme
entrapment has not yet been realized.
In this work, we used mesoporous silica with large pores that
were subsequently functionalized to thereby yield high protein
loading and enhanced enzyme activity simultaneously. Surprisingly,
by introducing only 2% coverage of HOOC-CH2-CH2- groups on
the internal silanol wall, the specific activity of organophosphorus
hydrolase (OPH) entrapped could reach more than 2 times as high
as that of the free enzyme prior to immobilization. The original
unfunctionalized mesoporous silica (UMS), prepared by using
nonionic block copolymer surfactant as the template,7 had a pore
size of 30 nm by the Barrett-Joyner-Halenda method,8 while the
surface area was as high as 533 m2/g with an average bead size of
12-15 m. We also used normal porous silica (NPS) for comparison experiments throughout this work.9 The N2 adsorption
isotherms suggest that NPS had very wide pore size distribution,
while the surfactant templated mesoporous silica had very narrow
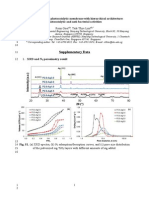
pore size distribution. Figure 1a shows the transmission electron
microscopy (TEM) image of NPS made of silica particles partially
sintered together. In comparison, the TEM image of the mesoporous
silica (Figure 1b) reveals a uniform cagelike porous structure.7
To prepare FMS, we mixed Tris-(methoxy)carboxylethylsilane
(TMCES) or Tris-(methoxy)aminopropylsilane (TMAPS) with
mesoporous silica in an appropriate solvent.4,10 We selected TMCES
and TMAPS as functionalization reagent mainly because HOOCand NH2- would offer electrostatic, H-bond, and hydrophilic
interaction with the charged amino acid residues of the protein
molecules.
* To whom correspondence should be addressed. E-mail: Eric.Ackerman@pnl.gov,
Jliu@sandia.gov.
Current address: Sandia National Laboratories, Albuquerque, NM 87181.
11242
J. AM. CHEM. SOC. 2002, 124, 11242-11243
Figure 1. TEM images of (a) normal porous silica, (b) mesoporous silica.
Figure 2. Comparison of different porous silica support for OPH
immobilization in pH 7.5, 20 mM HEPES at 21 ( 1 C.
We selected OPH for this work since it has been widely
investigated for biosensing and decontamination of poisonous
agents.11 OPH has a well-known crystal structure (a dimer with
two identical monomers) and dimensions of about 92 56
40 .12 By incubating FMS in OPH stock solution, the enzyme
was spontaneously sequestered into the functionalized mesopores.13
Figure 2 shows the protein amount and activity of OPH
immobilized with 1 mg of each silica support. The isoelectric point
(pI) of OPH is 8.3; thus, the overall charge of the protein is positive
at pH 7.5.13 As shown, the positively charged OPH could be
entrapped in UMS with a protein loading of 3.1% (w/w) due to
electrostatic interaction with the negatively charged silanol surface.
However, it displayed a low specific activity of 935 units/mg of
entrapped protein (calculated from Figure 2, see relative heights).
In contrast, higher protein loading and much higher specific activity
were obtained with the negatively charged HOOC-FMS. When a
2% surface coverage of carboxylic groups was introduced to the
mesoporous silica, a higher protein loading of 4.7% (w/w) with
significantly enhanced specific activity of 4182 units/mg was
obtained, showing an exceptional high immobilization efficiency
of more than 200% (the ratio of the specific activity of the entrapped
OPH with that of the free OPH in solution).12 These results
demonstrate that the organic functionalization (-CH2-CH2-COOH)
provided the enzyme with a benign surrounding microenvironment,
showing higher affinity for the protein and inducing a dramatic
change of specific activity. With 20% coverage of HOOC-FMS, a
high specific activity of 4109 units/mg was also achieved, but the
total protein loading and activity were reduced. We speculate that
10.1021/ja026855o CCC: $22.00 2002 American Chemical Society
COMMUNICATIONS
conventional approaches yield far lower immobilization efficiency.1,3,11 It has been reported that confinement from molecular
crowding in biological cells can both stabilize and induce orderof-magnitude enhancements in catalytic reaction rates compared
to enzymes in solution.14 We hope that rational design of organically
functionalized nanoporosity will lead to new approaches to entrap
and stabilize biomolecules for a wide range of applications.
Acknowledgment. We thank L. Koriazova for help making the
OPH expression vector. This work was supported by the U.S.
Department of Energy under Contract DE-AC06-RLO1830.
Figure 3. Stability of OPH entrapped in 2% HOOC-FMS and free OPH
in pH 7.5, 20 mM HEPES. The first 47 days of storage was carried out at
4 ( 0.2 C, and the following 98 days was at 21 ( 1 C.
the reduction of the loading amount might be attributed to the
decrease in the effective surface area by more close-packed
functional groups.
In contrast, positively charged OPH was almost completely
repelled from the positively charged 20% NH2-FMS, with a very
low protein loading (0.25%, w/w) and nearly zero activity.
Therefore, selective entrapment could be achieved by rational choice
and displacement of the functional groups in relation to the overall
charge of the protein. Unexpectedly, the specific activity with 2%
NH2-FMS was as high as 2691 units/mg with a protein loading
rate of 3.8% (w/w). This phenomenon could be explained by the
fact that the small coverage of amino groups is less closely packed4
and there are still plenty of local, negatively charged silanol groups.
Its overall environment still favored the entrapment of positively
charged protein. Some negatively charged amino acid residues on
the overall positively charged protein could interact with the small
coverage of the positively charged functional group as well. The
fact that even 2% NH2-FMS entrapped OPH more effectively than
negatively charged UMS demonstrated again that the organic
functionalization (-CH2-CH2-CH2-NH2) of mesoporous silica enhanced the affinity of the nanopores to the enzyme and induced an
increase of the specific activity. From these results, we can conclude
that fuctionalization of mesoporous silica and the type and coverage
of functional group are paramount for both high loading amount
and immobilization efficiency.
For comparison, the simple adsorption of OPH on NPS with or
without functionalization displayed lower protein loading and less
specific activity (Figure 2), demonstrating the great advantage of
rigid and uniformly open porous FMS over NPS with the nonopen pores and low surface area. The 2% HOOC-NPS displayed
only about 0.1 of OPH activity entrapped with the same amount of
2% HOOC-FMS.
As shown in Figure 3, the specific activity of the free OPH in
solution was reduced 77% after 145 days of storage. In contrast,
the enhanced specific activity of OPH entrapped in 2% HOOCFMS decreased only 38% after the same storage, which was still
134% of the initial specific activity of the free OPH. In fact, after
such long-term storage, the activity and protein amount in the
supernatant was still undetectable.13 This suggests that the decrease
of the enzyme activity was attributed to protein denaturation, rather
than the enzyme leaching from the FMS.
In conclusion, for the first time, we have demonstrated that as a
result of the unique mesoporous structure and surface chemistry,
the organically functionalized nanoporosity of HOOC-FMS provided both high affinity for the charged protein molecules and a
favored microenvironment that resulted in exceptionally high
immobilization efficiency (>200%) with enhanced stability, while
Supporting Information Available: OPH expression and purification, charged surface amino acid distributions, and structural dimensions
(PDF). This material is available free of charge via the Internet at http://
pubs.acs.org.
References
(1) Bickerstaff, G. F. (Ed.) Immobilization of Enzymes and Cells; Humana
Press: Totowa, NJ, 1997.
(2) Livage, J.; Coradin. T.; Roux, C. J. Phys. Condes. Matter. 2001, 13,
R673-691.
(3) (a) Braun, S.; Rappoport, S.; Zusman, R. Mater. Lett. 1990, 10, 1-5. (b)
Wei, Y.; Xu, J.; Feng, Q.; Dong, H.; Lin, M. Mater. Lett. 2000, 44, 6-11.
(4) (a) Feng, X.; Fryxell, G. E.; Wang, L.-Q.; Kim, A. Y.; Liu, J.; Kemner,
K. M. Science 1997, 276, 923-926. (b) Liu, J.; Feng, X.; Fryxell, G. E.;
Wang, L.-Q.; Kim, A. Y.; Gong, M. AdV. Mater. 1998, 10, 161-165.
(5) Yiu, H. H. P.; Wright, P. A.; Botting, N. P. Microporous Mesoporous
Mater. 2001, 44-45, 763-768.
(6) Yiu, H. H. P.; Wright, P. A.; Botting, N. P. J. Mol. Catal. B: Enzym.
2001, 15, 81-92.
(7) Zhao, D.; Feng, J.; Huo, Q.; Melosh, N.; Fredrickson, G. H.; Chmelka,
B. F.; Stucky, G. D. Science 1998, 279, 548-552.
(8) Linsen, B. G.; van den Heuvel, A. The Solid Gas Interface; Flood, E. A.,
Ed.; Mercel Dekker: New York, 1967; Vol. 2, p 1025.
(9) Normal porous silica is uncoated silica bulk material with a pore size of
30 nm with a 12-m bead size from PolyLC Inc., USA, item no. BMSI
1203.
(10) A controlled hydration and condensation reaction was used to introduce
functional groups into UMS and NPS.4 The amount of trimethoxy silanes
needed was calculated on the basis of the fraction of the surface area to
be occupied by silane molecules; e.g., a coverage of 2% HOOC-FMS or
NH2-FMS means 2% of the total available surface area of the mesoporous
silica would be silanized with the trimethoxy silane with the functional
group HOOC- or NH2-. For UMS (pore diameter, 30 nm), the coverage
of 2% corresponds to 0.09 mmol silane/g assuming 5 1018 molecules/
m2 in a fully dense monolayer coverage, as indicated by previous solidstate NMR studies.4
(11) (a) Singh, A. K.; Flounders, A. W.; Volponi, J. V.; Ashley, C. S.; Walley,
K.; Schoeniger, J. S. Biosens. Bioelectron. 1999, 98, 703-713. (b)
LeJeune, K. E.; Wild, J. R.; Russel, A. J. Nature 1998, 395, 27-28. (c)
LeJeune, K. E.; Russell, A. J. Biotechnol. Bioeng. 1996, 51, 450-457.
(12) OPH was cloned, expressed, purified, and stored at -80 oC. It was thawed
at 4 C before use and dialysed in pH 7.2, 0.1 M HEPES. The activity of
OPH was measured in 1 mM paraoxon in pH 9.0, 0.15 M CHES buffer
at 25 C, in which one OPH unit is the active amount allowing 1.0 mol
paraoxon to be hydrolyzed per minute. Protein amounts were measured
using Pierce BCA Assay Kit (Pierce, product no. 23227). OPH stock
solution, which was diluted 20-50 times by pH 7.5, 20 mM HEPES for
activity measurements, had an initial specific activity of 1928 units/mg
of protein.
(13) Generally, an aliquot of 2-4 mg of FMS or other silica support in a 1.8mL tube was added for incubation with 0.2-1.2 mL of the OPH stock
solution, shaking at a speed of 1400 min-1 on an Eppendorf Thermomixer
5436 at 25 C for 2 h, and then centrifuging at 12000 rpm for 10 min.
Repeatedly, the resulting OPH-FMS deposit was shaken vigorously and
washed in pH 7.5, 20 mM HEPES (g10 1.0 mL) at room temperature
(21 ( 1 C) and centrifuged to remove any nonfirmly immobilized
enzyme, which was monitored in later cycles until both the protein and
activity were undetectable in the supernatant. Finally, the OPH-FMS
composite was thoroughly resuspended in the same washing buffer with
100 L of the buffer per mg of FMS for further protein and activity
measurement. To avoid any scattering during spectrophotometric measurement of protein amounts, a centrifugation step was taken to remove the
silica particles after incubation of the OPH-FMS composite with the
working reagent. The errors for both the protein amount and activity
measurements were less than 5%.
(14) (a) Zhou, H. -X.; Dill, K. A. Biochemistry 2001, 40, 11289-11293. (b)
Minton, A. P. J. Biol. Chem. 2001, 276, 10577-10580 and references
therein.
JA026855O
J. AM. CHEM. SOC.
VOL. 124, NO. 38, 2002 11243
Você também pode gostar
- (SuppInfo) Effect of Nature and Location of Defects On Bandgap Narrowing in Black TiO2 Nanoparticles PDFDocumento15 páginas(SuppInfo) Effect of Nature and Location of Defects On Bandgap Narrowing in Black TiO2 Nanoparticles PDFchuotchu012024Ainda não há avaliações
- (SuppInfo) Ag-Decorated TiO2 Photocatalytic Membrane With Hierarchical Architecture. Photocatalytic and Anti-Bacterial ActivitiesDocumento5 páginas(SuppInfo) Ag-Decorated TiO2 Photocatalytic Membrane With Hierarchical Architecture. Photocatalytic and Anti-Bacterial Activitieschuotchu012024Ainda não há avaliações
- Enzyme-Functionalized Mesoporous Silica For Bioanalytical ApplicationsDocumento12 páginasEnzyme-Functionalized Mesoporous Silica For Bioanalytical Applicationschuotchu012024Ainda não há avaliações
- Enzyme Immobilization. An Overview On Techniques and Support MaterialsDocumento9 páginasEnzyme Immobilization. An Overview On Techniques and Support Materialschuotchu012024Ainda não há avaliações
- (SuppInfo) Entrapping Enzyme in A Functionalized Nanoporous SupportDocumento4 páginas(SuppInfo) Entrapping Enzyme in A Functionalized Nanoporous Supportchuotchu012024Ainda não há avaliações
- The Yellow House: A Memoir (2019 National Book Award Winner)No EverandThe Yellow House: A Memoir (2019 National Book Award Winner)Nota: 4 de 5 estrelas4/5 (98)
- Hidden Figures: The American Dream and the Untold Story of the Black Women Mathematicians Who Helped Win the Space RaceNo EverandHidden Figures: The American Dream and the Untold Story of the Black Women Mathematicians Who Helped Win the Space RaceNota: 4 de 5 estrelas4/5 (895)
- The Subtle Art of Not Giving a F*ck: A Counterintuitive Approach to Living a Good LifeNo EverandThe Subtle Art of Not Giving a F*ck: A Counterintuitive Approach to Living a Good LifeNota: 4 de 5 estrelas4/5 (5794)
- The Little Book of Hygge: Danish Secrets to Happy LivingNo EverandThe Little Book of Hygge: Danish Secrets to Happy LivingNota: 3.5 de 5 estrelas3.5/5 (399)
- Devil in the Grove: Thurgood Marshall, the Groveland Boys, and the Dawn of a New AmericaNo EverandDevil in the Grove: Thurgood Marshall, the Groveland Boys, and the Dawn of a New AmericaNota: 4.5 de 5 estrelas4.5/5 (266)
- Elon Musk: Tesla, SpaceX, and the Quest for a Fantastic FutureNo EverandElon Musk: Tesla, SpaceX, and the Quest for a Fantastic FutureNota: 4.5 de 5 estrelas4.5/5 (474)
- Never Split the Difference: Negotiating As If Your Life Depended On ItNo EverandNever Split the Difference: Negotiating As If Your Life Depended On ItNota: 4.5 de 5 estrelas4.5/5 (838)
- A Heartbreaking Work Of Staggering Genius: A Memoir Based on a True StoryNo EverandA Heartbreaking Work Of Staggering Genius: A Memoir Based on a True StoryNota: 3.5 de 5 estrelas3.5/5 (231)
- The Emperor of All Maladies: A Biography of CancerNo EverandThe Emperor of All Maladies: A Biography of CancerNota: 4.5 de 5 estrelas4.5/5 (271)
- The World Is Flat 3.0: A Brief History of the Twenty-first CenturyNo EverandThe World Is Flat 3.0: A Brief History of the Twenty-first CenturyNota: 3.5 de 5 estrelas3.5/5 (2259)
- The Hard Thing About Hard Things: Building a Business When There Are No Easy AnswersNo EverandThe Hard Thing About Hard Things: Building a Business When There Are No Easy AnswersNota: 4.5 de 5 estrelas4.5/5 (344)
- Team of Rivals: The Political Genius of Abraham LincolnNo EverandTeam of Rivals: The Political Genius of Abraham LincolnNota: 4.5 de 5 estrelas4.5/5 (234)
- The Unwinding: An Inner History of the New AmericaNo EverandThe Unwinding: An Inner History of the New AmericaNota: 4 de 5 estrelas4/5 (45)
- The Gifts of Imperfection: Let Go of Who You Think You're Supposed to Be and Embrace Who You AreNo EverandThe Gifts of Imperfection: Let Go of Who You Think You're Supposed to Be and Embrace Who You AreNota: 4 de 5 estrelas4/5 (1090)
- The Sympathizer: A Novel (Pulitzer Prize for Fiction)No EverandThe Sympathizer: A Novel (Pulitzer Prize for Fiction)Nota: 4.5 de 5 estrelas4.5/5 (121)
- SPS 370S - 2024 - Chapter 2Documento39 páginasSPS 370S - 2024 - Chapter 2ziziphomkosana2003Ainda não há avaliações
- Hydrogen Production With Carbon Dioxide Capture by Reforming of Natural Gas Using Chemical-Looping TechnologiesDocumento61 páginasHydrogen Production With Carbon Dioxide Capture by Reforming of Natural Gas Using Chemical-Looping TechnologiesFlorencia CapurroAinda não há avaliações
- Chestnut Oak Shells Activated CarbonDocumento41 páginasChestnut Oak Shells Activated CarbonVũ Văn NguyênAinda não há avaliações
- Deutschmann NatGasCS01Documento8 páginasDeutschmann NatGasCS01vazzoleralex6884Ainda não há avaliações
- Mto AdsorptionDocumento2 páginasMto AdsorptionnandiniAinda não há avaliações
- Journal Pre-Proof: Journal of Analytical and Applied PyrolysisDocumento90 páginasJournal Pre-Proof: Journal of Analytical and Applied PyrolysisMadhanAinda não há avaliações
- Catalysis and Catalytic EngineeringDocumento20 páginasCatalysis and Catalytic Engineeringhala hayderAinda não há avaliações
- Torres - FoodChem Week 11 To 13Documento1 páginaTorres - FoodChem Week 11 To 13john buenafeAinda não há avaliações
- Polyethylenimine Applications in Carbon Dioxide Capture and Separation: From Theoretical Study To Experimental WorkDocumento12 páginasPolyethylenimine Applications in Carbon Dioxide Capture and Separation: From Theoretical Study To Experimental WorkFrida Octavia PurnomoAinda não há avaliações
- Design of Fixed Bed Adsorption Columns: CENG 4710 Environmental ControlDocumento17 páginasDesign of Fixed Bed Adsorption Columns: CENG 4710 Environmental ControlchetanAinda não há avaliações
- PSA 50 Paper PDFDocumento5 páginasPSA 50 Paper PDFshashi kant kumarAinda não há avaliações
- TDS of AxSorb DDocumento1 páginaTDS of AxSorb DJohan KhaeriAinda não há avaliações
- Removal of Benzene by Vapor StrippingDocumento18 páginasRemoval of Benzene by Vapor StrippingMichał KisielewskiAinda não há avaliações
- Advance Separation Techniques: Pressure Swing AdsorptionDocumento26 páginasAdvance Separation Techniques: Pressure Swing AdsorptionJaykumar Bhupendrabhai PatelAinda não há avaliações
- Packed Bed ReactorDocumento6 páginasPacked Bed ReactorCik Tiem Ngagiman89% (9)
- Triblock Copolymer Syntheses of Mesoporous Silica With Periodic 50 To 300 Angstrom PoresDocumento6 páginasTriblock Copolymer Syntheses of Mesoporous Silica With Periodic 50 To 300 Angstrom PoresAneesh KumarAinda não há avaliações
- CV Alan ChaffeeDocumento2 páginasCV Alan Chaffeefajar hadi ramadanaAinda não há avaliações
- Using Nanotechnology in Bleaching Vegetable OilsDocumento8 páginasUsing Nanotechnology in Bleaching Vegetable OilsLeonardo RestrepoAinda não há avaliações
- Sodium and Potassium Silicates Brochure Eng Oct 2004 PDFDocumento16 páginasSodium and Potassium Silicates Brochure Eng Oct 2004 PDFandrei12320003181Ainda não há avaliações
- PrathibhaDocumento120 páginasPrathibhap.eswaraiahAinda não há avaliações
- Zinc Removal by Iron Nanoparticle Synthesized Using Mangifera Indica Seed Kernel As Capping AgentDocumento70 páginasZinc Removal by Iron Nanoparticle Synthesized Using Mangifera Indica Seed Kernel As Capping AgentEJ TanAinda não há avaliações
- Multifunctional Carbon Fibers From Chemical Upcycling of Mask WasteDocumento10 páginasMultifunctional Carbon Fibers From Chemical Upcycling of Mask WasteBarath SAinda não há avaliações
- CASE STUDY 1 - Catalytic Oxidation of SO2Documento15 páginasCASE STUDY 1 - Catalytic Oxidation of SO2LuthandoAinda não há avaliações
- Solar Cooling (Case Study)Documento146 páginasSolar Cooling (Case Study)2767141100% (2)
- Thesis Final PhD-ZairaDocumento421 páginasThesis Final PhD-ZairaArriane Faith A. EstoniloAinda não há avaliações
- Laboratory Investigation of Porosity and PermeabilDocumento7 páginasLaboratory Investigation of Porosity and PermeabilSanjay singhAinda não há avaliações
- Surface Chemistry QuestionsDocumento2 páginasSurface Chemistry QuestionsSindhu VelayudhamAinda não há avaliações
- Fouling and Cleaning of Ultra Filtration MembranesDocumento22 páginasFouling and Cleaning of Ultra Filtration Membranesfatima ezzahra rafiAinda não há avaliações
- Advantages and Disadvantages of Techniques Used For WastewaterDocumento11 páginasAdvantages and Disadvantages of Techniques Used For Wastewaterafham zulhusmiAinda não há avaliações
- BAEC Annual Report 14-15 - PressDocumento161 páginasBAEC Annual Report 14-15 - PressSazzad Hossain LemonAinda não há avaliações